
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 09 August 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.931507
 Jiaxing Song1†
Jiaxing Song1† Zhou Yu2†
Zhou Yu2† Jian Wang3†
Jian Wang3† Xiaojun Luo4
Xiaojun Luo4 Jie Du5
Jie Du5 Zhengxuan Tian6
Zhengxuan Tian6 Shunyu Yang7
Shunyu Yang7 Weihua Xie8
Weihua Xie8 Yuqi Peng9
Yuqi Peng9 Jinlin Mu10
Jinlin Mu10 Wenjie Zi1
Wenjie Zi1 Shuchun Huang11*
Shuchun Huang11* Mei Yang12*
Mei Yang12*Background: This study aimed to investigate the clinical outcomes of endovascular treatment (EVT) for distal basilar artery occlusion (BAO) and compare them with the outcomes of standard medical treatment (SMT) in daily clinical practice.
Methods: Patients with distal BAO enrolled in the BASILAR study from January 2014 to May 2019 were included. Differences in clinical outcomes were analyzed using Pearson's chi-square test and multivariable logistic regression. Clinical outcomes were evaluated using the modified Rankin Scale (mRS) score at 90 days, the mortality at 90 days, and the occurrence of symptomatic intracranial hemorrhage within 48 h.
Results: Among the 267 patients with distal BAO (222 patients in the EVT group and 45 patients in the SMT group), compared with the SMT group, the EVT group was associated with a favorable outcome (mRS 0–3; 40.1 vs. 15.6%; aOR 5.44; 95% CI, 1.68–17.66; P = 0.005) and decreased mortality (44.6 vs. 71.1%, aOR 0.32, 95% CI, 0.13–0.77; P = 0.012). In the EVT group, multivariable analysis showed that the initial National Institutes of Health Stroke Scale (NIHSS) score and posterior circulation-Alberta Stroke Program Early CT Score (pc-ASPECTS) were associated with favorable functional outcomes and mortality.
Conclusion: Our study suggests that, compared with SMT, EVT is technically feasible and safe for patients with distal BAO.
Acute basilar artery occlusion (BAO) accounts for approximately 1% of all acute ischemic strokes (1, 2). Although the acute BAO incidence is low, it is associated with poor outcomes and high fatality rates (3). Endovascular treatment (EVT) is the standard therapy for acute ischemic stroke in the anterior circulation. Recently, therapy decisions regarding BAO were affected by negative results of several randomized controlled trials comparing standard medical therapy with EVT (3, 4). Although the effectiveness of EVT for acute BAO still lacks strong evidence, clinical neurologists are more likely to choose EVT for acute BAO under real-world conditions.
Our previous multicenter, prospective cohort study indicated that EVT significantly improves the prognosis of patients with BAO, relative to standard medical therapy alone (5). In addition, the ATTENTION Registry also indicated that EVT was associated with significantly better functional outcomes (6). However, the vascular anatomy, clinical presentation, and severity of neurological deficits in patients with BAO in that study were different from those in patients with anterior circulation strokes.
For descriptive purposes, BAO is defined as complete blood flow occlusion in the proximal, middle, or distal basilar artery segment (7). Usually, the anatomy, etiology, and collateral status differ by the affected basilar artery segment, and cerebrovascular accidents involving specific territories supplied by the basilar artery may result in characteristic clinical syndromes, notably the locked-in syndrome and top-of-the-basilar syndrome (2, 8). During EVT, distinct therapeutic strategies may be applied to BAO patients with different occlusion sites. Several studies have focused on the impact of different occlusion locations on ischemic strokes in the anterior circulation. However, only a few reported that the occlusion site is a prognostic factor for BAO; these comprised subgroup analyses and case reports (9, 10).
Thus far, the occlusion site of the distal basilar artery has rarely been the focus of research, and systematic studies or randomized clinical trials in patients with distal BAO undergoing EVT are still lacking. In this multicenter cohort study, we aimed to explore the relationship between the distal BAO location and the clinical prognosis in patients with EVT.
Patients were selected from the BASILAR registry. BASILAR was a nationwide cohort study between January 2014 and May 2019 that included 47 comprehensive stroke centers from 15 provinces in China. All stroke centers were obliged to enroll consecutive patients to avoid selection bias. All participants or their authorized legal representatives provided written informed consent. BASILAR was registered in the Chinese Clinical Trial Registry (https://www.chictr.org.cn; ChiCTR1800014759).
Inclusion criteria were as follows: (1) age ≥18 years; (2) presentation within 24 h of the estimated time of BAO; (3) BAO confirmed by computed tomographic angiography (CTA), magnetic resonance angiography (MRA), or digital subtraction angiography (DSA); (4) intravenous recombinant tissue plasminogen activator (rt-PA) treatment initiation within 4.5 h or intravenous urokinase within 6.0 h of the estimated time of BAO; (5) occlusion site in the distal basilar artery [i.e., between the top of the basilar artery to the superior cerebellar artery (SCA)]; and (6) ability to provide informed consent. For the EVT group, EVT had to be initiated within 24 h of the estimated time of BAO. The exclusion criteria were as follows: (1) a clinically significant preexisting disability with a modified Rankin scale (mRS) score of >2; (2) neuroimaging evidence of cerebral hemorrhage on presentation; (3) lack of follow-up information on outcomes at 90 days; (4) current pregnancy or lactation; (5) a serious, advanced, or terminal illness; and (6) incomplete baseline imaging and time-metric data.
Patients with distal BAO were divided into two groups, namely, standard medical treatment group (SMT group) and EVT plus SMT group (EVT group). Patients in the SMT group received SMT as described in the guidelines for the management of acute ischemic stroke (antiplatelet drugs, anticoagulation drugs, intravenous thrombolysis therapy, or their combinations). In the EVT group, patients underwent SMT plus EVT, including mechanical thrombectomy therapies (stent retrievers, thrombo-aspiration, balloon angioplasty, intra-arterial thrombolysis, or their combinations).
Baseline characteristics, stroke risk factors, laboratory data, estimated time of BAO, imaging performance, and functional outcomes at 90 days were recorded (Tables 1, 2).
The causative mechanism of stroke was assessed based on the Trial of ORG10172 in Acute Stroke Treatment (TOAST) classification (11). The National Institutes of Health Stroke Scale (NIHSS) was used to assess neurological deficits at the time of treatment, with a higher NIHSS score indicating more severe neurological disability. The posterior circulation Alberta Stroke Program Early Computed Tomography Score (pc-ASPECTS; range, 0–10, a lower score indicating more extensive ischemia) was used to quantify ischemic changes on baseline images. The collateral circulation status was assessed using the posterior circulation-collateral score (PC-CS). The modified thrombolysis in cerebral infarction (mTICI), wherein a grade of 2b or 3 indicates successful reperfusion after EVT, was also used. Based on angiographic findings (CTA, MRA, or DSA), we classified the occlusion site into the distal basilar artery (distal to the SCA), middle basilar artery (from the anterior inferior cerebellar artery (AICA) to the SCA), and proximal basilar artery (from the vertebrobasilar junction to the AICA) (7).
The primary clinical efficacy outcome was a favorable outcome, defined as an mRS score (range, 0–6, with 0 indicating no disability, 3 indicating moderate disability, and 6 indicating death) of 0–3 at 90 days. The secondary clinical efficacy outcome was the rate of functional outcomes, defined by an mRS score of 0 to 2 or 0 to 1 (indicating an ability to walk unassisted) at 90 days.
Safety outcomes included symptomatic intracerebral hemorrhage (sICH) within 48 h as confirmed by neuroimaging (CT or MRI) and mortality at 90 days. sICH was defined according to the Heidelberg Bleeding Classification (hemorrhagic transformation of infarcted brain tissue, intracerebral hemorrhage both within and outside the infarcted brain tissue, intracerebral hemorrhage outside the infarcted brain tissue, or intracranial extracerebral hemorrhage, and an increase of ≥4 points in the NIHSS score or an increase of ≥2 points in one of the 11 NIHSS subcategories).
We compared baseline characteristics and outcomes between the SMT and EVT groups. Categorical variables were described as proportions and compared using the χ2 test or Fisher's exact test; continuous variables were described as mean (SD) or median (IQR) and compared using the t-test or equivalent nonparametric tests.
To adjust for confounders, we used a multivariable logistic regression analysis model and entered covariates such as age, sex, atrial fibrillation, IVT, NIHSS score, pc-ASPECTS, collateral status, and stroke etiology. Odds ratios (ORs) were calculated with 95% confidence intervals (CIs) to indicate statistical precision. To generate margin effect curves related to the treatment modalities, outcome-specific predicted probabilities for continuous age values were computed by setting other variables in the model to their mean values. The Kaplan-Meier method and log-rank test were used to analyze the mortality.
For propensity score matching analyses, we performed 1:2 matching based on the nearest-neighbor matching algorithm with a caliper width of 0.2 of the propensity score with age and intravenous thrombolysis as covariates (12). Furthermore, supportive analyses used the propensity score and computed based on multivariable regression models accounting for additional explanatory variables were performed. The significance level was set to P < 0.05, and all tests of the hypotheses were two-sided. Since we excluded patients with missing essential data from our analysis, we did not impute for missing data. Statistical analyses were performed using SPSS (version 26.0; IBM, Armonk, NY, USA) and R version 4.1.0 (R packages: tableone, MatchIt, ggeffects, forestplot, ggplot2, survminer, R Foundation for Statistical Computing, Vienna, Austria).
In total, 829 patients with acute ischemic stroke at our study between January 2014 and May 2019 were included. The proximal basilar artery was affected in 121 patients, the middle basilar artery in 295 patients, and the vertebral artery-V4 segment in 146 patients. Therefore, the distal BAO cohort comprised 267 patients, with 45 and 222 patients in the SMT and EVT groups, respectively (Supplementary Figure I).
The baseline characteristics are summarized in Table 1. The median age was 69 years (IQR, 59–76), and 96 patients (36.0%) were women. The median NIHSS score was 29 (IQR, 20–34), with no between-group difference. Patients in the EVT group had a pc-ASPECTS [7 (IQR, 6–9) vs. 8 (IQR, 7–10); P = 0.02]. There was no imbalance in time intervals between groups. Other baseline characteristics were balanced between groups.
The study outcomes are presented in Figure 1A and Table 2. Figure 1A shows the distribution of mRS scores at 90 days in the two groups. In univariable analysis, 89 of 222 patients (40.1%) in the EVT group and seven of 45 patients (15.6%) in the SMT group had a favorable functional outcome (P = 0.003). Mortality at 90 days was 44.6% in the EVT group and 71.1% significantly higher in the SMT group (P = 0.002). The rate of symptomatic intracranial hemorrhage was 6.9% in the EVT group but 0% in the SMT group (P = 0.002). In addition, there was a significant difference in survival rate between patients with EVT and SMT during the follow-up of 1 year (Figure 2). In the multivariable analysis with adjusted confounders, EVT was found to be significantly positively correlated with favorable functional outcomes (mRS score 0–3 at 90 days; aOR, 5.44; 95% CI, 1.68–17.66; P = 0.005). Likewise, EVT was positively correlated with good functional outcomes (mRS score 0–2 at 90 days; aOR, 3.69; 95% CI, 1.12–12.09; P = 0.031). Additionally, EVT was found to be negatively correlated with mortality (aOR, 0.32; 95% CI, 0.13–0.77; P = 0.012; Table 2). Among patients in both EVT and SMT groups, the NIHSS score and pc-ASPECTS were independently associated with the clinical outcomes. The predicted probability curves of clinical outcomes by NIHSS score and pc-ASPECTS in patients with distal BAO and EVT are shown in Figure 3.
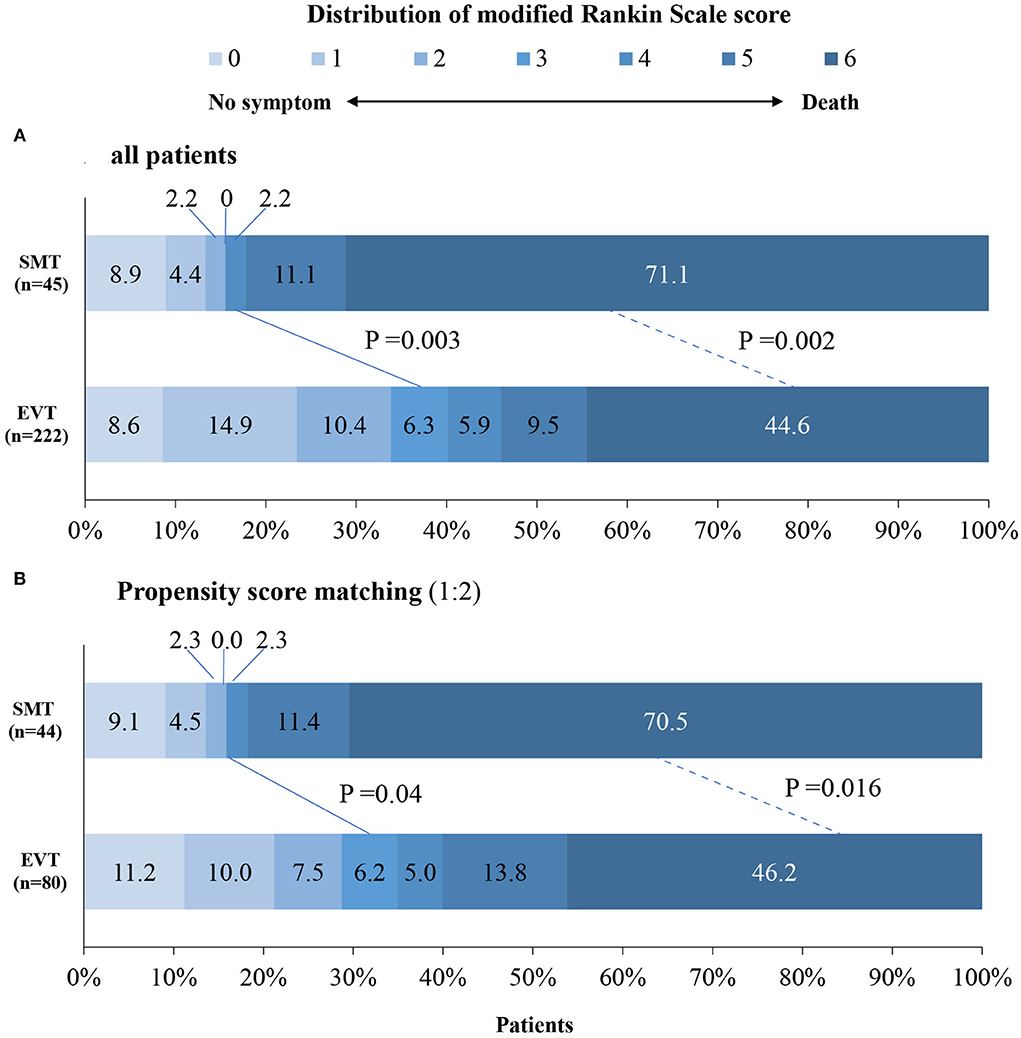
Figure 1. Distribution of modified Rankin scale scores at day 90 in patients with distal BAO. The distribution of the modified Rankin scale scores at day 90 in patients with distal BAO is shown. Clinical outcomes at day 90 follow-up in patients in the SMT group vs. those of the EVT group (A) and clinical outcomes at day 90 follow-up after propensity score matching (B). BAO, basilar artery occlusion; EVT, endovascular treatment; SMT, standard medical treatment.
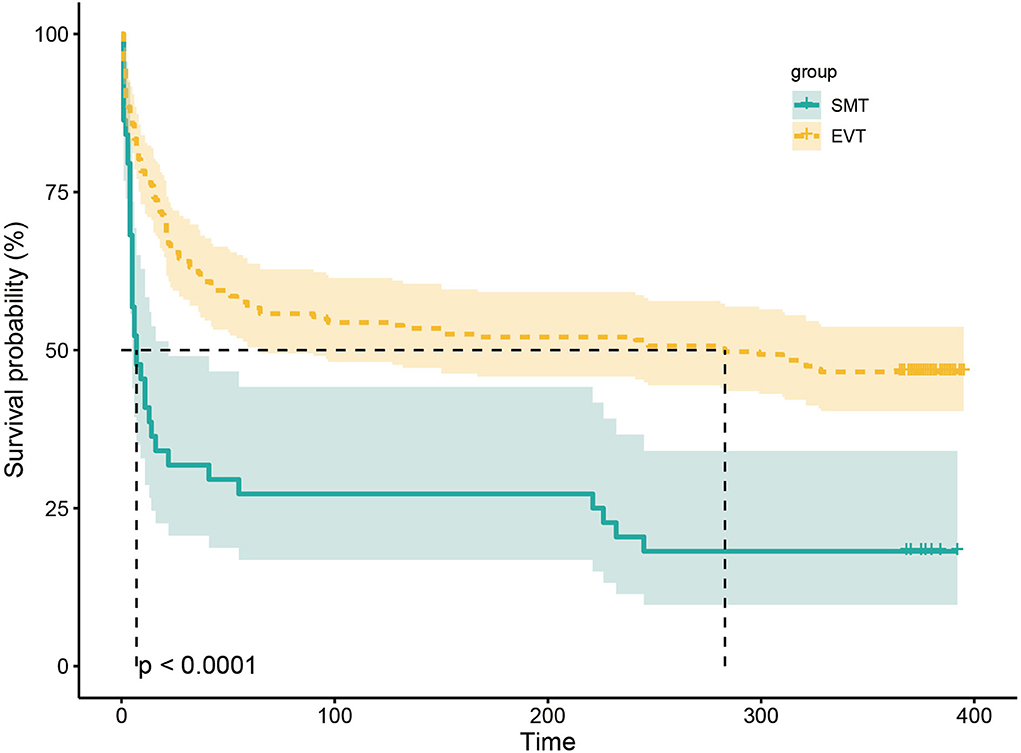
Figure 2. Kaplan–Meier curve estimates of the probability of death during the 1-year follow-up. The probability of death during the 1-year follow-up in SMT and EVT groups. SMT, standard medical treatment; EVT, endovascular treatment.
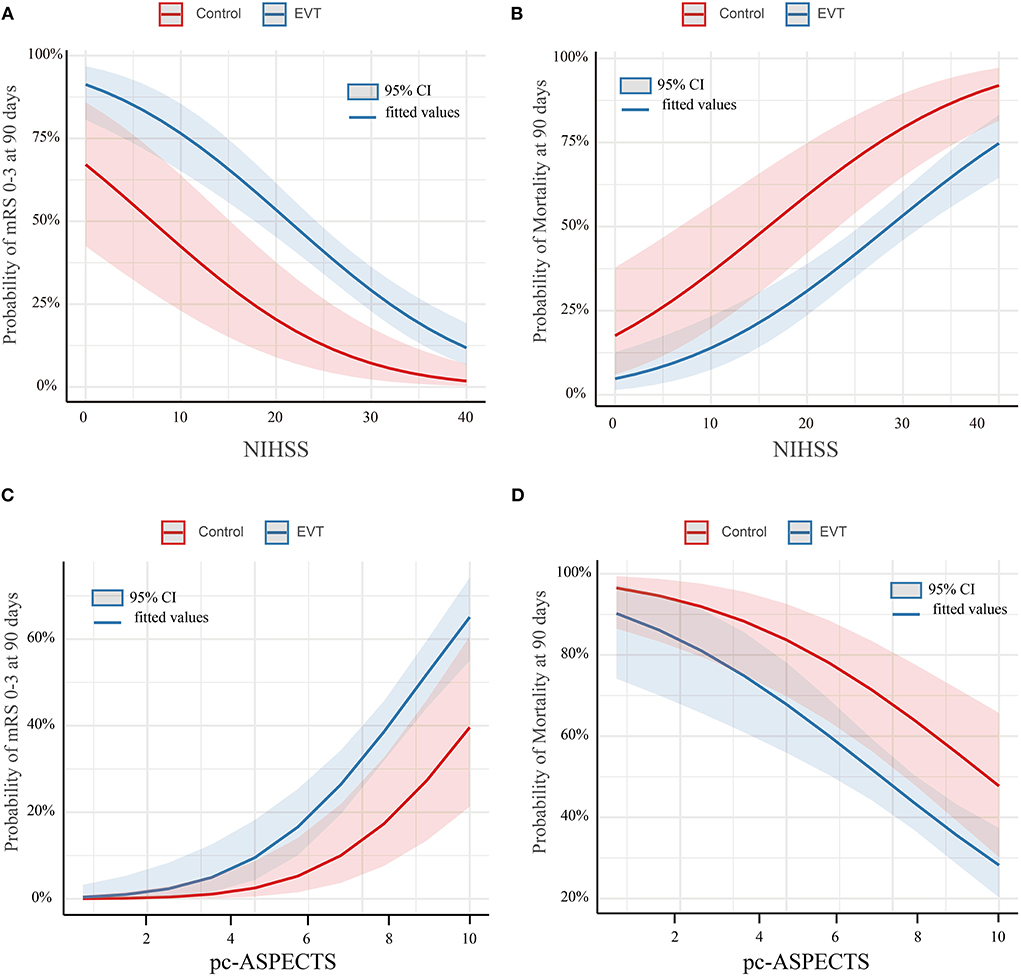
Figure 3. Predicted probability of clinical outcomes by NIHSS score and pc-ASPECTS in patients with distal BAO. Predicted probability of a favorable outcome (A) and mortality (B) by NIHSS in patients with distal BAO and the predicted probability of a favorable outcome (C) and mortality (D) by pc-ASPECTS in patients with distal BAO. Curves showed that among patients with distal BAO, the EVT group showed a higher predicted rate of favorable functional outcome and a lower predicted rate of mortality than the SMT group. Shading indicates the 95% confidence interval. BAO, basilar artery occlusion; EVT, endovascular treatment; NIHSS, National Institutes of Health Stroke Scale; pc-ASPECTS, posterior circulation-Alberta Stroke Program Early CT Score; SMT, standard medical treatment.
After 1:1 propensity score matching, the baseline characteristics of the two groups achieved a good balance, and the details are presented in Table 1 and Supplementary Methods. The proportion of favorable functional outcomes was significantly higher in the EVT group (35.0 vs. 15.9%; P = 0.04). Mortality at 90 days occurred in 37 of 80 patients (46.2%) in the EVT group and 31 of 44 patients (70.5%) in the SMT group (P = 0.016). The rate of symptomatic intracerebral hemorrhage was 6.2% (5 of 80 patients) in the EVT group and 0% in the SMT group (0 of 44 patients; P = 0.224). The results are shown in Figure 1B and Table 2. A subgroup analysis was performed to investigate the relationship between the two groups and 90-day functional outcomes (mRS score 0–3). The cutoff values and subgroup categories were based on the median values, including those based on age, sex, NIHSS score, diabetes mellitus, pc-ASPECTS, and intravenous thrombolysis. The results showed no significant heterogeneity across any of the prespecified subgroups (Figure 4).
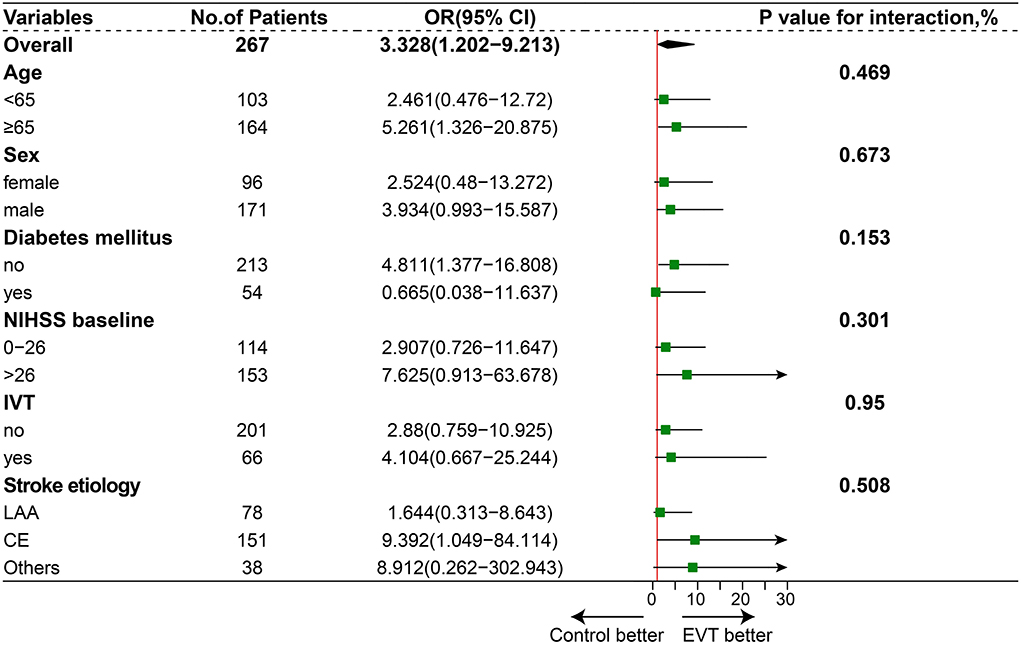
Figure 4. Subgroup analysis. The forest plot shows the difference in odds ratio (OR) for favorable outcomes (defined as a modified Rankin scale score of 0–3) at day 90 in the subgroup. Adjusted variables were age, sex, National Institutes of Health Stroke Scale (NIHSS) score, diabetes mellitus (DM), and intravenous thrombolysis (IVT), stroke etiology.
To identify the predictors of outcome in the EVT group, 222 patients with distal BAO were dichotomized by functional outcome (favorable vs. poor; Supplementary Table I) and mortality (living vs. dead) to determine the factors to be adjusted. In the multivariable analysis with adjusted confounders, baseline NIHSS score (aOR, 0.91; 95% CI, 0.87–0.95; P < 0.001), baseline pc-ASPECTS (aOR, 1.65; 95% CI, 1.30–2.08; P < 0.001), Pneumonia (aOR, 0.38; 95% CI, 0.17–0.85; P = 0.019), and PTR (aOR, 0.99; 95% CI, 0.98–1.00; P = 0.039) were associated with favorable functional outcome. Additionally, baseline SBP (aOR, 1.01; 95% CI, 1.00–1.03; P = 0.036), baseline NIHSS score (aOR, 1.11; 95% CI, 1.06–1.16; P < 0.001), baseline pc-ASPECTS (aOR, 0.76; 95% CI, 0.63–0.91; P = 0.003), PTR (aOR, 1.01; 95% CI, 1.003–1.017; P = 0.039), and successful reperfusion (aOR, 0.29; 95% CI, 0.11–0.78; P = 0.014) were associated with mortality (Table 3).
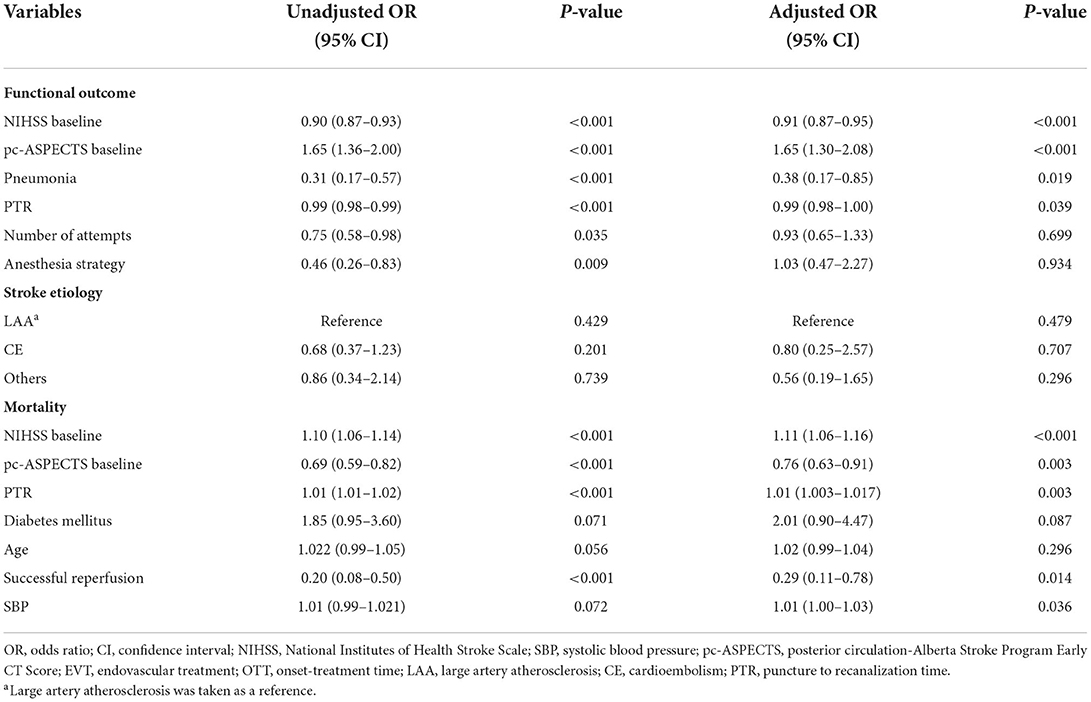
Table 3. Multivariable analysis: predictors of clinical outcome (mRS 0–3, mortality) in the EVT group.
To the best of our knowledge, this study is the first to determine the impact of distal BAO on patient prognosis. We investigated the efficacy and safety of EVT compared with those of SMT in patients with distal BAO. The outcomes were significantly different between patients who received EVT for distal BAO initiated within 24 h after stroke onset and those who received only medical therapy.
The incidence of a favorable outcome in patients with distal BAO was higher in the EVT group than in the SMT group. Mortality and symptomatic intracranial hemorrhage rates did not differ between groups. Moreover, after propensity score matching, the results still indicated that endovascular treatment is technically feasible and safe for distal BAO. In line with our findings, previous studies have reported favorable outcomes for additional EVT in patients with distal BAO (13, 14).
Thus, our study demonstrates the benefit of endovascular treatment for distal BAO. From the perspective of anatomical characteristics, the basilar artery, which is the main vessel of the posterior circulation, supplies most of the brainstem and occipital lobes, as well as parts of the cerebellum and thalamus (2). An ischemic stroke occurring at the top of the basilar artery often causes infarction of the midbrain, thalamus, and portions of the temporal and occipital lobes supplied by the posterior communicating and posterior cerebral branches of the basilar artery. These territories regulate the consciousness level, vision, and other functions (7, 8), and patients with distal BAO can show various clinical signs and symptoms, including consciousness disorder, hemianopia, oculomotor disorders, and behavioral abnormalities rather than prominent motor dysfunction. In addition, the corticospinal tracts of these patients usually remain intact (8, 15). Therefore, patients may achieve better therapeutic outcomes with additional EVT of the distal basilar artery than with SMT alone.
As reported previously, the stroke mechanism has a major influence on favorable outcomes. Similar to ischemic stroke, BAO can be caused by various stroke mechanisms. The two most common mechanisms underlying BAO are cardioembolism and large artery atherosclerosis (16, 17). Some studies have shown that atherosclerosis often leads to occlusion of the proximal and middle segments of the basilar artery, whereas embolic BAO often manifests in the distal segment (2, 18, 19). It is easy to conceive that a cardiac embolus leads to acute BAO, resulting in the sudden onset of neurological deficits. In previous studies, large artery atherosclerosis was found to be more frequent in patients with progressive presentations (2, 17). Thus, in distal BAO caused by emboli, patients often have abrupt courses without premonitory symptoms and are more likely to arrive at the hospital in time; this offers a better therapeutic time window.
Patients with large artery atherosclerosis often have poor clinical outcomes following EVT (18). Patients with atherosclerosis require a longer procedural time than those with emboli (20). Moreover, atherosclerotic lesions, such as plaque rupture, endothelial damage, or irritation, and local platelet activation-prone situations frequently cause acute arterial reocclusion after EVT (21, 22); this reocclusion requires repetitive thrombectomy procedures and additional rescue treatments. In contrast, the proportion of reocclusion in the embolism group is lower than that in the atherosclerosis group due to mild or no arterial wall injury, less damage to perforating branches, and reduced thrombectomy time. Similar to previous findings, our results suggest that patients with distal occlusion have a more favorable outcome following EVT (18, 23, 24).
Recently, a subgroup analysis of the Basilar Artery International Cooperation Study (BASICS) trial showed that EVT has no treatment benefit compared with SMT alone for distal BAO (3). First, in the SMT group, the median NIHSS score was 27 in our study, whereas this score was 22 in the BASICS trial, suggesting that the overall disease severity was lower in the BASICS trial. Second, the proportion of intravenous thrombolysis was 20% in our study and approximately 80% in the BASICS trial. In this case, in addition to antiplatelet or anticoagulant therapy, patients can receive more intravenous thrombolysis therapy, which may result in better clinical efficacy in the medical care group and abolish the differences between the two groups in terms of the prognosis. Third, since recruitment was lesser than anticipated, the BASICS trial was underpowered for some analyses, including this subgroup analysis. Finally, clinical patterns and healthcare policy decision-making differ between developed and developing countries; developing countries have a higher stroke burden and more limited healthcare resources.
Our study has several limitations. First was the nonrandomized design. Second, treatment allocation in the participating centers was based on medical conditions at local hospitals and individual decisions of the treating physicians; therefore, bias cannot be excluded. Moreover, the treatment groups were not balanced. Third, propensity score matching or multivariable analyses could not adjust completely for systematic differences between treatment groups, which is the aim of randomization in clinical trials. Therefore, we suggested that a randomized controlled trial in patients with acute BAO is of high priority. However, our study provides a good representation of daily clinical practice for patients with acute distal BAO, and despite its limitations, it still provides one of the best available datasets regarding the outcomes of distal BAO treatment.
Our study suggests that, compared with SMT, EVT is technically feasible and safe for distal BAO. In the future, the efficacy of EVT compared with that of SMT for distal BAO needs to be assessed in randomized controlled trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Xinqiao Hospital Affiliated to Army Medical University. ID: 201308701. The patients/participants provided their written informed consent to participate in this study.
SH, MY, and JS contributed to the conception and design of this study. JS, ZY, JW, XL, JD, ZT, SY, WX, YP, JM, and WZ contributed to the acquisition and analysis of data. JS and ZY contributed to drafting the text or preparing the figures. All authors critically reviewed and approved the manuscript.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this study. This study was supported by the National Natural Science Foundation of China (No. 82071323), the Chongqing Natural Science Foundation (cstc2020jcyj-msxmX0926), the Army Medical University Clinical Medical Research Talent Training Program (2019XLC2008), and the Clinical Medical Research Talents Training Program of Army Military Medical University (2018XLC1005).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.931507/full#supplementary-material
1. Meinel TR, Kaesmacher J, Chaloulos-Iakovidis P, Panos L, Mordasini P, Mosimann PJ, et al. Mechanical thrombectomy for basilar artery occlusion: efficacy, outcomes, and futile recanalization in comparison with the anterior circulation. J Neurointerv Surg. (2019) 11:1174–80. doi: 10.1136/neurintsurg-2018-014516
2. Mattle HP, Arnold M, Lindsberg PJ, Schonewille WJ, Schroth G. Basilar artery occlusion. Lancet Neurol. (2011) 10:1002–14. doi: 10.1016/S1474-4422(11)70229-0
3. Langezaal LCM, van der Hoeven E., Mont'Alverne FJA, de Carvalho JJF, Lima FO, Dippel DWJ, et al. Endovascular therapy for stroke due to basilar-artery occlusion. N Engl J Med. (2021) 384:1910–20. doi: 10.1056/NEJMoa2030297
4. Liu X, Dai Q, Ye R, Zi W, Liu Y, Wang H, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. (2020) 19:115–22. doi: 10.1016/S1474-4422(19)30395-3
5. Zi W, Qiu Z, Wu D, Li F, Liu H, Liu W, et al. Assessment of endovascular treatment for acute basilar artery occlusion via a nationwide prospective registry. JAMA Neurol. (2020) 77:561–73. doi: 10.1001/jamaneurol.2020.0156
6. Tao C, Qureshi AI, Yin Y, Li J, Li R, Xu P, et al. Endovascular treatment versus best medical management in acute basilar artery occlusion strokes: results from the ATTENTION multicenter registry. Circulation. (2022) 146:6–17. doi: 10.1161/CIRCULATIONAHA.121.058544
7. Archer CR, Horenstein S. Basilar artery occlusion: clinical and radiological correlation. Stroke. (1977) 8:383–90. doi: 10.1161/01.STR.8.3.383
9. Kwak HS, Park JS. Mechanical thrombectomy in basilar artery occlusion: clinical outcomes related to posterior circulation collateral score. Stroke. (2020) 51:2045–50. doi: 10.1161/STROKEAHA.120.029861
10. Wu L, Rajah GB, Cosky EE, Wu X, Li C, Chen J, et al. Outcomes in endovascular therapy for basilar artery occlusion: intracranial atherosclerotic disease vs. embolism Aging Dis. (2021) 12:404–14. doi: 10.14336/AD.2020.0704
11. Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
12. Kurth T, Walker AM, Glynn RJ, Chan KA, Gaziano JM, Berger K, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. (2006) 163:262–70. doi: 10.1093/aje/kwj047
13. Jung S, Mono ML, Fischer U, Galimanis A, Findling O, De Marchis GM, et al. Three-month and long-term outcomes and their predictors in acute basilar artery occlusion treated with intra-arterial thrombolysis. Stroke. (2011) 42:1946–51. doi: 10.1161/STROKEAHA.110.606038
14. Cross DT 3rd, Moran CJ, Akins PT, Angtuaco EE, Derdeyn CP, Diringer MN. Collateral circulation and outcome after basilar artery thrombolysis. AJNR Am J Neuroradiol. (1998) 19:1557–63.
15. Sparaco M, Ciolli L, Zini A. Posterior circulation ischaemic stroke-a review part I: anatomy, aetiology and clinical presentations. Neurol Sci. (2019) 40:1995–2006. doi: 10.1007/s10072-019-03977-2
16. Schonewille WJ, Wijman CA, Michel P, Rueckert CM, Weimar C, Mattle HP, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): a prospective registry study. Lancet Neurol. (2009) 8:724–30. doi: 10.1016/S1474-4422(09)70173-5
17. Buchman SL, Merkler AE. Basilar artery occlusion: diagnosis and acute treatment. Curr Treat Options Neurol. (2019) 21:45. doi: 10.1007/s11940-019-0591-0
18. Baik SH, Park HJ, Kim JH, Jang CK, Kim BM, Kim DJ. Mechanical thrombectomy in subtypes of basilar artery occlusion: relationship to recanalization rate and clinical outcome. Radiology. (2019) 291:730–7. doi: 10.1148/radiol.2019181924
19. Lee YY, Yoon W, Kim SK, Baek BH, Kim GS, Kim JT, et al. Acute basilar artery occlusion: differences in characteristics and outcomes after endovascular therapy between patients with and without underlying severe atherosclerotic stenosis. AJNR Am J Neuroradiol. (2017) 38:1600–4. doi: 10.3174/ajnr.A5233
20. Kim YW, Hong JM, Park DG, Choi JW, Kang DH, Kim YS, et al. Effect of intracranial atherosclerotic disease on endovascular treatment for patients with acute vertebrobasilar occlusion. AJNR Am J Neuroradiol. (2016) 37:2072–8. doi: 10.3174/ajnr.A4844
21. Kang DH, Kim YW, Hwang YH, Park SP, Kim YS, Baik SK. Instant reocclusion following mechanical thrombectomy of in situ thromboocclusion and the role of low-dose intra-arterial tirofiban. Cerebrovasc Dis. (2014) 37:350–5. doi: 10.1159/000362435
22. Bang OY. Intracranial atherosclerosis: current understanding and perspectives. J Stroke. (2014) 16:27–35. doi: 10.5853/jos.2014.16.1.27
23. Mehler MF. The rostral basilar artery syndrome: diagnosis, etiology, prognosis. Neurology. (1989) 39:9–16. doi: 10.1212/WNL.39.1.9
Keywords: stroke, distal basilar artery occlusion, endovascular treatment (EVT), occlusion site, acute basilar artery occlusion
Citation: Song J, Yu Z, Wang J, Luo X, Du J, Tian Z, Yang S, Xie W, Peng Y, Mu J, Zi W, Huang S and Yang M (2022) Endovascular treatment for distal basilar artery occlusion stroke. Front. Neurol. 13:931507. doi: 10.3389/fneur.2022.931507
Received: 29 April 2022; Accepted: 12 July 2022;
Published: 09 August 2022.
Edited by:
Pierre Seners, Fondation Ophtalmologique Adolphe de Rothschild, FranceReviewed by:
Chao Zhou, University of Pittsburgh, United StatesCopyright © 2022 Song, Yu, Wang, Luo, Du, Tian, Yang, Xie, Peng, Mu, Zi, Huang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuchun Huang, OTc2MDc5NjYzQHFxLmNvbQ==; Mei Yang, ZGx6Y3p6a3p4QDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.