- 1University Hospital St. Ivan Rilski, Sofia, Bulgaria
- 2Alexandrovska Hospital, Sofia, Bulgaria
Thought to be benign anatomical variants, cerebral infundibular dilatations (ID) are most commonly encountered at the junction of the internal carotid artery (ICA) and the posterior communicating artery (PcomA). The true nature of this entity remains controversial, as some literature reports suggest they should be considered preaneurysmal lesions and a potential source of devastating subarachnoid hemorrhage. This report describes cases of presumably ruptured IDs and their therapeutic endovascular management. We retrospectively reviewed and analyzed patients with isolated subarachnoid hemorrhage (SAH) where the only potential cause was ruptured cerebral IDs, treated or not, between January 2012 and June 2021. Morphological and radiological features, treatment and procedural considerations, clinical and angiographic outcomes were also reviewed. Natural history of the ID is poorly understood, and its relation to SAH remains controversial. Ruptured cerebral IDs can be the suspected cause of bleeding if no other vascular lesion is present during multimodal examinations. Endovascular flow diversion stenting is safe and effective for the proper treatment of ruptured IDs. Pending further validations with longitudinal data are needed to legitimate the natural course of these mysterious lesions.
Introduction
The clinical significance of an infundibular dilatation (ID) at the origin of an intracranial artery remains a matter of debate. Those junctional dilatations have been postulated to have specific radiological characteristics with qualitative parameters including a maximum diameter of 3 mm, teardrop bulging or funnel-like shapes, and a lack of an aneurysmal-like neck (1). An infundibulum usually can be seen at branching sites within the intracranial circulation, typically at the internal carotid artery (ICA) and the posterior communicating artery orifice (2). However, IDs can less frequently occur at the level of the anterior choroidal artery, ophthalmic artery, anterior communicating artery, or superior cerebellar artery, or across the middle cerebral artery (Figure 1) (3, 4).
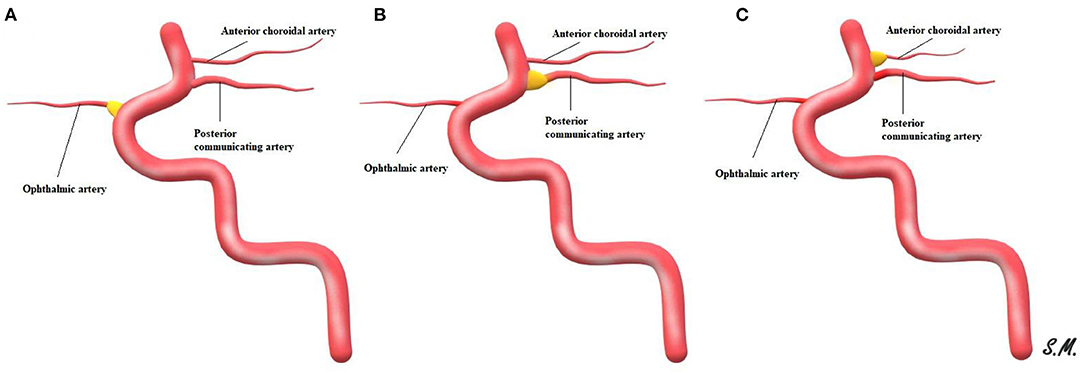
Figure 1. Schematic representation of ID with the adjacent origin of an intracranial artery, ophthalmic artery ID (A), posterior communicating artery ID (B) and ID at anterior choroidal artery (C).
Although little is known regarding the actual natural courses of these entities, intracranial arterial infundibulums are commonly encountered during neurovascular clinical examinations (5). The reported incidence varies between 2.2 and 24.6% at the population level, and these entities may be misdiagnosed as aneurysms on non-invasive imaging (6, 7). IDs are commonly accepted to be benign lesions or normal anatomic variations whose clinical significance is unclear or non-existent (8). However, multiple reports have described ruptures with clinical sequelae and aneurysmal transformations of an ID (9–12). A diagnostic and therapeutic dilemma may occur only after a junctional dilatation is identified in the setting of unexplained subarachnoid hemorrhage (SAH) (13). The available literature is scarce and insufficiently consistent to entirely resolve the clinical uncertainties of IDs. Herein, we aim to emphasize and report the experience of a moderate-volume single center regarding the diagnosis and treatment management of ruptured IDs in the setting of otherwise unexplained SAH.
Methods
Patients
An institutional review board-approved retrospective review was performed to identify and analyze patients with isolated and non-aneurysmal SAH. The only objective cause was ruptured cranial IDs, either treated or untreated, between January 2012 and June 2021. A search of the institutional medical record database revealed eight patients with IDs as a potential source of SAH. Among them, we treated six patients whose cases had not been previously published or reported.
IDs were defined as lesions meeting the following explicit morphological criteria: symmetrical cone-like outpouching at the level of the arterial ostium, with no distinct neck and a maximum diameter <3 mm. SAH due to ruptured ID was considered a diagnosis if no other vascular lesions were present during multimodal radiological examinations and delayed (7 days after onset) angiographic imaging. Three-dimensional rotational angiography (3DRA) was used to rule out blister aneurysms or dissecting aneurysms involving the PcomA origin that might mimic an ID.
The infundibulum's geometric ratios and morphological characteristics, and the involved parent artery were assessed during diagnosis, treatment, and radiological follow-up.
The patients' demographics, clinical and neurological status, and radiological records were obtained and reviewed. The clinical outcomes were measured and evaluated with the modified Rankin Scale at discharge. The parents' arterial vascular remodeling and ID occlusion were assessed according to the O'Kelly-Marotta scale during the endovascular procedure and 3–12 months after flow diversion (14).
Treatment Considerations
According to our local institutional policy for all patients diagnosed with an acute SAH due to a possible ID rupture, all cases were discussed in neurovascular multidisciplinary team meetings. Concerns regarding the diagnosis itself, treatment indications, and modalities were fundamental aspects of each case analysis. The treatment decisions were based on all the aforementioned medical criteria, including the anticipated rebleeding prevention.
In general, the medical management of these patients did not significantly diverge from commonly accepted therapeutic protocols (15, 16). The only protocol deviation was the mandatory antiplatelet therapy on the conducted flow diverter stent (FD) implantation. The deployment of the flow modulation stent was aimed to fully cover IDs and the ostium of the involved parent artery.
Management of Antiplatelet Therapy
All patients undergoing endovascular intervention received either a loading dose (per os) of aspirin (300 mg) and prasugrel (40 mg) 7 h before the procedure or an intraprocedural bolus dosage of glycoprotein IIb/IIIa inhibitor. In the post-treatment phase, a standard daily dose of 1 × 10 mg of prasugrel and 100 mg of aspirin were maintained for at least 6 months. Adequate therapy response was measured with VerifyNow assays (Accumetrics, San Diego, CA, USA) with a desired PRU < 120.
Results
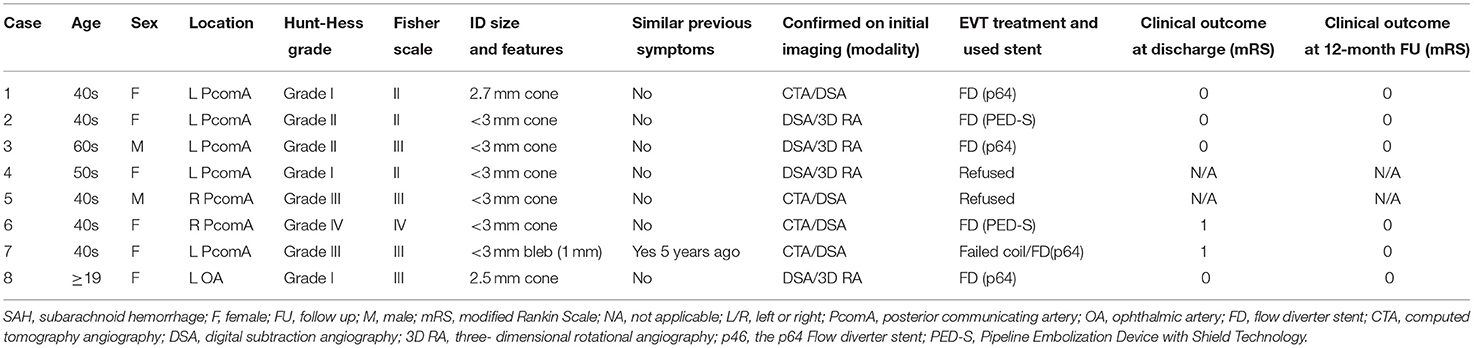
Patients' Baseline and Clinical Characteristics
The demographics and clinical details of the reviewed patients are summarized and presented in Table 1. We identified eight patients (six of whom were women), whose mean age was 44.5 years (range 19–60). Most IDs were located at the level of the PcomA (n = 7), and only one was located on the arterial orifice of the ophthalmic artery (OA). All patients reported in our series presented with radiologically confirmed acute SAH. Only one (12.5%) patient experienced SAH after a re-rupture earlier in life. Pretreatment Hunt and Hess (H&H) grade I and II were observed in five (62.5%) patients. However, two (25%) patients experienced severe and diffuse SAH resulting in H&H grade III, and one had an H&H score of IV.
In all cases, the initial computed tomography angiography (CTA) did not indicate that the presence of an aneurysm or any other vascular lesion was responsible for the SAH. However, in four cases, unilateral PcomA IDs were noted. In those cases, the SAH distribution from the non-contrast-enhanced CT scan was located predominantly near the anatomical location of the observed PcomA IDs into the origin of the ipsilateral suprasellar cistern and Sylvian fissure. One patient, patient No. 7, had a prior history of aneurysmal negative SAH 5 years prior.
The four IDs detected in the CTA were verified through subsequent digitally subtracted angiography (DSA). In general, DSA and preoperative 3DRA detected all IDs and indicated an absence of any other vascular pathology associated with the SAH. Delayed DSA examinations 7 days after onset confirmed the above findings in all eight patients. Endovascular therapy (EVT) of the ruptured ID was performed in six (75%) patients. Two patients (25%) rejected the therapeutic intervention because of their individual beliefs.
Treatment Modality and Feasibility
The technical, procedural, and clinical results are summarized in Table 2.
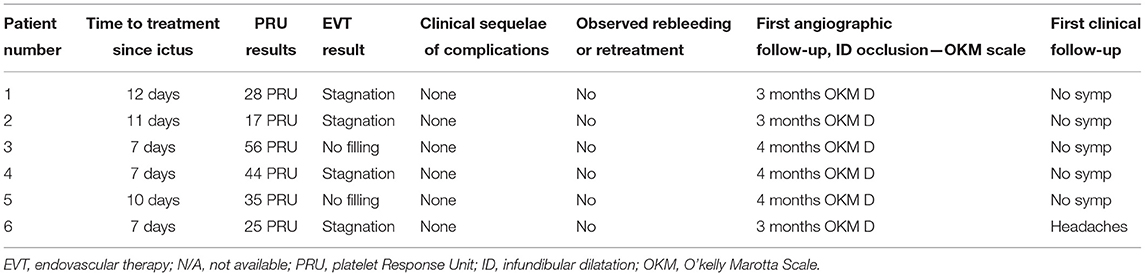
Table 2. Procedural, clinical, and follow-up angiographic results after the endovascular intervention.
Of the eight patients with SAH due to a ruptured ID, six underwent EVT in the acute phase of SAH. For the patients undergoing EVT, treatment of the ruptured ID was performed via implantation of an FD across the documented focal lesion. In all patients, only one FD was used; of the FDs used, two (33.3%) had additional antithrombotic surface coating. In all procedures, the attempted implantation of the FD was feasible. No unexpected procedure-related complications were encountered. No cases of recurrent bleeding resulted from the endovascular intervention or the mandatory antiplatelet medication. SAH related complications were observed, and all six patients demonstrated favorable outcomes, with modified Rankin Scale scores of 0 or 1 at discharge.
Angiographic and Clinical Follow-Up
At least two cross-sectional radiological and clinical follow-ups were available for all patients who underwent EVT. The two patients who refused treatment were lost to follow-up after the initial presentation and discharge. The initial angiographic follow-up was conducted after a median of 3 months (range 3–4 months) after the intervention. No patients reported any post-treatment clinical and neurological symptoms associated with ID rerupture during the follow-up examinations. No mortality or morbidity was observed in this group.
Illustrative Cases
Case 1
A 46-year-old woman was admitted to another hospital for a sudden thunderclap headache (Figure 2). CT revealed diffuse SAH with increased blood collection in the left suprasellar cistern and left-sided Sylvian fissure. Subsequent CTA did not confirm the presence of any cerebrovascular findings that could be responsible for the acute SAH. DSA did not detect any intracranial aneurysms but revealed an ID at the origin of the left PcomA. The delayed 3DRA/DSA 7 days after onset identified a possible rupture bleb on the lateral side of the left PcomA ID. The patient had a history of an identical episode occurring 5 years earlier, but the radiological examinations did not confirm any intracranial hemorrhage. The patient's medical records included trombangiitis obliterans (Buerger disease), idiopathic thrombocytopenic purpura, and uncontrolled hypertonia. On the basis of the above findings, we concluded that the tiny bleb on the PcomA ID was responsible for the intracranial hemorrhage. Endovascular coil embolization of the ruptured infundibular bleb was performed but was unsuccessful. Coiling of the ruptured dilatation was not possible because the coils protruded toward the parent artery's lumen. Bailout flow diversion was considered appropriate for this case. The patient received a bolus dosage of glycoprotein IIb/IIIa–tirofiban and received a p64 (phenox, Bochum, Germany) FD. Contrast stagnation inside the ruptured bleb was observed on the final angiograph. The patient was discharged after 14 days with no new neurological deficits detected.
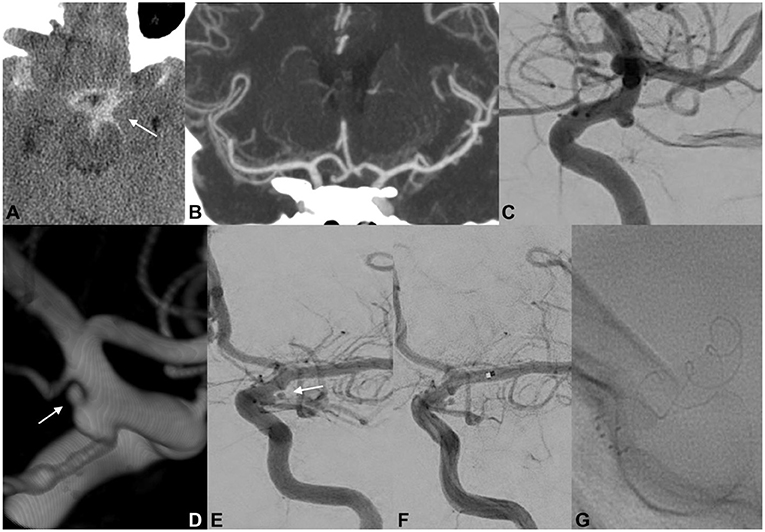
Figure 2. EVT of a ruptured PcomA ID. An initial CT scan demonstrated the presence of SAH predominantly distributed across the left basal cisterns (A; white arrow). Cranial CTA did not document any cerebrovascular findings that could have caused the SAH (B). At 6 h after onset, catheter angiography confirmed the absence of a ruptured aneurysm but revealed a typical ID at the origin of the left PcomA (C). Delayed 3DRA/DSA (D,E; white arrows) 7 days after onset identified a possible rupture bleb on the lateral side of the left PcomA ID. Endovascular coil embolization of the ruptured bleb was unsuccessful, and a p64 FD was carefully deployed across the C7 segment of the left ICA (F,G). Contrast stagnation inside the ruptured ID bleb was seen on the delayed angiographic phase (not provided).
Case 2
A 19-year-old woman was found unconscious at home (Figure 3). Her last memory was of an extreme burning sensation engulfing her neck and scalp. CT revealed a thin SAH mainly distributed in the left suprasellar cistern and left hemispheral subarachnoid spaces. CT cranial angiography and initial DSA did not indicate any aneurysmal dilatations or other vascular findings. Subsequently, 3DRA revealed the presence of an ID at the level of the orifice of the left ophthalmic artery. The secondary delayed DSA indicated the presence of a focal irregularity adjacent to the OA ID. The patient repeatedly reported having observed increasing flashes of light and bright spots in her left eye during this period. A ruptured OA ID was hypothesized to have been responsible for the cerebral hemorrhage, and the patient was scheduled for EVT with an FD stent. The patient was premedicated with a loading dose of DAP, and the implantation of the endoluminal flow modulation device was performed uneventfully. The patient was discharged without any related procedural complications. Follow-up angiography demonstrated no infundibular widening and the complete preservation of the OA.
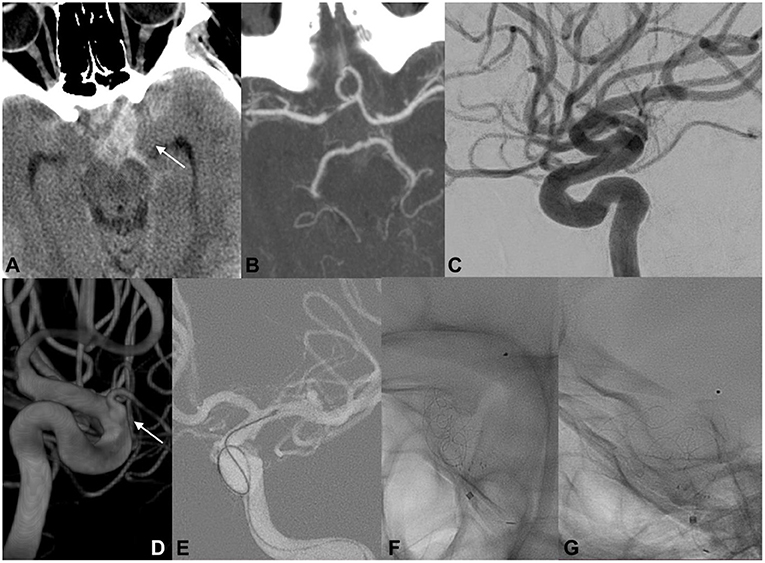
Figure 3. EVT of ruptured OA ID in a young woman. The initial radiological work-up indicated intracranial hemorrhage, suggesting the rupture of an intracranial aneurysm (A; white arrow). Cranial CTA and initial DSA did not reveal any cerebrovascular pathologies that could be associated with the hemorrhage (B,C). Three-dimensional rotational angiography demonstrated the presence of an ID at the level of the orifice of the left ophthalmic artery (D; white arrow). Endovascular FD deployment was performed with complete coverage of the OA infundibular orifice (E–G).
Case 3
A 60-year-old man was admitted to the hospital with severe headache, nausea, vomiting, and prominent neck stiffness (Figure 4). He reported that the headache intensity had worsened in the prior 7 days and did not respond to analgesics. CT demonstrated the presence of a diffuse SAH with a focal distribution mainly into the chiasmatic, interpeduncular, and left crural cisterns. CTA did not reveal any cerebrovascular pathology but confirmed the presence of an ID at the level of the left PcomA. The patient underwent catheter angiography, which yielded similar observations to those of the initial and delayed DSA examinations. Considering the above findings, we assumed that the most probable cause of the SAH was the rupture of the PcomA ID on the left. A loading dose of DAP was administered, and the patient underwent uneventful FD placement across the C7 segment of the left ICA. Noticeable contrast stagnation was found in the PcomA ID after stent implantation. The patient was discharged after 2 weeks of conservative management with no neurological deficits. Both mid- and long-term follow-up radiological examinations confirmed the complete remodeling of the PcomA with total obliteration of the treated ID.
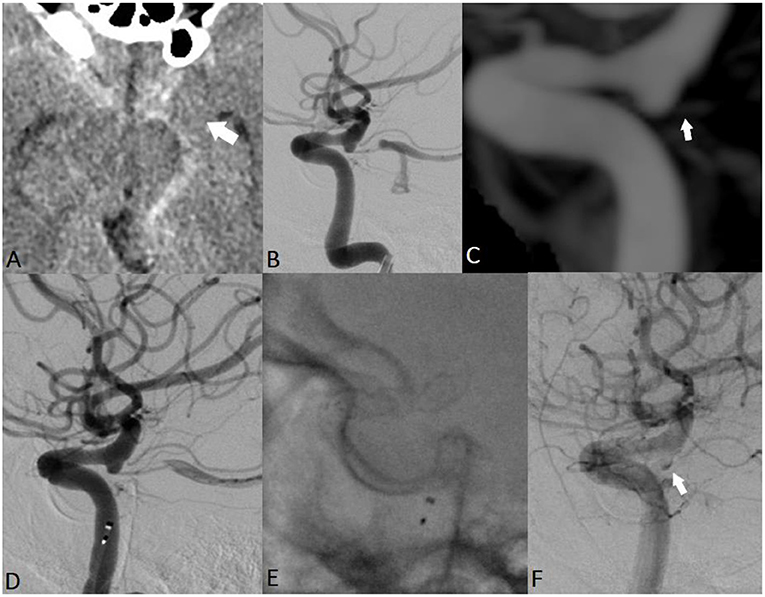
Figure 4. EVT of a ruptured PcomA ID in a 60-year-old man. Non-contrast cranial CT revealed a thin hemorrhage in the chiasmatic, interpeduncular, and left crural cisterns (A; white arrow). Subsequent repeated radiological examinations did not reveal any cerebrovascular pathology but confirmed the presence of an ID at the level of the left PcomA (B,C; white arrow). The assumed rupture site was the observed ID, and the patient received EVT (D,E). Noticeable contrast stagnation was observed in the PcomA ID after stent implantation (F).
Discussion
Pathogenesis and Histological Considerations
Arterial IDs are benign lesions commonly believed to be present in as many as one-quarter of cerebral angiograms (7, 13). Although these findings are considered normal vascular variants, whether they should be considered aneurysmal precursors, and candidates for clinical surveillance and potential treatment, remains a matter of debate (8, 17). We cannot fully determine whether this paradoxical understanding of IDs was initially derived from a divergence among published histological findings. Histologically, one of the first assumptions promoted by Hassler and Saltzmann was that cerebral infundibulum might resemble an aneurysm (18). Degenerative changes and arterial wall alterations were also noted in later studies histologically examining ID samples (19, 20). However, neither abnormalities nor structural arterial defects were observed in patients with junctional dilatations in the histological studies performed by Epstein et al. (21), who proposed that these conical dilatations might merely be developmental vessel remnants with non-conditional clinical significance.
Regardless of whether IDs exhibit degenerative changes in the internal elastic lamina, the lytic process of elastase has been demonstrated to play a role in the degenerative changes directly leading to aneurismal development (22). Small defects and structural anomalies within the internal elastic lamina have been documented in post-mortem analyses of ruptured ID (23). These biological events might result from focal disruption of the homeostatic balance in the affected arterial vessel wall, as a result of increased circumferential wall stress (24). Coupe et al. (25) have reported a surgically treated case of a ruptured ID with a macroscopically evident perforation site, thus suggesting that the ID bleeding might have resulted from wall weakness without prerequired aneurysmal transformation. The very geometrical definition of arterial infundibulum or funnel-shaped widenings at the branching sites of major cerebral arteries may lead to local fluid dynamics that result in further ID dilatation (26). Marshman et al. (27) have suggested that these hydraulic distentions, conditioned by Bernoulli's principle, might be directly associated with later aneurysmal transformation of an ID.
Clinical Significance
Although arterial IDs are present in up to 25% of cranial angiographs, fully distinguishing them from their aneurysmal counterparts remains difficult. Currently, non-invasive radiological imaging modalities, i.e., CTA and magnetic resonance angiography, have become the primary screening techniques used to detect, differentiate, and evaluate cerebral aneurysms. Superioinferior projections of cerebral CTA can sometimes aid in revealing the horizontal direction of the infundibulum. CTA has a considerably lower spatial resolution than DSA, thus resulting in poor and limited visualization of small vessels. In the case of hypoplastic but present PcomA, CTA may not aid in differentiating ID from an aneurysm, particularly at the ICA-PComA junction. According to Min et al., the sensitivity, specificity, and accuracy of CTA in differentiating cerebral aneurysms from IDs remain considerably high (28). Magnetic resonance imaging techniques with time-of-flight and volume rendering have been reported to have similarly high sensitivity and accuracy in detecting cerebral IDs (29). Furthermore, geometric parameters on magnetic resonance angiography axial source images can provide added value in the diagnostic process. Magnetic resonance imaging enables easy visualization of silhouettes of tiny or hypoplastic vessels adjacent to the infundibulum with fast spin-echo and fast imaging by using steady-state acquisition sequences. With fusion imaging, Satoh et al. have viewed the outer wall configuration of the ipsilateral ICA-PcomA junction to distinguish and differentiate both lesions successfully (30).
Despite being promoted as the “gold standard,” DSA has several diagnostic and technical limitations in flow alterations and poor contrast filling of the PcomA during cerebrovascular catheter examinations. Recent technological improvements and software implementations with sophisticated smoothing and visualization algorithms have indicated the value of 3DRA, whose superior diagnostic properties to those of two-dimensional DSA imaging for evaluating cerebral IDs have been described (31).
Anecdotally, differentiating an aneurysm from an ID might not be the only dilemma faced by physicians. Labeling a “negative” radiological result as SAH in cases in which cerebral ID is present might be an equally difficult decision. The incidence of “normal” radiological findings in patients with SAH has been frequently reported in the literature. However, case reports are providing increasing evidence suggesting that these entities are not necessarily benign in nature (1, 29, 32–35). Particular radiological patterns, such as predominantly distributed SAH close to the observed infundibulum, may suggest the rupture site and the cause of the hemorrhage. For example, Yu et al. have found concentrated blood clots on CT located across the ipsilateral suprasellar cistern, interhemispheric cistern, and ipsilateral Sylvian fissure (33). Post-mortem reports have confirmed diagnoses of SAH associated with ID ruptures, thus underscoring the diagnostic challenges. Therefore, the question remains as to how often cerebral ID related hemorrhages are being neglected and misdiagnosed.
No defined guidelines or established clinical protocols exist regarding radiological follow-up of IDs in young patients. Because arterial IDs appear to be active lesions, radiological surveillance might be justified in these patients. In the literature, isolated case reports and case series have described progressive aneurysmal formation from ID (21, 26, 36, 37). Recently, Lee et al. have reported seven patients with ruptured PcomA IDs: two patients experienced directly ruptured IDs, whereas the other five experienced ruptured aneurysms originating from IDs. Only IDs of the PcomA had been incidentally identified during previous aneurysm treatment. However, the mean time between the identification of a PcomA ID and the rupture event was 9.2 ± 4.8 years. Fischer et al. have documented the objective transformation of a PcomA ID to a saccular aneurysm during follow-up angiography 7 years after the initial identification of the conical dilatation (38). Donauer et al. (39) have described a tiny acutely ruptured aneurysm (<2 mm fundus diameter) located on the infundibulum surface in a 62-year-old woman—an exemplary case demonstrating the feasibility and limitations of microvascular and endovascular approaches in managing these lesions.
In the present study, we reported our experience in diagnostic and endovascular management of ruptured cerebral IDs. Over more than 9 years, we identified eight patients with direct rupture of a cerebral ID. Multimodality imaging and specific radiological patterns drawn from the available literature allowed us to carefully conclude that an ID was the cause of the SAH in each case. In our experience, 3DRA remains the best diagnostic tool to successfully resolve any diagnostic discrepancies. Using post-processing reconstructions, we identified 0.5–0.7 mm blebs suspected to be ruptured sites. We strongly recommend performing at least one delayed radiological examination before planning any further action. Careful neuroprotection and blood pressure monitoring can meanwhile be successfully maintained.
Our experience in managing acutely ruptured complex and wide-necked aneurysms allowed us to successfully address these lesions. We consider endoluminal flow diversion to be an excellent stand-alone treatment candidate with high efficacy and a good safety profile (40). We acknowledge that acute SAH poses specific challenges to the safe use of FDs. Nevertheless, we suggest that careful analyses and strict selection of patients who could benefit from this integrated treatment are critical for its success.
It is essential to highlight that this study builds up around the SAH hypothesis due to ruptured IDs. The study case examples could only demonstrate that rupture can occur from an ID, but the inherent limitations of the design and the small sample size are not enough to the true nature of these lesions. Last but not least, with the more advanced FD technology, the available arsenal of stents, and the increased operator experience, we prove that such entities could be successfully adressed with a good safety profile. Tailoring a patient-specific approach with preoperative planning, adequate platelet inhibition and PRU testing, and competent operator experience should yield successful treatment results.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
SM, KS, KC, MP, and DM creating and writing the manuscript. MK, KM, KN, and VK participating in cases and review of the manuscript. SS performing the cases and final review. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pool JL, Potts DG. Aneurysms and Arteriovenous Anomalies of the Brain : Diagnosis and Treatment. New York, NY: Harper & Row (1965).
2. Taveras JM, Wood EH. Diagnostic Neuroradiology. 2d ed. Baltimore, MD: Williams & Wilkins Co. (1976).
3. Kim HS, Kim EJ, Lee KM, Choi WS. Aneurysm and infundibular dilatation at an unusual origin of the ophthalmic artery. J Korean Soc Radiol. (2014) 71:164. doi: 10.3348/jksr.2014.71.4.164
4. Miyasaka K, Wolpert SM, Prager RJ. The association of cerebral aneurysms, infundibula, and intracranial arteriovenous malformations. Stroke. (1982) 13:196–203. doi: 10.1161/01.STR.13.2.196
5. Yuan J, Li Z, Jiang X, et al. Hemodynamic and morphological differences between unruptured carotid-posterior communicating artery bifurcation aneurysms and infundibular dilations of the posterior communicating artery. Front Neurol. (2020) 11:741. doi: 10.3389/fneur.2020.00741
6. Baskaya M, Coscarella E, Gomez F, Morcos J. Surgical and angiographic anatomy of the posterior communicating and anterior choroidal arteries. Neuroanatomy. (2004) 3:38–42. doi: 10.1016/S0531-5131(03)01408-0
7. Ebina K, Ohkuma H, Iwabuchi T. An angiographic study of incidence and morphology of infundibular dilatation of the posterior communicating artery. Neuroradiology. (1986) 28:23–9. doi: 10.1007/BF00341761
8. Griffin AS, Oppong MD, Hauck EF. Infundibular dilations and subarachnoid hemorrhage: to treat or not to treat? World Neurosurg. (2019) 123:188–92. doi: 10.1016/j.wneu.2018.12.007
9. Kuwahara S, Uga S, Mori K. Successful treatment of a ruptured enlarged infundibular widening of the posterior communicating artery—case report. Neurol Med Chir. (2001) 41:25–8. doi: 10.2176/nmc.41.25
10. Koike G, Seguchi K, Kyoshima K, Kobayashi S. Subarachnoid hemorrhage due to rupture of infundibular dilation of a circumflex branch of the posterior cerebral artery: case report. Neurosurgery. (1994) 34:1075–7. doi: 10.1227/00006123-199406000-00020
11. Radulovic D, Nestorovic B, Rakic M, Janosevic V. Enlargement to a saccular aneurysm and subsequent rupture of infundibular widening of posterior communicating artery. Neurochirurgie. (2006) 52:525–8. doi: 10.1016/S0028-3770(06)71360-1
12. Lee W, Han HJ, Kim J, Choi JY, Park KY, Kim YB, et al. Ruptured Infundibular dilatation of the posterior communicating artery. Acta Neurochir. (2021) 163:797–803. doi: 10.1007/s00701-021-04716-3
13. Saltzman GF. Infundibular widening of the posterior communicating artery studied by carotid angiography. Acta Radiol. (1959) 51:415–21. doi: 10.3109/00016925909171114
14. O'Kelly CJ, Krings T, Fiorella D, Marotta TR. A Novel Grading Scale for the angiographic assessment of intracranial aneurysms treated using flow diverting stents. Interv Neuroradiol. (2010) 16:133–7. doi: 10.1177/159101991001600204
15. Connolly ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
16. Maher M, Schweizer TA, Macdonald RL. Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke. (2020) 51:1326–32. doi: 10.1161/STROKEAHA.119.025997
17. Chen CJ, Moosa S, Ding D, et al. Infundibular dilations of the posterior communicating arteries: pathogenesis, anatomical variants, aneurysm formation, and subarachnoid hemorrhage. J NeuroIntervent Surg. (2016) 8:791–5. doi: 10.1136/neurintsurg-2015-011827
18. Hassler O, Saltzman GF. Angiographic and histologic changes in infundibular widening of the posterior communicating artery. Acta Radiol Diagn. (1963) 1:321–7. doi: 10.1177/028418516300100225
19. Stehbens WE. Histopathology of cerebral aneurysms. Arch Neurol. (1963) 8:272–85. doi: 10.1001/archneur.1963.00460030056005
20. Archer CR, Silbert S. Infundibula may be clinically significant. Neuroradiology. (1978) 15:247–51. doi: 10.1007/BF00342917
21. Epstein F, Ransohoff J, Budzilovich GN. The clinical significance of junctional dilatation of the posterior communicating artery. J Neurosurg. (1970) 33:529–31. doi: 10.3171/jns.1970.33.5.0529
22. Kim Ch, Cervós-Navarro J, Kikuchi H, Hashimoto N, Hazama F. Degenerative changes in the internal elastic lamina relating to the development of saccular cerebral aneurysms in rats. Acta Neurochir. (1993) 121:76–81. doi: 10.1007/BF01405187
23. Stuntz JT, Ojemann GA, Alvord EC. Radiographic and histologic demonstration of an aneurysm developing on the infundibulum of the posterior communicating artery: case report. J Neurosurg. (1970) 33:591–5. doi: 10.3171/jns.1970.33.5.0591
24. Kataoka H, Yagi T, Ikedo T, Imai H, Kawamura K, Yoshida K, et al. Hemodynamic and histopathological changes in the early phase of the development of an intracranial aneurysm. Neurol Med Chir. (2020) 60:319–28. doi: 10.2176/nmc.st.2020-0072
25. Coupe NJ, Athwal RK, Marshman LAG, Brydon HL. Subarachnoid hemorrhage emanating from a ruptured infundibulum. Surg Neurol. (2007) 67:204–6. doi: 10.1016/j.surneu.2006.05.066
26. Martins C, Macanovic M, Silva IEC e, Griz F, Azevedo-Filho HRC. Progression of an arterial infundibulum to aneurysm: case report. Arq Neuro-Psiquiatr. (2002) 60(2B):478–80. doi: 10.1590/S0004-282X2002000300026
27. Marshman LA, Ward PJ, Walter PH, Dossetor RS. The progression of an infundibulum to aneurysm formation and rupture: case report and literature review. Neurosurgery. (1998) 43:1445–9. doi: 10.1097/00006123-199812000-00107
28. Min KJ, Yoon DY, Kim HC, Lee JY, Cho BM. Infundibular dilation and aneurysm at the origin of the posterior communicating artery: differential diagnosis by CT angiography. Neuroradiology. (2014) 56:917–23. doi: 10.1007/s00234-014-1400-9
29. Sun ZK Li YD, Li MH, Chen SW, Tan HQ. Detection of infundibula using three-dimensional time-of-flight magnetic resonance angiography with volume rendering at 3. 0 Tesla compared to digital subtraction angiography. J Clin Neurosci. (2011) 18:504–8. doi: 10.1016/j.jocn.2010.07.128
30. Satoh T, Omi M, Ohsako C, Fujiwara K, Tsuno K, Sasahara W, et al. Differential diagnosis of the infundibular dilation and aneurysm of internal carotid artery: assessment with fusion imaging of 3D MR cisternography/angiography. AJNR Am J Neuroradiol. (2006) 27:306–12.
31. Shi WY Li YD, Li MH, Gu BX, Gu JP. Differential diagnosis of infundibular dilation versus a small aneurysm of the internal carotid artery: assessment by three-dimensional rotational angiography with volume rendering. Neurol Sci. (2013) 34:1065–70. doi: 10.1007/s10072-012-1182-y
32. Yu J, Wang H, Xu K, Wang B, Luo Q. Endovascular embolization of ruptured infundibular dilation of posterior communicating artery: a case report. Case Rep Med. (2010) 2010:1–5. doi: 10.1155/2010/210397
33. Yu J, Xu B, Liu Y, Xu B, Xu K. Progress in treating ruptured infundibular dilatation at the origin of the intracranial posterior communicating artery. Int J Clin Exp Med. (2015) 8:17080–7.
34. Ohyama T, Ohara S, Momma F. Fatal subarachnoid hemorrhage due to ruptured infundibular widening of the posterior communicating artery —case report. Neurol Med Chir. (1994) 34:172–5. doi: 10.2176/nmc.34.172
35. Trasi S, Vincent LM, Zingesser LH. Development of aneurysm from infundibulum of posterior communicating artery with documentation of prior hemorrhage. AJNR Am J Neuroradiol. (1981) 2:368–70.
36. Jang WY, Joo SP, Kim TS, Kim JH. An aneurysm developing on the infundibulum of posterior communicating artery : case report and literature review. J Korean Neurosurg Soc. (2006) 40:293–5.
37. Patrick D, Appleby A. Infundibular widening of the posterior communicating artery progressing to true aneurysm. Br J Radiol. (1983) 56:59–60. doi: 10.1259/0007-1285-56-661-59
38. Fischer S, Hopf N, Henkes H. Evolution from an infundibulum of the posterior communicating artery to a saccular aneurysm. Clin Neuroradiol. (2011) 21:87–90. doi: 10.1007/s00062-010-0038-1
39. Donauer E, Jedi F, Jangid N, Juergens M, Terstegge K, Henkes H. Posterior communicating artery aneurysm: subarachnoid hemorrhage from a small aneurysm located on an infundibulum of the posterior communicating artery; partial clipping of the aneurysm, followed by endovascular flow diversion, with good clinical outcome. In: Henkes H, Lylyk P, Ganslandt O, editors. The Aneurysm Casebook. Berlin, Heidelberg: Springer International Publishing (2020), p. 333–45. doi: 10.1007/978-3-319-77827-3_96
Keywords: infundibular dilatation, posterior communicating arteries, subarachnoid hemorrhage, flow diverter, anterior choroidal artery
Citation: Matanov S, Sirakova K, Chupetlovksa K, Penkov M, Monov D, Krupev M, Minkin K, Ninov K, Karakostov V and Sirakov S (2022) Flow Diversion for the Management of Ruptured Intracranial Arterial Infudibular Dilatation: Proof of Principle and Therapeutic Protocol. Front. Neurol. 13:913879. doi: 10.3389/fneur.2022.913879
Received: 06 April 2022; Accepted: 06 May 2022;
Published: 24 May 2022.
Edited by:
Xianli Lv, Tsinghua University, ChinaReviewed by:
Marie-Sophie Schüngel, University Hospital in Halle, GermanyVictoria Hellstern, Klinikum Stuttgart, Germany
Copyright © 2022 Matanov, Sirakova, Chupetlovksa, Penkov, Monov, Krupev, Minkin, Ninov, Karakostov and Sirakov. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stanimir Sirakov, c3NpcmFrb3ZAYnN1bml2ZXJzLmNvbQ==
 Svetozar Matanov1
Svetozar Matanov1 Kristina Sirakova
Kristina Sirakova Dimitar Monov
Dimitar Monov Krasimir Minkin
Krasimir Minkin Kristian Ninov
Kristian Ninov Stanimir Sirakov
Stanimir Sirakov