- 1Department of Neurosurgery, Shanghai University of Medicine and Health Sciences Affiliated Jia Ding Hospital, Shanghai, China
- 2Department of Neurosurgery, Shanghai Minhang District Central Hospital, Shanghai, China
- 3Department of Neurosurgery, Fuyang Fifth People's Hospital, Anhui, China
Objective: Spontaneous intracerebral hemorrhage (sICH) is a frequently encountered neurosurgical disease. The purpose of this study was to evaluate the relationship between modified Graeb Score (mGS) at admission and clinical outcomes of sICH and to investigate whether the combination of ICH score could improve the accuracy of outcome prediction.
Methods: We retrospectively reviewed the medical records of 511 patients who underwent surgery for sICH between January 2017 and June 2021. Patient outcome was evaluated by the Glasgow Outcome Scale (GOS) score at 3 months following sICH, where a GOS score of 1–3 was defined as a poor prognosis. Univariate and multivariate logistic regression analyses were conducted to determine risk factors for unfavorable clinical outcomes. Receiver operating characteristic (ROC) curve analysis was performed to detect the optimal cutoff value of mGS for predicting clinical outcomes. An ICH score combining mGS was created, and the performance of the ICH score combining mGS was assessed for discriminative ability.
Results: Multivariate analysis demonstrated that a higher mGS score was an independent predictor for poor prognosis (odds ratio [OR] 1.207, 95% confidence interval [CI], 1.130–1.290, p < 0.001). In ROC analysis, an optimal cutoff value of mGS to predict the clinical outcome at 3 months after sICH was 11 (p < 0.001). An increasing ICH-mGS score was associated with increased poor functional outcome. Combining ICH score with mGS resulted in an area under the curve (AUC) of 0.790, p < 0.001.
Conclusion: mGS was an independent risk factor for poor outcome and it had an additive predictive value for outcome in patients with sICH. Compared with the ICH score and mGS alone, the ICH score combined with mGS revealed a significantly higher discriminative ability for predicting postoperative outcome.
Introduction
Spontaneous intracerebral hemorrhage (sICH) is defined as non-traumatic bleeding into the brain parenchyma and accounts for 10–30% of all strokes (1, 2), with a high rate of disability and mortality (3). Surgical treatment is one of the main treatments for ICH. At present, multiple surgical techniques are utilized to treat ICH, including craniotomy, minimally invasive surgery, and decompressive craniectomy (4). The ICH score is a clinical grading method that can be used to predict 30-day mortality in patients with ICH. This score, which consists mainly of the Glasgow Coma Scale (GCS) score, patient age, and neuroimaging features, is effective in predicting the short-term risk of death or poor outcome in patients with ICH (5).
It has been reported in the literature that ICH is often complicated by intraventricular hemorrhage (IVH), which can further exacerbate brain damage and thus influence the prognosis (6). The modified Graeb Score (mGS) is used as an indicator to assess the severity of IVH and is calculated based on three factors, namely, location of IVH, hematoma volume in each ventricle, and ventricular dilatation, as described by Morgan et al. (7), and has a high predictive value for the prognosis of IVH.
However, the relationship between mGS and sICH has not been widely recognized, so the aim of this study was to evaluate the relationship between mGS at admission and clinical outcomes of sICH and to investigate whether the combination of ICH score could improve the accuracy of outcome prediction.
Methods
Patient population
We retrospectively reviewed the medical records of 511 patients who underwent surgery for sICH between January 2017 and June 2021, in two hospitals (Shanghai University of Medicine and Health Sciences Affiliated Jia Ding Hospital in Shanghai and Fuyang Fifth People's Hospital in Anhui Province, China). The inclusion criteria were as follows: 1. all patients were older than 18 years. 2. patients had ICH with or without IVH on head computed tomography (CT) scan. 3. patients underwent a hematoma evacuation; and 4. patients had complete documentation. The exclusion criteria were as follows: 1. ICH caused by head trauma, brain tumor, cerebral aneurysm, and vascular malformation. 2. missing imaging data. 3. previous history of ICH or other neurological diseases such as ischemic stroke. 4. other systematic diseases such as renal dysfunction, hepatic dysfunction, cancer, hematological disorders, and heart diseases; and 5. patients who were lost to follow-up at 3 months. This study was performed in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Fuyang Fifth People's Hospital.
Data collection
Data collected included gender, age, ICH location, hypertension and/or diabetes, blood pressure at admission, time from symptom onset to baseline CT scan, antiplatelet agents use, GCS score at admission, and preoperative findings such as cerebral hernia, presence of obstructive hydrocephalus, IVH, mGS, and ICH score. An operative method such as external ventricular drainage (EVD) was considered for analysis. The postoperative findings including acute cerebral infarction and intracranial infection were investigated.
The diagnosis of intracranial infection defined according to the standards issued by the National Ministry of Health is as follows: 1) presence of clinical manifestation of intracranial infection, including temperature higher than 38°C or lower than 36°C, positive signs of meningeal irritation (nuchal rigidity, Brudzinski sign, and Kernig sign), vomiting, and headache. 2) positive changes in cerebrospinal fluid specimens: white blood cell count > 1,000 × 106 cells/L; glucose levels < 2.25 mmol/L; chloride < 120 mmol/L, and protein > 0.45 g/L; and 3) positive results for bacteria in cerebrospinal fluid culture (8). Cerebral infarction is defined as radiologically proven new infarcts.
Imaging
Two experienced neurologists were employed to independently review all the CT scans in a blinded manner. All patients underwent baseline CT within 6 h after onset of symptoms, and follow-up CT scan was performed within 24 h after the baseline CT scan. Hematoma volumes were calculated by the ABC/2 method (9). In this study, we defined hematoma growth as an increase in hematoma volume of >33% or >6 ml at follow-up CT scan (10, 11). In addition, IVH growth was defined as either any newly occurring intraventricular bleeding on follow-up CT scan in patients without baseline IVH or an increase in IVH volume ≥1 ml on follow-up CT scan in patients with initial IVH (12).
Outcome assessment
The mGS and ICH scores at admission were calculated to assess the severity of ICH. All included patients with sICH were treated surgically, of which 215 cases received EVD. All patients with sICH were treated stringently following the guidelines for the management of sICH (13). Patient outcome was evaluated by the Glasgow Outcome Scale (GOS) score at 3 months following sICH. Good outcomes were defined as GOS score of 4–5, and score of 1–3 was deemed a poor outcome (14).
Statistical analysis
Statistical analysis was performed using SPSS version 26.0 (SPSS, Inc., Chicago, USA). Categorical variables were presented as numbers with percentages and analyzed with an χ2 test or Fisher's exact test, whereas continuous variables were expressed as mean ± standard deviation and analyzed using Student's t-test or Mann–Whitney U test. Univariate and multivariate logistic regression analyses were conducted to determine the risk factors for unfavorable clinical outcomes. Variance inflation factors (VIFs) were used to examine independent variables for potentially strong contributions to multicollinearity in a regression model, and it was suggested that predictors with values above a VIF > 5 or a tolerance index < 0.20 could be contributing considerably to multicollinearity (15). Receiver operating characteristic (ROC) curve analysis was performed to detect the optimal cutoff value of mGS for predicting clinical outcomes. To assess the prognostic capability of ICH score combining mGS (ICH-mGS score), the area under the curve (AUC) was calculated. Z-test was used to compare the difference of AUC between the ICH score and ICH-mGS score. ROC analysis was also used to estimate the predictive accuracy of the ICH-mGS score on the IVH growth of patients with sICH. Statistical significance was defined as p < 0.05 for all tests.
Results
A total of 511 patients were included in this study. There were 268 men and 243 women, with an average age of 62.86 ± 9.66 years. ICH location was 465 (91.0%) supratentorial and 46 (9.0%) infratentorial. The admission GCS score was 7.86 ± 2.92. Notably, 102 (20.0%) patients had cerebral hernia, 84 (16.4%) patients had obstructive hydrocephalus, and 300 (58.7%) patients had associated IVH. Of note, 157 (30.7%) patients with ICH experienced hematoma expansion (HE), and 124 (24.3%) patients had IVH growth on follow-up CT scan. The admission mGS score was 7.91 ± 8.18 and the ICH score was 1.88 ± 0.92. Notably, 215 (42.1%) patients underwent EVD placement. The clinical characteristics, in detail, of the 511 patients are listed in Table 1.
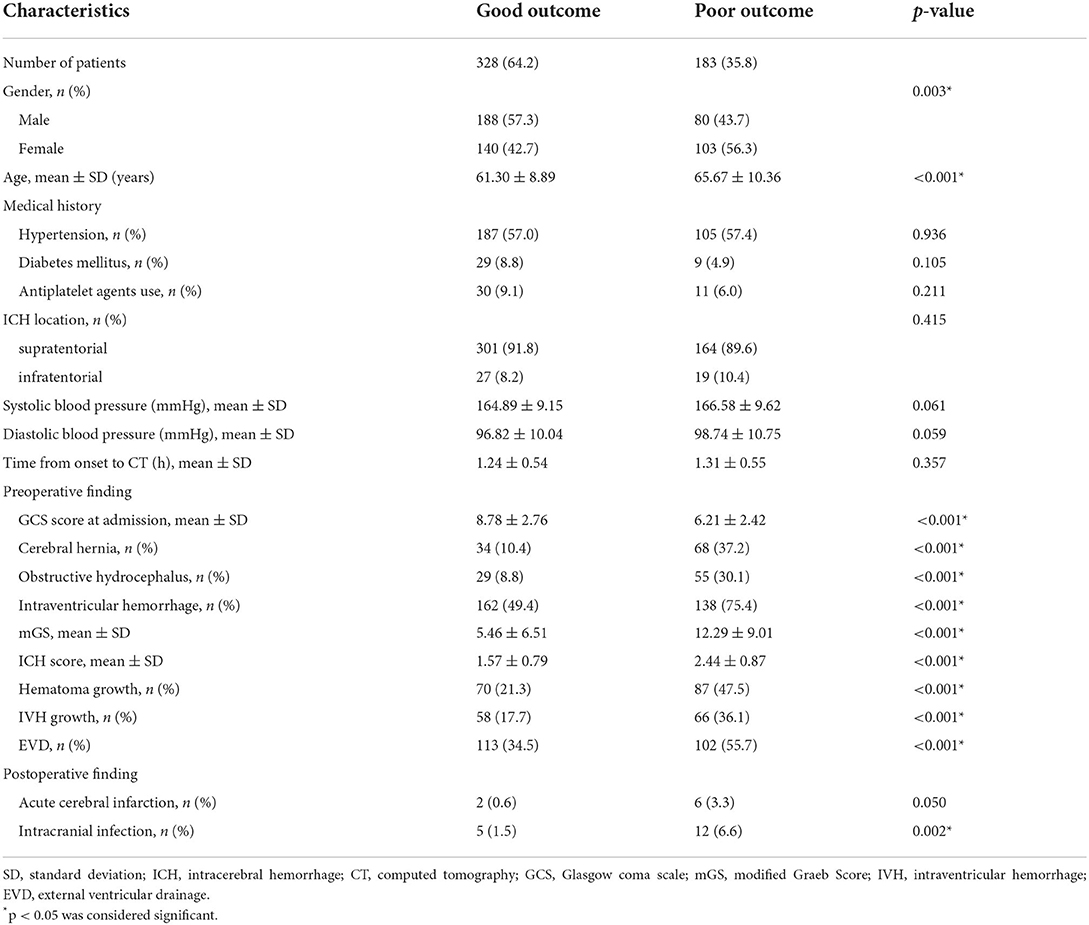
At 3 months after sICH, an unfavorable clinical outcome was found in 183 (35.8%) patients and a favorable prognosis was found in 328 (64.2%) patients. The poor outcome group showed lower initial GCS and higher rate of women than the good outcome group. The poor outcome group had higher rates of those with cerebral hernia, obstructive hydrocephalus, IVH, hematoma growth, IVH growth, EVD, and intracranial infection than the good outcome group. The poor outcome group had higher age, mGS, and ICH score than the good outcome group. Between groups, there were no significant differences in ICH location, medical history, antiplatelet agents use, admission blood pressure, time from symptom onset to baseline CT scan, and postoperative cerebral infarction (Table 2).
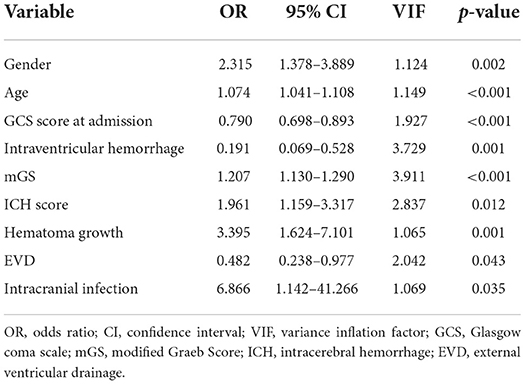
Multicollinearity was not observed between the independent variables studied and outcome. A multivariate logistic regression analysis revealed that gender [odds ratio (OR), 2.315; 95% confidence interval (CI), 1.378–3.889; p = 0.002], age (OR, 1.074; 95% CI, 1.041–1.108; p < 0.001), GCS score at admission (OR, 0.790; 95% CI, 0.698–0.893; p < 0.001), IVH (OR, 0.191; 95% CI, 0.069–0.528; p = 0.001), mGS (OR, 1.207; 95% CI, 1.130–1.290; p < 0.001), ICH score (OR, 1.961; 95% CI, 1.159–3.317; p = 0.012), hematoma growth (OR, 3.395; 95% CI, 1.624–7.101; p = 0.001), EVD (OR, 0.482; 95% CI, 0.238–0.977; p = 0.043), and intracranial infection (OR, 6.866; 95% CI, 1.142–41.266; p = 0.035) were independent risk factors for poor outcome of sICH (Table 3).
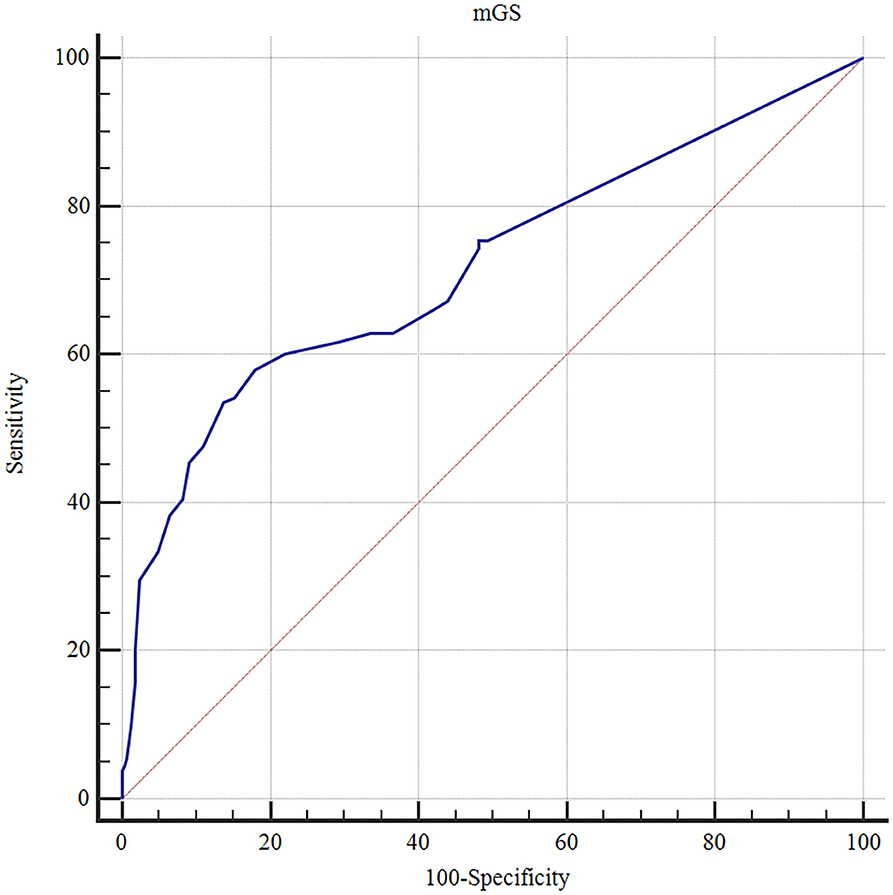
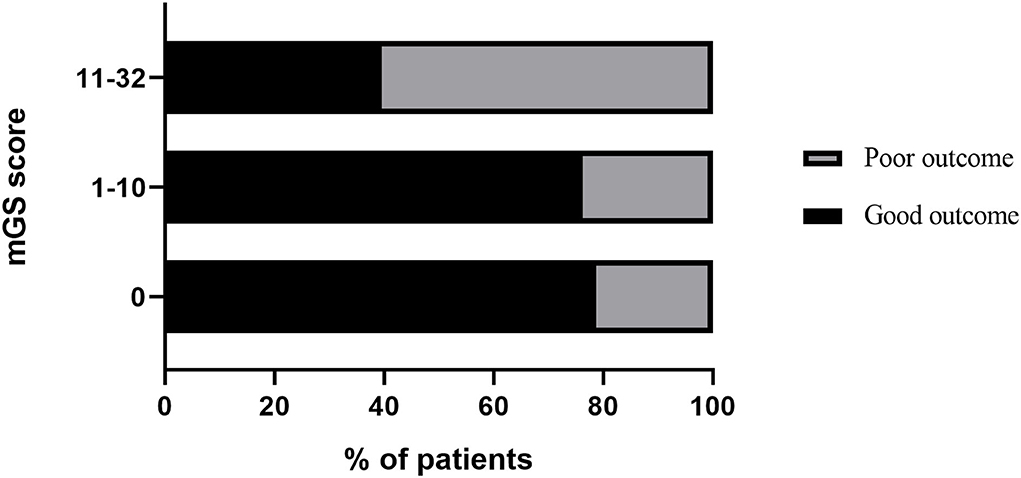
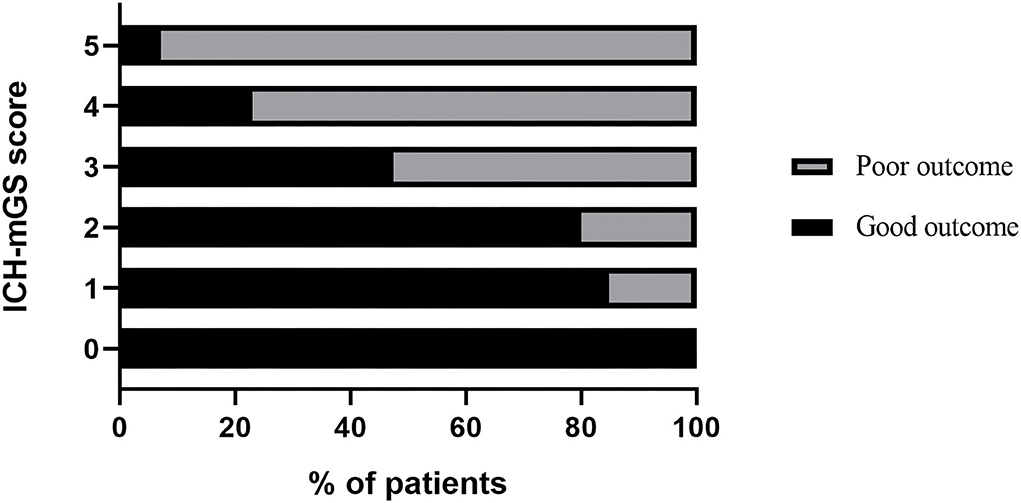
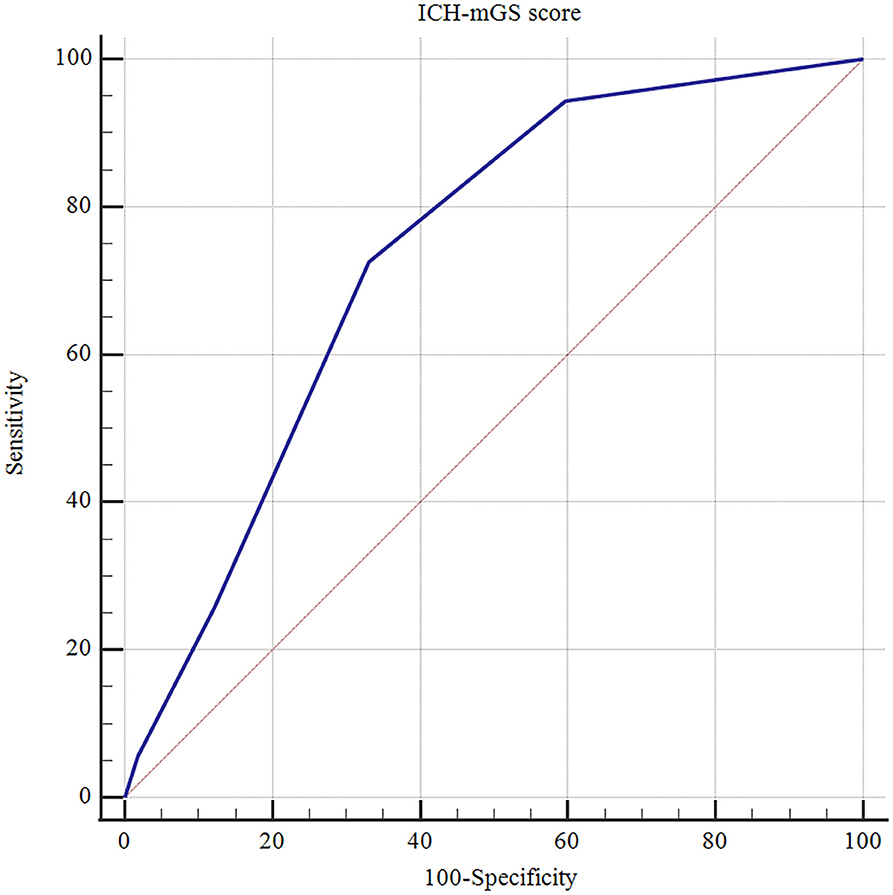
The ROC curves analysis showed that the optimal cutoff value of mGS to predict the clinical outcome at 3 months after sICH was 11 (AUC: 0.718; 95% CI, 0.677–0.756; p < 0.001) (Figure 1). The probability of poor functional outcome at 3 months for mGS score is shown in Figure 2. We found that in the different mGS score groups, the higher the mGS score, the higher the proportion of patients with poor outcome.
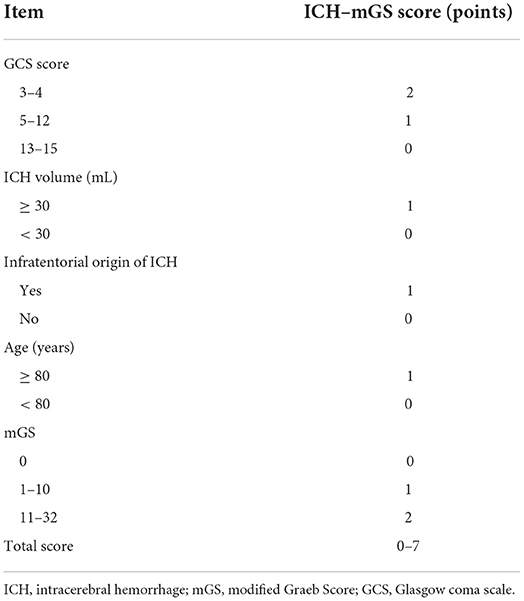
The mGS scores were divided into 3 subgroups (0, 1–10, and 11–32) and assigned 0, 1, or 2 points, respectively, to assess their additive predictive ability to the ICH score. We replaced IVH in the ICH score with mGS and designed an ICH score combining mGS with a total score ranging from 0 to 7 points (Table 4). The probability of poor functional outcome at 3 months for the ICH-mGS score is shown in Figure 3. Poor outcomes were observed, respectively, in 0.00%, 15.22%, 20.00%, 52.52%, 76.92%, and 92.86% of the patients. ROC curves were created to assess the ability of ICH-mGS score to predict poorer functional outcome. In ROC analysis, the AUC of ICH-mGS score predicted poor outcome of sICH was 0.790 (cutoff value = 2; 95% CI, 0.752–0.824; p < 0.001), and AUC of ICH score was 0.751 (cutoff value = 2; 95% CI, 0.712–0.788; p < 0.001) (Figure 4). Compared with the ICH score alone, the ICH-mGS score was significantly better for predicting an unfavorable outcome (Z = 3.795, p < 0.001).
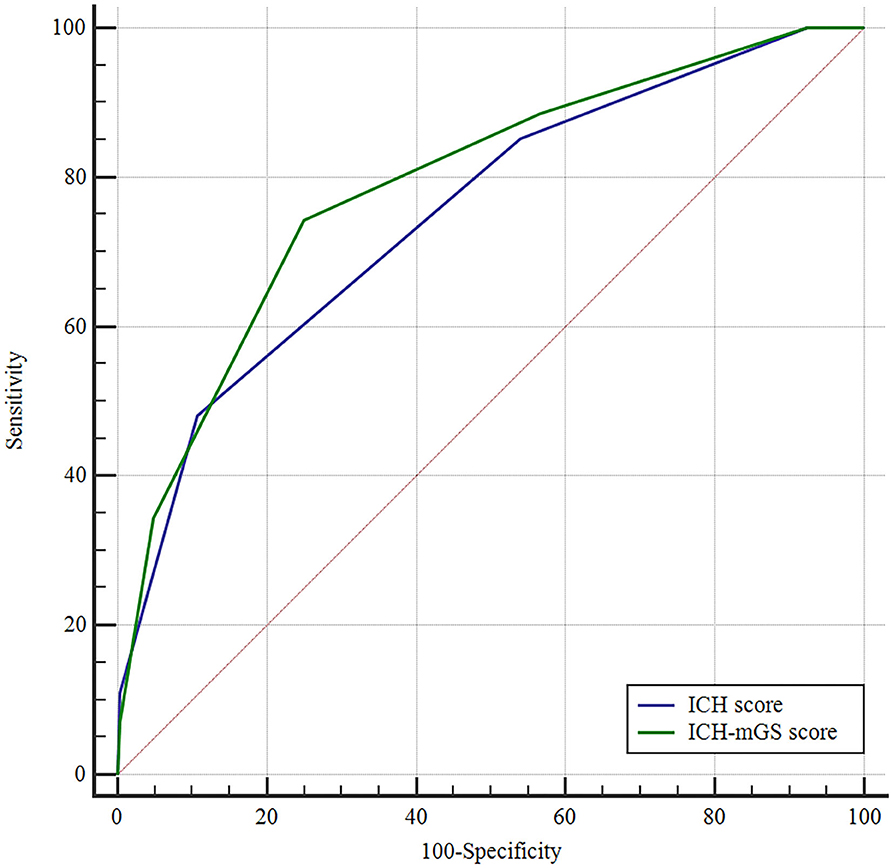
Figure 4. Receiver operating characteristic (ROC) curves of ICH score, ICH-mGS score on the poor outcome of sICH.
An ROC curve analysis was also made to estimate the predictive ability of the ICH-mGS score for IVH growth. An ICH-mGS score of 2 was observed to have the best cutoff value with AUC of 0.734 (95% CI, 0.693–0.772; p < 0.001), sensitivity of 72.58%, and specificity of 66.93% (Figure 5).
Discussion
This study demonstrated that a higher mGS score at admission was an independent predictor of the prognosis of sICH, mGS had significant prognostic value for unfavorable outcomes following a surgical treatment of sICH, and combining mGS and ICH score was of greater value in predicting poor prognosis.
Intracerebral hemorrhage is a common critical disease in neurosurgery. Surgical intervention is one of the main treatments for ICH. Traditionally, open craniotomy and hematoma aspiration were considered the mainstay of surgical management of ICH. In recent years, some of the surgical approaches that have been studied include stereotactic guidance aspiration and thrombolysis, image-guided stereotactic endoscopic aspiration, ultrasound-induced thrombolysis, stereotactic intracerebral hemorrhage underwater blood aspiration technique, and minimally invasive surgery (16). Increasing evidence suggests that newer surgical techniques have positive therapeutic effects on ICH and can be more effective than the conventional treatments (17, 18). The annual incidence rate of ICH is found as high as 35 persons per 100,000 individuals per year (19), and its high disability and mortality rates impose a huge burden on families and society (20). An identification of risk factors associated with poor outcome of ICH is of paramount importance for the treatment. Factors such as age, initial GCS, ICH volume, and diabetes mellitus are commonly associated with ICH (21–24). IVH is one of the common complications of ICH and is an independent predictor of worse outcome and neurological deterioration (25–27); the reason for this is that prolonged exposure of the ventricles to blood may lead to altered consciousness and, at the tissue level, inflammation, fibrosis, and hydrocephalus (28, 29).
Thurim et al. (30) first demonstrated that IVH volume was related to higher 30-day mortality rates. Young et al. (27) identified that IVH volume of 20 ml was a predictive value for poor outcome. In addition, Hwang et al. (31) reported that admission IVH volume of 6 ml was associated with a significant increase in the likelihood of poor functional outcome after sICH. Thus, this suggested that the amount of IVH was a key factor in the prognosis of ICH. The mGS score is used as a semiquantitative scale for IVH volume measurement with a total score of 0–32 and is similarly predictive of outcome to actual IVH volume (7). There is another method available for the assessment of IVH volume, and the Graeb scale is highly accurate to prognosticate the risk of mortality and long-term disability in patients with ICH (6). In contrast, compared to the original Graeb Scale (oGS), mGS is more reliable in predicting poor prognosis, following ICH. Hansen et al. (32) found that the mGS improved outcome prediction after supratentorial ICH beyond other established prognostic factors in a broad ICH population. Morgan et al. (7) reported that mGS was more closely related to change in IVH volume and outcome than the oGS. In this study, the mGS score was significantly higher in the GOS poor outcome group than in the good outcome group, the optimal cutoff value of mGS was 11 to predict the clinical outcome (AUC: 0.718, 95% CI: 0.677–0.756, p < 0.001), and from univariate and multivariate regression analyses, mGS was a risk factor for poor prognosis. Therefore, the mGS score serves as an assessment tool for IVH severity and can be readily used to predict the poor clinical outcomes of patients with sICH after surgical treatment.
The ICH score is a prognostic tool commonly used in clinical practice that includes GCS score, age ≥ 80 years, infratentorial location, hematoma volume, and presence of IVH, and is used to evaluate 30-day mortality of patients with ICH. The ICH score ranges from 0 to 6, with 30-day mortality rates increasing from 0% for a score of 0 to 100% for score of 5 or 6 (5). This study first combines mGS and ICH score and sets a new ICH-mGS score for evaluating poor outcome in patients with sICH. In our study, both mGS and ICH score were found to be independent predictors for poor outcome in sICH, an increasing ICH-mGS score was associated with increased poor functional outcome, and in ROC analysis, the AUC for the combined mGS and ICH score was better than that of each parameter alone, suggesting that the ICH-mGS score showed a potential advantage to predict poorer functional outcome relative to ICH score and mGS.
Hematoma expansion (HE) is a common early and severe complication of ICH (33, 34). Several studies indicate that HE occurs in approximately one-third of patients with ICH and is associated with in-hospital mortality and poor outcome (35–37). At present, the relevant imaging markers have been used to predict HE after ICH, including spot sign, leakage sign, island sign, and intra-hematomal hypodensity on non-contrast CT (38). IVH growth is most often defined as either the development of delayed IVH or an increase in IVH volume, individually. Previous studies have reported that IVH growth is an independent predictor of death and poor functional outcome in patients with ICH (12, 39, 40). Li et al. (12) reported that IVH growth occurred at 19.5% and was an independent predictor of poor functional outcome at 3 months after ICH. Dowlatshahi et al. (41) demonstrated that patients (6.1%) without HE had ≥2 ml IVH expansion, which was associated with poor outcome. In addition, other studies have shown that IVH growth is strongly associated with early HE (6). Patients who had severe HE could develop delayed IVH subsequently, which ultimately led to poor clinical outcomes (42). We argue that IVH growth might be a complication of HE, and IVH growth together with hematoma growth can result in a worse outcome in patients with ICH. In this study, we found that HE was a risk factor for poor prognosis; however, IVH growth was not independently associated with an unfavorable outcome following ICH. ROC curve was further applied, and the results showed that the ICH-mGS score could also significantly predict the IVH growth. Thus, our findings suggest that the ICH-mGS score reveals a higher discriminative ability for predicting the IVH growth and poor outcome in patients with sICH undergoing surgical treatment.
Consistent with the previous reports, we found that the admission GCS score was associated with poor clinical outcome. Øie et al. (43) reported that a GCS score of < 9 on admission was a risk factor for poor prognosis at 3 months in patients with ICH. Wang et al. (44) also found that patients with a GCS score of 3 to 4 had a significantly higher 30-day mortality than those with a GCS score of 5 to 15. In our study, multivariate analysis showed that a low GCS score was associated with poor outcome of patients with sICH after hematoma evacuation. In addition, we found that gender, age, IVH, EVD, and intracranial infection were related to poor outcome of sICH.
Neuroinflammation has been linked to neurological diseases, especially cerebrovascular disease (45). Previous studies have revealed that inflammatory response plays a pivotal role in the pathologic mechanism of ICH and is associated with poor functional outcome (46, 47). A number of biochemical factors, including white blood cell count, C-reactive protein, tumor necrosis factor alpha, vascular endothelial growth factor, homocysteine, and neutrophil-to-lymphocyte ratio (NLR), are known as inflammatory markers in ICH (48–52). There is increasing evidence that easily available serum biomarkers of inflammation can be reliable predictors of outcome in patients with ICH and can improve the outcome prediction when added to validated prognostic scales (53, 54). Lattanzi et al. (53) reported that NLR was associated with 30-day mortality and morbidity after ICH, and improved the accuracy of outcome prediction when added to the modified ICH score. Zhou et al. (55) demonstrated that elevated plasma D-dimer level was an independent risk factor for poor functional outcome, and the ICH outcome score combining D-dimer level could effectively evaluate and predict mortality at 3 months after ICH. Taken together, we propose that a combination of clinical grading scales and serum inflammatory biomarkers could aid to more exhaustively explain and refine the ICH prognosis and should be further analyzed for its potential to predict the outcome after ICH.
Our study has several limitations. First, this was a retrospective study, the selection bias may exist, and given the subselection of only surgical patients, which may affect the catholicity of the study results. Second, this study did not assess the role of hematological parameters in the prognosis of sICH. Third, the number of patients available for analysis was small, and no patients showed the ICH-mGS score of 6–7, making the assessment of outcome less reliable. Further studies with a prospective and multicenter trial are required to confirm these findings.
Conclusion
Our study showed that mGS was an independent risk factor for poor outcome and it had an additive predictive value for outcome in patients with sICH undergoing surgical treatment. Compared with the ICH score and mGS alone, the ICH score combined with mGS exhibited a better predictive ability for the functional prognosis of patients with sICH undergoing surgical treatment.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Fuyang Fifth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception or design of the study: SW and RW. Data collection: SW, QY, and RW. Data analysis and interpretation: SW, XX, and CH. Drafting this article: SW. Critical revision of this article: RW. Others (study supervision, funding, materials): HH and RW. All authors reviewed the results and approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
sICH, spontaneous intracerebral hemorrhage; GCS, Glasgow coma scale; IVH, intraventricular hemorrhage; mGS, modified Graeb Score; CT, computed tomography; EVD, external ventricular drainage; GOS, Glasgow outcome scale; VIF, variance inflation factor; ICH-mGS score, ICH score combining mGS; ROC, receiver operating characteristic; AUC, area under the curve; OR, odds ratio; CI, confidence interval; oGS, original Graeb Scale; HE, hematoma expansion; NLR, neutrophil-to-lymphocyte ratio.
References
1. Brainin M, Bornstein N, Boysen G, Demarin V. Acute neurological stroke care in Europe: results of the European Stroke Care Inventory. Eur J Neurol. (2000) 7:5–10. doi: 10.1046/j.1468-1331.2000.007001005.x
2. de Oliveira Manoel AL, Goffi A, Zampieri FG, Turkel-Parrella D, Duggal A, Marotta TR, et al. The critical care management of spontaneous intracranial hemorrhage: a contemporary review. Crit Care. (2016) 20:272. doi: 10.1186/s13054-016-1432-0
3. Sun T, Chen S, Wu K, Sun M, Zhang X, You C. Trends in incidence and mortality of stroke in China from 1990 to 2019. Front Neurol. (2021) 12:759221. doi: 10.3389/fneur.2021.759221
4. Mitchell P, Gregson BA, Vindlacheruvu RR, Mendelow AD. Surgical options in Ich including decompressive craniectomy. J Neurol Sci. (2007) 261:89–98. doi: 10.1016/j.jns.2007.04.040
5. Hemphill JC. 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ich score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. (2001) 32:891–7. doi: 10.1161/01.STR.32.4.891
6. Trifan G, Arshi B, Testai FD. Intraventricular hemorrhage severity as a predictor of outcome in intracerebral hemorrhage. Front Neurol. (2019) 10:217. doi: 10.3389/fneur.2019.00217
7. Morgan TC, Dawson J, Spengler D, Lees KR, Aldrich C, Mishra NK, et al. The modified graeb score: an enhanced tool for intraventricular hemorrhage measurement and prediction of functional outcome. Stroke. (2013) 44:635–41. doi: 10.1161/STROKEAHA.112.670653
8. Yu Y, Li HJ. Diagnostic and prognostic value of procalcitonin for early intracranial infection after craniotomy. Braz J Med Biol Res. (2017) 50:e6021. doi: 10.1590/1414-431x20176021
9. Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, et al. The Abcs of measuring intracerebral hemorrhage volumes. Stroke. (1996) 27:1304–5. doi: 10.1161/01.STR.27.8.1304
10. Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the Ct-angiography spot sign (Predict): a prospective observational study. Lancet Neurol. (2012) 11:307–14. doi: 10.1016/S1474-4422(12)70038-8
11. Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. (2011) 76:1238–44. doi: 10.1212/WNL.0b013e3182143317
12. Li Q, Li R, Zhao LB, Yang XM, Yang WS, Deng L, et al. Intraventricular hemorrhage growth: definition, prevalence and association with hematoma expansion and prognosis. Neurocrit Care. (2020) 33:732–9. doi: 10.1007/s12028-020-00958-8
13. Hemphill JC. 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2015) 46:2032–60. doi: 10.1161/STR.0000000000000069
14. Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the glasgow outcome scale and the extended glasgow outcome scale: guidelines for their use. J Neurotrauma. (1998) 15:573–85. doi: 10.1089/neu.1998.15.573
15. Marcoulides KM, Raykov T. Evaluation of variance inflation factors in regression models using latent variable modeling methods. Educ Psychol Meas. (2019) 79:874–82. doi: 10.1177/0013164418817803
16. Bhatia K, Hepburn M, Ziu E, Siddiq F, Qureshi AI. Modern approaches to evacuating intracerebral hemorrhage. Curr Cardiol Rep. (2018) 20:132. doi: 10.1007/s11886-018-1078-4
17. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (mistie): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. (2016) 15:1228–37.
18. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. (2012) 43:2923–30. doi: 10.1161/STROKEAHA.112.667535
19. Pantazis G, Tsitsopoulos P, Mihas C, Katsiva V, Stavrianos V, Zymaris S. Early Surgical Treatment Vs Conservative Management for Spontaneous Supratentorial Intracerebral Hematomas: A Prospective Randomized Study. Surg Neurol. (2006) 66:492–501. doi: 10.1016/j.surneu.2006.05.054
20. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. (2001) 344:1450–60. doi: 10.1056/NEJM200105103441907
21. Arboix A, Massons J, García-Eroles L, Oliveres M, Targa C. Diabetes is an independent risk factor for in-hospital mortality from acute spontaneous intracerebral hemorrhage. Diabetes Care. (2000) 23:1527–32. doi: 10.2337/diacare.23.10.1527
22. Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. (2009) 40:394–9. doi: 10.1161/STROKEAHA.108.523209
23. Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. a powerful and easy-to-use predictor of 30-day mortality. Stroke. (1993) 24:987–93. doi: 10.1161/01.STR.24.7.987
24. Chen HS, Hsieh CF, Chau TT, Yang CD, Chen YW. Risk factors of in-hospital mortality of intracerebral hemorrhage and comparison of ich scores in a Taiwanese population. Eur Neurol. (2011) 66:59–63. doi: 10.1159/000328787
25. Mustanoja S, Satopää J, Meretoja A, Putaala J, Strbian D, Curtze S, et al. Extent of secondary intraventricular hemorrhage is an independent predictor of outcomes in intracerebral hemorrhage: data from the helsinki ich study. Int J Stroke. (2015) 10:576–81. doi: 10.1111/ijs.12437
26. Witsch J, Bruce E, Meyers E, Velazquez A, Schmidt JM, Suwatcharangkoon S, et al. Intraventricular hemorrhage expansion in patients with spontaneous intracerebral hemorrhage. Neurology. (2015) 84:989–94. doi: 10.1212/WNL.0000000000001344
27. Young WB, Lee KP, Pessin MS, Kwan ES, Rand WM, Caplan LR. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology. (1990) 40:616–9. doi: 10.1212/WNL.40.4.616
28. Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: part 2. in vivo safety study of intraventricular Urokinase. Neurosurgery. (1986) 19:547–52. doi: 10.1227/00006123-198610000-00009
29. Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke. (2009) 40:1533–8. doi: 10.1161/STROKEAHA.108.535419
30. Tuhrim S, Horowitz DR, Sacher M, Godbold JH. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med. (1999) 27:617–21. doi: 10.1097/00003246-199903000-00045
31. Hwang BY, Bruce SS, Appelboom G, Piazza MA, Carpenter AM, Gigante PR, et al. Evaluation of intraventricular hemorrhage assessment methods for predicting outcome following intracerebral hemorrhage. J Neurosurg. (2012) 116:185–92. doi: 10.3171/2011.9.JNS10850
32. Hansen BM, Morgan TC, Betz JF, Sundgren PC, Norrving B, Hanley DF, et al. Intraventricular extension of supratentorial intracerebral hemorrhage: the modified graeb scale improves outcome prediction in lund stroke register. Neuroepidemiology. (2016) 46:43–50. doi: 10.1159/000442575
33. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. (2012) 11:101–18. doi: 10.1016/S1474-4422(11)70264-2
34. Kazui S, Naritomi H, Yamamoto H, Sawada T, Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage. incidence and time course. Stroke. (1996) 27:1783–7. doi: 10.1161/01.STR.27.10.1783
35. Al-Shahi Salman R, Frantzias J, Lee RJ, Lyden PD, Battey TWK, Ayres AM, et al. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. (2018) 17:885–94. doi: 10.1016/S1474-4422(18)30253-9
36. Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. (2006) 66:1175–81. doi: 10.1212/01.wnl.0000208408.98482.99
37. Wang X, Arima H, Al-Shahi Salman R, Woodward M, Heeley E, Stapf C, et al. Clinical prediction algorithm (brain) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. (2015) 46:376–81. doi: 10.1161/STROKEAHA.114.006910
38. Nehme A, Ducroux C, Panzini MA, Bard C, Bereznyakova O, Boisseau W, et al. Non-Contrast Ct markers of intracerebral hematoma expansion: a reliability study. Eur Radiol. (2022). doi: 10.1007/s00330-022-08710-w
39. Maas MB, Nemeth AJ, Rosenberg NF, Kosteva AR, Prabhakaran S, Naidech AM. Delayed intraventricular hemorrhage is common and worsens outcomes in intracerebral hemorrhage. Neurology. (2013) 80:1295–9. doi: 10.1212/WNL.0b013e31828ab2a7
40. Moullaali TJ, Sato S, Wang X, Rabinstein AA, Arima H, Carcel C, et al. Prognostic significance of delayed intraventricular haemorrhage in the interact studies. J Neurol Neurosurg Psychiatry. (2017) 88:19–24. doi: 10.1136/jnnp-2015-311562
41. Dowlatshahi D, Deshpande A, Aviv RI, Rodriguez-Luna D, Molina CA, Blas YS, et al. do intracerebral hemorrhage nonexpanders actually expand into the ventricular space? Stroke. (2018) 49:201–3. doi: 10.1161/STROKEAHA.117.018716
42. Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. Hematoma growth and outcomes in intracerebral hemorrhage: the interact1 study. Neurology. (2012) 79:314–9. doi: 10.1212/WNL.0b013e318260cbba
43. Øie LR, Madsbu MA, Solheim O, Jakola AS, Giannadakis C, Vorhaug A, et al. Functional outcome and survival following spontaneous intracerebral hemorrhage: a retrospective population-based study. Brain Behav. (2018) 8:e01113. doi: 10.1002/brb3.1113
44. Wang CW, Liu YJ, Lee YH, Hueng DY, Fan HC, Yang FC, et al. Hematoma shape, hematoma size, glasgow coma scale score and ich score: which predicts the 30-day mortality better for intracerebral hematoma? PLoS ONE. (2014) 9:e102326. doi: 10.1371/journal.pone.0102326
45. Xanthos DN, Sandkühler J. Neurogenic neuroinflammation: inflammatory Cns reactions in response to neuronal activity. Nat Rev Neurosci. (2014) 15:43–53. doi: 10.1038/nrn3617
46. Wang J, Doré S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. (2007) 27:894–908. doi: 10.1038/sj.jcbfm.9600403
47. Morotti A, Phuah CL, Anderson CD, Jessel MJ, Schwab K, Ayres AM, et al. Leukocyte count and intracerebral hemorrhage expansion. Stroke. (2016) 47:1473–8. doi: 10.1161/STROKEAHA.116.013176
48. Yu S, Arima H, Heeley E, Delcourt C, Krause M, Peng B, et al. White Blood cell count and clinical outcomes after intracerebral hemorrhage: the interact2 trial. J Neurol Sci. (2016) 361:112–6. doi: 10.1016/j.jns.2015.12.033
49. Bernstein JE, Savla P, Dong F, Zampella B., Wiginton JGt, Miulli DE, et al. Inflammatory markers and severity of intracerebral hemorrhage. Cureus. (2018) 10:e3529. doi: 10.7759/cureus.3529
50. Sobrino T, Arias S, Rodríguez-González R, Brea D, Silva Y., de la Ossa NP, et al. High serum levels of growth factors are associated with good outcome in intracerebral hemorrhage. J Cereb Blood Flow Metab. (2009) 29:1968–74. doi: 10.1038/jcbfm.2009.182
51. Yang G, Shao GF. Elevated Serum Il-11, Tnf, and Vegf expressions contribute to the pathophysiology of hypertensive intracerebral hemorrhage (hich). Neurol Sci. (2016) 37:1253–9. doi: 10.1007/s10072-016-2576-z
52. Zhang F, Ren Y, Fu W, Yang Z, Wen D, Hu X, et al. Predictive accuracy of neutrophil-to-lymphocyte ratio on long-term outcome in patients with spontaneous intracerebral hemorrhage. World Neurosurg. (2019) 125:e651–e7. doi: 10.1016/j.wneu.2019.01.143
53. Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. (2018) 387:98–102. doi: 10.1016/j.jns.2018.01.038
54. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-Lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. (2017) 8:57489–94. doi: 10.18632/oncotarget.15423
Keywords: modified Graeb score, intracerebral hemorrhage score, surgical treatment, outcome, spontaneous intracerebral hemorrhage
Citation: Wang S, Xu X, Yu Q, Hu H, Han C and Wang R (2022) Combining modified Graeb score and intracerebral hemorrhage score to predict poor outcome in patients with spontaneous intracerebral hemorrhage undergoing surgical treatment. Front. Neurol. 13:915370. doi: 10.3389/fneur.2022.915370
Received: 07 April 2022; Accepted: 11 July 2022;
Published: 29 July 2022.
Edited by:
Alastair Webb, University of Oxford, United KingdomReviewed by:
Simona Lattanzi, Marche Polytechnic University, ItalyQi Li, The First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2022 Wang, Xu, Yu, Hu, Han and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruhai Wang, drwangruhai@163.com
 Shen Wang1
Shen Wang1 Ruhai Wang
Ruhai Wang