- 1Department of Neurology, Osaka Neurological Institute, Toyonaka, Japan
- 2Department of Neurology, Kyoto Prefectural Rehabilitation Hospital for the Disabled, Nakaashihara, Kyoto, Japan
- 3Department of Neurology, Itsuki Hospital, Tokushima, Japan
- 4Department of Pharmacology, School of Pharmacy and Pharmaceutical Sciences, Mukogawa Women's University, Hyogo, Japan
- 5Department of Clinical Neuroscience, Institute of Biomedical Sciences, Tokushima University, Tokushima, Japan
- 6Center for Drug Discovery and Development Sciences, Research Organization of Science and Technology, Ritsumeikan University, Kyoto, Japan
Impairment of balanced activity between dopamine D1 and D2 receptor functions in the striatum, particularly in striatal functional subdivisions (i.e., striosome and matrix compartments), has been proposed to underlie dystonia genesis. This study was undertaken to examine the therapeutic effect of dual dopaminergic modulation with L-3,4-dihydroxyphenylalanine (L-DOPA) and chlorpromazine (CPZ) in patients with blepharospasm, a focal dystonia. For this purpose, Dopacol tablets™ (L-DOPA 50 mg plus carbidopa 5 mg) and Wintermin™ (CPZ phenolphthalinate 180 mg/g) were used. Clinical evaluations were performed before and after an 8-week drug treatment interval using the Visual Analog Scale (VAS), Blepharospasm Disability Index (BSDI), modified VAS (mVAS), and Jankovic Rating Scale (JRS). The data were analyzed using non-parametric statistics. Results showed that in patients (n = 7) with blepharospasm, dystonia symptoms were significantly alleviated by the administration of both Dopacol tablets™ (one tablet × 3/day) and CPZ (5 mg × 3/day), as determined using the VAS, BSDI, mVAS, and JRS. In contrast, there was no improvement of dystonia symptoms in patients (n = 7) who ingested Dopacol tablets™ (one tablet × 3/day) alone, nor in those (n = 7) who ingested CPZ (5 mg × 3/day) alone. Thus, dual pharmacotherapy with L-DOPA and CPZ can exert a therapeutic effect on blepharospasm, suggesting that dystonia symptoms can be attenuated through dopaminergic modulation with inducing an increase in striatal D1-signals. Since dopamine D1 receptors are heavily enriched in the striosome compartment in the “human” striatum, our results also suggest that striosomal loss of D1-signaling may be important in the pathogenesis of dystonia.
Introduction
Blepharospasm is the most frequent phenotype of focal dystonia in adults and manifests as excessive blinking and spasms of the eyes (1–3). The reported prevalence of blepharospasm ranges from 20 to 133 cases per million individuals (4).
The symptoms of blepharospasm are often severe enough to result in functional blindness (5–7). Botulinum toxin (BTX) injection into the orbicularis oculi muscles is now considered as the first-line treatment for blepharospasm (8); however, it often produces unsatisfactory results (9, 10). Administration of anticholinergics, benzodiazepines, baclofen, or tetrabenazine can also be therapeutic options (11), but these frequently cause serious side effects as well as failure of therapy (12, 13). The currently available oral pharmacotherapy can be limited by the common occurrence of adverse effects, which contribute to a decrease in compliance or discontinuation even before benefits are evident (11). Therefore, the development of alternative or adjunct pharmacotherapy for the treatment of blepharospasm is required (3, 11).
Impairment of balanced activity between dopamine D1- and D2-like receptor (D1R and D2R) functions in the striatum, which consists of two functional subdivisions called the striosome (patch) and matrix compartments (14), has been proposed to underlie dystonia genesis (15–18). The striosome and matrix dopamine systems play central roles in cortico-thalamo-basal ganglia circuits and are thought to underlie the genesis of multiple movement and behavioral disorders (18–20). A recent modular computational model of the basal ganglia network suggested that striosomal dysfunction may induce inappropriate “motor action selection” and promote specific repetitive, stereotyped behaviors, including dystonia symptoms (21). This hypothesis is supported by the functional anatomy observed in several human disease models. For instance, mutations in the GNAL gene, which encodes the stimulatory α subunit of the G-protein (Gαolf), cause primary torsion dystonia (22), known as DYT25 dystonia (Figure 1, DYT25). Gαolf is highly expressed in the striatum (23, 24), where it couples D1Rs in direct pathway medium spiny neurons (MSNs) and adenosine A2A receptors in indirect pathway MSNs to increase 3′,5′-cyclic AMP (cAMP) through activation of adenylyl cyclase type 5 (23, 24). This indicates that in DYT25, loss of Gαolf function induces a decrease in the cAMP level in both the D1-direct and D2-indirect pathway MSNs, leading to the reduced activity of D1Rs in direct pathway MSNs and enhanced activity of D2Rs in indirect pathway MSNs. Given that both D1Rs and Gαolf are highly concentrated in striosomes (18, 25), DYT25 dystonia also represents a loss of striosomal D1-signal activity. Furthermore, in patients with X-linked dystonia-parkinsonism, also known as DYT3 (Figure 1, DYT3), postmortem analyses revealed a predominant loss of D1R-expressing MSNs in the striosomes relative to the matrix compartment in the early disease phase when dystonia symptoms occur (15, 17). Thus, loss of D1-signaling in the striosome compartment may cause dystonia symptoms, at least in part, in dystonia syndrome.
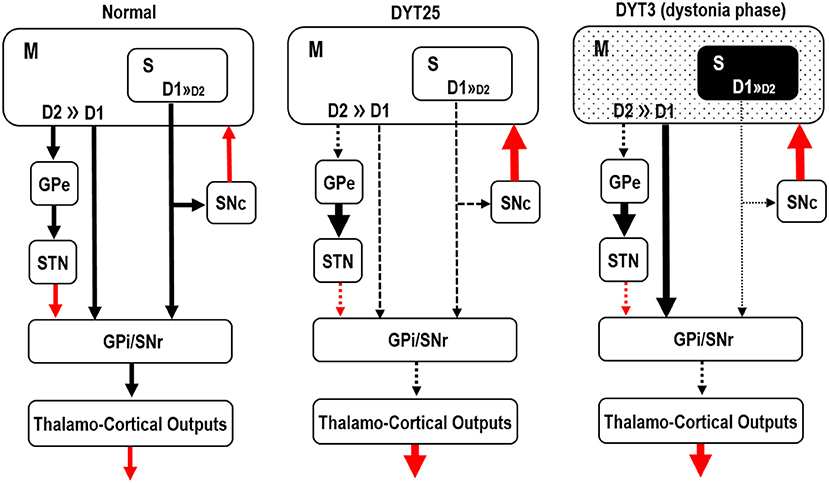
Figure 1. Hypothetical functional anatomy of the basal ganglia in DYT25 and DYT3. A schematic representation of the basal ganglia circuit is presented. Black and red arrows indicate inhibitory and excitatory striatal output projections, respectively. The striatum consists of two functional subdivisions: striosome (S) and matrix (M) compartments. Three major pathways are emphasized: the direct pathway originating from medium spiny striatal projection neurons (MSNs) possessing D1-receptors (D1), the indirect pathway from MSNs possessing D2-receptors (D2), and the striosomal pathway from the striosome (S) compartment, which is heavily enriched in D1-receptors. In DYT 25, mutations in the GNAL gene cause a loss of function of Gαolf, leading to reduced activity of D1-receptors in striosomal pathway MSNs. In DYT3, severe degeneration of striosomal MSNs that possess D1-receptors is found in the early disease phase when dystonia symptoms occur (dystonia phase). M, matrix compartment; S, striosome compartment; GPe, globus pallidus externa; STN, subthalamic nucleus; SNc, substantia nigra pars compacta; GPi, globus pallidus internus; SNr, substantia nigra pars reticulata.
This study was undertaken to examine whether dystonia symptoms can be attenuated through dopaminergic modulation, which induces an increase in striosomal D1-signaling. In line with our immunohistochemical studies on human autopsied brains, we have shown that in the neostriatum, D1R proteins are heavily enriched in striosomes, while these are modestly distributed in the matrix compartment (26). This indicates that when D1 agonists are administered orally, they preferentially act on the striosome compartment in the “human” striatum. Here, we sought to determine whether dual dopaminergic therapy with L-3,4-dihydroxyphenylalanine (L-DOPA) and chlorpromazine (CPZ) exerts a therapeutic effect on blepharospasm. L-DOPA is the direct precursor of dopamine (27), the full agonist of both D1Rs and D2Rs, whereas CPZ is an effective antagonist of D2Rs (28).
Subjects and methods
This randomized clinical trial was approved by the Institutional Ethics Committee. The study was registered with the International Committee of Medical Journal Editors recognized registry, the UMIN Clinical Trials Registry (number: UMIN00027430; date of permission: May 21, 2017).
Subjects
This study enrolled 21 patients with blepharospasms (six men and fifteen women) with an age range of 51–79 years (mean age average, 68.7 ± 7.8 years). Blepharospasm was diagnosed according to the criteria of Albanese et al. (2). Blepharospasm are characterized by focal involuntary contractions that interfere with physiological opening or closing of the eyelids, and those are caused by dystonic contractions of the orbicularis oculi often accompanied by contractions of the procerus and corrugator muscles. Onset is usually insidious, with eye irritation or dryness followed by excessive blinking, especially in bright light. All participants were examined by a single qualified neurologist, movement disorder specialist (S.M.) who performed general physical and neurological examinations to confirm the absence of neurological abnormalities other than blepharospasm. We carefully excluded the patients with dementia or apparent psychiatric disorders and/or those who taking medications that might affect dopamine signaling.
Brain magnetic resonance imaging and laboratory and genetic tests were performed to exclude hereditary and secondary dystonia. For genetic tests, we screened for pathogenic variants in known dystonia genes using whole-exome sequencing (OMIM Phenotypic Series PS128100).
All patients who had received BTX type A (BTX-A) treatment were instructed to undergo a 3-month washout period from the last BTX-A injection until the start of this clinical trial. In all of them, the medications except for L-DOPA and CPZ were unchanged from 3 months prior to the start of the clinical trial to the end of it. Video analyses were performed before and after the drug challenge tests (Figure 2).
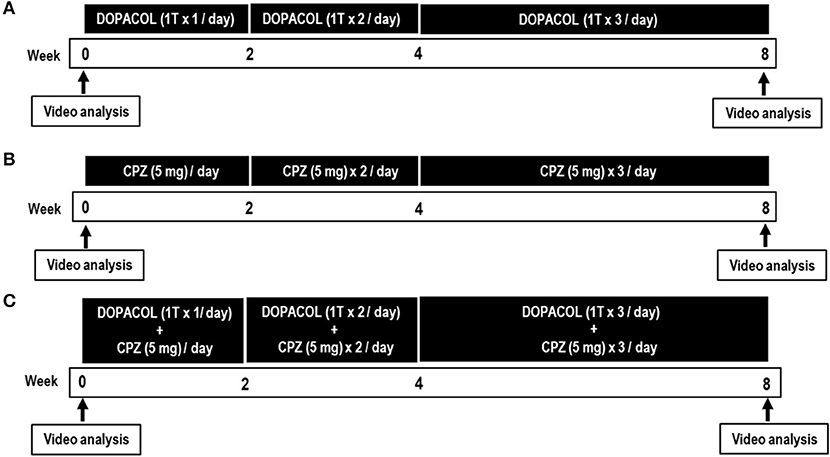
Figure 2. Timetable for the drug challenge tests with Dopacol tablets L50™ (DOPACOL) (A), chlorpromazine (CPZ) (B), and DOPACOL + CPZ (C). T, tablet. Clinical evaluations and video recordings were performed 1 day before (pre-treatment) and 8 weeks after (post-treatment) the initial administration of the drugs.
Drugs
We used Dopacol tablets L50™ (DOPACOL) (50 mg of L-DOPA plus 5 mg of carbidopa; Nichi-Iko Pharmaceutical Co., Ltd. Toyama, Japan) and Wintermin fine granules (10%; 180 mg of chlorpromazine phenolphthalinate per gram; Shionogi & Co., Ltd. Osaka, Japan).
Patient sorting and drug administration
There were 415 patients with blepharospasm who attended our hospital by the end of 2021. All of them had received BTX-A injection into the orbicularis oculi muscles as a first-line treatment, but some of them were refractory. Several studies have reported that some patients have not responded to BTX-A therapy (29–32), categorized as primary non-responders (33). In one study, 9.1 and 7.5% of patients were thought to have “primary resistance” and “secondary resistance,” respectively (30). Patients who poorly responded to BTX-A treatment were randomly assigned to the following three groups (L-DOPA, CPZ, and L-DOPA + CPZ groups) in the order of consent. In a double-blind fashion, participants and evaluators were not informed of the identification of each group.
Although we happened to have had unequal sex distribution among the groups after randomized patient sorting (Table 1), it is well documented that blepharospasm shows a female-to-male preponderance in prevalence, with a reported male-to-female ratio between 1:2 and 1:8 (4, 34, 35).
L-DOPA group
This group included seven patients with blepharospasms (three men and four women) who ingested DOPACOL alone (Figure 2A, Table 1). Their age range was 51–79 years (age average, 66.0 ± 10.1 years), and their mean disease duration was 10.7 ± 9.5 years. We prescribed DOPACOL (one tablet per day) for the first 2 weeks, DOPACOL (one tablet × 2/day) for the next 2 weeks, and DOPACOL (one tablet × 3/day) for the last 4 weeks.
CPZ group
This group included seven patients with blepharospasm (one man and six women) who ingested CPZ alone (Figure 2B, Table 1). Their age range was 60–72 years (age average, 66.4 ± 3.9 years), and their mean disease duration was 8.4 ± 5.9 years. We prescribed CPZ (5 mg/day) for the first 2 weeks, CPZ (5 mg × 2/day) for the next 2 weeks, and CPZ (5 mg × 3/day) for the last 4 weeks.
L-DOPA + CPZ group
This group included seven patients with blepharospasms (two men and five women) who ingested both DOPACOL and CPZ (Figure 2C, Table 1). Their age range was 66–78 years (age average, 73.5 ± 5.9 years), and their mean disease duration was 8.0 ± 7.6 years. We prescribed DOPACOL (one tablet/day) with CPZ (5 mg/day) for the first 2 weeks, DOPACOL (one tablet × 2/day) with CPZ (5 mg × 2/day) for the next 2 weeks, and DOPACOL (one tablet × 3/day) with CPZ (5 mg × 3/day) for the last 4 weeks.
Clinical assessments and measures
As shown in Figure 2, clinical evaluations and video recordings were performed 1 day before (pre-treatment) and 8 weeks after (post-treatment) the initial administration of the drugs. The severity of blepharospasm was evaluated using the Visual Analog Scale (VAS) (36), Blepharospasm Disability Index (BSDI) (37), modified VAS (mVAS) (38), and Jankovic Rating Scale (JRS) (37, 39). The VAS and BSDI were used for subjective signs, and the mVAS and JRS were used for objective signs.
Pre-treatment period symptom severities and participant backgrounds
Comparison of gender, age and disease duration at the pre-treatment period revealed no significant group differences among three treatment groups (P > 0.05, Kruskal–Wallis test).
Comparison of subjective (VAS and BSDI) and objective (mVAS and JRS) measures at the pre-treatment period revealed significant group differences only in mVAS (P < 0.05, Kruskal–Wallis test). Therefor we performed Mann–Whitney U-test on mVAS between each two groups, and it showed a significant difference between “LDOPA” and “CPZ” groups (P < 0.05), but neither between “LDOPA” and “LDOPA + CPZ” groups (P > 0.05) nor between “CPZ” and “LDOPA + CPZ” groups (P > 0.05). Thus, no apparent differences were found between the “LDOPA + CPZ” group and the other two groups. This indicates that there is no problem in determining the therapeutic efficacy in “LDOPA + CPZ” group.
Statistical analyses
All values are expressed as mean ± SD. Statistical significance was evaluated using the non-parametric methods that include Wilcoxon signed-rank, Kruskal–Wallis, and Mann-Whitney U-tests. Statistical significance was set at P < 0.05. Statistical analyses were performed using SPSS statistical software (version 11.0; IMB Corp., Armonk, NY, USA).
Results
In the L-DOPA group (Figure 3; top), symptom severity was significantly increased after the drug trial, as determined by the mVAS (P < 0.05, r = 0.59), but not by the VAS, BSDI, and JRS (Table 1). In the CPZ group (Figure 3; middle), there was no apparent change in symptom severity after the drug trial, as determined by the VAS, BSDI, mVAS, and JRS (Table 1). In the L-DOPA+CPZ group (Figure 3; bottom), symptom severity was significantly decreased by administration of L-DOPA with CPZ in all subjective and objective signs, as determined by the VAS (P < 0.05, r = 0.53), BSDI (P < 0.05, r = 0.64), mVAS (P < 0.05, r = 0.59), and JRS (P < 0.05, r = 0.64) (Table 1).
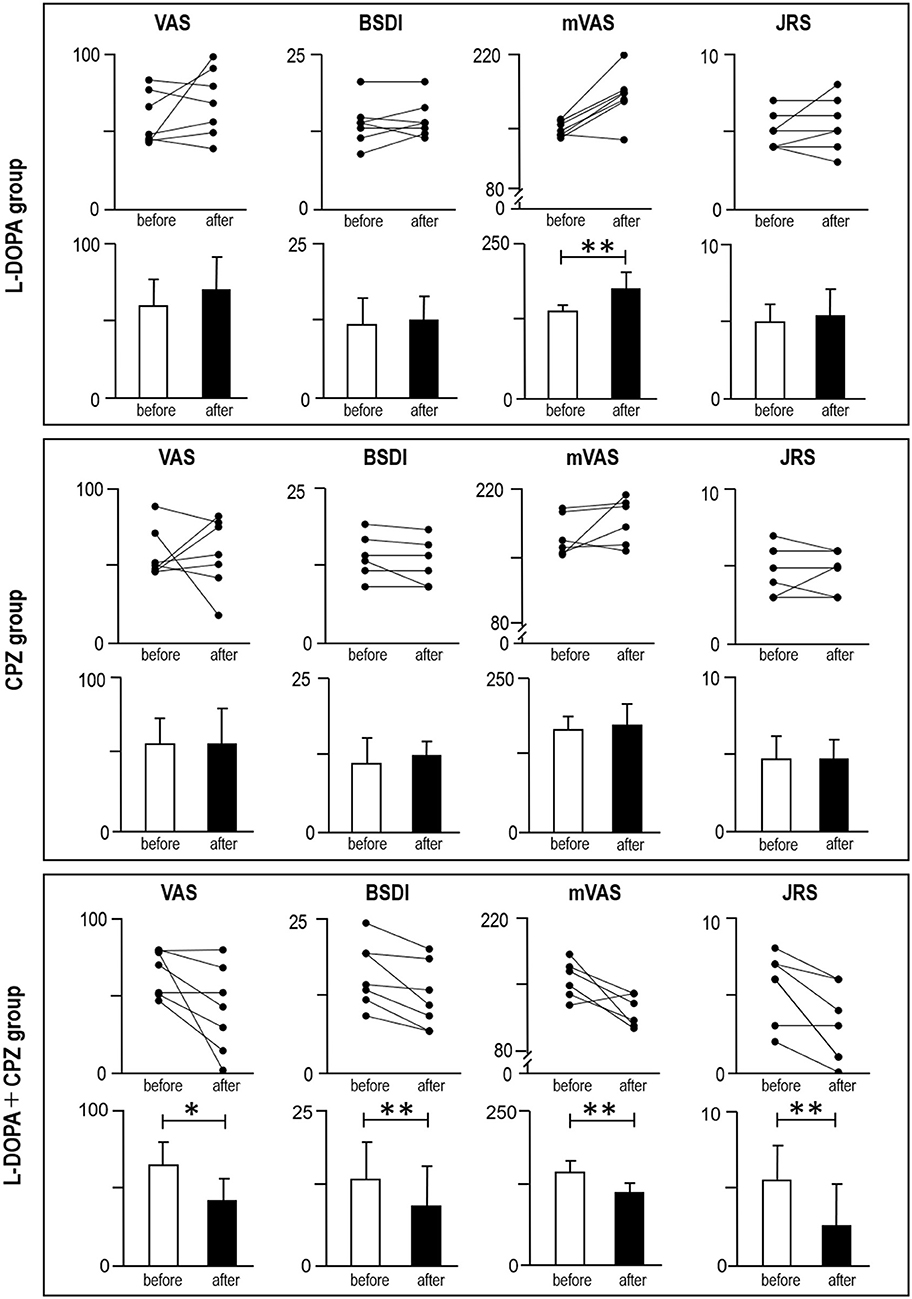
Figure 3. Measurements of blepharospasm severities before and after the drug challenge test. Symptom severities were determined by using visual analog scale (VAS), Blepharospasm Disability Index (BSDI), modified VAS (mVAS), and Jankovic Rating Scale (JRS). Line plots (upper graphs) and average plots (lower graphs) are shown in the “L-DOPA group (n = 7)” (top panel), “CPZ group (n = 7)” (middle panel), and “L-DOPA + CPZ group (n = 7)” (bottom panel). *P < 0.05, **P < 0.01 (“before” vs. “after”, Wilcoxon signed-rank test).
No apparent neuropsychiatric and neurobehavioral adverse effects were found in the L-DOPA, CPZ, and L-DOPA + CPZ groups and no patients dropped out during the drug challenge tests. Thus, the dual use of DOPACOL (one tablet × 3/day) and CPZ (5 mg × 3/day), but not the administration of DOPACOL (one tablet × 3/day) alone or CPZ (5 mg × 3/day) alone, can give rise to a therapeutic effect on blepharospasm (for a reference see Supplementary Video 1).
Discussion
The present study showed that the symptoms of blepharospasm, a type of focal dystonia, could be alleviated by dual dopaminergic therapy using both L-DOPA and CPZ, with dosages lower than the usual in clinical practice. In this study, we used CPZ phenolphthalinate (15 mg/day) and L-DOPA (150 mg/day) with carbidopa (15 mg/day). In contrast, the usual dosage of CPZ in adults is 30–100 mg/day, and, for psychiatric use, 50–450 mg/day (40, 41), while the standard maintenance dose of L-DOPA with carbidopa for advanced Parkinson's disease is ~600–750 mg/day (42).
Since L-DOPA is the prodrug of dopamine while CPZ is a D2-antagonist, our results suggest that dystonia symptoms could be attenuated through dopaminergic modulation, which induces an increase in striatal D1-signaling. Based on the evidence that D1Rs are highly concentrated in the striosome compartment in the “human” striatum (26), we also suggest that striosomal loss of D1 signaling may be important in the pathogenesis of dystonias (15, 16, 18, 21).
According to the classical D1-direct/D2-indirect pathway model (the so called “Matrix Model”; for reference, see Figure 1 “M, Matrix”), both matrix D1Rs and D2Rs are influenced by excessive dopamine signaling, causing abnormalities in individual firing rates and/or firing patterns in downstream structures (43–46). It is hypothesized that excessive dopamine signals could cause the activated D1-direct pathway and inhibited D2-indirect pathway to induce disinhibition and hyperexcitation of the thalamus and primary motor cortex, respectively, both of which result in hyperkinetic disorders such as dystonia (47, 48). Based on the classical matrix model, a D2-antagonist may improve dystonia symptoms via the D2-indirect pathway. However, the present study showed that the administration of CPZ (5 mg × 3/day) alone had no effect on blepharospasm symptoms. This is likely because the dose of CPZ used here was not high enough to affect dystonia symptoms.
One may say that even with the low dosage used here, administration of CPZ has a potential risk of causing tardive dystonia due to its D2-antagonistic action (49, 50). Because L-DOPA is a prodrug for dopamine that acts as both a D1 and D2 agonist (27), we consider that in dual therapy with L-DOPA and CPZ, simultaneous administration of L-DOPA can also dampen the D2-antagonism caused by CPZ and reduce the risk of tardive dystonia.
In conclusion, dual dopaminergic therapy with L-DOPA and CPZ can exert a therapeutic effect on blepharospasm, which is a focal dystonia. Our results suggest that dopaminergic modulation inducing an increase in striatal D1-signaling may attenuate dystonia symptoms, which is in accordance with the hypothesis that a loss of D1-signaling in the striosome compartment may underlie dystonia. Since the present study had a relatively small sample size, independent replications with a larger sample size may be warranted. It would be necessary to determine the optimal dosages for the most effective treatment of blepharospasm, because we have done this first clinical trial with relatively low doses of “L-DOPA and CPZ.” It is also necessary to determine if the dual dopaminergic therapy used here could give rise to long-term and sustained benefits in patients with dystonias. It is currently under investigation to assess its therapeutic effects on the various types of focal dystonias (e.g. cervical dystonia, writer's cramp and other occupational dystonias), and the other types of idiopathic and/or secondary dystonias of which involves segmental or generalized body parts.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This randomized clinical trial was approved by the Institutional Ethics Committee of the Osaka Neurological Institute. The study was registered with the International Committee of Medical Journal Editors recognized registry, the UMIN Clinical Trials Registry (number: UMIN00027430). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SM: research project—conception, organization, and execution, statistical analysis—design, execution, and review and critique, and manuscript preparation—writing of the first draft. HK: research project—organization and execution. HS, RK, and SG: research project—conception, statistical analysis—design, execution, and review and critique, and manuscript preparation—review and critique. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.922333/full#supplementary-material
Supplementary Video 1. Therapeutic effects of pharmacotherapy with Dopacol tablets L50™ (one tablet × 3/day) and chlorpromazine (5 mg × 3/day) on dystonia symptoms in a patient with blepharospasm.
Abbreviations
L-DOPA, L-3, 4-dihydroxyphenylalanine; CPZ, Chlorpromazine; VAS, Visual Analog Scale; BSDI, Blepharospasm Disability Index; mVAS, Modified Visual Analog Scale; JRS, Jankovic Rating Scale; BTX, Botulinum Toxin; D1R, dopamine D1 like receptor; Gαolf, olfactory type G-protein α subunit; MSNs, Medium Spiny Neurons; cAMP, 3′, 5′-cyclic AMP; D1Rs and D2Rs, dopamine D1- and D2-like receptors; DOPACOL, Dopacol tablets L50 ™; (50 mg of L-DOPA plus 5 mg of carbidopa, ; Nichi-Iko Pharmaceutical Co., Ltd. Toyama, Japan); D2R, dopamine D2 like receptor.
References
1. Fahn S, Marsden CD, Calne DB. Classification and investigation of dystonia. In: Marsden CD, Fahn S, editors, Movement Disorders. London: Butterworths (1987). p. 332–58.
2. Albanese A, Bhatia K, Bressman SB, Delong MR, Fahn S, Fung VS, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord. (2013) 28:863–73. doi: 10.1002/mds.25475
3. Albanese A, Barnes MP, Bhatia KP, Fernandez-Alvarez E, Filippini G, Gasser T, et al. A systematic review on the diagnosis and treatment of primary (idiopathic) dystonia and dystonia plus syndromes: report of an EFNS/MDS-ES Task Force. Eur J Neurol. (2006) 13:433–44. doi: 10.1111/j.1468-1331.2006.01537.x
4. Defazio G, Livrea P. Epidemiology of primary blepharospasm. Mov Disord. (2002) 17:7–12. doi: 10.1002/mds.1275
5. Barbosa P, Warner TT. Dystonia. Handb Clin Neurol. (2018) 159:229–36. doi: 10.1016/B978-0-444-63916-5.00014-8
6. Coscarelli JM. Essential blepharospasm. Semin Ophthalmol. (2010) 25:104–8. doi: 10.3109/08820538.2010.488564
7. Nicoletti AG, Aoki L, Nahas TR, Matayoshi S. Essential blepharospasm: literature review. Arq Bras Oftalmol. (2010) 73:469–73. doi: 10.1590/S0004-27492010000500018
8. Simpson DM, Hallett M, Ashman EJ, Comella CL, Green MW, Gronseth GS, et al. Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. (2016) 86:1818–26. doi: 10.1212/WNL.0000000000002560
9. Ramirez-Castaneda J, Jankovic J. Long-term efficacy, safety, and side effect profile of botulinum toxin in dystonia: a 20-year follow-up. Toxicon. (2014) 90:344–48. doi: 10.1016/j.toxicon.2014.07.009
10. Streitová H, Bareš M. Long-term therapy of benign essential blepharospasm and facial hemispasm with botulinum toxin A: retrospective assessment of the clinical and quality of life impact in patients treated for more than 15 years. Acta Neurol Belg. (2014) 114:285–91. doi: 10.1007/s13760-014-0285-z
11. Sy MAC. Fernandez HH. Dystonia and leveraging oral pharmacotherapy. J Neural Transm. (2021) 128:521–9. doi: 10.1007/s00702-021-02339-7
12. Tolosa ES, Lai C. Meige disease: striatal dopaminergic preponderance. Neurology. (1979) 29:1126–30. doi: 10.1212/WNL.29.8.1126
13. Greene P, Shale H, Fahn S. Analysis of open-label trials in torsion dystonia using high dosages of anticholinergics and other drugs. Mov Disord. (1988) 3:46–60. doi: 10.1002/mds.870030107
14. Graybiel AM, Ragsdale CW Jr. Histochemically distinct compartments in the striatum of human, monkeys, and cat demonstrated by acetylthiocholinesterase staining. Proc Natl Acad Sci USA. (1978) 75:5723–6. doi: 10.1073/pnas.75.11.5723
15. Goto S, Lee LV, Munoz EL, Tooyama I, Tamiya G, Makino S, et al. Functional anatomy of the basal ganglia in X-linked recessive dystonia-parkinsonism. Ann Neurol. (2005) 58:7–17. doi: 10.1002/ana.20513
16. Sato K, Sumi-Ichinose C, Kaji R, Ikemoto K, Nomura T, Nagatsu I, et al. Differential involvement of striosome and matrix dopamine systems in a transgenic model of dopa-responsive dystonia. Proc Natl Acad Sci USA. (2008) 105:12551–6. doi: 10.1073/pnas.0806065105
17. Goto S, Kawarai T, Morigaki R, Okita S, Koizumi H, Nagahiro S, et al. Defects in the striatal neuropeptide Y system in X-linked dystonia-parkinsonism. Brain. (2013) 136:1555–67. doi: 10.1093/brain/awt084
18. Crittenden JR, Graybiel AM. Basal ganglia disorders associated with imbalances in the striosome and matrix compartments. Front Neuroanat. (2011) 5:59. doi: 10.3389/fnana.2011.00059
19. Graybiel AM, Canales JJ, Capper-Loup C. Levodopa-induced dyskinesias and dopamine-dependent stereotypies: a new hypothesis. Trends Neurosci. (2000) 23:S71–7. doi: 10.1016/S1471-1931(00)00027-6
20. Graybiel AM. Habits, rituals, and the evaluative brain. Annu Rev Neurosci. (2008) 31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851
21. Amemori KI, Gibb LG, Graybiel AM. Shifting responsibly: the importance of striatal modularity to reinforcement learning in uncertain environments. Front Hum Neurosci. (2011) 5:47. doi: 10.3389/fnhum.2011.00047
22. Fuchs T, Saunders-Pullman R, Masuho I, Luciano MS, Raymond D, Factor S. et al. Mutations in GNAL cause primary torsion dystonia. Nat Genet. (2013) 45:88–92. doi: 10.1038/ng.2496
23. Hervé D. Identification of a specific assembly of the g protein golf as a critical and regulated module of dopamine and adenosine-activated cAMP pathways in the striatum. Front Neuroanat. (2011) 5:48. doi: 10.3389/fnana.2011.00048
24. Goto S. Striatal Gα olf/cAMP signal-dependent mechanism to generate levodopa-induced dyskinesia in Parkinson's disease. Front Cell Neurosci. (2017) 11:364. doi: 10.3389/fncel.2017.00364
25. Sako W, Morigaki R, Nagahiro S, Kaji R, Goto S. Olfactory type G-protein α subunit (Gαolf) in striosome-matrix dopamine systems in adult mice. Neuroscience. (2010) 170:497–502. doi: 10.1016/j.neuroscience.2010.06.072
26. Morigaki R, Goto S. Postsynaptic density protein 95 in the striosome and matrix compartments of the human neostriatum. Front Neuroanat. (2015) 9:154. doi: 10.3389/fnana.2015.00154
28. Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Pavel Mohr P, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. (2006) 20:389–409. doi: 10.2165/00023210-200620050-00004
29. Dressler D. Clinical presentation and management of antibody-induced failure of botulinum toxin therapy. Mov Disord. (2004) 19(Suppl 8):S92–100. doi: 10.1002/mds.20022
30. Hsiung GY, Das SK, Ranawaya R, Lafontaine AL, Suchowersky O. Long-term efficacy of botulinum toxin A in treatment of various movement disorders over a 10-year period. Mov Disord. (2002) 17:1288–93. doi: 10.1002/mds.10252
31. Ramirez-Castaneda J, Jankovic J. Long-term efficacy and safety of botulinum toxin injections in dystonia. Toxins. (2013) 5:249–66. doi: 10.3390/toxins5020249
32. Naumann M, Boo LM, Ackerman AH, Gallagher CJ. Immunogenicity of botulinum toxins. J Neural Transm. (2013) 120:275–90. doi: 10.1007/s00702-012-0893-9
33. Bellows S. Jankovic J. Immunogenicity associated with botulinum toxin treatment. Toxins. (2019) 11:491. doi: 10.3390/toxins11090491
34. Yang J, Zhang L, Hou Y, Wei Q, Ou R, Lin J. et.al. Sex related differences in nonmotor symptoms of patients with idiopathic blepharospasm. Sci Rep. (2021) 11:17856. doi: 10.1038/s41598-021-97289-1
35. Cossu G, Mereu A, Deriu M, Melis M, Molari A, Melis G, et al. Prevalence of primary blepharospasm in Sardinia, Italy: a service-based survey. Mov Disord. (2006) 21:2005–8. doi: 10.1002/mds.21084
36. Matsumoto S, Murakami N, Koizumi H, Takahashi M. Izumi Y, Kaji R. Evaluation of the edrophonium challenge test for cervical dystonia. Intern Med. (2017) 56:2415–21. doi: 10.2169/internalmedicine.8555-16
37. Jankovic J, Kenney C, Grafe S, Goertelmeyer R, Comes G. Relationship between various clinical outcome assessments in patients with blepharospasm. Mov Disord. (2009) 24:407–13. doi: 10.1002/mds.22368
38. Matsumoto S, Murakami N, Koizumi H, Takahashi M, Izumi Y, Kaji R. Edrophonium challenge test for blepharospasm. Front Neurosci. (2016) 10:226. doi: 10.3389/fnins.2016.00226
39. Jankovic J, Orman J. Botulinum A toxin for cranial-cervical dystonia: a double-blind, placebo-controlled study. Neurology. (1987) 37:616–23. doi: 10.1212/WNL.37.4.616
40. Adams CE, Awad GA, Rathbone J, Thornley B, Soares-Weiser K. Chlorpromazine versus placebo for schizophrenia. Cochrane Database Syst Rev. (2014) 6:CD000284. doi: 10.1002/14651858.CD000284.pub3
41. Liu X, De Haan S. Chlorpromazine dose for people with schizophrenia. Cochrane Database Syst Rev. (2009) 15:CD007778. doi: 10.1002/14651858.CD007778
42. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
43. Simonyan K, Cho H, Hamzehei SA, Rubien-Thomas E, Hallett M. The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain. (2017) 140:3179–90. doi: 10.1093/brain/awx263
44. Ribot B, Aupy J, Vidailhet M, Mazere J, Pisani A, Bezard E, et al. Dystonia and dopamine: From phenomenology to pathophysiology. Prog Neurobiol. (2019) 182:101678. doi: 10.1016/j.pneurobio.2019.101678
45. Magill PJ, Bolam JP, Bevan MD. Dopamine regulates the impact of the cerebral cortex on the subthalamic nucleus-globus pallidus network. Neuroscience. (2001) 106:313–30. doi: 10.1016/S0306-4522(01)00281-0
46. Singh A, Liang L, Kaneoke Y, Cao X, Papa SM. Dopamine regulates distinctively the activity patterns of striatal output neurons in advanced parkinsonian primates. J Neurophysiol. (2015) 113:1533–44. doi: 10.1152/jn.00910.2014
47. Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. (1990) 13:266–71. doi: 10.1016/0166-2236(90)90107-L
48. DeLong MR, Wichmann T. Basal ganglia circuits as targets for neuromodulation in Parkinson disease. JAMA Neurol. (2015) 72:1354–60. doi: 10.1001/jamaneurol.2015.2397
49. Morigaki R, Mure H, Kaji R, Nagahiro S, Goto S. Therapeutic perspective on tardive syndrome with special reference to deep brain stimulation. Front Psychiatry. (2016) 7:207. doi: 10.3389/fpsyt.2016.00207
Keywords: blepharospasm, dystonia, L-DOPA, chlorpromazine, dopamine D1 receptor, striatum, striosome compartment, patients
Citation: Matsumoto S, Koizumi H, Shimazu H, Kaji R and Goto S (2022) A dual dopaminergic therapy with L-3,4-dihydroxyphenylalanine and chlorpromazine for the treatment of blepharospasm, a focal dystonia: Possible implications for striosomal D1 signaling. Front. Neurol. 13:922333. doi: 10.3389/fneur.2022.922333
Received: 17 April 2022; Accepted: 05 July 2022;
Published: 25 July 2022.
Edited by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceReviewed by:
Stephen Louis Aita, Dartmouth College, United StatesVeronica Alexandra Antipova, Medical University of Graz, Austria
Jian Qu, Central South University, China
Copyright © 2022 Matsumoto, Koizumi, Shimazu, Kaji and Goto. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoshi Goto, c2dvdG8wMzI2QG91dGxvb2suanA=; c2dvdG9AdG9rdXNoaW1hLXUuYWMuanA=
 Shinichi Matsumoto
Shinichi Matsumoto Hidetaka Koizumi
Hidetaka Koizumi Hideki Shimazu
Hideki Shimazu Ryuji Kaji
Ryuji Kaji Satoshi Goto
Satoshi Goto