- Department of Neurosurgery, First Hospital of Jilin University, Changchun, China
The paraophthalmic segment of the internal carotid artery (ICA) originates from the distal border of the cavernous ICA and terminates at the posterior communicating artery. Aneurysms arising from the paraophthalmic segment represent ~5–10% of intradural aneurysms. Due to the advent of endovascular treatment (EVT) techniques, specifically flow-diverting stents (FDSs), EVT has become a good option for these aneurysms. A literature review on EVT for paraophthalmic segment aneurysms is necessary. In this review, we discuss the anatomy of the paraophthalmic segment, classification of the paraophthalmic segment aneurysms, EVT principle and techniques, and prognosis and complications. EVT techniques for paraophthalmic segment aneurysms include coil embolization, FDSs, covered stents, and Woven EndoBridge devices. Currently, coiling embolization remains the best choice for ruptured paraophthalmic segment aneurysms, especially to avoid long-term antiplatelet therapy for young patients. Due to the excessive use of antiplatelet therapy, unruptured paraophthalmic segment aneurysms that are easy to coil should not be treated with FDS. FDS is appropriate for uncoilable or failed aneurysms. Other devices cannot act as the primary choice but can be useful auxiliary tools. Both coiling embolization and FDS deployment can result in a good prognosis for paraophthalmic segment aneurysms. The overall complication rate is low. Therefore, EVT offers promising treatments for paraophthalmic segment aneurysms. In addition, surgical clipping continues to be a good choice for paraophthalmic segment aneurysms in the endovascular era.
Introduction
Traditionally, the ophthalmic segment of the internal carotid artery (ICA) has been defined as extending from the origin of the ophthalmic artery (OphA) to the origin of the posterior communicating artery (PcomA) (1). Recently, from an endovascular treatment (EVT) perspective, the paraophthalmic segment was proposed to optimize the description of the ICA originating from the distal border of the cavernous ICA and terminating at the PcomA origin (2). This nomenclature recognizes intrinsic uncertainty in the precise angiographic localization of aneurysms adjacent to the dural rings, regarding all lesions distal to the cavernous ICA as potentially intradural, which emphasizes their common features, and such lesions are increasingly addressed by endovascular means (2).
Aneurysms arising from paraophthalmic segments represent ~5–10% of intradural aneurysms (3, 4). Surgical clipping of these aneurysms poses challenges given complex nearby structures and a higher rate of visual complications (5, 6). Due to the advent of EVT techniques and devices, specifically flow-diverting stents (FDSs), EVT has become a good option for these aneurysms (7, 8). However, EVT may obviate some challenges. Currently, a complete review of the current status of EVT for aneurysms of the paraophthalmic segment is unavailable. Therefore, a review of the literature from a PubMed search is necessary. Additionally, we provide important images and educational cases in this review to increase reading interest.
Anatomy of the paraophthalmic segment
The definition of the ophthalmic segment of the ICA is inaccurate because the OphA can arise from the low clinoid segment or high supraclinoid segment of the ICA (9). Shapiro et al. (2) proposed the influential NYU (New York University) segmentation system, which divides the ICA into seven segments: cervical, petrous, cavernous, paraophthalmic, PcomA, choroidal, and terminal segments. The paraophthalmic segment that originated from the distal border of the cavernous ICA incorporates the clinoid segments of the classifications of the ICA by Bouthillier and Ziyal (Figure 1) (10, 11). The clinoid segment is a short and variable-length portion of the ICA limited to the area between the distal dural ring and the proximal ring (carotid-oculomotor membrane) (9, 12).
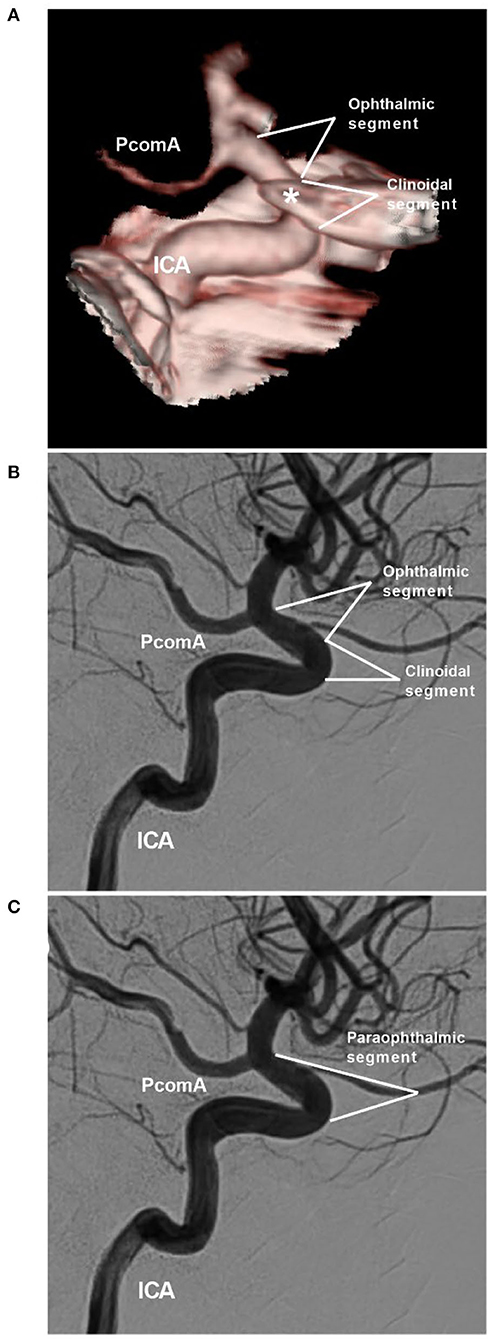
Figure 1. NYU paraophthalmic segment. (A,B) CTA (A) and DSA of the ICA (B) show the clinoidal and ophthalmic segments of the ICA. The segmentation is according to the classifications by Bouthillier and Ziyal. The asterisk in image (A) indicates the anterior clinoid process. (C) DSA shows the NYU segmentation. The clinoidal and ophthalmic segments of the ICA are combined into the paraophthalmic segment. CTA, computed tomography angiography; DSA, digital subtraction angiography; ICA, internal carotid artery; NYU, New York University; PcomA, posterior communicating artery.
Anatomically, the paraophthalmic segment of the ICA has two bends, of which the first is located at the ICA siphon where the vessel penetrates the dura, while the second is located near its termination; the length of this segment averages 1 cm (1). Regarding branches, the OphA is the largest branch of the paraophthalmic segment of the ICA; several large perforating vessels arise from the medial or inferomedial surface of the ICA and from the carotid cave of the ICA, and the number of perforators averages 3.6, of which the largest perforator is the superior hypophyseal artery (SHA) (1, 12).
Classification of the aneurysms
Previously, according to their relationship to adjacent anatomical landmarks, aneurysms of the paraophthalmic segment of the ICA have had various names and types, such as clinoid, paraclinoid, supraclinoid, and ICA dorsal/ventral aneurysms (13). Therefore, a clear, unified classification has been necessary. In this review, peripheral-type aneurysms originating from the OphA were excluded because they are not located on the ICA (14). Traumatic aneurysms were also excluded because they do not share the same pathogenesis (15).
Classification according to location
Aneurysms of the paraophthalmic segment are regarded as clearly or potentially intradural and include the categories of clinoid, carotid cave, OphA, and SHA aneurysms (2). Four classes can be proposed. The first type includes the clinoid and carotid cave families, which are totally or mainly located at the clinoid segment of the ICA (Figures 2A,B) (9, 16, 17). Carotid cave aneurysms belong to the clinoid segment and they share similar anatomic characteristics as clinoid aneurysms (9). However, carotid cave aneurysms are a unique subtype located in the carotid cave, which is a natural space medial to the clinoid segment of the ICA (12). The second type is OphA aneurysms (Figures 2C,D). The third type is SHA aneurysms (Figures 2E,F). The fourth type is the blood blister-like aneurysm (BBA), which originates at the perforator-free part of the anteromedial wall of the supraclinoid ICA (Figure 3) (4).
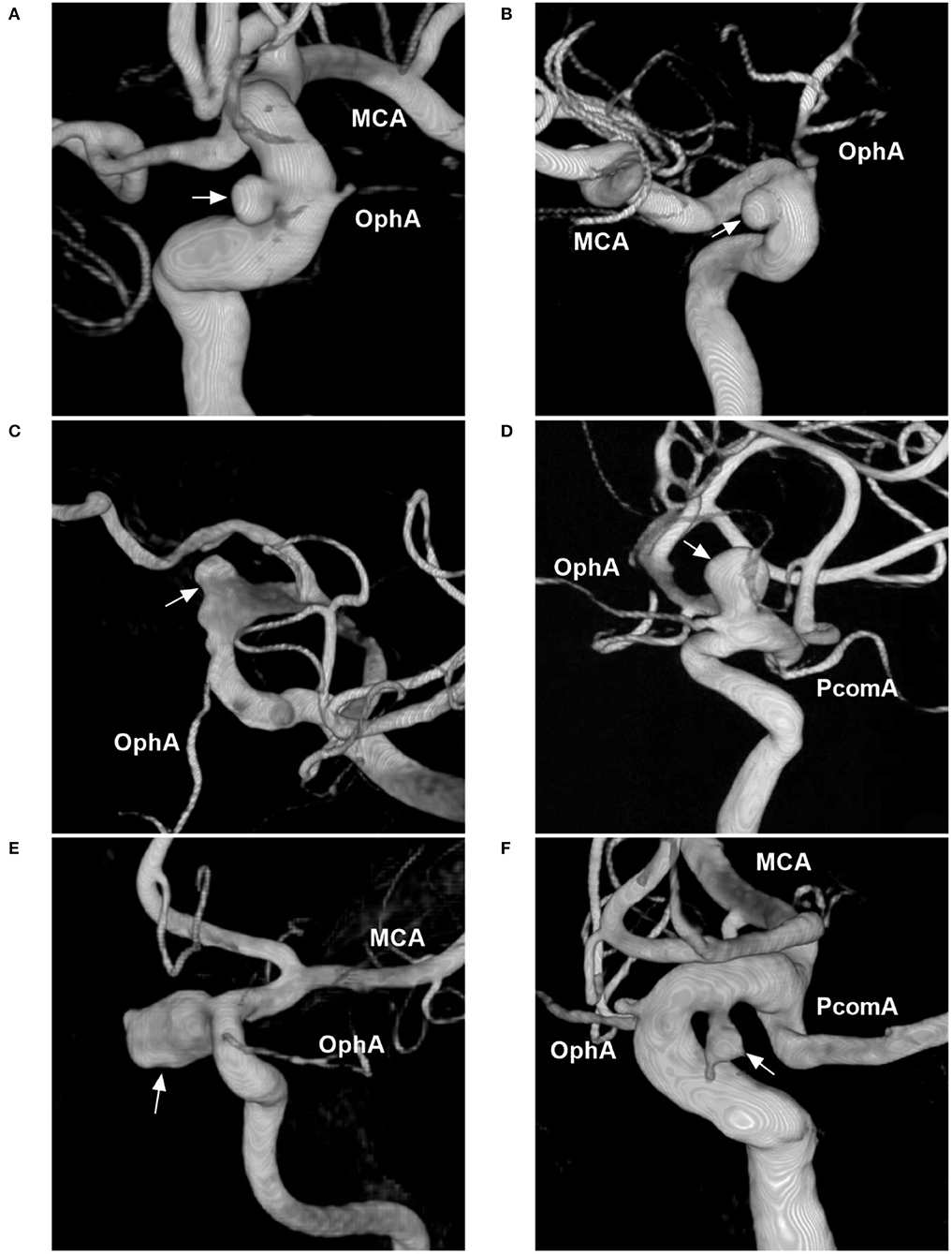
Figure 2. Classification of aneurysms of the paraophthalmic segment according to their locations. (A) Three-dimensional DSA shows a carotid cave aneurysm (arrow). (B) Three-dimensional DSA shows a clinoidal aneurysm (arrow) opposite the carotid cave. (C) Three-dimensional DSA shows an OphA aneurysm (arrow); the OphA was located away from the aneurysm neck. (D) Three-dimensional DSA shows an OphA aneurysm (arrow); the OphA arose from both the aneurysmal and ICA walls. (E) Three-dimensional DSA shows a suprasellar SHA aneurysm (arrow). (F) Three-dimensional DSA shows a paraclinoid SHA aneurysm (arrow). DSA, digital subtraction angiography; MCA, middle cerebral artery; OphA, ophthalmic artery; SHA, superior hypophyseal artery; PcomA, posterior communicating artery.
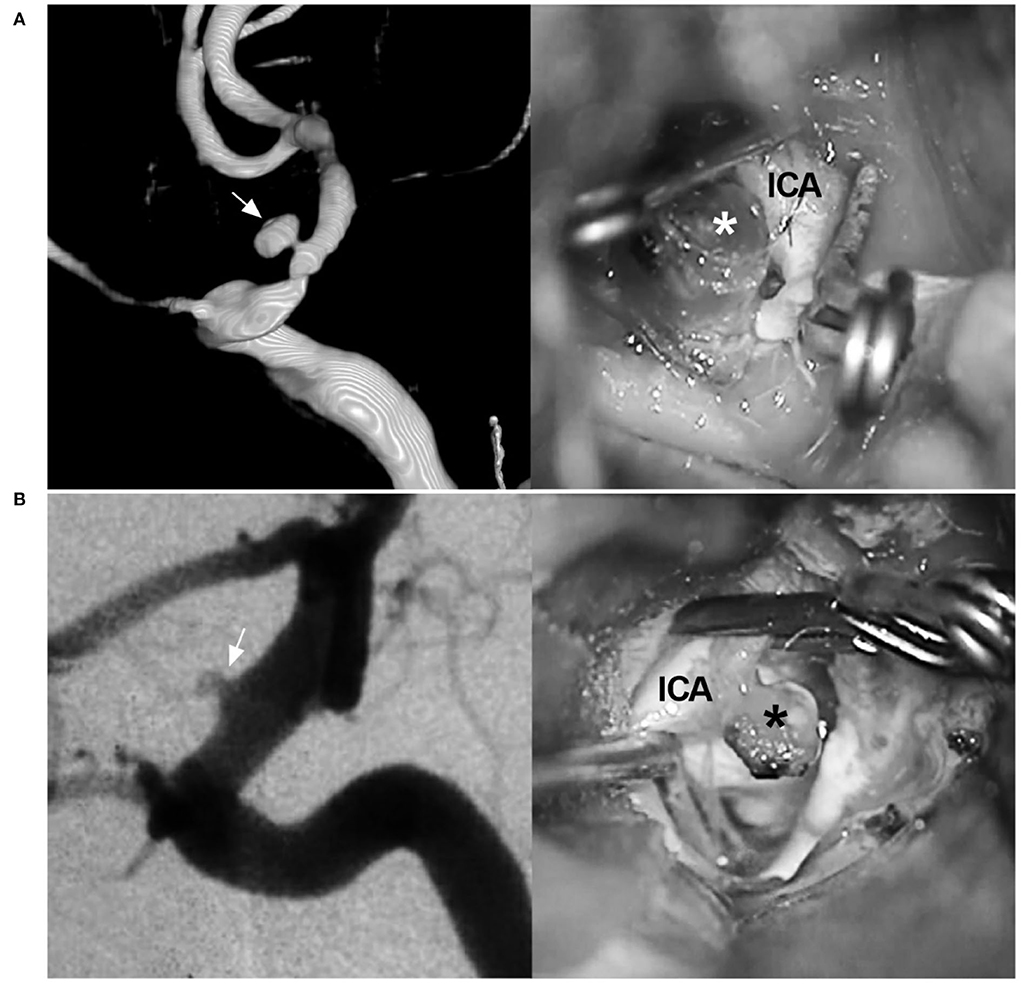
Figure 3. BBA and its differential diagnosis. (A) Left: Three-dimensional DSA of the ICA shows a mushroom-shaped BBA (arrow) on the paraophthalmic segment; Right: intraoperative image shows the fragile BBA without a definite neck (asterisk). (B) Left: DSA of the ICA shows an aneurysm (arrow) exactly like the BBA on the paraophthalmic segment; Right: intraoperative image shows that the aneurysm is saccular with a definite and stable neck (asterisk). BBA, blood blister-like aneurysm; DSA, digital subtraction angiography; ICA, internal carotid artery.
OphA aneurysms often arise from the ICA just distal to the OphA, pointing superiorly or superomedially. The locations of OphA aneurysms vary because although most OphAs originate from the intradural ICA, 2–8% of OphAs originate from the extradural ICA (18, 19). OphA aneurysms can be divided into separate and shared types (20). A separate-type aneurysm is defined as an OphA originating completely from the ICA wall, away from the aneurysmal neck (Figure 2C); in a shared-type aneurysm, the OphA originates from both the aneurysm and the ICA (Figure 2D). Separate-type OphA aneurysms are common (21).
SHA aneurysms arise from the ICA medial wall at the site of perforator origin, including suprasellar and paraclinoid variants (22). The suprasellar variant arises from the medial ICA wall and expands directly into the suprasellar space (Figure 2E). The paraclinoid variant arises from the inferomedial ICA and burrows down toward the carotid cave (Figure 2F). Because the origin of SHA may be in the carotid cave, when SHA aneurysms are large, they may involve the carotid cave (12, 23).
Saccular, fusiform (dissecting), and blood blister-like types
Based on their morphology and nature, aneurysms of the paraophthalmic segment can be divided into saccular, fusiform, and BBA types. Saccular aneurysms originate as branch-related aneurysms at the OphA and SHA. When the paraophthalmic segment is dilated, if the diameter of the dilatation is 1.5 times that of the normal ICA, fusiform aneurysms have formed, and they may be from dissection (Figure 4A) (24). Fusiform aneurysms often involve the long paraophthalmic segment and are called transitional aneurysms (25, 26).
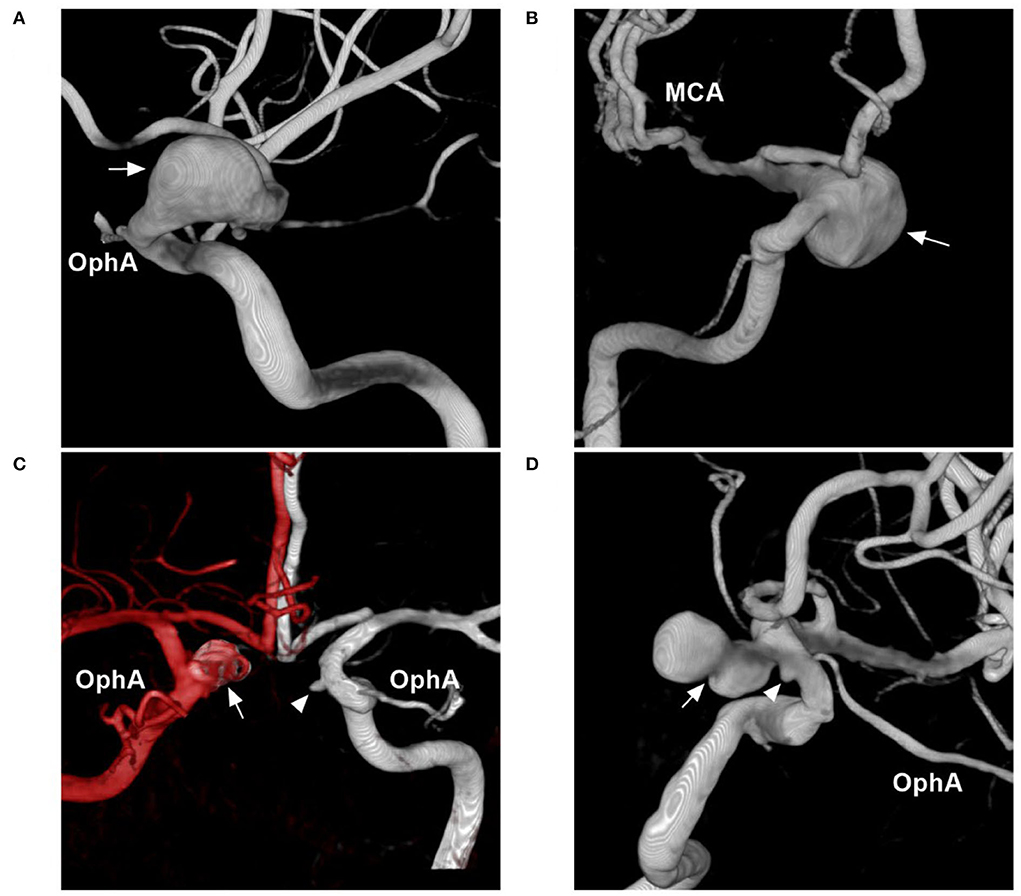
Figure 4. Other classifications of aneurysms of the paraophthalmic segment. (A) Three-dimensional DSA shows a fusiform aneurysm (arrow) of the paraophthalmic segment, which also belongs to dissection. (B) Three-dimensional DSA shows a large SHA aneurysm (arrow). (C) Three-dimensional DSA shows bilateral aneurysms: one is a coiled SHA aneurysm (arrow) and the other is a carotid cave aneurysm (arrowhead). (D) Three-dimensional DSA shows two tandem aneurysms: the large aneurysm is an SHA aneurysm (arrow), and the small aneurysm is a carotid cave aneurysm (arrowhead). DSA, Digital subtraction angiography; MCA, middle cerebral artery; OphA, ophthalmic artery; SHA, superior hypophyseal artery.
In nature, BBAs are often located at the anteromedial wall of the supraclinoid ICA; they are false, consisting of a platelet plug covering a thin layer of adventitia overlying a defect in intima and media (27). BBAs have unique characteristics, such as hemispheric and broad-based appearances, and lack a neck. However, the identification of BBAs should be confirmed via microsurgery because some aneurysms that are initially indistinguishable from BBAs are not actually blood blister-like (Figure 3) (28, 29).
Other classifications
Aneurysms of the paraophthalmic segment also have other classification systems, such as ruptured or unruptured; small (<7 mm), medium (7–12 mm), large (13–24 mm) (Figure 4B), or giant (>25 mm), and single or multiple (Figures 4C,D) (30–32).
Principle and techniques of EVT
EVT for aneurysms of the paraophthalmic segment includes the use of coil embolization, FDS, covered stents, and a Woven EndoBridge (WEB) device (MicroVention-Terumo, Aliso Viejo, California) (Figure 5). These aneurysms can also be treated with ICA occlusion, but FDS has eliminated the technique (33).
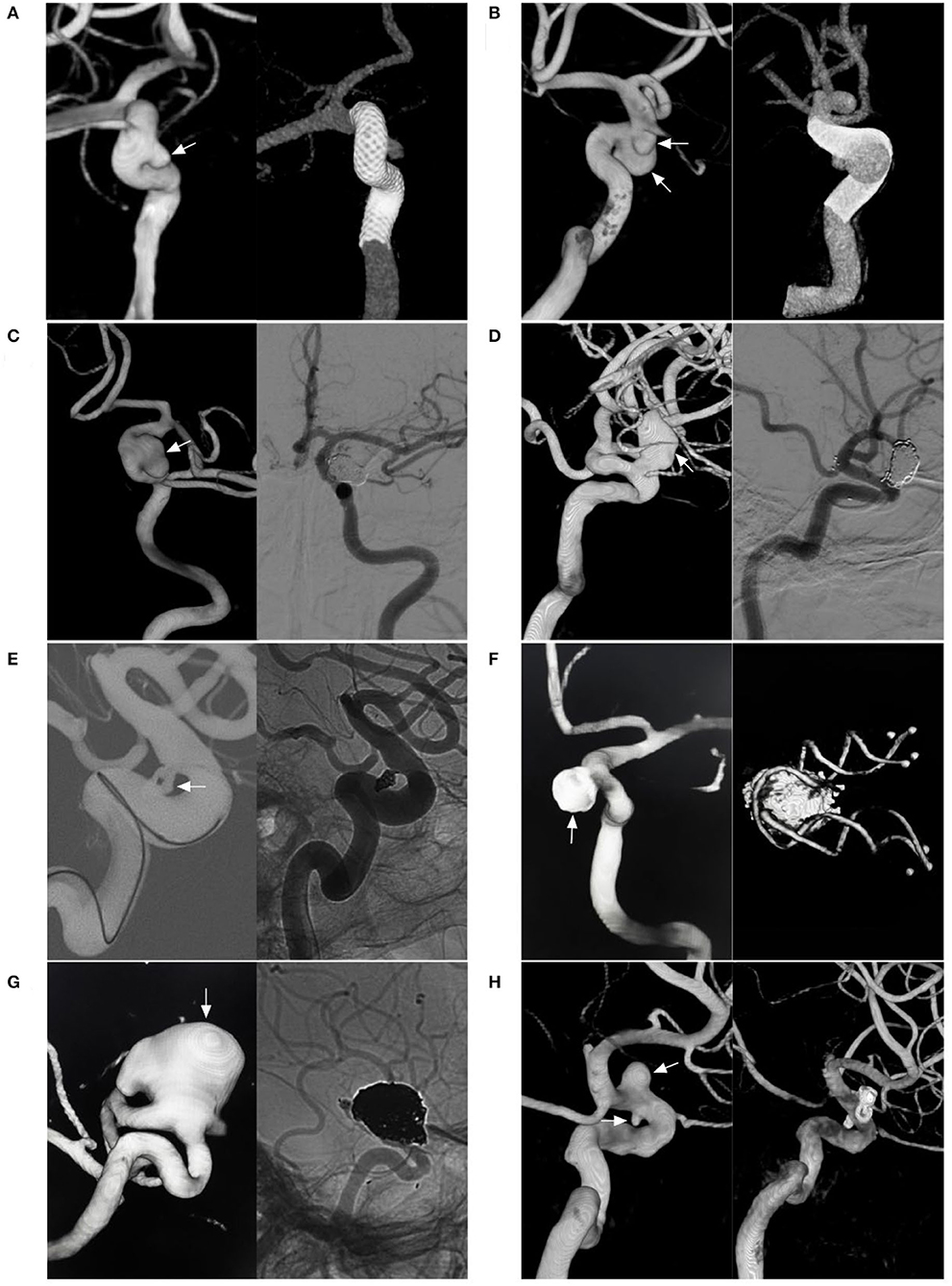
Figure 5. Various EVT techniques for aneurysms of the paraophthalmic segment. (A) Left, three-dimensional DSA shows a carotid artery aneurysm (arrow); right: Vaso CT shows the FDS covering the aneurysm. (B) Left, three-dimensional DSA shows two aneurysms (arrows) of the paraophthalmic segment; right: Vaso CT shows the FDS covering two aneurysms. (C) Left, three-dimensional DSA shows a clinoidal aneurysm (arrow); right: follow-up DSA shows that the aneurysms were coiled completely. (D) Left, three-dimensional DSA shows an OphA aneurysm (arrow); right: follow-up DSA shows that the aneurysm was coiled under stenting assistance. (E) Left, road map DSA shows a microcatheter into a paraclinoid-type SHA aneurysm (arrow); right: DSA shows the aneurysm was coiled. (F) Left, postoperative three-dimensional DSA shows a coiled suprasellar-type SHA aneurysm (arrow); right: Vaso CT shows the coils and stent. (G) Left, three-dimensional DSA shows a large OphA aneurysm (arrow); right: unsubtracted follow-up DSA shows that the aneurysm was coiled under stenting assistance. (H) Left, three-dimensional DSA shows OphA and SHA aneurysms (arrows); right: follow-up three-dimensional DSA shows that two aneurysms were coiled under stenting assistance. CT, computed tomography; DSA, digital subtraction angiography; EVT, endovascular treatment; FDS, flow-diverting stent; OphA, ophthalmic artery; SHA, superior hypophyseal artery; Vaso, vascular space occupancy.
EVT consideration based on natural history
Paraophthalmic segment aneurysms often grow slowly and are less likely to rupture than aneurysms in other categories. In a study by Jeon et al. (34) 524 patients harbored a total of 568 small unruptured paraclinoid aneurysms ( ≤ 5 mm). During the follow-up of 1675.5 aneurysm-years, the annual rupture rate and growth rates were 0.12 and 1.01%, respectively, and risk factors included lesions >4 mm in size, branch-related lesions, and multiple lesions. Close monitoring was only necessary for aneurysms with the above risk factors. Regarding the natural history of carotid cave aneurysms, Kalluri et al. reported 290 small (<4 mm) carotid cave aneurysms over 17 years, and no instances of aneurysm rupture or growth were found (35). Therefore, for these small carotid cave aneurysms, a watchful waiting strategy is feasible.
Therefore, EVT should only be indicated in large symptomatic aneurysms with mass effects on cranial nerves (36). In addition, due to aneurysms with regrowth or irregular shapes that confer a higher risk of rupture, EVT is necessary (37). Certainly, for ruptured aneurysms, EVT is mandatory (38–40).
EVT consideration based on the collateral circulation of the OphA
Collateral circulation between external carotid artery (ECA) branches and OphA may be apparent or potential, varying from 36 to 89% of cases with ICA occlusion (41, 42). The balloon occlusion test (BOT) can identify collateral circulation (43). In the report by Kim et al. (42) on EVT for unruptured paraophthalmic segment aneurysms, after the BOT, intact collateral circulation was demonstrated in 92.9% of patients.
During the BOT, the ICA should be occluded, or the OphA orifice should be covered. HyperForm, HyperGlide (Medtronic Inc., Irvine, CA, USA), or Scepter balloons (MicroVention-Terumo, Aliso Viejo, California) can be chosen. After the balloon is inflated, contrast medium is injected into the common carotid artery. Positive BOT is defined as retrograde filling of the OphA with choroidoretinal blush. BOT may provide useful information to predict visual outcomes once OphA is threatened by EVT (42, 44). However, in OphA aneurysms with a positive BOT, intentional OphA occlusion should be avoided.
Coiling with/without stent or balloon assistance
For paraophthalmic segment aneurysms, the main difficulties in EVT are accurate positioning and stable support of the microcatheter (Figure 6). Due to the curvature of the ICA siphon and the aneurysms from the ICA sidewall, the sharp upturning of the microcatheter from the ICA to the aneurysm is difficult, stressful, risky, and sometimes impossible. The difficulty of catheterization is similar to that of EVT in the first segment of anterior cerebral artery aneurysms (45). For aneurysms of the carotid cave and clinoid on the sidewall of the ICA, the microcatheter is often most difficult to access for the aneurysm sac. The OphA and SHA aneurysms leave the ICA siphon and often have an upward or downward direction, and it is relatively easy for the microcatheter to access the aneurysms.
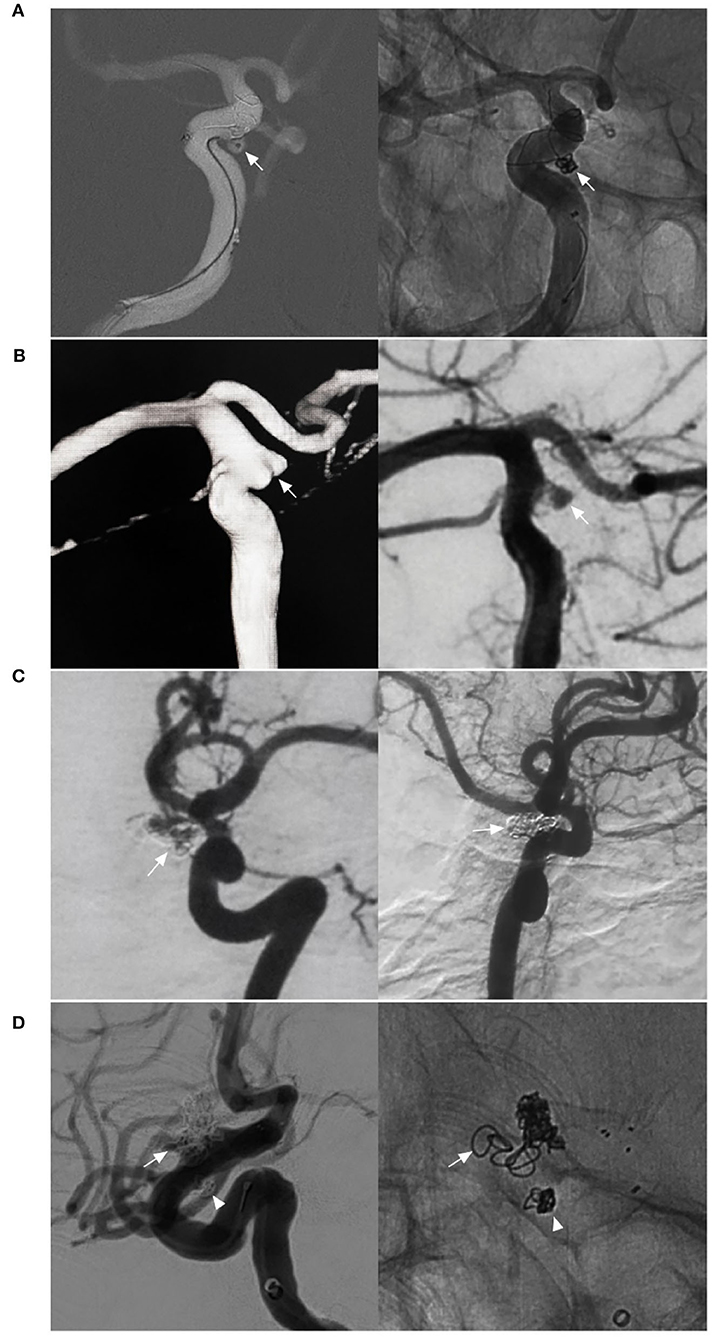
Figure 6. EVT difficulty in aneurysms of the paraophthalmic segment. (A) Left, Road map navigation shows the microcatheter tip (arrow) positioned in the aneurysm sac with the assistance of stent semi-deployment. The stent sealed a part of the aneurysm neck and provided the opportunity for the microcrater to enter the aneurysm; right: unsubtracted DSA shows the coils in the aneurysm (arrow). (B) Left, three-dimensional DSA shows a wide-necked lobulated carotid cave aneurysm (arrow); right: DSA shows the loose packing of the coiling in the aneurysm because the microcatheter was knocked off the aneurysm, but contrast agent retention can be seen in the aneurysm (arrow). (C) Left, DSA shows a recanalized aneurysm due to low-density packing (arrow); right: follow-up DSA shows the aneurysm had a complete embolization (arrow) after repeated coiling. (D) Left, DSA shows a recanalized lobulated upper aneurysm (arrow) and a completely embolized lower aneurysm (arrowhead); right: X-ray film shows that the upper aneurysm had low-density packing due to difficult catheterization (arrow), and the lower aneurysm had satisfactory embolization (arrowhead). DSA, Digital subtraction angiography; EVT, endovascular treatment.
For access to aneurysms, microcatheter shaping is very important. The microcatheter shapes can be classified as straight, curved (45 and 90 degrees, J and C), pigtail (simple, right and left), and S-shaped (simple, right and left) (46). An S-shaped or straight microcatheter is helpful in superiorly directed aneurysms; a pigtail shape is useful in medially directed aneurysms (43). During microcatheter navigation, antegrade/retrograde shift, wire steering, looping, and coil or guidewire guidance can be used (46, 47). Despite these choices of microcatheter shaping, during coiling, the microcatheter tends to be knocked off the aneurysm, resulting in partial or low-density coil packing. In addition, due to OphA incorporation by aneurysms, efforts to save the OphA may result in incomplete coiling.
Single coiling can be used in narrow-necked aneurysms (31). However, for wide-necked aneurysms with a neck diameter > 4 mm or a dome-to-neck ratio <2, stent or balloon assistance is required (48). Old stents are challenging to deploy due to the acute curve of the ICA siphon; stents may kink or twist and have a defective wall attachment, which results in in-stent thrombosis (49). Currently, the low-profile Neuroform Atlas stent (Stryker Neurovascular, Fremont, California, USA) may be appropriate for deployment in the ICA siphon (50).
Coiling embolization with balloon assistance is a good choice due to the lack of antiplatelet therapy, which benefits ruptured aneurysms. HyperForm, HyperGlide, and Scepter balloons are useful. The balloon should be inflated while coiling. When finished, the microcatheter should be removed under balloon protection (51). Balloon inflation is limited to no more than 5 min at a time, alternating with at least 1 min of balloon deflation. In addition, a Scepter balloon can be used to release the Neuroform Atlas stent, which has additional advantages (52).
Regarding the need for antiplatelet use with stent-assisted coiling, a loading dose of oral aspirin (300 mg) and clopidogrel (300 mg) can be given at least 3 h before EVT for ruptured aneurysms, while a 3- to 5-day regimen of oral aspirin (100 mg/day) and clopidogrel (75 mg/day) is sufficient for unruptured aneurysms (50, 53). After EVT, dual antiplatelet therapy with oral aspirin (100 mg/day) and clopidogrel (75 mg/day) should be continued for 1–6 months depending on the stent (50). Dual antiplatelet therapy for 1 to 2 months is sufficient for the Neuroform Atlas stent due to low metal coverage, 3 to 6 months of dual antiplatelet therapy is necessary for the Solitaire stent (Medtronic, Irvine, California, USA) and Enterprise stent (Codman Neurovascular, Raynham, MA, USA), and 6 months of dual antiplatelet therapy is necessary for LVIS (MicroVention, Tustin, California, USA) and LEO stent (Balt Extrusion, Montmorency, France). Then, aspirin can continue to be administered daily at a dose of 100 mg for 3–6 months.
FDS deployment
FDS can disrupt blood flow into aneurysms and act as a scaffold for endothelial cell proliferation (54). For aneurysms of the paraophthalmic segment, FDS is effective, as demonstrated by the Pipeline for Uncoilable or Failed Aneurysms trial (55). In addition, an FDS cannot be deployed to access the aneurysm sac, which reduces iatrogenic rupture from catheterization and coiling (21). However, FDS deployment in tortuous ICA siphons is not easy, and a good support system is important. After FDS deployment, the morphology and hemodynamics of the ICA siphon change, which may improve aneurysm healing (56). As technologies continue to evolve, FDSs will eventually become soft and pliable enough to deploy (57). FDSs will become easy to use in the ICA siphon.
For large fusiform aneurysms, adjunctive coiling can reduce FDS prolapse and act as a scaffold to organize thrombi, and the FDS should be supported by coiling until it is completely opened (Figure 7) (58). However, for giant aneurysms with mass effects, adjunctive coiling is not necessary because the optic nerve can be compromised by coiling via progressive mass effects or local inflammation (Figure 8) (59).
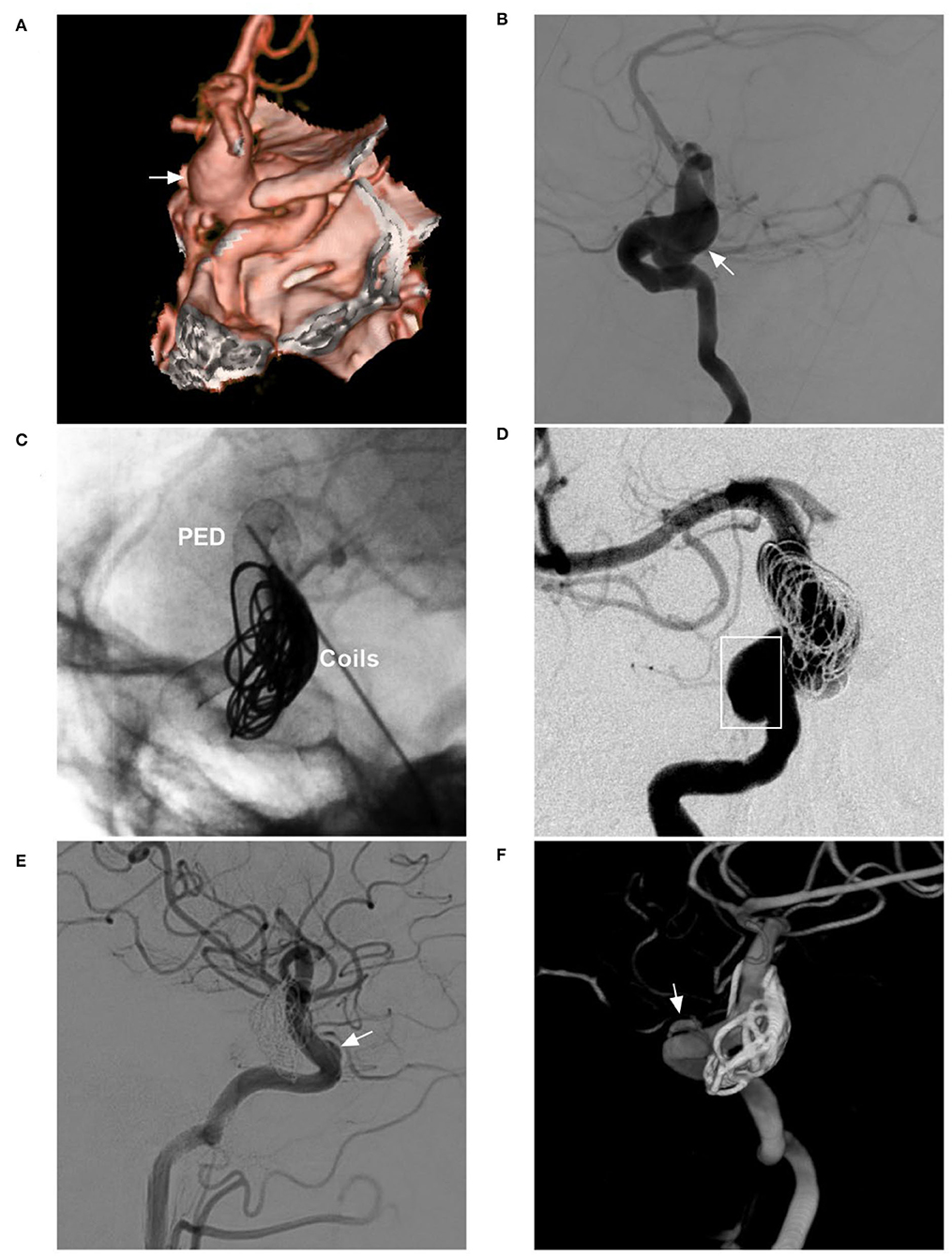
Figure 7. FDS deployment in a fusiform aneurysm under coiling assistance. (A,B) CTA (A) and DSA (B) show a large fusiform aneurysm (arrows) of the paraophthalmic segment. (C) X-ray shows the FDS deployment (PED) under the assistance of coiling (coils). (D) Postoperative DSA shows that the distal part of the aneurysm was coiled, and the proximal part (frame) was left. (E,F) Follow-up DSA at 6 months (E) and three-dimensional reconstructive DSA (F) show nearly complete occlusion; the arrows indicate the remnant neck. CTA, computed tomography angiography; DSA, digital subtraction angiography; FDS, flow-diverting stent; PED, pipeline embolization device.

Figure 8. FDS deployment in a giant aneurysm without coiling. (A) Sagittal MRI shows a giant aneurysm with jet flow in the middle (asterisk). (B) DSA shows the aneurysm sac. (C) X-ray shows that contrast agent retention (asterisk) can be seen after FDS (PED) deployment. (D) Postoperative DSA shows reduced blood flow into the aneurysm. (E) Postoperative 1-week CTA shows the location of the PED. (F) Follow-up DSA at 6 months shows that most of the aneurysm was occluded and that complete occlusion occurred, and the arrow indicates the remnant part. CTA, computed tomography angiography; DSA, digital subtraction angiography; FDS, flow-diverting stent; MRI, magnetic resonance imaging; PED, pipeline embolization device.
After FDS deployment, aneurysm healing is gradual. In a study of 44 patients with 46 unruptured aneurysms of the paraophthalmic segment by Burrows et al., the complete occlusion rates were 65, 78, and 96% at 6 months, 1 year, and 3 years, respectively (60). Therefore, FDSs do not result in immediate aneurysm closure for ruptured aneurysms. Ruptured aneurysms may not have sufficient time to wait for healing because the treatment effect is gradual. In fact, patients are still at risk of hemorrhaging after FDS deployment, and this is the main issue why separate, non–coiled-assisted FDSs are not suitable for acute cases (61). Due to the excessive use of antiplatelet therapy, unruptured paraophthalmic segment aneurysms that are easy to coil should not be treated with FDS (62).
Regarding the need for antiplatelet treatment with FDS deployment for unruptured aneurysms, patients should receive 100 mg of aspirin and 75 mg of clopidogrel daily for 5–7 days prior to the intervention (60). Platelet function assays should be performed to identify clopidogrel response. If the patient has been identified as a clopidogrel non-responder, ticagrelor may be selected instead. After EVT, dual antiplatelet therapy should be continued for 6 months; later, a daily dose of aspirin of 100 mg is used for life or at least for another half year.
Covered stent placement
A covered stent can immediately lead to complete occlusion of aneurysms of the paraophthalmic segment (63). In the report by Yan et al. (64) of 49 intracranial aneurysms, 77.6% of aneurysms were large and located at the paraophthalmic segment; after covered stent (MicroPort, Shanghai, China) deployment, complete occlusion was achieved in 89.5% of aneurysms. However, stiff-covered stents have difficulty navigating through the ICA siphon, and procedure-related complications are non-negligible, including stent navigation failure, vasospasm, acute in-stent thrombosis, endoleak, and OphA, PcomA, or anterior choroidal artery occlusion (65, 66).
Regarding antiplatelet therapy, patients should be administered a preoperative double-antiplatelet regimen (100 mg aspirin and 75 mg clopidogrel) for at least 3 days (64). The postoperative double-antiplatelet regimen should continue for 6 months, and then a single-antiplatelet regimen (100 mg aspirin) should be continued for life (64). Currently, an FDS can replace a covered stent in most aneurysms of the paraophthalmic segment with fewer complications, especially when the side branches can be preserved (66).
Woven EndoBridge (WEB) device
The WEB device is a self-expanding, retrievable, electrothermally detachable braided nitinol device that is placed within the aneurysm sac (67). It disrupts blood flow at the aneurysm neck and induces intra-aneurysmal thrombosis and offers a flat proximal surface that potentially supports the neoendothelium (68). In general, the WEB device in the aneurysm sac obviates the need for potent antiplatelet therapy if it does not protrude into the parent artery, which makes the WEB device appealing for ruptured and unruptured aneurysms (69).
The WEB device was initially designed to treat wide-necked bifurcation aneurysms. The use of the WEB device requires <45 degrees between the aneurysm and parent artery (70). Most aneurysms of the paraophthalmic segment are not appropriate for deploying the WEB device due to their directions. However, in selected aneurysms of the paraophthalmic segment, if the angle between the ICA and the aneurysm axil is <45 degrees and the proximal ICA near the aneurysm is not excessively tortuous, a WEB can be applied effectively (71).
EVT for BBA of the paraophthalmic segment
For BBAs of the supraclinoid ICA, the optimal EVT has yet to be defined. Current EVT techniques include multiple overlapping stents with coiling, FDSs, and covered stents (72).
Stent-assisted coiling BBAs facilitate the placement of coils, but the complete occlusion rate is low. In a meta-analysis by Rouchaud et al. (73) of stent-assisted coiling BBA, complete occlusions were 33% initially and ~70% at mid- to long-term follow-up. FDS can be used to treat BBAs (Figure 9). FDS deployment for BBA of the supraclinoid ICA is also hindered by a low initial occlusion rate (36%) and its complication rate (17%), early rebleeding rate (7%), morbidity rate (13%), and mortality rate (9%) (73). Multilayer FDSs appear to be a promising strategy for BBAs, but sufficient evidence from trials is unavailable. A major disadvantage of FDS is dual antiplatelet therapy in the acute phase of ruptured BBAs (74).
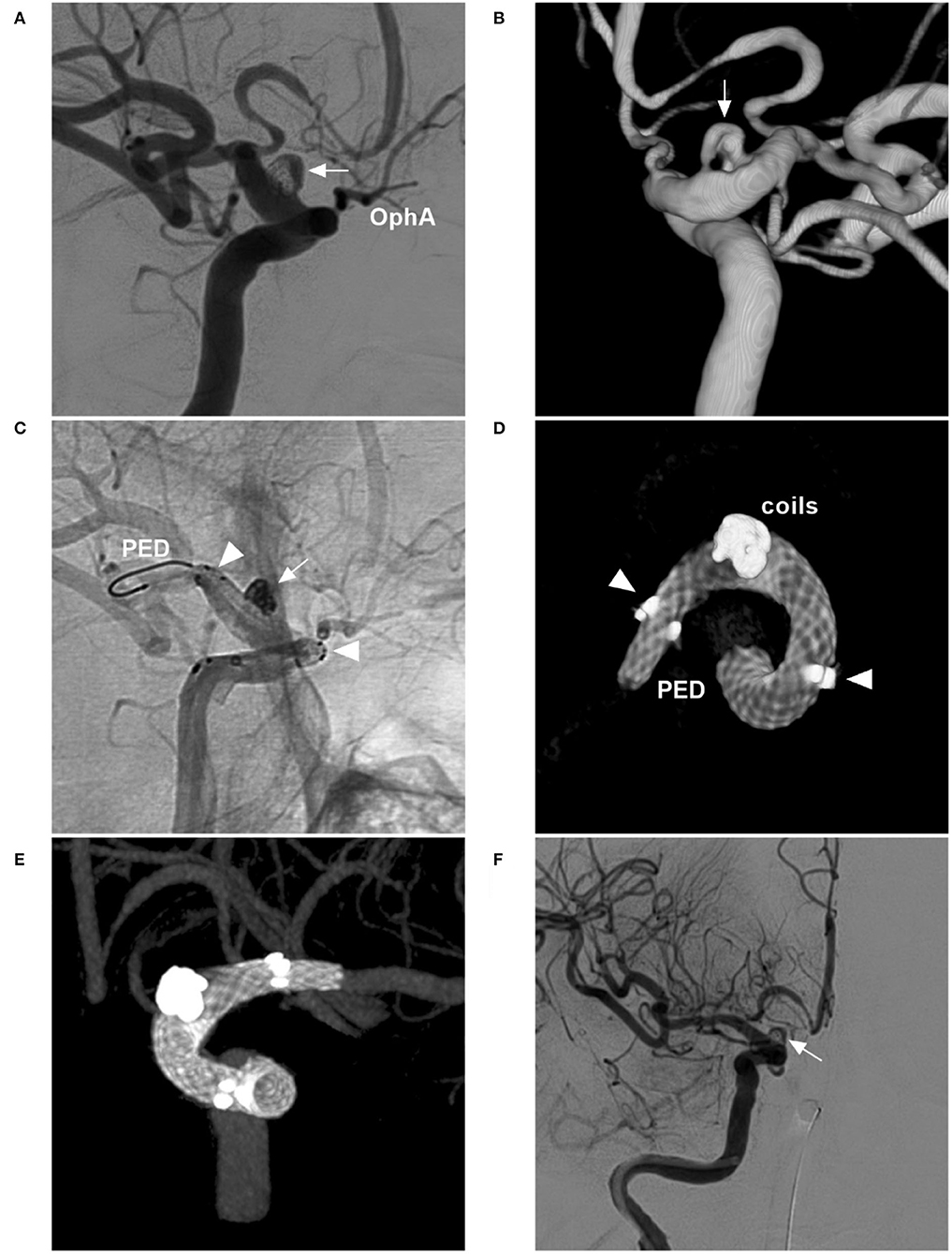
Figure 9. FDS deployment in a BBA with failed previous stent-assisted coiling. (A,B) Two-dimensional DSA (A) and three-dimensional DSA (B) show a BBA (arrows) with incomplete embolization of stent-assisted coiling. (C) Unsubtracted DSA shows the FDS (PED) crossing the BBA (arrow) and the FDS running in the stent (arrowheads). (D,E) Vaso CT without the vessel (D) and shows the FDS opened and covering the BBA in the stent (arrowheads). (F) Postoperative DSA shows the BBA without immediate complete occlusion; complete occlusion was expected by long-term follow-up. BBA, blister-like aneurysm; DSA, digital subtraction angiography; FDS, flow-diverting stent; PED, pipeline embolization device; Vaso, vascular space occupancy.
In a recent meta-analysis by Lee et al. comparing stent-assisted coiling and FDS in the management of BBAs, the long-term complete occlusion rate was higher in the FDS group (89.3%) than in the stent-assisted coiling group (70.2%), and the rate of aneurysm recanalization was lower in the FDS group (4.5%) than in the stent-assisted coiling group (25.4%); however, the rates of mortality, favorable functional outcome, procedural complications, and rebleeding showed no differences between the two groups (75). Therefore, although FDS was associated with more favorable angiographic outcomes, FDS had similar complications and clinical outcomes as stent-assisted coiling, indicating little advantage in using FDS to improve BBA treatment.
In theory, covered stents can be used to treat BBAs of the supraclinoid ICA (76). However, due to the drawbacks mentioned above, especially intraoperative BBA rupture and endoleak, covered stents are not an ideal treatment for BBAs (77, 78). However, we look forward to advanced covered stents with good compliance, which will involve EVT for BBAs of the supraclinoid ICA.
In addition, the identification of true BBAs should be confirmed via microsurgery. Some putative BBAs of the supraclinoid ICA that were treated successfully by various EVT techniques in previous reports may not have been true BBAs but merely routine aneurysms (Figure 3) (79, 80).
Prognosis and complications
For paraophthalmic segment aneurysms, the functional outcome is recorded using the modified Rankin Scale (mRS) score; aneurysm occlusion by coiling is categorized using the Raymond Roy Occlusion Classification; aneurysm occlusion of FDS deployment is categorized by angiography as complete, near complete (>90%), or incomplete (<90%) (81). Regarding the prognosis and complications, only coiling embolization and FDS deployment have been discussed, and other techniques are discussed in the corresponding sections.
Prognosis
Both coiling embolization and FDS deployment can result in a good prognosis for paraophthalmic segment aneurysms. In a report by Adeeb et al. (81) good functional outcomes were 96.6 and 94.7% in-stent coiling and FDS, respectively. For paraophthalmic segment aneurysms with visual symptoms, coil embolization and FDS deployment can improve vision due to a reduction in mass effects and aneurysm pulsation (82, 83).
Regarding angiographic occlusion of the aneurysms, FDS deployment had a better outcome. In a meta-analysis by Touze et al. the complete occlusion rate for ophthalmic aneurysms after FDS deployment was 85% (54). For separate-type OphA aneurysms, the complete occlusion rate reached 89.5% (21). After the discontinuation of the second antiplatelet agent, the complete occlusion rate will increase (4).
Regarding coiling embolization, Wisniewski et al. reported that the overall recanalization rate ranged from 37.5 to 53% (4). Stent-assisted coiling lowers the recanalization rate; in long-term follow-up, the complete occlusion rate in-stent-assisted coiling may reach the rate of FDS deployment (84). In a report by Adeeb et al. on EVT for paraophthalmic segment aneurysms, the complete occlusion rate was 75.9% in-stent coiling compared with 81.1% in FDS deployment, and no significant difference was found (81).
Complications
For EVT of paraophthalmic segment aneurysms, hemorrhagic and thromboembolic events, as well as visual deficits, must be considered (3, 85, 86). Aneurysm size influences complications; thromboembolic events are found more often in large aneurysms (7.4%) than in small ones (1%), whereas hemorrhagic complications are found in 0.7% of small aneurysms and no large aneurysms (53).
Thromboembolic events included in-stent thrombosis and distal cerebral ischemia (87). In the report by Di Maria et al., the overall rates in the coiling group and FDS group were 6.6 and 7.8%, respectively, and the rates of permanent morbidity in the coiling group and FDS group were 1.6 and 3.9%, respectively, and no significant difference was found between groups (88).
Regarding visual deficits, in coil embolization, the rate ranged from 4 to 10% (20). In FDS covering the OphA origin, the rate was 3% (54). One possibility is OphA occlusion with insufficient collateral circulation. The other is from thrombotic material, which is produced in aneurysms and migrates to the OphA (44). During EVT, acknowledging that tiny thrombi occlude the central retinal artery regardless of the collateral flow, intra-arterial injection of the glycoprotein IIb–IIIa inhibitor tirofiban can be helpful (89).
For large or giant paraophthalmic segment aneurysms, after FDS deployment, aneurysm volume can increase, resulting in a worse mass effect or delayed rupture due to thrombosis (24, 34, 88, 90). In addition, coiling embolization for large or giant aneurysms can be associated with the progression of mass effect and/or perianeurysmal inflammation, resulting in acute or delayed visual impairment (50, 91–93).
OphA fate after FDS deployment
After FDS deployment, the blood flow of the OphA may be impeded or even occluded (94). In a report by Chalouhi et al. (95) with 95 patients whose OphA was covered by an FDS, during a follow-up of 7.5 months, OphA showed diminished flow in 4% of patients and occluded flow in 7% of patients; OphA occlusion was 8.6% when covered by one device vs. 21% when covered by two devices. Therefore, minimizing the number of FDSs across the OphA is a crucial factor to preserve its patency.
After FDS deployment, if the pressure gradient between the ICA and OphA is large and creates an aspiration effect that allows the continuation of blood flow, the OphA will always be patent, or the OphA will be occluded (96). In situations of robust collateral circulation, the OphA can obtain sufficient blood from the ECA, the pressure gradient may be abolished, and the OphA will be occluded (97). Due to the existence of sufficient collaterals, OphA occlusion usually has no overt consequences in the majority of cases (86).
Surgical clipping
For paraophthalmic segment aneurysms, surgical clipping is still a therapeutic option and should always be considered (98). In a meta-analysis by Falk et al. (99) EVT and surgical clipping were compared, and no significant difference in clinical outcome was found between them for ruptured ophthalmic aneurysms. Aneurysms causing visual symptoms can be considered candidates for surgical clipping to improve visual function, and the recovery of visual function can often be expected when surgical clipping is performed rapidly before the visual dysfunction becomes irreversible (100). Moreover, surgical clipping is still a valuable option in younger patients to avoid long-term antiplatelet therapy (101).
Summary
For paraophthalmic segment aneurysms, EVT techniques include coil embolization, FDSs, covered stents, and WEB devices. Coiling embolization remains the best choice for ruptured aneurysms. FDS is appropriate specifically for uncoilable or failed aneurysms. Due to the excessive use of antiplatelet therapy, aneurysms that are easy to coil should not be treated by FDS deployment. Both coiling embolization and FDS deployment can result in a good prognosis. For EVT of paraophthalmic segment aneurysms, the overall complication rate is low. Therefore, current EVT techniques offer promising treatment prospects for paraophthalmic segment aneurysms. Certainty surgical clipping continues to be a good choice in the endovascular era.
Author contributions
JY contributed to the conception and design of the manuscript and critically revised the manuscript. YW wrote the manuscript and collected the medical records of the patients. Both authors approved the final version of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gibo H, Lenkey C, Rhoton AL Jr. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. (1981) 55:560–74. doi: 10.3171/jns.1981.55.4.0560
2. Shapiro M, Becske T, Riina HA, Raz E, Zumofen D, Jafar JJ, et al. Toward an endovascular internal carotid artery classification system. AJNR Am J Neuroradiol. (2014) 35:230–6. doi: 10.3174/ajnr.A3666
3. Bhogal P, Aguilar Perez M, Sauder G, Bazner H, Ganslandt O, Henkes H. Management of paraophthalmic aneurysms: review of endovascular treatment strategies. Ophthalmologe. (2018) 115:114–22. doi: 10.1007/s00347-017-0497-8
4. Wisniewski K, Tomasik B, Bobeff EJ, Stefanczyk L, Hupalo M, Jaskolski DJ. Predictors for ophthalmic segment aneurysms recanalization after coiling and flow diverter embolization in 6- and 12-month follow-up. J Clin Neurosci. (2019) 68:151–7. doi: 10.1016/j.jocn.2019.07.007
5. De Jesus O, Sekhar LN, Riedel CJ. Clinoid and paraclinoid aneurysms: surgical anatomy, operative techniques, and outcome. Surg Neurol. (1999) 51:477–87. doi: 10.1016/S0090-3019(98)00137-2
6. Catapano JS, Koester SW, Srinivasan VM, Labib MA, Majmundar N, Nguyen CL, et al. A comparative propensity-adjusted analysis of microsurgical versus endovascular treatment of unruptured ophthalmic artery aneurysms. J Neurosurg. (2021) 136:1–6. doi: 10.3171/2021.5.JNS211149
7. Yadla S, Campbell PG, Grobelny B, Jallo J, Gonzalez LF, Rosenwasser RH, et al. Open and endovascular treatment of unruptured carotid-ophthalmic aneurysms: clinical and radiographic outcomes. Neurosurgery. (2011) 68:1434–43. doi: 10.1227/NEU.0b013e31820b4f85
8. Aboukais R, Zairi F, Bourgeois P, Thines L, Kalsoum E, Leclerc X, et al. Clinical and imaging follow-up after surgical or endovascular treatment in patients with unruptured carotid-ophthalmic aneurysm. Clin Neurol Neurosurg. (2014) 125:155–9. doi: 10.1016/j.clineuro.2014.08.006
9. Revilla-Pacheco F, Escalante-Seyffert MC, Herrada-Pineda T, Manrique-Guzman S, Perez-Zuniga I, Rangel-Suarez S, et al. Prevalence of incidental clinoid segment saccular aneurysms. World Neurosurg. (2018) 115:e244–e51. doi: 10.1016/j.wneu.2018.04.027
10. Bouthillier A, van Loveren HR, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. (1996) 38:425–32. doi: 10.1227/00006123-199603000-00001
11. Ziyal IM, Ozgen T, Sekhar LN, Ozcan OE, Cekirge S. Proposed classification of segments of the internal carotid artery: anatomical study with angiographical interpretation. Neurol Med Chir. (2005) 45:184–90. doi: 10.2176/nmc.45.184
12. Joo W, Funaki T, Yoshioka F, Rhoton AL Jr. Microsurgical anatomy of the carotid cave. Neurosurgery. (2012) 70(2 Suppl Operative):300–11. doi: 10.1227/NEU.0b013e3182431767
13. Barami K, Hernandez VS, Diaz FG, Guthikonda M. Paraclinoid carotid aneurysms: surgical management, complications, and outcome based on a new classification scheme. Skull Base. (2003) 13:31–41. doi: 10.1055/s-2003-37551
14. Kawaguchi S, Sakaki T, Okuno S, Uchiyama Y, Nishioka T. Peripheral ophthalmic artery aneurysm. Report of two cases. J Neurosurg. (2001) 94:822–5. doi: 10.3171/jns.2001.94.5.0822
15. Tang K, Zhang C, Wu JL, Gao C. Microsurgical suturing and a wrapped clipping of traumatic internal carotid-ophthalmic artery aneurysm: a case report. Asian J Surg. (2021) 44:1135–6. doi: 10.1016/j.asjsur.2021.04.029
16. Sheikh B, Ohata K, El-Naggar A, Hong B, Tsuyuguchi N, Hakuba A. Contralateral approach to carotid cave aneurysms. Acta Neurochir. (2000) 142:33–7. doi: 10.1007/s007010050004
17. Pritz MB. Intraclinoidal ophthalmic artery aneurysm. Acta Neurochir. (1998) 140:102–4. doi: 10.1007/s007010050067
18. Kyoshima K, Oikawa S, Kobayashi S. Interdural origin of the ophthalmic artery at the dural ring of the internal carotid artery. Report of two cases. J Neurosurg. (2000) 92:488–9. doi: 10.3171/jns.2000.92.3.0488
19. Huynh-Le P, Natori Y, Sasaki T. Surgical anatomy of the ophthalmic artery: its origin and proximal course. Neurosurgery. (2005) 57(4 Suppl):236–41. doi: 10.1227/01.NEU.000177442.96517.3D
20. Michishita S, Ishibashi T, Yuki I, Urashima M, Karagiozov K, Kodama T, et al. Visual complications after coil embolization of internal carotid artery aneurysms at the ophthalmic segment. Interv Neuroradiol. (2021) 27:622–30. doi: 10.1177/1591019921996886
21. Griessenauer CJ, Ogilvy CS, Foreman PM, Chua MH, Harrigan MR, Stapleton CJ, et al. Pipeline embolization device for small paraophthalmic artery aneurysms with an emphasis on the anatomical relationship of ophthalmic artery origin and aneurysm. J Neurosurg. (2016) 125:1352–9. doi: 10.3171/2015.12.JNS152499
22. Day AL. Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg. (1990) 72:677–91. doi: 10.3171/jns.1990.72.5.0677
23. Kim JM, Romano A, Sanan A, van Loveren HR, Keller JT. Microsurgical anatomic features and nomenclature of the paraclinoid region. Neurosurgery. (2000) 46:670–80. doi: 10.1097/00006123-200003000-00029
24. Guo Y, Song Y, Hou K, Yu J. Intracranial fusiform and circumferential aneurysms of the main trunk: therapeutic dilemmas and prospects. Front Neurol. (2021) 12:679134. doi: 10.3389/fneur.2021.679134
25. Sherif C, Gruber A, Dorfer C, Bavinzski G, Standhardt H, Knosp E. Ruptured carotid artery aneurysms of the ophthalmic (C6) segment: clinical and angiographic long term follow-up of a multidisciplinary management strategy. J Neurol Neurosurg Psychiatry. (2009) 80:1261–7. doi: 10.1136/jnnp.2008.170860
26. al-Rodhan NR, Piepgras DG, Sundt TM Jr. Transitional cavernous aneurysms of the internal carotid artery. Neurosurgery. (1993) 33:993–6. doi: 10.1227/00006123-199312000-00006
27. Ji T, Guo Y, Huang X, Xu B, Xu K, Yu J. Current status of the treatment of blood blister-like aneurysms of the supraclinoid internal carotid artery: a review. Int J Med Sci. (2017) 14:390–402. doi: 10.7150/ijms.17979
28. Kim JH, Kwon TH, Kim JH, Park YK, Chung HS. Internal carotid artery dorsal wall aneurysm with configurational change: are they all false aneurysms? Surg Neurol. (2006) 66:441–3. doi: 10.1016/j.surneu.2005.12.030
29. Owen CM, Montemurro N, Lawton MT. Blister aneurysms of the internal carotid artery: microsurgical results and management strategy. Neurosurgery. (2017) 80:235–47. doi: 10.1227/NEU.0000000000001259
30. Wiebers DO, Whisnant JP, Huston J. 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
31. Durst CR, Starke RM, Gaughen J, Nguyen Q, Patrie J, Jensen ME, et al. Vision outcomes and major complications after endovascular coil embolization of ophthalmic segment aneurysms. AJNR Am J Neuroradiol. (2014) 35:2140–5. doi: 10.3174/ajnr.A4032
32. Date I, Ogihara K, Tamiya T, Ohmoto T. “Kissing” bilateral large carotid-ophthalmic aneurysms. A case report Neurosurg Rev. (1998) 21:281–3. doi: 10.1007/BF01105786
33. Zanaty M, Chalouhi N, Barros G, Schwartz EW, Saigh MP, Starke RM, et al. Flow-diversion for ophthalmic segment aneurysms. Neurosurgery. (2015) 76:286–9. doi: 10.1227/NEU.0000000000000607
34. Jeon JS, Ahn JH, Huh W, Son YJ, Bang JS, Kang HS, et al. A retrospective analysis on the natural history of incidental small paraclinoid unruptured aneurysm. J Neurol Neurosurg Psychiatry. (2014) 85:289–94. doi: 10.1136/jnnp-2013-305019
35. Kalluri AG, Sukumaran M, Nazari P, Golnari P, Ansari SA, Hurley MC, et al. Retrospective review of 290 small carotid cave aneurysms over 17 years. J Neurosurg. (2019) 5:1–5. doi: 10.3171/2019.7.JNS191471
36. Hauck EF, Welch BG, White JA, Replogle RE, Purdy PD, Pride LG, et al. Stent/coil treatment of very large and giant unruptured ophthalmic and cavernous aneurysms. Surg Neurol. (2009) 71:19–24. doi: 10.1016/j.surneu.2008.01.025
37. Wang JT, Yang HC, Lin CF, Guo WY, Luo CB, Chen MH, et al. Bilobulated paraclinoid aneurysm mimics double aneurysms: a comparison of endovascular coiling and surgical clipping treatments. J Chin Med Assoc. (2014) 77:544–7. doi: 10.1016/j.jcma.2010.09.002
38. Horiuchi T, Kusano Y, Yako T, Murata T, Kakizawa Y, Hongo K. Ruptured anterior paraclinoid aneurysms. Neurosurg Rev. (2011) 34:49–55. doi: 10.1007/s10143-010-0272-7
39. Loumiotis I, D'Urso PI, Tawk R, Cloft HJ, Kallmes DF, Kairouz V, et al. Endovascular treatment of ruptured paraclinoid aneurysms: results, complications, and follow-up. AJNR Am J Neuroradiol. (2012) 33:632–7. doi: 10.3174/ajnr.A2825
40. Oh SY, Kim MJ, Kim BM, Lee KS, Kim BS, Shin YS. Angiographic characteristics of ruptured paraclinoid aneurysms: risk factors for rupture. Acta Neurochir. (2013) 155:1493–9. doi: 10.1007/s00701-013-1794-x
41. Hou K, Wu W, Liu Y, Qu L, Xu B, Yu J. Role of the ophthalmic artery in the endovascular treatment for intracranial vascular diseases. Acta Neurol Belg. (2021) 121:321–30. doi: 10.1007/s13760-020-01576-z
42. Kim B, Jeon P, Kim K, Yang N, Kim S, Kim H, et al. Endovascular treatment of unruptured ophthalmic artery aneurysms: clinical usefulness of the balloon occlusion test in predicting vision outcomes after coil embolization. J Neurointerv Surg. (2016) 8:696–701. doi: 10.1136/neurintsurg-2015-011800
43. Ahn JH, Cho YD, Kang HS, Kim JE, Cho WS, Jung SC, et al. Endovascular treatment of ophthalmic artery aneurysms: assessing balloon test occlusion and preservation of vision in coil embolization. AJNR Am J Neuroradiol. (2014) 35:2146–52. doi: 10.3174/ajnr.A3999
44. Yu JW, Shim YS, Lee JW, Kim DJ, Kim BM, Lim YC, et al. Vision outcomes of endovascular treatment for unruptured ophthalmic artery aneurysms. World Neurosurg. (2018) 116:e1223–e9. doi: 10.1016/j.wneu.2018.05.238
45. Hou K, Li G, Guo Y, Yu J. Endovascular treatment for aneurysms at the A1 segment of the anterior cerebral artery: current difficulties and solutions. Acta Neurol Belg. (2021) 121:55–69. doi: 10.1007/s13760-020-01526-9
46. Kwon BJ, Im SH, Park JC, Cho YD, Kang HS, Kim JE, et al. Shaping and navigating methods of microcatheters for endovascular treatment of paraclinoid aneurysms. Neurosurgery. (2010) 67:34–40. doi: 10.1227/01.NEU.0000370891.67129.2F
47. Cho YD, Rhim JK, Park JJ, Jeon JS, Yoo RE, Kang HS, et al. Microcatheter looping to facilitate aneurysm selection in coil embolization of paraclinoid aneurysms. Korean J Radiol. (2015) 16:899–905. doi: 10.3348/kjr.2015.16.4.899
48. Zaidat OO, Hanel RA, Sauvageau EA, Aghaebrahim A, Lin E, Jadhav AP, et al. Pivotal trial of the neuroform atlas stent for treatment of anterior circulation aneurysms: one-year outcomes. Stroke. (2020) 51:2087–94. doi: 10.1161/STROKEAHA.119.028418
49. Boet R, Wong GK, Poon WS, Lam JM, Yu SC. Aneurysm recurrence after treatment of paraclinoid/ophthalmic segment aneurysms–a treatment-modality assessment. Acta Neurochir. (2005) 147:611–6. doi: 10.1007/s00701-005-0524-4
50. Hou K, Yu J. Application of the neuroform atlas stent in intracranial aneurysms: current status. Front Neurol. (2022) 13:9143. doi: 10.3389/fneur.2022.829143
51. Chaohui L, Yu ZG, Kai H. Balloon-assisted coils embolization for ophthalmic segment aneurysms of the internal carotid artery. Front Neurol. (2021) 12:658661. doi: 10.3389/fneur.2021.658661
52. Martinez-Galdamez M, Orlov K, Kadziolka K, Puthuran M, Kalousek V, Pabon B, et al. Safety and efficacy of intracranial aneurysm embolization using the “combined remodeling technique”: low-profile stents delivered through double lumen balloons: a multicenter experience. Neuroradiology. (2019) 61:1067–72. doi: 10.1007/s00234-019-02240-x
53. Shimizu K, Imamura H, Mineharu Y, Adachi H, Sakai C, Sakai N. Endovascular treatment of unruptured paraclinoid aneurysms: single-center experience with 400 cases and literature review. AJNR Am J Neuroradiol. (2016) 37:679–85. doi: 10.3174/ajnr.A4577
54. Touze R, Gravellier B, Rolla-Bigliani C, Touitou V, Shotar E, Lenck S, et al. Occlusion rate and visual complications with flow-diverter stent placed across the ophthalmic artery's origin for carotid-ophthalmic aneurysms: a meta-analysis. Neurosurgery. (2020) 86:455–63. doi: 10.1093/neuros/nyz202
55. Sahlein DH, Fouladvand M, Becske T, Saatci I, McDougall CG, Szikora I, et al. Neuroophthalmological outcomes associated with use of the pipeline embolization device: analysis of the PUFS trial results. J Neurosurg. (2015) 123:897–905. doi: 10.3171/2014.12.JNS141777
56. Waihrich E, Clavel P, Mendes G, Iosif C, Kessler IM, Mounayer C. Influence of anatomic changes on the outcomes of carotid siphon aneurysms after deployment of flow-diverter stents. Neurosurgery. (2018) 83:1226–33. doi: 10.1093/neuros/nyx618
57. Kim LJ, Tariq F, Levitt M, Barber J, Ghodke B, Hallam DK, et al. Multimodality treatment of complex unruptured cavernous and paraclinoid aneurysms. Neurosurgery. (2014) 74:51–61. doi: 10.1227/NEU.0000000000000192
58. Zhou Y, Wu X, Tian Z, Yang X, Mu S. Pipeline embolization device with adjunctive coils for the treatment of unruptured large or giant vertebrobasilar aneurysms: a single-center experience. Front Neurol. (2020) 11:522583. doi: 10.3389/fneur.2020.522583
59. Heran NS, Song JK, Kupersmith MJ, Niimi Y, Namba K, Langer DJ, et al. Large ophthalmic segment aneurysms with anterior optic pathway compression: assessment of anatomical and visual outcomes after endosaccular coil therapy. J Neurosurg. (2007) 106:968–75. doi: 10.3171/jns.2007.106.6.968
60. Burrows AM, Brinjikji W, Puffer RC, Cloft H, Kallmes DF, Lanzino G. Flow diversion for ophthalmic artery aneurysms. AJNR Am J Neuroradiol. (2016) 37:1866–9. doi: 10.3174/ajnr.A4835
61. Dornbos D III, Pillai P, Sauvageau E. Flow diverter assisted coil embolization of a very small ruptured ophthalmic artery aneurysm. J Neurointerv Surg. (2016) 8:e2–4. doi: 10.1136/neurintsurg-2013-010876.rep
62. D'Urso PI, Karadeli HH, Kallmes DF, Cloft HJ, Lanzino G. Coiling for paraclinoid aneurysms: time to make way for flow diverters? AJNR Am J Neuroradiol. (2012) 33:1470–4. doi: 10.3174/ajnr.A3009
63. Lu D, Ma T, Zhu G, Zhang T, Wang N, Lei H, et al. Willis covered stent for treating intracranial pseudoaneurysms of the internal carotid artery: a multi-institutional study. Neuropsychiatr Dis Treat. (2022) 18:125–35. doi: 10.2147/NDT.S345163
64. Yan P, Zhang Y, Ma C, Liang F, Zhu H, Jiang C. Application of the Willis covered stent in the treatment of intracranial unruptured aneurysms in internal carotid artery: a retrospective single-center experience. J Clin Neurosci. (2020) 78:222–7. doi: 10.1016/j.jocn.2020.04.045
65. Zhang Y, Zhang Y, Liang F, Jiang C. Procedure-related complication of willis covered stent in the treatment of blood blister-like aneurysm: stent detachment from dilating balloon. Front Neurol. (2017) 8:639. doi: 10.3389/fneur.2017.00639
66. Liu LX, Zhang CW, Lin S, Wu C, Wang T, Zhou LX, et al. Application of the willis covered stent in the treatment of ophthalmic artery segment aneurysms: a single-center experience. World Neurosurg. (2019) 122:e546–e52. doi: 10.1016/j.wneu.2018.10.098
67. Pierot L. Ten years of clinical evaluation of the woven endobridge: a safe and effective treatment for wide-neck bifurcation aneurysms. Neurointervention. (2021) 16:211–21. doi: 10.5469/neuroint.2021.00395
68. Dmytriw AA, Diestro JDB, Dibas M, Phan K, Sweid A, Cuellar-Saenz HH, et al. International study of intracranial aneurysm treatment using woven endobridge: results of the worldwideweb consortium. Stroke. (2022) 53:e47–e9. doi: 10.1161/STROKEAHA.121.037609
69. Gajera J, Maingard J, Foo M, Ren Y, Lamanna A, Nour D, et al. The woven endobridge device for the treatment of intracranial aneurysms: initial clinical experience within an australian population. Neurointervention. (2022) 17:28–36. doi: 10.5469/neuroint.2021.00430
70. Monteiro A, Lazar AL, Waqas M, Rai HH, Baig AA, Cortez GM, et al. Treatment of ruptured intracranial aneurysms with the woven Endobridge device: a systematic review. J Neurointerv Surg. (2022) 14:366–70. doi: 10.1136/neurintsurg-2021-017613
71. Recker MJ, Rajah GB, Tso MK, Dossani RH, Levy EI. Treatment of carotid ophthalmic aneurysm with woven endoBridge (WEB SL): 2-dimensional operative video. Oper Neurosurg. (2020) 19:E424–E5. doi: 10.1093/ons/opaa098
72. Meling TR. What are the treatment options for blister-like aneurysms? Neurosurg Rev. (2017) 40:587–93. doi: 10.1007/s10143-017-0893-1
73. Rouchaud A, Brinjikji W, Cloft HJ, Kallmes DF. Endovascular treatment of ruptured blister-like aneurysms: a systematic review and meta-analysis with focus on deconstructive vs. reconstructive and flow-diverter treatments. AJNR Am J Neuroradiol. (2015) 36:2331–9. doi: 10.3174/ajnr.A4438
74. Lang ST, Assis Z, Wong JH, Morrish W, Mitha AP. Rapid delayed growth of ruptured supraclinoid blister aneurysm after successful flow diverting stent treatment. J Neurointerv Surg. (2017) 9:e16. doi: 10.1136/neurintsurg-2016-012506.rep
75. Lee J, Kim DH, Lee SH, Moon JH, Yang SY, Cho KT, et al. Stent-assisted coiling vs. flow diverter for treating blood blister-like aneurysms: a proportion meta-analysis. Clin Neuroradiol. (2022). doi: 10.1007/s00062-022-01160-3
76. Liu LX, Zhang CW, Xie XD, Wang CH. Application of the willis covered stent in the treatment of blood blister-like aneurysms: a single-center experience and systematic literature review. World Neurosurg. (2019) 123:e652–e60. doi: 10.1016/j.wneu.2018.11.245
77. Chang H, Shen Y, Li Z, Lin C, Chen H, Lu H. Safety and efficacy of endovascular therapy for blood blister-like aneurysms: willis covered stents and double stents assistant coils-a single center cohort study. Front Neurol. (2021) 12:606219. doi: 10.3389/fneur.2021.606219
78. Qi Y, Xu T, Jiang C, Wang Y, Liu H. Application of the Willis covered stent in the treatment of internal carotid artery blood blister-like aneurysms. Neurosurg Rev. (2021). doi: 10.1007/s10143-021-01666-3
79. Gross BA, Du R. Microsurgical treatment of ophthalmic segment aneurysms. J Clin Neurosci. (2013) 20:1145–8. doi: 10.1016/j.jocn.2012.11.005
80. Ogawa A, Suzuki M, Ogasawara K. Aneurysms at non-branching sites in the surpaclinoid portion of the internal carotid artery: internal carotid artery trunk aneurysms. Neurosurgery. (2000) 47:578–83. doi: 10.1227/00006123-200009000-00008
81. Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Motiei-Langroudi R, Chua MH, et al. Comparison of stent-assisted coil embolization and the pipeline embolization device for endovascular treatment of ophthalmic segment aneurysms: a multicenter cohort study. World Neurosurg. (2017) 105:206–12. doi: 10.1016/j.wneu.2017.05.104
82. Silva MA, See AP, Khandelwal P, Mahapatra A, Frerichs KU, Du R, et al. Comparison of flow diversion with clipping and coiling for the treatment of paraclinoid aneurysms in 115 patients. J Neurosurg. (2018) 34:1–8. doi: 10.3171/2018.1.JNS171774
83. Patel S, Fargen KM, Peters K, Krall P, Samy H, Hoh BL. Return of visual function after bilateral visual loss following flow diversion embolization of a giant ophthalmic aneurysm due to both reduction in mass effect and reduction in aneurysm pulsation. J Neurointerv Surg. (2015) 7:e1. doi: 10.1136/neurintsurg-2013-010960.rep
84. Colby GP, Paul AR, Radvany MG, Gandhi D, Gailloud P, Huang J, et al. A single center comparison of coiling versus stent assisted coiling in 90 consecutive paraophthalmic region aneurysms. J Neurointerv Surg. (2012) 4:116–20. doi: 10.1136/jnis.2011.004911
85. Lanzino G, Crobeddu E, Cloft HJ, Hanel R, Kallmes DF. Efficacy and safety of flow diversion for paraclinoid aneurysms: a matched-pair analysis compared with standard endovascular approaches. AJNR Am J Neuroradiol. (2012) 33:2158–61. doi: 10.3174/ajnr.A3207
86. Heller RS, Lawlor CM, Hedges TR. 3rd, Bababekov YJ, Safain MG, Malek AM. Neuro-ophthalmic effects of stenting across the ophthalmic artery origin in the treatment of intracranial aneurysms. J Neurosurg. (2014) 121:18–23. doi: 10.3171/2014.3.JNS131493
87. Brasiliense LB, Stanley MA, Grewal SS, Cloft HJ, Sauvageau E, Lanzino G, et al. Silent ischemic events after Pipeline embolization device: a prospective evaluation with MR diffusion-weighted imaging. J Neurointerv Surg. (2016) 8:1136–9. doi: 10.1136/neurintsurg-2015-012091
88. Di Maria F, Pistocchi S, Clarencon F, Bartolini B, Blanc R, Biondi A, et al. Flow diversion vs. standard endovascular techniques for the treatment of unruptured carotid-ophthalmic aneurysms. AJNR Am J Neuroradiol. (2015) 36:2325–30. doi: 10.3174/ajnr.A4437
89. Miller TR, Wessell A, Jindal G, Malhotra A, Simard JM, Gandhi D. The utility of platelet inhibition testing in patients undergoing Pipeline embolization of intracranial aneurysms. J Neurointerv Surg. (2022) 14:7681. doi: 10.1136/neurintsurg-2021-017681
90. Hou K, Li G, Lv X, Xu B, Xu K, Yu J. Delayed rupture of intracranial aneurysms after placement of intra-luminal flow diverter. Neuroradiol J. (2020) 33:451–64. doi: 10.1177/1971400920953299
91. Turner RD, Byrne JV, Kelly ME, Mitsos AP, Gonugunta V, Lalloo S, et al. Delayed visual deficits and monocular blindness after endovascular treatment of large and giant paraophthalmic aneurysms. Neurosurgery. (2008) 63:469–74. doi: 10.1227/01.NEU.0000324730.37144.4B
92. Dehdashti AR, Thines L, Willinsky RA, Tymianski M. Symptomatic enlargement of an occluded giant carotido-ophthalmic aneurysm after endovascular treatment: the vasa vasorum theory. Acta Neurochir (Wien). (2009) 151:1153–8. doi: 10.1007/s00701-009-0270-0
93. Ashour R, Johnson J, Ebersole K, Aziz-Sultan MA. “Successful” coiling of a giant ophthalmic aneurysm resulting in blindness: case report and critical review. Neurosurg Rev. (2013) 36:661–5. doi: 10.1007/s10143-013-0472-z
94. Durst CR, Starke RM, Clopton D, Hixson HR, Schmitt PJ, Gingras JM, et al. Endovascular treatment of ophthalmic artery aneurysms: ophthalmic artery patency following flow diversion versus coil embolization. J Neurointerv Surg. (2016) 8:919–22. doi: 10.1136/neurintsurg-2015-011887
95. Chalouhi N, Daou B, Kung D, Zanaty M, Phillips JL, Tjoumakaris S, et al. Fate of the ophthalmic artery after treatment with the pipeline embolization device. Neurosurgery. (2015) 77:581–4. doi: 10.1227/NEU.0000000000000887
96. Puffer RC, Kallmes DF, Cloft HJ, Lanzino G. Patency of the ophthalmic artery after flow diversion treatment of paraclinoid aneurysms. J Neurosurg. (2012) 116:892–6. doi: 10.3171/2011.11.JNS111612
97. Rouchaud A, Leclerc O, Benayoun Y, Saleme S, Camilleri Y, D'Argento F, et al. Visual outcomes with flow-diverter stents covering the ophthalmic artery for treatment of internal carotid artery aneurysms. AJNR Am J Neuroradiol. (2015) 36:330–6. doi: 10.3174/ajnr.A4129
98. Pahl FH, de Oliveira MF, Brock RS, Lucio JE, Rotta JM. Surgical clipping is still a good choice for the treatment of paraclinoid aneurysms. Arq Neuropsiquiatr. (2016) 74:314–9. doi: 10.1590/0004-282X20150215
99. Falk Delgado A, Andersson T, Falk Delgado A. Ruptured carotid-ophthalmic aneurysm treatment: a non-inferiority meta-analysis comparing endovascular coiling and surgical clipping. Br J Neurosurg. (2017) 31:345–9. doi: 10.1080/02688697.2017.1297371
100. Date I, Asari S, Ohmoto T. Cerebral aneurysms causing visual symptoms: their features and surgical outcome. Clin Neurol Neurosurg. (1998) 100:259–67. doi: 10.1016/S0303-8467(98)00047-X
Keywords: paraophthalmic segment, internal carotid artery, endovascular treatment, aneurysm, review
Citation: Wang Y and Yu J (2022) Endovascular treatment of aneurysms of the paraophthalmic segment of the internal carotid artery: Current status. Front. Neurol. 13:913704. doi: 10.3389/fneur.2022.913704
Received: 06 April 2022; Accepted: 30 August 2022;
Published: 16 September 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Kamil Krystkiewicz, Copernicus Memorial Hospital, PolandAhmed Mohamed Elhfnawy, Uniklinikum Giessen und Marburg, Germany
Copyright © 2022 Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlu Yu, amx5dUBqbHUuZWR1LmNu; amlubHV5dUBob3RtYWlsLmNvbQ==
†ORCID: Jinlu Yu orcid.org/0000-0003-2329-7946
 Yiheng Wang
Yiheng Wang Jinlu Yu
Jinlu Yu