- 1Department of Respiratory and Critical Care Medicine, Sleep Center, The Second Affiliated Hospital of Soochow University, Soochow University, Suzhou, China
- 2Department of Pulmonary and Critical Care Medicine Affiliated Kunshan Hospital of Jiangsu University, Suzhou, China
Objective: The main aim of this meta-analysis was to evaluate the predictors of the efficacy of continuous positive airway pressure (CPAP) in ameliorating excessive daytime sleepiness (EDS) in patients with obstructive sleep apnea (OSA).
Methods: Randomized controlled trials (RCTs) published between January 1994 and October 2021 were searched in the PubMed, EMBASE, and Cochrane Library databases. The weighted mean differences (WMDs) for the Epworth Sleepiness Scale (ESS) scores, the Multiple Sleep Latency Test (MSLT), and the Maintenance of Wakefulness Test (MWT) were pooled in STATA.
Results: A total of 41 RCTs involving 7,332 patients were included. CPAP therapy was found to be significantly associated with changes in ESS (WMD = −2.14, P < 0.001), MSLT (WMD = 1.23, P < 0.001), and MWT (WMD = 1.6, P < 0.001). Meta-regression analysis and subgroup analysis indicated that in mild OSA, the efficacy of CPAP therapy for subjective EDS was limited to patients <50 years of age, with a baseline body mass index (BMI) of ≥30 kg/m2, baseline ESS score of ≥11, therapy adherence for ≥3 h/night, and treatment duration of ≥2 months. In moderate OSA, significant differences were observed in the changes in ESS among groups stratified by baseline ESS score (P = 0.005), adherence (P < 0.001), treatment duration (P = 0.009), and trial design type (P = 0.001). In severe OSA, this difference was observed among groups stratified by baseline BMI (P = 0.028), baseline ESS score (P = 0.001), and adherence (P = 0.047). Patients with moderate-severe OSA but not mild OSA showed significant improvements in MSLT. Patients with the age <50 years or BMI ≥33 kg/m2 had a more significant increase in MWT.
Conclusion: Continuous positive airway pressure therapy improved subjective and objective sleepiness in patients with OSA. Age, baseline BMI, baseline ESS score, adherence, and duration of treatment may predict the effects of CPAP on EDS in patients with OSA. Notably, the baseline ESS scores and adherence were stable predictors regardless of OSA severity.
Introduction
Obstructive sleep apnea (OSA) is among the most common sleep breathing disorders and is characterized by repeated obstruction of the upper airway during sleep, thus causing sleep fragmentation and intermittent hypoxia (1). Excessive daytime sleepiness (EDS), the most frequently self-reported symptom of OSA, may negatively affect mood, cognitive ability, and quality of life (2). EDS has been reported to affect 40.5%−58% of patients with OSA and is a risk factor for motor vehicle accidents (3). In recent years, EDS has received increased attention in the field of sleep medicine, and several clinical assessment tools are now available for EDS evaluation, including objective daytime sleepiness based on the multiple sleep latency test (MSLT) (4), the maintenance of wakefulness test (MWT) (5), and the subjective daytime sleepiness measured with the Epworth Sleepiness Scale (ESS) (6).
Continuous positive airway pressure (CPAP) therapy is the main treatment for OSA, particularly in moderate to severe cases (7). Previous meta-analyses have demonstrated that CPAP significantly decreases subjective but not objective sleepiness in patients with OSA (8, 9). Although EDS can be decreased with CPAP therapy, a substantial number of patients still experience EDS after treatment. Between 9 and 22% of patients still experience residual EDS after CPAP treatment (10). The efficacy of CPAP therapy for EDS is inconsistent due to several potential confounding factors, including heterogeneity in age, obesity, adherence, duration of CPAP therapy, and OSA severity. Given the heterogeneity and complexity of OSA, accurate CPAP therapy should involve a combination of several dimensions, including demographic characteristics, appropriate CPAP protocols, and pathophysiology. Therefore, clinical factors associated with CPAP efficacy regarding daytime sleepiness in patients with OSA must be identified. The purpose of this meta-analysis was to explore the relationship between different clinical subtypes of OSA and the efficacy of CPAP therapy for EDS.
Methods
Literature Search
We conducted a literature search of the PubMed, EMBASE, and Cochrane Library databases for articles published between January 1994 and October 2021. Combinations of Medical Subject Heading (MeSH) terms and free-text words were searched according to the Population, Intervention, Control, Outcome, and Study Design (PICOS) principle. Search terms for the population category were sleep apnea, obstructive (MeSH) OR (OSA syndrome) OR (OSA) OR (OSAHS) OR (sleep apnea-hypopnea syndrome) OR (syndrome, OSA) OR (upper airway resistance sleep apnea syndrome) OR (syndrome, upper airway resistance, and sleep apnea). Search terms for the intervention category were continuous positive airway pressure (MeSH) OR (CPAP ventilation) OR (nasal CPAP) OR (nCPAP ventilation) OR (biphasic CPAP) OR [bi-level positive airway pressure (BiPAP)] OR (bilevel CPAP). Search terms for the control category were (oral placebo) OR (sham CPAP) OR (placebo CPAP) OR (conservative treatment). Search terms for the outcome category were (the ESS) OR (subjective sleepiness) OR (the MSLT) OR (objective sleepiness) OR (the MWT) OR (objective wakefulness). Search terms for the study design category were (randomized controlled trial) OR (randomized) OR (placebo).
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: (1) study design: randomized controlled trials for CPAP therapy vs. sham control; (2) participants: OSA diagnosed with an apnea-hypopnea index (AHI) ≥5 events/h on the basis of polysomnography; (3) study reported outcomes: assessment of at least one of the sleepiness indicators, ESS, MSLT, or MWT. The exclusion criteria were as follows: repeat studies, abstracts, case reports, reviews, letters, studies with invalid data, and patients <18 years of age.
Quality Assessment
The JADAD scale (11) and Cochrane risk bias assessment tools (12) were used to evaluate the quality of RCTs and were independently completed and verified by two researchers. A modified JADAD score of 4–7 represented high-quality research and 1–3 signified low-quality research. All literature included in the meta-analysis was scored 4–7, indicative of high-quality research (Table 1). The Cochrane risk bias assessment tool (Supplementary Figure 1) was applied to evaluate seven important sources of bias (random sequence generation, allocation concealment, blindness of subjects and researchers, blindness of outcome evaluation, incomplete data, selective reporting of results, and other biases). Green color represents low risk, yellow color represents medium risk, and red color represents high risk.
Statistical Analyses
This meta-analysis was performed according to the PRISMA guidelines (54). The mean differences and standard errors in treatment efficacy between the CPAP and control groups were pooled in Stata 12.0. Heterogeneity between studies was assessed with Q-tests, and the I2-value indicated the degree of heterogeneity among therapeutic effects. Substantial heterogeneity between trials was indicated by P < 0.1 and I2 > 50%. A two-tailed test was selected (α = 0.05). Meta-regression and subgroup analyses were used to explore potential sources of heterogeneity. Meta-regression models were performed to assess changes in the efficacy of CPAP, including the effects of basic study and patient characteristics, such as RCT design type (parallel or crossover), treatment duration, age, BMI, AHI, ESS scores, adherence, and comorbidity. Begg's and Egger's tests were used to quantitatively evaluate publication bias, and a P-value > 0.5 was considered to indicate no publication bias. Sensitivity analyses were performed by individually eliminating each study and recalculating the pooled weighted mean difference (WMD) and 95% CI to determine the reliability of the results.
Results
Literature Selection
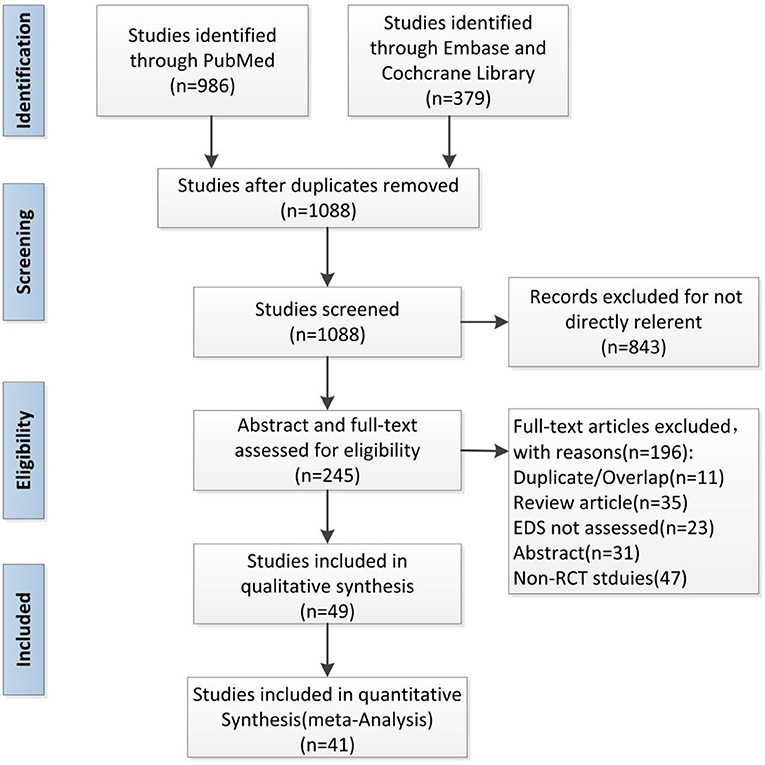
A total of 1,365 articles were retrieved by independent assessors, with the substantive agreement. Overall, 267 duplicate publications were excluded. The remaining 245 RCTs were obtained after the removal of 843 irrelevant articles. We excluded 196 studies according to the inclusion and exclusion criteria and removed eight articles with invalid data. Finally, 41 RCTs, representing 7,332 patients, were integrated into our meta-analysis (Figure 1).
Data Extraction and Quality Assessment
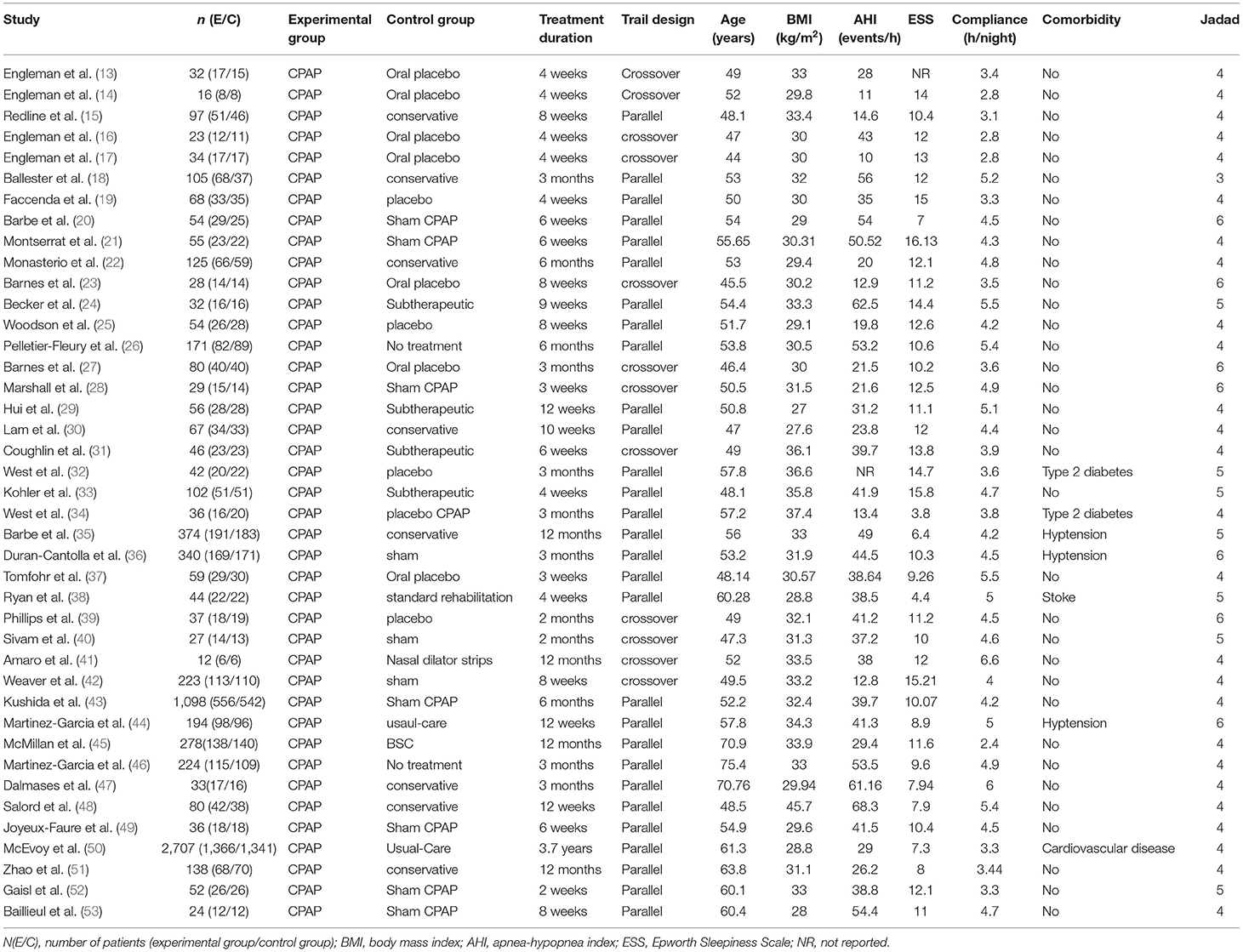
Baseline characteristics of the included studies and patients are shown in Table 1. The first author, year of publication, number of patients, intervention and control groups, RCT design, treatment duration, baseline age, BMI, AHI, ESS score, adherence, comorbidity, and outcome data (ESS/MSLT/MWT) were extracted. The studies included 29 parallel RCTs and 12 crossover RCTs. The treatment duration lasted from 1 month to 3.7 years. The patients' baseline age, BMI, AHI, ESS score, and adherence were 44.0–75.4 years, 27.2–45.7 kg/m2, 10.0–68.3 events/h, 4.4–15.8 points, and 2.8–6.6 h/night, respectively. Engleman et al. (13) did not report baseline ESS scores, and West 2007 (32) did not report baseline AHI. Forty studies reported ESS (14–53), eight studies reported MSLT (13–17, 20, 22, 23), and six studies reported MWT (17, 27, 28, 32, 34, 43).
Overall Pooled Effect Sizes of ESS, MSLT, and MWT (Meta-Analysis of CPAP for Ameliorated Sleepiness)
We pooled the effect sizes to assess the effects of CPAP therapy on sleepiness. After controlling for the placebo effect, CPAP treatment was found to decrease the ESS scores by 2.14 points (95% CI: −2.20 to −2.08, P < 0.001), I2 = 95% (Figure 2), prolong MSLT by 1.23 min (95% CI: 0.90 to 1.55, P < 0.001), I2 = 69.7% (Figure 3), and increase MWT by 1.6 min (95% CI: 1.32 to 1.88, P < 0.001), I2 = 92.1% (Figure 4). The severity of OSA was classified as mild (5 ≤ AHI <15 events/h), moderate (15 ≤ AHI <30 events/h), or severe (AHI ≥ 30 events/h). In mild, moderate, and severe OSA, the ESS scores decreased by 1.29 points (95% CI: −1.89 to −0.70, P < 0.001), I2 = 53.2%, 2.03 points (95% CI: −2.11 to −1.96, P < 0.001), I2 = 95.5%, and 2.58 points (95% CI: −2.72 to −2.45, P < 0.001), I2 = 95.7%, respectively.
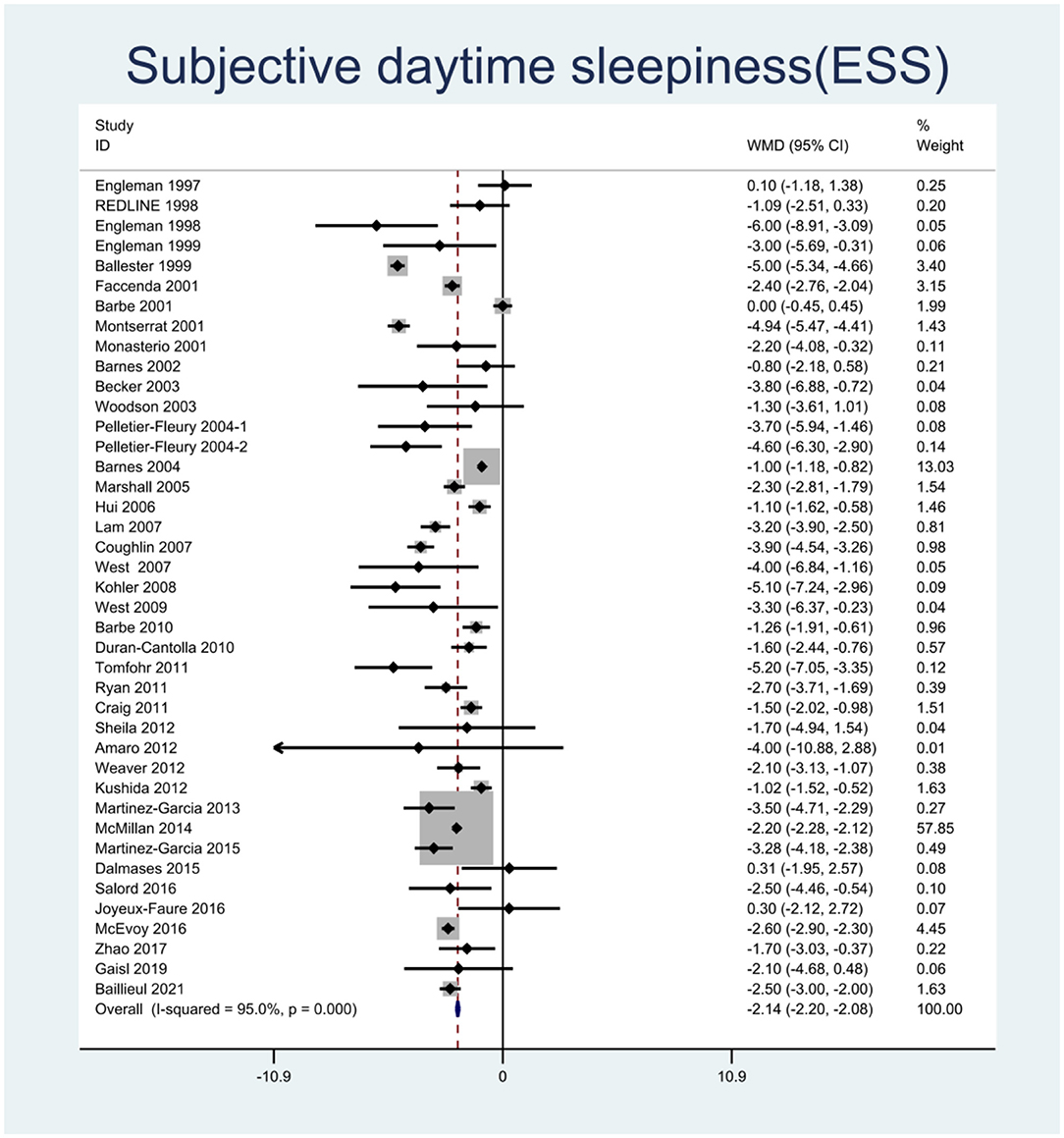
Figure 2. Forest plot showing pooled weighted mean difference (WMD) for Epworth Sleepiness Scale (ESS) in studies on continuous positive airway pressure (CPAP), compared with control group.
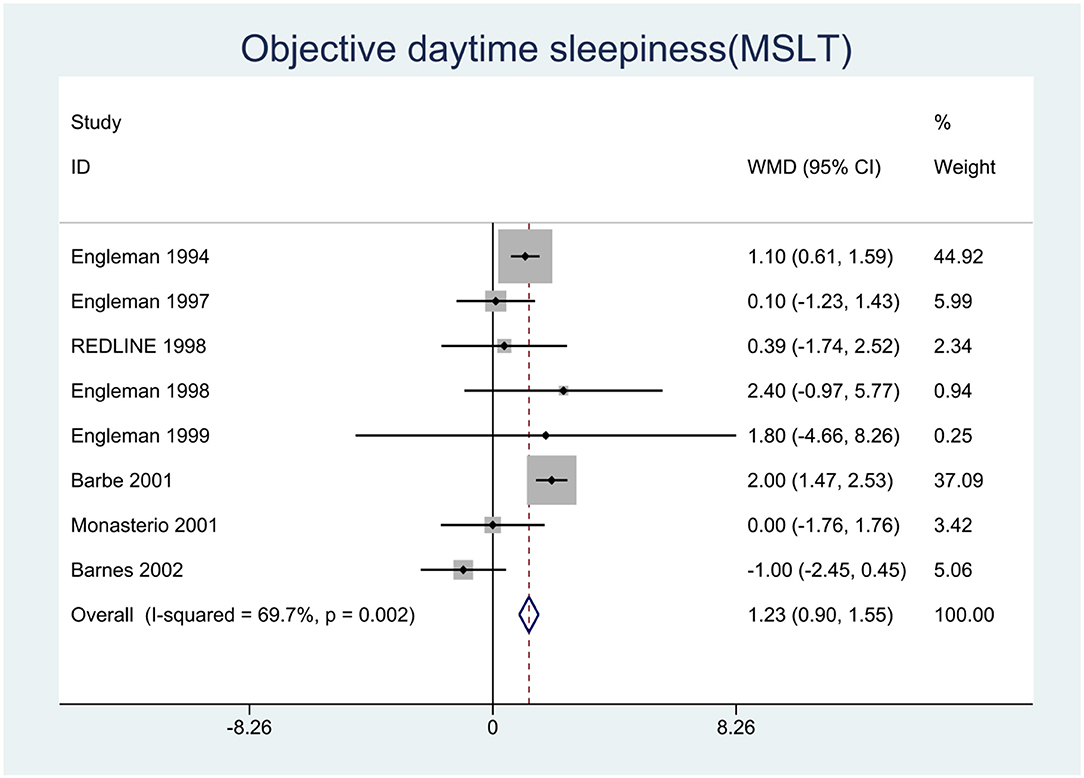
Figure 3. Forest plot showing pooled weighted mean difference (WMD) for Multiple Sleep Latency Test (MSLT) in studies on continuous positive airway pressure (CPAP), compared with control group.
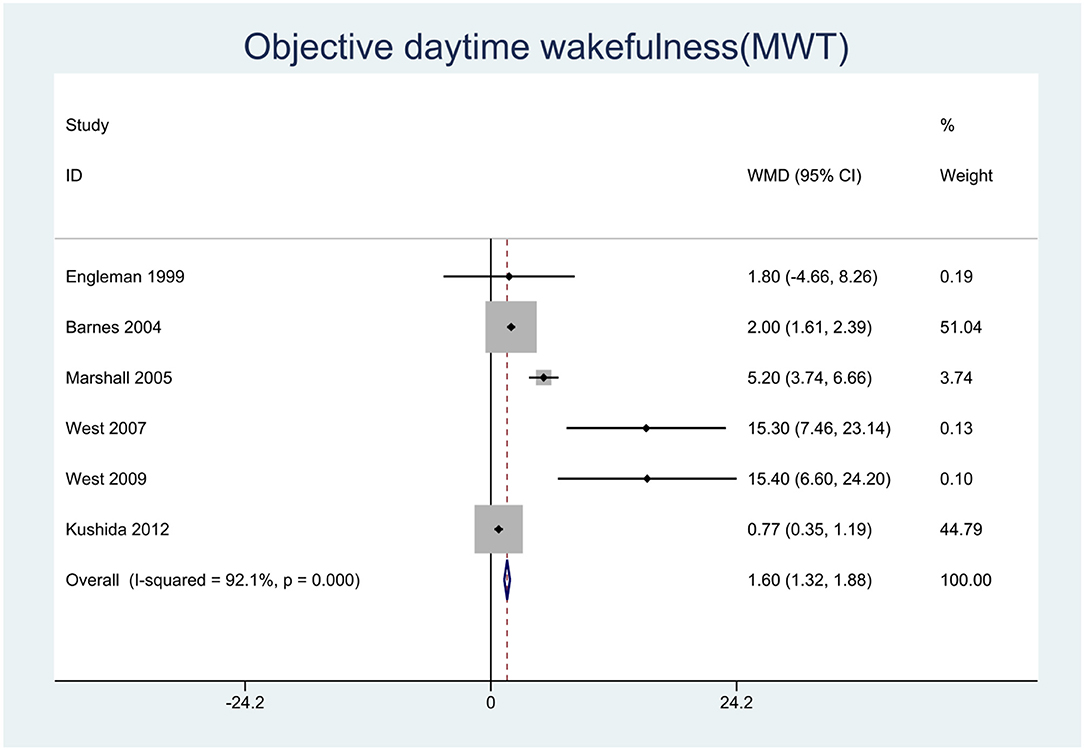
Figure 4. Forest plot showing pooled weighted mean difference (WMD) for Maintenance of Wakefulness Test (MWT) in studies on continuous positive airway pressure (CPAP), compared with control group.
Meta-Regression and Subgroup Analysis for Heterogeneity in the Change in Subjective EDS
The results of ESS scores in mild OSA are summarized in Table 2 and Supplementary Figure 4. Meta-regression demonstrated that the efficacy of CPAP therapy was significantly associated with BMI (segmentation point of BMI: 30 kg/m2) (P = 0.007) and adherence (P = 0.029) after adjustment for age. Subgroup analysis indicated that the efficacy of CPAP therapy was limited to patients <50 years of age (<50: WMD = −1.6, P < 0.001 vs. ≥50: WMD = −0.4, P = 0.505), with BMI ≥30 kg/m2 (<30: WMD = 0.1, P = 0.878 vs. ≥30: WMD = −1.68, P < 0.001), ESS scores ≥11 points (<11: WMD = −1.09, P = 0.132 vs. ≥11: WMD = −1.34, P < 0.001), adherence ≥3 h/night (<3 h: WMD = −0.47, P = 0.424 vs. ≥3 h: WMD = −1.59, P < 0.001), and treatment duration = 2–3 months (1 month: −0.47, P = 0.424 vs. 2 months: WMD = −1.5, P < 0.001 vs. 3 months: WMD = −3.3, P = 0.035).
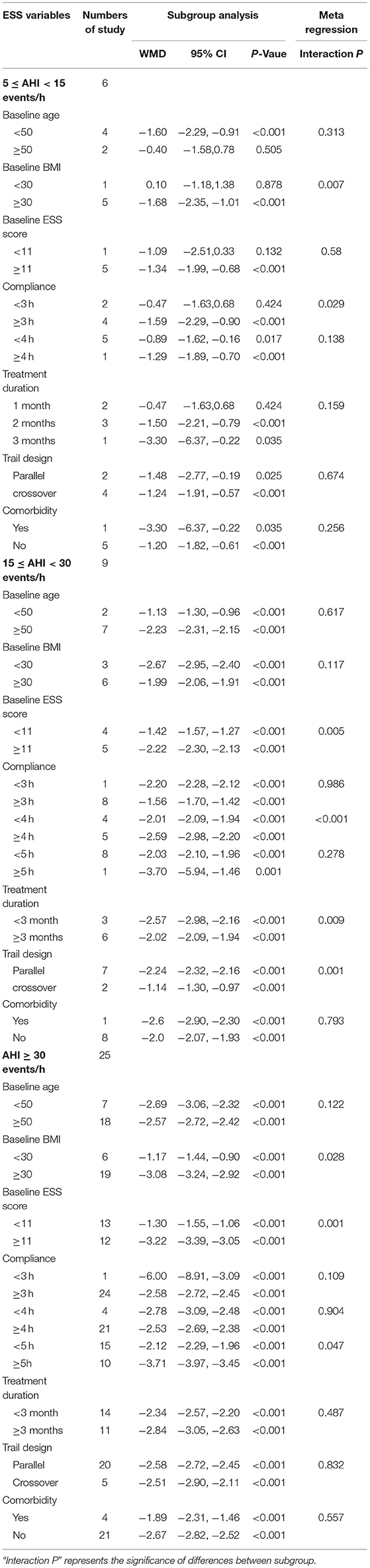
Table 2. Meta-regression and subgroup analysis for subjective excessive daytime sleepiness (EDS) based on Basic characteristics of patients and studies in different severity of OSA.
The results of the ESS scores in moderate OSA are summarized in Table 2 and Supplementary Figure 5. Meta-regression demonstrated that the efficacy of CPAP therapy was significantly associated with ESS score (P = 0.005), adherence (P <0.001), treatment duration (P = 0.009), and trial design (P = 0.001) after adjustment for age, BMI, and AHI. Subgroup analysis revealed significant differences according to the ESS score [ <11: WMD = −1.42, 95% CI (−1.57, −1.27) vs. ≥11: WMD = −2.22, 95% CI (−2.30, −2.13)], adherence [<4 h: WMD = −2.01, 95% CI (−2.09, −1.94) vs. ≥4 h: WMD = −2.59, 95% CI (−2.98, −2.20)], treatment duration [<3 months: WMD = −2.57, 95% CI (−2.98, −2.16) vs. ≥3 months: WMD = −2.02, 95% CI (−2.09, −1.94)], and trial design [parallel: WMD = −2.24, 95% CI (−2.32, −2.16) vs. crossover: WMD = −1.14, 95% CI (−1.30, −0.97)].
The results of meta-regression and subgroup analysis of ESS scores in severe OSA are summarized in Table 2 and Supplementary Figure 6. Meta-regression demonstrated that the efficacy of CPAP therapy was significantly associated with BMI (P = 0.028), ESS score (P = 0.001), and adherence (P = 0.047) after adjustment for age, BMI, and AHI. Subgroup analysis indicated significant differences according to BMI [<30: WMD = −1.17, 95% CI (−1.44, −0.90) vs. ≥30: WMD = −3.08, 95% CI (−3.24, −2.92)], ESS score [<11: WMD = −1.3, 95% CI (−1.55, −1.06) vs. ≥11: WMD = −3.22, 95% CI (−3.39, −3.05)], adherence [<5 h: WMD = −0.47, 95% CI (−1.63, −0.68) vs. ≥5 h: WMD = −2.12, 95% CI (−2.29, −1.96)], and treatment duration [<3 months: WMD = −2.34, 95% CI (−2.57, −2.20) vs. ≥3 months: WMD = −2.84, 95% CI (−3.05, −2.63)].
Meta-Regression and Subgroup Analysis of Heterogeneity in the Change in Objective EDS
As shown in Table 3 and Supplementary Figure 7, meta-regression revealed that the AHI explained the heterogeneity of the change in MSLT very well (P = 0.004). Subgroup analysis based on OSA severity indicated that in mild, moderate, and severe OSA, the changes in MSLT were −0.23 min (95% CI: −1.11, 0.66), I2 = 0%, 1.02 min (95% CI: 0.55, 1.49), I2 = 28.1%, and 2.01 min (95% CI: 1.48, 2.54), I2 = 0%, respectively. These results indicated no significant effects of CPAP in mild OSA, whereas significant effects were observed in moderate and severe OSA, particularly the latter.
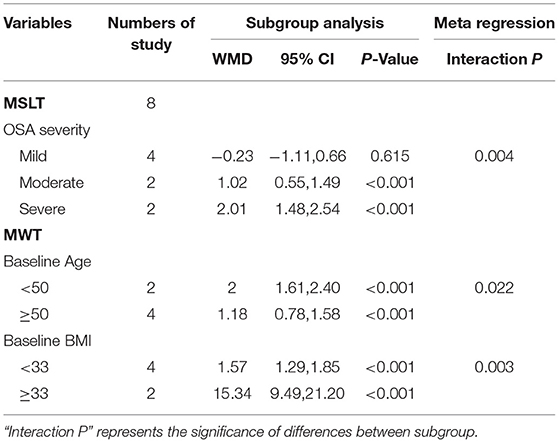
Table 3. Meta-regression and subgroup analysis for obejective excessive daytime sleepiness (EDS) based on Basic characteristics of patients and studies in different severity of OSA.
As shown in Table 3 and Supplementary Figures 8, 9, meta-regression to assess the sources of heterogeneity revealed that age (P = 0.022) and BMI (P = 0.003) were the factors responsible for the change in MWT. Subgroup analysis indicated that patients <50 years of age [<50: WMD = 2.00, 95% CI (1.61, 2.40) vs. ≥50: WMD = 1.18, 95% CI (0.78, 1.58)] with BMI ≥33 kg/m2 [<33: WMD = 1.57, 95% CI (1.29, 1.85) vs. ≥33: WMD = 15.34, 95% CI (9.49, 21.20)] were more likely to show improvements in MWT.
Publication Bias and Sensitivity Analysis
Begg's test and Egger's test for the evaluation of publication bias in terms of the ESS score returned P-values of 0.973 and 0.477, respectively, thus indicating an absence of publication bias. Begg's funnel plot and egger's publication bias plot are shown in Supplementary Figures 1, 2. Sensitivity analysis revealed that the overall effect size did not change when either study was removed. Therefore, the results of our meta-analysis were considered stable.
Discussion
In this study, first, we conducted a meta-analysis of the efficacy of CPAP treatment, compared with placebo, in ameliorating EDS in patients with OSA. In total, 41 RCTs in 7,332 patients were included in our meta-analysis. A total of 40 studies reported ESS, demonstrating clinically significantly lower subjective sleepiness, by 2.14 points, among participants undergoing CPAP therapy than controls. In mild, moderate, and severe OSA, the ESS scores were 1.29 points, 2.03 points, and 2.58 points lower than those in controls, respectively. The above conclusions were consistent with the results reported by Marshall et al. (8) and Patil et al. (9). Second, eight studies reported MSLT and demonstrated a clinically significant prolongation of objective sleepiness by 1.23 min with CPAP therapy; however, this effect was not observed in the meta-analyses by Marshall et al. (8)and Patil et al. (9). This inconsistent result might be associated with the discordant severity of OSA among the patients enrolled. Therefore, we conducted a subgroup analysis and found that patients with mild OSA did not show improvements in MSLT with CPAP therapy, thus further confirming our hypothesis. Finally, six studies reported MWT, and the results demonstrated a clinically significant increase in objective wakefulness by 1.6 min. Subgroup analysis indicated that patients <50 years of age with a BMI of >33 kg/m2 may benefit more from CPAP therapy.
The efficacy of CPAP therapy in ameliorating subjective sleepiness was highly heterogeneous, at 95%. To explore the source of this heterogeneity, we performed a subgroup analysis based on OSA severity. The heterogeneity remained high in patients with mild, moderate, and severe OSA, at 53.2, 95.5, and 95.7%, respectively. Therefore, we conducted further subgroup analysis and meta-regression based on the characteristics of the study and mild, moderate, or severe OSA status. In mild OSA, heterogeneity in ESS was associated with baseline age, BMI, ESS score, compliance, and duration of treatment; patients ≥50 years of age with a BMI <30 kg/m2, ESS scores <11, adherence <3 h/night, and treatment duration <2 months showed no significant improvements. In moderate OSA, heterogeneity in ESS was associated with the baseline ESS score, compliance, duration of treatment, and type of trial design; patients with ESS scores ≥11, adherence ≥4 h/night, treatment duration <3 months, and parallel RCTs showed significant improvement. In severe OSA, heterogeneity in ESS was associated with baseline BMI, ESS score, compliance, and duration of treatment; patients with BMI ≥30 kg/m2, ESS scores ≥11, adherence ≥5 h/night, and treatment duration ≥3 months showed greater changes in ESS. Overall, our results extend the previous meta-analysis reported by Marshall et al. (8) and Patil et al. (9) by meta-regression analyzing that treatment of OSA with CPAP results in more significant efficacy in EDS in patients who were sleepier and younger at baseline and who had higher BMI and good adherence.
Our subgroup analysis based on the basic clinical characteristics of patients revealed the following findings. Younger patients with OSA may be more likely to benefit from CPAP therapy. Patients ≥50 years of age with mild OSA may not benefit from CPAP therapy for subjective sleepiness, and patients <50 years of age show more significant improvements in objective wakefulness. Older patients appear to respond less well to CPAP therapy—an interesting finding that we speculate might be due to drowsiness in older patients, under the influence of potential confounding factors beyond OSA, such as sleep rhythm disorders, cerebrovascular diseases, and concomitant use of sleeping medications (55). Therefore, the underlying cause of drowsiness in patients with OSA with EDS must be comprehensively evaluated before CPAP therapy. In addition, obese patients showed a more favorable response to CPAP treatment than nonobese patients. Some studies have shown that BMI has no effect on the efficacy of CPAP therapy (56, 57). However, others have concluded that BMI does affect the efficacy of CPAP therapy (58, 59), in agreement with our results. Tangugsorn's research (60) based on cephalometric analyses has shown that nonobese patients with OSA tend to have craniofacial bone structure malformations, whereas obese patients with OSA primarily show abnormalities in upper airway soft tissue. This finding also suggests that different treatment options should be provided for obese and nonobese patients with OSA. First, obese patients may respond well to CPAP therapy, in contrast to nonobese patients, who may benefit more from surgical treatment. Second, obesity may contribute to daytime sleepiness by increasing the production of pro-inflammatory somatic adipose tissue-derived cytokines, such as interleukin (IL)-6 and plasminogen activator inhibitor (PAI)-1 (61). CPAP therapy effectively decreases the inflammatory response in obese patients with OSA, thereby improving EDS. Moreover, patients with low BMI may not respond well to CPAP therapy. A study comparing obese OSA with nonobese OSA has indicated that nonobese OSA is associated with a lower arousal threshold for airway stenosis, which may limit CPAP resistance and CPAP adherence, thus leading to poor outcomes (62). The combination of these factors may result in obese patients particularly benefitting from CPAP therapy to improve daytime function, such as EDS. Third, the baseline ESS score is a sensitive predictor of ameliorated subjective sleepiness in patients with OSA treated with CPAP. Regardless of the OSA severity, patients with a higher baseline ESS score were more likely to benefit from subjective sleepiness, whereas ESS scores did not predict the change in objective sleepiness or objective wakefulness. The MSLT, MMT test, and questionnaire (ESS) measured different objective and subjective aspects of sleepiness. Huang et al. (62) have found that, among untreated patients with OSA, the increase in subjective sleepiness is not significantly associated with a decrease in objective sleep latency and inability to remain awake. This result supports the findings of our meta-analysis, in which baseline ESS scores did not predict amelioration of objective sleepiness in patients with OSA treated with CPAP. Furthermore, although CPAP therapy significantly improves EDS, individuals do not respond equally to treatment. Baseline age, BMI, and ESS scores as clinical markers have potential clinical utility for identifying patients with OSA who are more likely to show EDS improvement. Further studies should be conducted to examine the links between these clinical markers and CPAP response and their underlying mechanisms.
On the basis of the subgroup analysis of CPAP compliance, treatment duration, and study characteristics, we obtained the following findings. Despite a consensus in which good CPAP adherence is defined as 4 h/night over 5 days/week, the optimal level of CPAP use appears to vary depending on the symptoms and the target treatment. Masa et al. (63) have demonstrated that subjective sleepiness symptoms normalize when compliance reaches 4 h/night, whereas Weaver et al. (64) has found that more than 5 h is required in severe OSA. How many hours of CPAP per night can significantly ameliorate daytime sleepiness at different levels of OSA severity? Our results demonstrated that CPAP compliance in ameliorating subjective sleepiness showed a dose-response effect depending on OSA severity. For instance, in mild OSA, CPAP use reaching 3 h/night can be beneficial, whereas for moderate or severe OSA, compliance should be increased to 4 or 5 h/night, respectively, to achieve better effects. Our results suggest that the traditional CPAP use threshold of 4 h/night does not perfectly normalize EDS. Therefore, we recommended that the minimum threshold for CPAP compliance be stratified according to OSA severity to control daytime symptoms in future clinical work. However, adherence to CPAP varies widely among individuals. In prior studies, 29%−83% of patients have been reported to use CPAP for <4 h/night (65). The effectiveness of CPAP therapy is often limited by suboptimal adherence, and strategies that can be implemented in clinical work to optimize adherence will be crucial for future research. In terms of treatment duration, mild to moderate OSA showed a good curative effect within 3 months, and severe OSA showed a good curative effect after more than 3 months. These findings suggested that the duration of CPAP therapy should be extended to more than 3 months in clinical practice for severe OSA. Finally, the trials in our meta-analysis included both parallel and crossover RCTs. Compared with parallel RCTs, crossover RCTs have the advantage of eliminating individual differences and improving statistical accuracy. Our subgroup analysis demonstrated that CPAP therapy significantly decreased subjective and objective sleepiness in both parallel and crossover RCTs, thus providing convincing results.
Our review has the following strengths: we included RCTs with a large sample size, which was high quality evaluated by the JADAD scale and Cochrane risk bias assessment tool, indicating that the quality of evidence provided by our meta-analysis is high. However, there are a few studies with reporting bias and attrition bias. Furthermore, the lack of detail on the randomization methods used in some studies leads to a high/unclear risk of bias, which may reflect reporting problems rather than genuine methodological flaws. We reported the first evidence that some subtypes with mild OSA may not achieve subjective sleepiness benefits from CPAP therapy, and CPAP therapy can ameliorate objective sleepiness in patients with moderate-severe OSA. We identified no publication bias, and the results of sensitivity analysis were stable. Although the heterogeneity was high, we identified the source of the heterogeneity and predictors of CPAP therapy efficacy through meta-regression and subgroup analysis. Our review also has several limitations, as follows: our meta-analysis was not registered, and there may be a small bias, but we still strictly followed the steps of systematic evaluation. Although tests for publication bias did not show statistical significance, it is always possible that there are unpublished negative trials that lead to overestimates of the efficacy of CPAP. We restricted eligibility to studies in English only, which potentially led to language bias. Meta-analysis of individual patient data (IPD meta-analysis) can make the results more realistic, but due to the large number of studies involved, obtaining original data would be challenging, so we did not perform an IPD meta-analysis. Some studies included OSA with other diseases (e.g., stroke, diabetes, or hypertension), thus potentially interfering with the results of our meta-analysis, although we performed meta-regression to correct for confounding factors. The RCTs included in our review included both parallel and crossover trial designs; therefore, we conducted a subgroup analysis according to trial design type and found that patients were more likely to benefit from CPAP therapy in parallel RCTs. The combination of parallel and crossover analyses is considered statistically irregular, given the different statistical methods used to calculate the standard error; this aspect remains a potential weakness of the review.
The American Academy of Sleep Medicine (AASM) practice parameters (66) recommended CPAP as the first-line treatment for moderate to severe OSA, and CPAP therapy may also be attempted for mild OSA with clinical symptoms. Our review could provide evidence in support of previously published AASM practice parameters regarding the efficacy of CPAP therapy for OSA. However, OSA is considered a complex and heterogeneous sleep disorder (67). Diagnosis, severity assessment, and treatment of OSA still often rely on a single indicator, the AHI, which is highly flawed (68). Previous studies (69, 70) have demonstrated that the severity of daytime sleepiness is poorly correlated with OSA severity. Some patients still have residual daytime sleepiness after CPAP therapy despite normalization of AHI. Other indicators must urgently be explored for OSA stratification, to improve understanding of the genetic and biological mechanisms and to identify OSA subtypes most suitable for CPAP therapy. Therefore, we conducted this meta-analysis and found that patients who were drowsy, obese, younger, and compliant appeared to be more likely to experience improvement in EDS after CPAP therapy. Overall, our meta-analysis appears to have captured prognostic heterogeneity, owing to some clinical characteristics of patients with OSA, such as age, BMI, EDS, and compliance. However, whether these represent true subtypes remains to be identified in future studies. The pattern of response to OSA treatment varies depending on the initial clinical subtype and CPAP adherence (71, 72). Our results suggest that the proposed subtype classification provides prognostic information related to CPAP treatment outcomes for daytime sleepiness that existing clinical criteria do not provide alone.
In summary, our meta-analysis highlight that age, BMI, ESS scores, and CPAP adherence are useful predictors to identify patients with OSA likely to respond to CPAP with a reduction in EDS. These results may help sleep specialists identify patients with OSA who have an advantage in CPAP therapy for EDS. Notably, this finding suggests that the identification of patients using clinical predictors may provide a new paradigm for understanding the treatment expectations of patients with OSA, thus facilitating the early identification and prognosis of OSA. Future research should focus on the identification of more effective clinical markers and explore the underlying mechanisms by which these predictors mediate CPAP response.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JW participated in data collection and verification. SC helped perform the analysis with constructive discussions. ZL performed the data analyses and wrote the manuscript. RC contributed to the conception of the study. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Natural Science Foundation of China (Grant numbers: NSFC81770085 and 82070095).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.911996/full#supplementary-material
References
1. Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. (2005) 28:472–7. doi: 10.1093/sleep/28.4.472
2. Sauter C, Asenbaum S, Popovic R, Bauer H, Lamm C, Klösch G, et al. Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome. J Sleep Res. (2000) 9:293–301. doi: 10.1046/j.1365-2869.2000.00211.x
3. Bjorvatn B, Lehmann S, Gulati S, Aurlien H, Pallesen S, Saxvig IW. Prevalence of excessive sleepiness is higher whereas insomnia is lower with greater severity of obstructive sleep apnea. Sleep Breath. (2015) 19:1387–93. doi: 10.1007/s11325-015-1155-5
4. Thorpy MJ. The clinical use of the Multiple Sleep Latency Test. The standards of practice committee of the american sleep disorders association. Sleep. (1992) 15:268–76. doi: 10.1093/sleep/15.3.268
5. Poceta JS, Timms RM, Jeong DU, Ho SL, Erman MK, Mitler MM. Maintenance of wakefulness test in obstructive sleep apnea syndrome. Chest. (1992) 101:893–7. doi: 10.1378/chest.101.4.893
6. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
7. Qaseem A, Holty JE, Owens DK, Dallas P, Starkey M, Shekelle P. Clinical guidelines committee of the American College of Physicians. Management of obstructive sleep apnea in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2013) 159:471–83. doi: 10.7326/0003-4819-159-7-201310010-00704
8. Marshall NS, Barnes M, Travier N, Campbell AJ, Pierce RJ, McEvoy RD, et al. Continuous positive airway pressure reduces daytime sleepiness in mild to moderate obstructive sleep apnoea: a meta-analysis. Thorax. (2006) 61:430–4. doi: 10.1136/thx.2005.050583
9. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an american academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. (2019) 15:301–34. doi: 10.5664/jcsm.7638
10. Pépin JL, Viot-Blanc V, Escourrou P, Racineux JL, Sapene M, Lévy P, et al. Prevalence of residual excessive sleepiness in CPAP-treated sleep apnoea patients: the French multicentre study. Eur Respir J. (2009) 33:1062–7. doi: 10.1183/09031936.00016808
11. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. (1996) 17:1–12. doi: 10.1016/0197-2456(95)00134-4
12. Higgins J Green S Cochrane Cochrane handbook for systematic reviews of interventions Version 5.1. 0. London: The Cochrane Collaboration (2011). Confidence intervals.
13. Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet. (1994) 343:572–5. doi: 10.1016/S0140-6736(94)91522-9
14. Engleman HM, Martin SE, Deary IJ, Douglas NJ. Effect of CPAP therapy on daytime function in patients with mild sleep apnoea/hypopnoea syndrome. Thorax. (1997) 52:114–9. doi: 10.1136/thx.52.2.114
15. Redline S, Adams N, Strauss ME, Roebuck T, Winters M, Rosenberg C. Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med. (1998) 157:858–65. doi: 10.1164/ajrccm.157.3.9709042
16. Engleman HM, Martin SE, Kingshott RN, Mackay TW, Deary IJ, Douglas NJ. Randomised placebo controlled trial of daytime function after continuous positive airway pressure (CPAP) therapy for the sleep apnoea/hypopnoea syndrome. Thorax. (1998) 53:341–5. doi: 10.1136/thx.53.5.341
17. Engleman HM, Kingshott RN, Wraith PK, Mackay TW, Deary IJ, Douglas NJ. Randomized placebo-controlled crossover trial of continuous positive airway pressure for mild sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. (1999) 159:461–7. doi: 10.1164/ajrccm.159.2.9803121
18. Ballester E, Badia JR, Hernández L, Carrasco E, de Pablo J, Fornas C, et al. Evidence of the effectiveness of continuous positive airway pressure in the treatment of sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. (1999) 159:495–501. doi: 10.1164/ajrccm.159.2.9804061
19. Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. (2001) 163:344–8. doi: 10.1164/ajrccm.163.2.2005037
20. Barbé F, Mayoralas LR, Duran J, Masa JF, Maimó A, Montserrat JM, et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. A randomized, controlled trial. Ann Intern Med. (2001) 134:1015–23. doi: 10.7326/0003-4819-134-11-200106050-00007
21. Montserrat JM, Ferrer M, Hernandez L, Farré R, Vilagut G, Navajas D, et al. Effectiveness of CPAP treatment in daytime function in sleep apnea syndrome: a randomized controlled study with an optimized placebo. Am J Respir Crit Care Med. (2001) 164:608–13. doi: 10.1164/ajrccm.164.4.2006034
22. Monasterio C, Vidal S, Duran J, Ferrer M, Carmona C, Barbé F, et al. Effectiveness of continuous positive airway pressure in mild sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. (2001) 164:939–43. doi: 10.1164/ajrccm.164.6.2008010
23. Barnes M, Houston D, Worsnop CJ, Neill AM, Mykytyn IJ, Kay A, et al. A randomized controlled trial of continuous positive airway pressure in mild obstructive sleep apnea. Am J Respir Crit Care Med. (2002) 165:773–80. doi: 10.1164/ajrccm.165.6.2003166
24. Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. (2003) 107:68–73. doi: 10.1161/01.CIR.0000042706.47107.7A
25. Woodson BT, Steward DL, Weaver EM, Javaheri S. A randomized trial of temperature-controlled radiofrequency, continuous positive airway pressure, and placebo for obstructive sleep apnea syndrome. Otolaryngol Head Neck Surg. (2003) 128:848–61. doi: 10.1016/S0194-5998(03)00461-3
26. Pelletier-Fleury N, Meslier N, Gagnadoux F, Person C, Rakotonanahary D, Ouksel H, et al. Economic arguments for the immediate management of moderate-to-severe obstructive sleep apnoea syndrome. Eur Respir J. (2004) 23:53–60. doi: 10.1183/09031936.03.00066903
27. Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, et al. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. (2004) 170:656–64. doi: 10.1164/rccm.200311-1571OC
28. Marshall NS, Neill AM, Campbell AJ, Sheppard DS. Randomised controlled crossover trial of humidified continuous positive airway pressure in mild obstructive sleep apnoea. Thorax. (2005) 60:427–32. doi: 10.1136/thx.2004.032078
29. Hui DS, To KW, Ko FW, Fok JP, Chan MC, Ngai JC, et al. Nasal CPAP reduces systemic blood pressure in patients with obstructive sleep apnoea and mild sleepiness. Thorax. (2006) 61:1083–90. doi: 10.1136/thx.2006.064063
30. Lam B, Sam K, Mok WY, Cheung MT, Fong DY, Lam JC, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. (2007) 62:354–9. doi: 10.1136/thx.2006.063644
31. Coughlin SR, Mawdsley L, Mugarza JA, Wilding JP, Calverley PM. Cardiovascular and metabolic effects of CPAP in obese males with OSA. Eur Respir J. (2007) 29:720–7. doi: 10.1183/09031936.00043306
32. West SD, Nicoll DJ, Wallace TM, Matthews DR, Stradling JR. Effect of CPAP on insulin resistance and HbA1c in men with obstructive sleep apnoea and type 2 diabetes. Thorax. (2007) 62:969–74. doi: 10.1136/thx.2006.074351
33. Kohler M, Pepperell JC, Casadei B, Craig S, Crosthwaite N, Stradling JR, et al. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. (2008) 32:1488–96. doi: 10.1183/09031936.00026608
34. West SD, Kohler M, Nicoll DJ, Stradling JR. The effect of continuous positive airway pressure treatment on physical activity in patients with obstructive sleep apnoea: a randomised controlled trial. Sleep Med. (2009) 10:1056–8. doi: 10.1016/j.sleep.2008.11.007
35. Barbé F, Durán-Cantolla J, Capote F, de la Peña M, Chiner E, Masa JF, et al. Long-term effect of continuous positive airway pressure in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. (2010) 181:718–26. doi: 10.1164/rccm.200901-0050OC
36. Durán-Cantolla J, Aizpuru F, Montserrat JM, Ballester E, Terán-Santos J, Aguirregomoscorta JI, et al. Continuous positive airway pressure as treatment for systemic hypertension in people with obstructive sleep apnoea: randomised controlled trial. BMJ. (2010) 341:c5991. doi: 10.1136/bmj.c5991
37. Tomfohr LM, Ancoli-Israel S, Loredo JS, Dimsdale JE. Effects of continuous positive airway pressure on fatigue and sleepiness in patients with obstructive sleep apnea: data from a randomized controlled trial. Sleep. (2011) 34:121–6. doi: 10.1093/sleep/34.1.121
38. Ryan CM, Bayley M, Green R, Murray BJ, Bradley TD. Influence of continuous positive airway pressure on outcomes of rehabilitation in stroke patients with obstructive sleep apnea. Stroke. (2011) 42:1062–7. doi: 10.1161/STROKEAHA.110.597468
39. Phillips CL, Yee BJ, Marshall NS, Liu PY, Sullivan DR, Grunstein RR. Continuous positive airway pressure reduces postprandial lipidemia in obstructive sleep apnea: a randomized, placebo-controlled crossover trial. Am J Respir Crit Care Med. (2011) 184:355–61. doi: 10.1164/rccm.201102-0316OC
40. Sivam S, Phillips CL, Trenell MI, Yee BJ, Liu PY, Wong KK, et al. Effects of 8 weeks of continuous positive airway pressure on abdominal adiposity in obstructive sleep apnoea. Eur Respir J. (2012) 40:913–8. doi: 10.1183/09031936.00177011
41. Amaro AC, Duarte FH, Jallad RS, Bronstein MD, Redline S, Lorenzi-Filho G. The use of nasal dilator strips as a placebo for trials evaluating continuous positive airway pressure. Clinics. (2012) 67:469–74. doi: 10.6061/clinics/2012(05)11
42. Weaver TE, Mancini C, Maislin G, Cater J, Staley B, Landis JR, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. (2012) 186:677–83. doi: 10.1164/rccm.201202-0200OC
43. Kushida CA, Nichols DA, Holmes TH, Quan SF, Walsh JK, Gottlieb DJ, et al. Effects of continuous positive airway pressure on neurocognitive function in obstructive sleep apnea patients: the Apnea Positive Pressure Long-term Efficacy Study (APPLES). Sleep. (2012) 35:1593–602. doi: 10.5665/sleep.2226
44. Martínez-García MA, Capote F, Campos-Rodríguez F, Lloberes P, Díaz de. Atauri MJ, Somoza M, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. (2013) 310:2407–15. doi: 10.1001/jama.2013.281250
45. McMillan A, Bratton DJ, Faria R, Laskawiec-Szkonter M, Griffin S, Davies RJ, et al. Continuous positive airway pressure in older people with obstructive sleep apnoea syndrome (PREDICT): a 12-month, multicentre, randomised trial. Lancet Respir Med. (2014) 2:804–12. doi: 10.1016/S2213-2600(14)70172-9
46. Martínez-García MÁ, Chiner E, Hernández L, Cortes JP, Catalán P, Ponce S, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. (2015) 46:142–51. doi: 10.1183/09031936.00064214
47. Dalmases M, Solé-Padullés C, Torres M, Embid C, Nuñez MD, Martínez-Garcia MÁ, et al. Effect of CPAP on cognition, brain function, and structure among elderly patients with OSA: a randomized pilot study. Chest. (2015) 148:1214–23. doi: 10.1378/chest.15-0171
48. Salord N, Fortuna AM, Monasterio C, Gasa M, Pérez A, Bonsignore MR, et al. A randomized controlled trial of continuous positive airway pressure on glucose tolerance in obese patients with obstructive sleep apnea. Sleep. (2016) 39:35–41. doi: 10.5665/sleep.5312
49. Joyeux-Faure M, Naegelé B, Pépin JL, Tamisier R, Lévy P, Launois SH. Continuous positive airway pressure treatment impact on memory processes in obstructive sleep apnea patients: a randomized sham-controlled trial. Sleep Med. (2016) 24:44–50. doi: 10.1016/j.sleep.2016.06.023
50. McEvoy RD, Antic NA, Heeley E, Luo Y, Ou Q, Zhang X, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. (2016) 375:919–31. doi: 10.1056/NEJMoa1606599
51. Zhao YY, Wang R, Gleason KJ, Lewis EF, Quan SF, Toth CM, et al. Effect of Continuous positive airway pressure treatment on health-related quality of life and sleepiness in high cardiovascular risk individuals with sleep apnea: best apnea interventions for research (BestAIR) Trial. Sleep. (2017) 40:zsx040. doi: 10.1093/sleep/zsx040
52. Gaisl T, Rejmer P, Thiel S, Haile SR, Osswald M, Roos M, et al. Effects of suboptimal adherence of CPAP therapy on symptoms of obstructive sleep apnoea: a randomised, double-blind, controlled trial. Eur Respir J. (2020) 55:1901526. doi: 10.1183/13993003.01526-2019
53. Baillieul S, Wuyam B, Pérennou D, Tamisier R, Bailly S, Benmerad M, et al. A randomized sham-controlled trial on the effect of continuous positive airway pressure treatment on gait control in severe obstructive sleep apnea patients. Sci Rep. (2021) 11:9329. doi: 10.1038/s41598-021-88642-5
54. Moher D, Liberati A, Tetzlaff J, Altman DG. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
55. Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. (2009) 5:263–76. doi: 10.5664/jcsm.27497
56. Florés M, Martinez-Alonso M. Sánchezde-la-Torre A, Aldomà A, Galera E, Barbé F, et al. Predictors of long-term adherence to continuous positive airway pressure in patients with obstructive sleep apnoea and acute coronary syndrome. J Thorac Dis. (2018) 10:S124–34. doi: 10.21037/jtd.2017.12.128
57. Lee CHK, Leow LC, Song PR Li H, Ong TH. Acceptance and adherence to continuous positive airway pressure therapy in patients with obstructive sleep apnea (OSA) in a Southeast Asian privately funded healthcare system. Sleep Sci. (2017) 10:57–63. doi: 10.5935/1984-0063.20170010
58. Luyster FS, Strollo PJ Jr, Thunström E, Peker Y. Long-term use of continuous positive airway pressure therapy in coronary artery disease patients with nonsleepy obstructive sleep apnea. Clin Cardiol. (2017) 40:1297–302. doi: 10.1002/clc.22827
59. Tsuyumu M, Tsurumoto T, Iimura J, Nakajima T, Kojima H. Ten-year adherence to continuous positive airway pressure treatment in patients with moderate-to-severe obstructive sleep apnea. Sleep Breath. (2020) 24:1565–71. doi: 10.1007/s11325-020-02033-0
60. Tangugsorn V, Krogstad O, Espeland L, Lyberg T. Obstructive sleep apnoea: multiple comparisons of cephalometric variables of obese and non-obese patients. J Craniomaxillofac Surg. (2000) 28:204–12. doi: 10.1054/jcms.2000.0147
61. Vgontzas AN, Bixler EO, Tan TL, Kantner D, Martin LF, Kales A. Obesity without sleep apnea is associated with daytime sleepiness. Arch Intern Med. (1998) 158:1333–7. doi: 10.1001/archinte.158.12.1333
62. Huang Y, Aumüller P, Fietze I, Penzel T, Veauthier C. Comparison of the Oxford sleep resistance test and the multiple sleep latency test. Physiol Meas. (2020) 41:104005. doi: 10.1088/1361-6579/ab9feb
63. Masa JF, Corral-Peñafiel J. Should use of 4 hours continuous positive airway pressure per night be considered acceptable compliance? Eur Respir J. (2014) 44:1119–20. doi: 10.1183/09031936.00121514
64. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. (2007) 30:711–9. doi: 10.1093/sleep/30.6.711
65. Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. (2008) 5:173–8. doi: 10.1513/pats.200708-119MG
66. Morgenthaler TI, Aurora RN, Brown T, Zak R, Alessi C, Boehlecke B, et al. Practice parameters for the use of autotitrating continuous positive airway pressure devices for titrating pressures and treating adult patients with obstructive sleep apnea syndrome: an update for 2007. An American Academy of Sleep Medicine report. Sleep. (2008) 31:141–7. doi: 10.1093/sleep/31.1.141
67. Ayas NT, Owens RL, Kheirandish-Gozal L. Update in sleep medicine 2014. Am J Respir Crit Care Med. (2015) 192:415–20. doi: 10.1164/rccm.201503-0647UP
68. Punjabi NM. Counterpoint: is the apnea-hypopnea index the best way to quantify the severity of sleep-disordered breathing? No Chest. (2016) 149:16–9. doi: 10.1378/chest.14-2261
69. Koch H, Schneider LD, Finn LA, Leary EB, Peppard PE, Hagen E, et al. Breathing disturbances without hypoxia are associated with objective sleepiness in sleep apnea. Sleep. (2017) 40:zsx152. doi: 10.1093/sleep/zsx152
70. Dündar Y, Saylam G, Tatar EÇ, Özdek A, Korkmaz H, Firat H, et al. Does AHI value enough for evaluating the obstructive sleep apnea severity? Indian J Otolaryngol Head Neck Surg. (2015) 67:16–20. doi: 10.1007/s12070-014-0722-6
71. Pien GW, Ye L, Keenan BT, Maislin G, Björnsdóttir E, Arnardottir ES, et al. Changing faces of obstructive sleep apnea: treatment effects by cluster designation in the Icelandic sleep apnea cohort. Sleep. (2018) 41:zsx201. doi: 10.1093/sleep/zsx201
Keywords: obstructive sleep apnea, continuous positive airway pressure, excessive daytime sleepiness, predictors, efficacy
Citation: Li Z, Cai S, Wang J and Chen R (2022) Predictors of the Efficacy for Daytime Sleepiness in Patients With Obstructive Sleep Apnea With Continual Positive Airway Pressure Therapy: A Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 13:911996. doi: 10.3389/fneur.2022.911996
Received: 04 April 2022; Accepted: 24 May 2022;
Published: 27 June 2022.
Edited by:
Ahmed S. BaHammam, King Saud University, Saudi ArabiaReviewed by:
Haitham Jahrami, Arabian Gulf University, BahrainOreste Marrone, National Research Council (CNR), Italy
Copyright © 2022 Li, Cai, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui Chen, Y2hlbnJ1aWdvb2RAMTI2LmNvbQ==
 Zhiqiang Li
Zhiqiang Li Sijie Cai1,2
Sijie Cai1,2 Rui Chen
Rui Chen