
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 11 July 2022
Sec. Neuro-Otology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.910062
This article is part of the Research TopicInsights in Neuro-Otology: 2021 and 2022View all 27 articles
 Jacob C. Lucas1*
Jacob C. Lucas1* Zack Arambula1
Zack Arambula1 Alexandra M. Arambula1
Alexandra M. Arambula1 Katherine Yu1
Katherine Yu1 Nathan Farrokhian1
Nathan Farrokhian1 Linda D'Silva2
Linda D'Silva2 Hinrich Staecker1
Hinrich Staecker1 Jennifer A. Villwock1
Jennifer A. Villwock1Background: Dysfunction in the olfactory, auditory, and vestibular systems are commonly seen in aging and are associated with dementia. The impact of sensory loss(es) on cognition is not well understood. Our aim was to assess the relationships between performance on objective multisensory testing and quantify the impact of dysfunction on cognition.
Methods: Patients presenting with subjective hearing loss presenting to a tertiary care otologic/audiologic clinic were identified and underwent multisensory testing using the Affordable, Rapid Olfactory Measurement Array (AROMA), pure tone audiometric evaluations, and the Timed “Up and Go” test. Cognitive impairment (CI) was assessed via the Montreal Cognitive Assessment (MoCA) was also administered.
Key Results: 180 patients were enrolled. Thirty one percentage (n = 57) screened positive for cognitive impairment. When evaluating single sensory impairments, we found that olfactory dysfunction, gait impairment, and sensorineural hearing loss were all statistically significantly (p < 0.05) associated with a higher risk of cognitive impairment (ORs 3.89, 3.49, and 2.78, respectively) for CI. Multisensory impairment was significantly associated with cognitive impairment. Subjects with dysfunction in all domains were at the highest risk for cognitive impairment (OR 15.7, p < 0.001) vs. those with impairment in 2 domains (OR 5.32, p < 0.001).
Conclusion: Dysfunction of the olfactory, auditory, and vestibular systems is associated with a significantly increased risk of CI. The dramatically increased risk of CI with multisensory dysfunction in all three systems indicated that MSD may synergistically contribute to CI.
Cognitive impairment (CI) and dementia have been linked to sensory impairments in multiple domains such as hearing loss, olfactory dysfunction, and vestibular dysfunction. Several large, epidemiologic studies suggest that impairments in more than one sensory domain may be additive with respect to subsequent risk of developing CI. Unlike other risk factors such as age and genetics, sensory deficits are potentially modifiable by techniques such as hearing amplification, olfactory training, and vestibular therapy. Emerging literature suggests that approaches to improve sensory processing, such as cochlear implantation, may reverse cognitive decline (1).
The concept of Multisensory Impairment has been broached in the literature and is an area of active study; several retrospective cohort studies have examined age-related sensory decline including the Epidemiology of Hearing Loss Study (EHLS) (2, 3), Health Aging and Body Composition (Health ABC) (4), National Social Life, Health and Aging Project (NSHAP) (5), and Baltimore Longitudinal Study of Aging (6). Dual impairments in hearing and vision have even been associated with increased risk of all-cause mortality (7). The additive effects of multiple sensory impairments is compounded when compared to loss of a single sense (2). Sensory domains examined include hearing, touch, taste, olfaction, and vision (4, 5, 7, 8). Balance, gait, and vestibular function are also implicated and associated with age-related cognitive decline (6, 8–10). In the context of multisensory impairment, no studies have examined the summative effects of hearing, balance and gait, and olfaction on incident cognitive impairment.
The objective of this study was to prospectively examine neurocognition, olfactory performance, and gait and balance in subjects presenting for audiologic evaluation to determine the incidence of multisensory impairment in this population and the correlation of sensory impairments with neurocognitive status.
Institutional review board approval was obtained prior to commencement of any study activities (IRB #145682). This was a cross-sectional case-control study with initial recruitment occurring over a 6-month period from February 2021 through June 2021.
Subjects were recruited from a patient pool presenting with chief complaint of “Hearing Loss” to an otology and audiology clinic. Subjects presenting for audiologic evaluation were screened for eligibility and provided with written informed consent to participate. Enrolled subjects underwent evaluation of hearing, gait and balance, olfaction, and cognitive function. Exclusion criteria were age <50, history of primary progressive neurological disease such as Parkinson's and Multiple Sclerosis, non-ambulatory patients, patients that were unable to follow instructions due to severe cognitive impairment, conductive hearing loss due to middle ear pathology, non-intact tympanic membrane, vestibular schwannoma or other central nervous system tumor, and recent COVID-19 infection. Hearing was assessed using a comprehensive audiologic evaluation including audiometric thresholds, pure tone average, speech discrimination, and tympanometry. Olfaction was assessed using the Affordable, Rapid, Olfactory Measurement Array (AROMA) test (11, 12). Gait and balance were assessed with the Timed Up-and Go assessment (13). Cognitive function was evaluated using the Montreal Cognitive Assessment (MoCA) (14). Baseline demographic data was also obtained.
Audiometry was performed in accordance with the American National Standards Institute ANSI/ASA S3.21-2004 (R 2019) standards (15) and the American Speech-Language-Hearing Association guidelines (16). Hearing level (HL) thresholds measured in decibels (dB) were obtained at 250, 500, 1,000, 2,000, 3,000, 4,000, 6,000, and 8,000 Hertz (Hz). Air and bone conduction thresholds were measured in both ears. Pure-tone averages (PTA) were calculated for each individually tested ear using 500, 1,000, 2,000, and 3,000 Hz, as recommended by the American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) (17) and the American Medical Association (18). If no recording existed of the 3000 Hz threshold, the mean of the 2,000 and 4,000 Hz thresholds was utilized; a PTA valuation using this average is within +/- 5 dB of the PTA utilizing 3,000 Hz in 99% of audiograms in one previous series of 2170 patients (19). Word recognition scores were also obtained. A PTA > 25 dB HL was considered to have a sensorineural hearing impairment. For purposes of analysis, the best hearing ear was used for subject classification.
The Timed “Up & Go” (13) test was chosen as a simple screening tool for balance impairment due to its ease of administration and well-established cut off scores for patients with vestibular dysfunction. It is a timed version of the older “Get Up and Go” test (20). The TUG consists of the average time of three trials: the patient is instructed to sit in a chair, a timer is started, the patient stands, walks 3 meters, then turns around and walks back to the chair, and finally sits back down (13). The TUG is well-studied and has excellent validity and reliability in a wide range of adult populations with various disabilities, including Parkinson's, cerebral palsy, and stroke. It has also been studied in otherwise “normal” elderly adult populations (21). It had a sensitivity of 80% in determining fall risk for patients with vestibular disorders, making it a reasonable choice for balance screening. Fall risk due to impairment was associated with a TUG score > 11 s (22). For this reason, subjects with TUG scores > 11 s were considered to have gait and balance impairment during analysis.
AROMA (11) is an essential oil-based test that comprises 14 scents and one negative control at four concentrations: 1X, 2X, 4X, and 8X. It is administered by trained research personnel. The full battery consists of four rounds of 15 inhalant sticks. The number of odorants and concentrations included in olfactory testing is dependent on each subject's baseline olfactory status; participants start at the 2X concentration and are presented all scents in randomized order. Patients select answers from a 4-item multiple-choice field. Incorrect responses trigger a higher concentration to be presented in the next round. Correct responses trigger the lower concentration to be presented. Due to this branching logic and the nested quality of the testing array, every test is unique, and requires administration on a tablet computer. All participants are asked to identify the 2X concentrations, but subsequent rounds are customized based on responses. This allows increased stratification of responses and development olfactory phenotypes. Preliminary data has identified unique testing phenotypes for normal, mild cognitive impairment, and Alzheimer's disease patients (12). Out of a total score of 100, patients were classified as normosmic (>75), hyposmic (< 75), and anosmic (<40). During multivariate modeling, anosmic and hyposmic patients were collapsed into a single category (Hyposmia) to allow for more straightforward interpretation of binary logistic regression. Related to cognitively impaired individuals, AROMA was validated in examining the relationship of olfactory dysfunction to Alzheimer's dementia, mild cognitive impairment, and cognitively unimpaired individuals. Other advantages of the AROMA are its re-usability, odorant levels that are dynamic, and ability to test both detection and identification of smell (11, 12).
The MoCA, as compared to the Mini Mental Status Exam (MMSE) is more sensitive in the detection of mild cognitive impairment (MCI) (23, 24). It is a multiple domain instrument that tests short term memory recall, working memory, visuospatial ability, abstraction, attention, concentration, and executive functioning (14). It has up to 90% sensitivity in MCI detection and takes ~10 min to administer, making it an ideal cognitive function screening test in the clinical setting. A score of 26 was used as the cutoff for cognitive impairment; (14) subjects with scores <26 were considered CI. The overall score was corrected based on education level–one additional point was added for patients who did not complete high school.
Using previous literature that has estimated the prevalence of various sensory impairments among an aging population, an approximately 30% prevalence of olfactory dysfunction was expected in the control group. Prior prospective cohort studies (2, 3) have demonstrated a Hazard Ratio (HR) of 1.5 for hearing loss and incident dementia. The Odds Ratio (OR) for hearing loss in Alzheimer's dementia was found to be 2.0 in a case-control study with similar design considerations to the present study (25). Risk of fall in a 12-month period for adults aged 65 and older was calculated at 28.7% (26, 27). For the present case-control study comparing multisensory impairment and odds of incident CI; we estimated a likely 0.3 proportion of CI in the control group (28), a 2.0 OR for multisensory impairment and CI, 0.95 confidence level (alpha = 0.05), and 0.8 power level. With these estimates, we determined a conservative sample size of n = 275 to uncover a moderate effect size. Patients were actively recruited from a clinical pool of patients presenting with hearing loss. The first author (JCL) screened scheduled clinic lists the day before enrollment for eligible subjects based on chart review. Some initially eligible subjects were ineligible after additional in-person screening. Some subjects declined participation. Some were unable to complete the entirety of testing and so were excluded from analysis. Of the theoretical 585 eligible subjects on preliminary screening during the enrollment period, 180 were included. Enrollment rate based on initial eligibility screening was ~31% and fell short of goal enrollment of 275 subjects.
Data was cleaned and wrangled using R (29), RStudio, and the Tidyverse (30) suite of packages. Statistical analysis was performed using base R generalized linear model functions. Plots were generated using the R ggplot2 (31) and audiometry (32) packages.
Wilcoxon Rank Sum, Pearson's Chi-squared, and Fisher's exact test were used where appropriate to evaluate sociodemographic differences between patients with cognitive impairment (Table 1). Univariate analysis was performed for all predictor variables with cognitive status as the outcome variable (Table 2), again utilizing Wilcoxon Rank Sum and Chi-squared testing where appropriate.
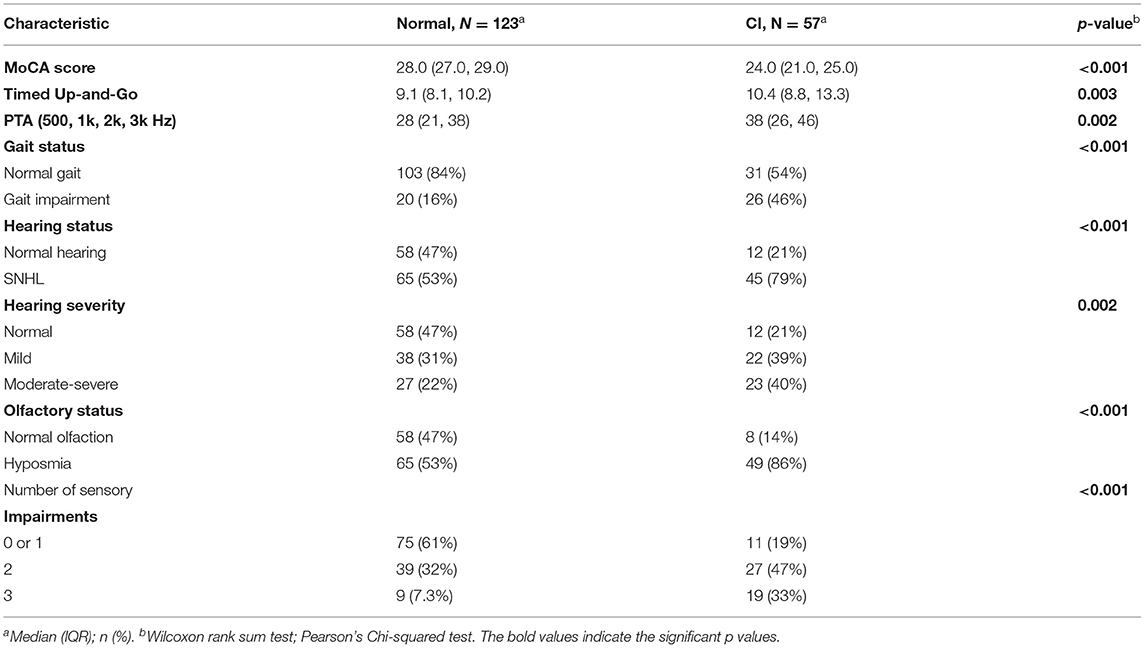
Table 2. Univariate analysis for all measured sensory impairments, with continuous and categorical variables analyzed with Wilcoxan rank-sum and Pearson's Chi-squared, respectively.
Binary logistic regression was chosen for multivariate analysis. Results of multisensory testing, age, gender, and education levels were submitted to binary logistic regression analysis using a stepwise selection procedure. Only items that were related (p < 0.10) to the outcome after adjusting for all other items were retained. Odds ratios (OR) for incident cognitive impairment with confidence intervals were calculated for each of two models. Predictor values included hearing impairment, balance impairment, and olfactory impairment, along with control variables for age and education. Each predictor variable was categorized as a binary value and the response variable (cognitive impairment) was also coded as binary, with cases having a MoCA score <26 and controls having scores >26. A first model comparing the outcome for each of three sensory deficits was created, demonstrated in Table 3; a second model comparing the outcome for patients with multiple sensory deficits sought to define the influence of multiple sensory impairments on incident CI demonstrated in Table 4.
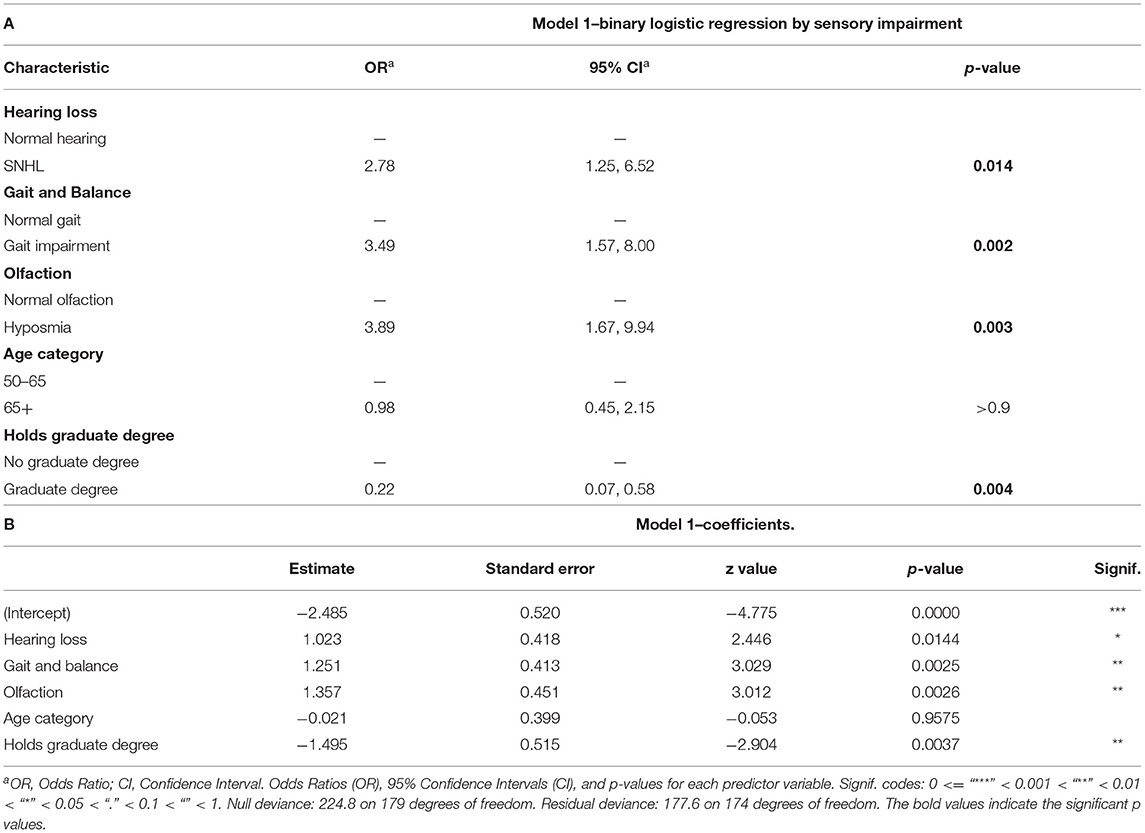
Table 3. Binary logistic regression model of all sensory impairments, age, and education level; (A) Odds Ratios (OR), 95% Confidence Intervals (CI), and p-values for each predictor variable. (B) Model coefficients with deviance values for the tested model.
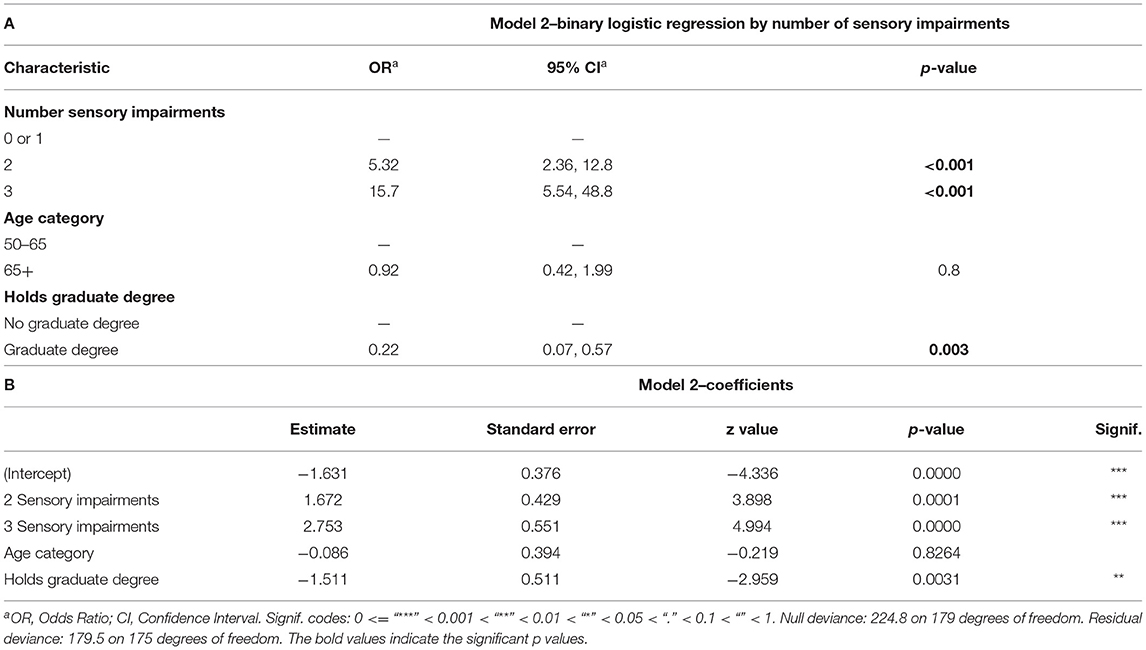
Table 4. Binary logistic regression model of number of sensory impairments, age, and education level; (A) Odds Ratios (OR), 95% Confidence Intervals (CI), and p-values for each predictor variable. (B) Model coefficients with deviance values for the tested model.
In demographic analysis, education level was found to have significant predictive value toward incident cognitive impairment. Additional stratification revealed significant differences between subjects with graduate-level education, and subjects without graduate-level education. Due to this finding, education was added as a control variable to the logistic regression model, with Odds Ratios reported separately. Age was also included as a control predictor variable, with patients stratified into either “50–65” and “65+” age categories.
One hundred and eighty subjects were enrolled after excluding 4 subjects with incomplete datasets. Fourteen subjects required an assistive device such as a cane or walker to perform the TUG examination. Subject demographic data are presented in Table 1. One hundred and twenty three subjects with normal cognition as defined on the MoCA were enrolled, and 57 subjects with CI were enrolled. The mean age at enrollment was 66 for normal subjects and 69 for CI subjects. Fifty four percentage of subjects were female with 46% male. Recruited subjects were predominantly Caucasian (89%). Education level and employment status were collected during enrollment. Education was found to be significantly associated with differences in incident cognitive impairment, as indicated by Fisher's exact test (two-tailed p < 0.001. Other sociodemographic data, including age, gender, race, and employment status were not associated with increased incidence of cognitive impairment.
Total MoCA scores from all subjects are demonstrated in Figure 1. Scores are ordinal and range from 0 to 30. The data is left-skewed with normal or near-normal scores heavily weighted. Cognitive impairment is defined as any score <26. Univariate analysis for all predictor variables on incident cognitive impairment is summarized in Table 2. All three sensory impairments demonstrated significant associations with incident cognitive impairment.
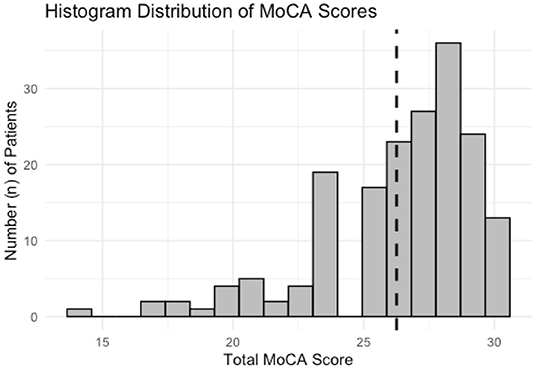
Figure 1. Histogram distribution of MoCA scores for all subjects enrolled for analysis. The data is left-skewed with a heavier distribution of normal and near-normal scores. Dashed line corresponds to a score of 26, scores below which are considered for analysis to have “Cognitive Impairment.”
Hearing-impaired subjects had higher proportions of CI, χ2(1, N = 180) = 11.2, p < 0.001. Figure 2 shows aggregate audiometric data for all subjects, with a negative correlation noted between MoCA score and PTA (R = −0.18, p = 0.018); subjects with impaired hearing on audiometry were more likely to score lower on the MoCA (p = 0.011). Composite audiograms for both “Normal” and “CI” subjects are displayed in Figure 2C. Figure 2D displays the minimum standard for reporting hearing loss as prescribed by the AAO-HNS (17). Hearing severity was also significantly associated with cognition, although severity categories were collapsed across moderate, moderately severe, and severe hearing due to a scarcity of subjects with hearing worse than the “Mild” classification. “Normal” and “Mild” hearing loss were heavily weighted in the dataset. No patients with profound hearing loss in the better hearing ear were recruited.

Figure 2. Aggregate audiometric data for patients with Normal Cognition and Cognitive Impairment (CI). (A) Scatterplot of PTA versus MoCA score. Horizontal dashed line correlates to MoCA score of 26. Vertical dashed line correlates to PTA of 25. Shaded area includes patients with co-incident hearing loss and CI, line of best fit included with R- and p-values for Pearson's correlation. (B) Box- and violin-plots of Normal Hearing and Hearing-Impaired (SNHL) individuals and the distribution of MoCA scores in each group. (C) Composite audiometric data, median scores for each threshold correspond to the dark line, error bars correspond to 1 SD. (D) AAO-HNS minimum reporting standards for raw data of PTA plotted against word recognition scores (WRS) (17).
A higher proportion of gait-impaired subjects had co-incident CI on Pearson's Chi Squared test, χ2(1, N = 180) = 17.6, p < 0.001. Gait impairment is depicted in Figure 3; impaired gait scores were associated with lower scores on the MoCA assessment (R = −0.34, p < 0.001).
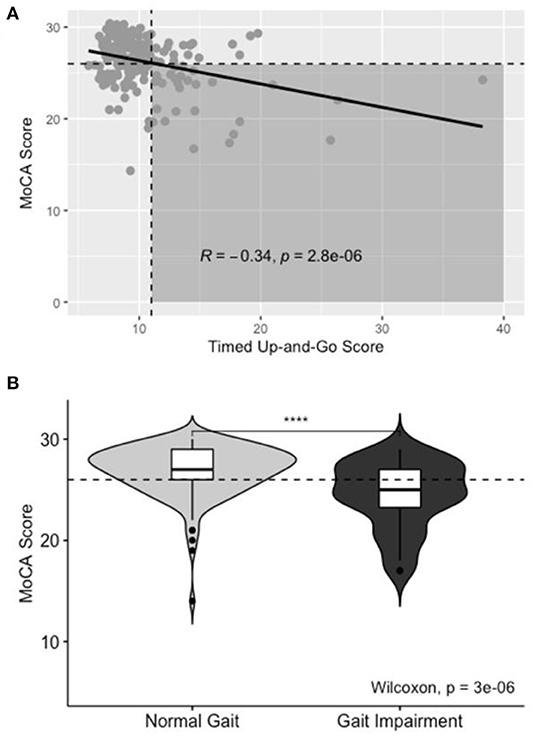
Figure 3. Aggregate TUG data for Normal and Gait-impaired individuals. (A) Scatterplot of TUG score vs. MoCA score. Horizontal dashed line correlates to MoCA score of 26. Vertical dashed line correlates to TUG of 11. Shaded area includes patients with co-incident gait impairment and CI, line of best fit included with R- and p-values for Pearson's correlation. (B) Box- and violin-plots of Normal and Gait-Impaired individuals and the distribution of MoCA scores in each group.
Olfactory impairment was similarly associated with co-incident CI on Pearson's Chi Squared test, χ2(1, N = 180) = 18.4, p < 0.001. Figure 4 shows the positive correlation between MoCA score and AROMA score (R = 0.41, p < 0.001). On univariate analysis of olfaction, distinct differences in MoCA score were noted between normosmic, hyposmic, and anosmic individuals; with anosmic individuals scoring lower than hyposmic individuals on the MoCA instrument (Figure 4B). For multivariate modeling using binary logistic regression, anosmic and hyposmic individuals were collapsed into a single “Hyposmia” group to simplify interpretation in the context of other confounding variables.
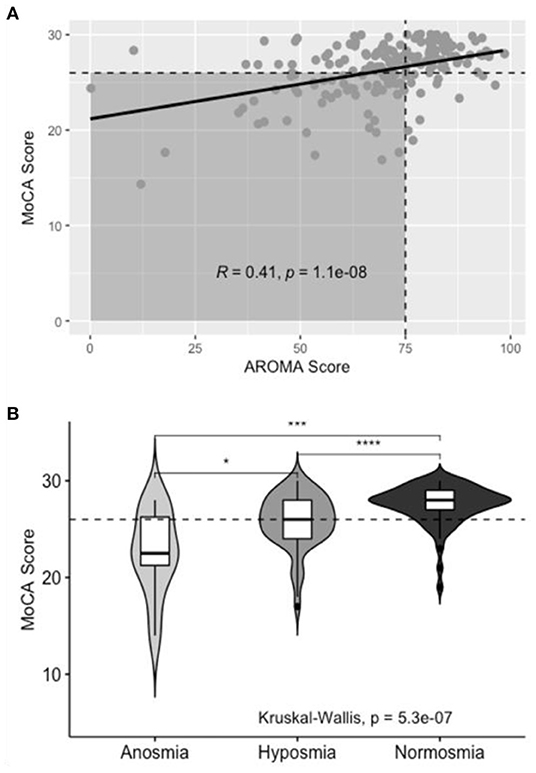
Figure 4. Aggregate AROMA data for subjects with normal and impaired olfaction. (A) Scatterplot of AROMA vs. MoCA score. Horizontal dashed line correlates to MoCA score of 26. Vertical dashed line correlates to AROMA score of 75. Shaded area includes patients with co-incident olfactory impairment and CI, line of best fit included with R- and p-values for Pearson's correlation. (B) Box- and violin-plots of Normal, Hyposmic, and Anosmic individuals and the distribution of MoCA scores in each group.
Analysis using binary logistic regression is shown in Tables 3, 4. Within initial analysis of sociodemographic data, education level was found to have a significant correlation to cognitive status. Further breakdown demonstrated the stratification to be primarily between individuals with and without graduate-level education. Due to this, education was included as a control variable in the models.
The multivariate influence of three sensory domains on incident cognitive impairment was examined in two separate models.
In the first model (Table 3A), the influence of each individual sensory impairment was examined on co-incident cognitive impairment. Hearing loss [OR = 2.78, 95% CI (1.25, 6.52), p = 0.014], gait and balance [OR = 3.49, 95% CI (1.57, 8.00), p = 0.002], and olfaction [OR = 3.89, 95% CI (1.67, 9.94), p = 0.003] were all significant predictors of co-incident cognitive impairment. Having a graduate-level education was associated with a lower odds of co-incident CI [OR = 0.22, 95% CI (0.07, 0.58), p = 0.004], while no statistical difference was seen between age groups. Table 3B reports coefficients for the model. Figure 5 depicts the OR values graphically.
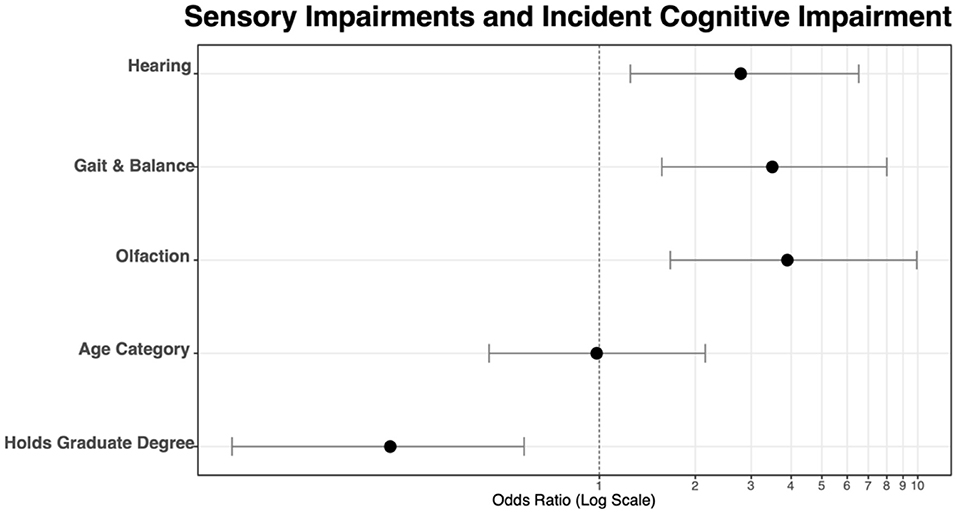
Figure 5. Odds ratios visualized for each of three sensory components and two control variables, age, and education level.
The second model sought to examine the additive effects of multiple sensory impairments on co-incident cognitive impairment. To account for the small number of subjects with zero sensory impairments and incident CI (n = 2), 0 and 1 sensory impairment categories were collapsed into a single level “0 or 1 Sensory Impairments.” Education and age were again included as control variables in the model. Table 4A displays odds ratios for increasing number of sensory impairments as predictors for co-incident CI. “0 or 1” sensory impairments were considered as the baseline condition. With 2 coexisting sensory impairments, the odds of CI increased [OR = 5.32, 95% CI (2.36, 12.8), p < 0.001]. When all 3 sensory impairments were present, the odds increased further [OR = 15.7, 95% CI (5.54, 48.8), p < 0.001]. Table 4B reports coefficients for the model.
Despite the well-established link between sensory deficits–many of which are potentially modifiable with training and targeted rehabilitation–and neurocognitive decline, the incidence and impact of multisensory impairments are not well understood. Sensorineural hearing loss increases the relative risk for dementia and has a weighted population attributable fraction of 8.2% among midlife (age 45–65) adults according to the recent Lancet Commission on Dementia Prevention (33), making it the number one modifiable risk factor in the prevention of dementia. Apart from hearing loss, there are no current guidelines related to the screening and treatment of sensory impairments in the context of cognitive decline. The objective of this study was to determine the incidence of hearing, olfactory, and/or balance impairment and determine the magnitude of the impact of single vs. multisensory dysfunction on cognitive performance. When evaluating single sensory impairments, we found that olfactory dysfunction, gait impairment, and sensorineural hearing loss were all statistically significantly associated with a higher incidence of cognitive impairment (OR 3.89, p = 0.003; 3.49, p = 0.002; and 2.78, p = 0.014, respectively) for cognitive impairment. Subjects with dysfunction in all domains were at the highest risk for cognitive impairment (OR 15.7, p < 0.001) vs. those with impairment in 2 domains (OR 5.32, p < 0.001). These findings underscore the need to comprehensively evaluate patients for multisensory impairment, particularly those who are at-risk for cognitive decline due to advancing age or other known risk factors. Additionally, the sensory domains studied are all amenable to rehabilitation. The dramatically increased risk of cognitive impairment in those with multisensory impairment highlights the potential of sensory rehabilitation, even if only in a single domain, to potentially improve outcomes.
The concept of multisensory impairment is a burgeoning area of active study. For example, several cohort studies have examined age-related sensory decline including the Epidemiology of Hearing Loss Study (EHLS), Beaver Dam Offspring Study (BOSS), Health Aging and Body Composition (Health ABC), National Social Life, Health and Aging Project (NSHAP), and Baltimore Longitudinal Study of Aging (2–6).
There are competing and parallel hypotheses for why sensory impairment contributes to the onset and trajectory of cognitive decline. Most of these use hearing loss as an illustrative example. The “cognitive load” theory postulates that loss of hearing places additional cognitive processing demands on the brain, resulting in diverting of limited neuroprocessing resources toward auditory processing, and away from other cognitive processes such as working memory (34, 35). The cumulative strain may contribute to loss of cognitive function over time. Social isolation due to hearing loss may also contribute to cognitive decline. Self-reported measures such as depression and “fair or poor” overall mental health are seen with a higher frequency in the hearing impaired (36). A common pathophysiologic mechanism underpinning both hearing loss and dementia such as microvascular insult could explain the apparent linkage as well. However, multiple large cohort studies controlling for cardiovascular disease have repeatedly shown hearing loss to be an independent risk factor for the development of dementia (2–5).
The neuroanatomy of sensory processing provides context for why multiple sensory impairments occur concomitantly in the context of cognitive impairment. Bilateral vestibular loss is linked to hippocampal atrophy detectable on MRI (37, 38). Olfaction and neurocognition are also linked anatomically and pathophysiologically, particularly concerning Alzheimer's disease and related dementias (39–41). Abnormal amyloid and tau proteins deposit in the olfactory bulb and tract prior to cognitive decline (42). As regional involvement increases, so does the extent of cognitive decline. As the disease progresses, additional neurofibrillary tangles develop in the entorhinal cortex and hippocampal-related structures (43). These are the same anatomic areas through which olfaction is processed. Olfactory identification deficits correlate with atrophy of the hippocampus, olfactory bulb, and entorhinal cortex (44, 45). Behavioral and functional data both indicate that activation of the primary olfactory cortex depends on attention (46, 47). The anterior cingulate cortex, which is activated when cognitive demand is high (48), and during working memory tasks is also activated by olfactory stimuli (49). The increased workload of maintaining appropriate attention and memory as neurocognitive decline progresses may occur at the expense of specific aspects of olfactory and other sensory performance (50). In the context of hearing loss, loss of high-SR auditory fibers, as occurs in age-related hearing loss, is implicated in the development of imbalances in excitation and inhibition in ascending central pathways. This imbalance may lead to a decrease in central gain, dysregulation of the hypothalamic-pituitary axis, decrease in hippocampal long-term potentiation, and an overall decrease in signal-noise ratio (51).
Prior studies have investigated multiple sensory domains including hearing, touch, olfaction, vision, and even taste (52), and found that subjects with multiple sensory impairments have worse neurocognitive outcomes. Our data are consistent in demonstrating the individual associations of hearing, balance, and olfaction with cognitive impairment. Additionally, the highest risk of neurocognitive impairment was in subjects with deficits in all three of the sensory domains tested. This suggests that the effects of multiple sensory impairments may be additive toward odds of CI.
The Epidemiology of Hearing Loss Study (EHLS) demonstrated that olfactory dysfunction predicted the development of cognitive impairment in a cohort of patients prospectively followed for a 5-year period (2). In addition to cognitive impairment, studies of community-dwelling elders have also shown increased morbidity and mortality in subjects with olfactory dysfunction (53–56).
Vestibular dysfunction has been shown to correlate strongly with CI and dementia (9, 57–59). The vestibular system has been independently studied in the context of aging and cognition. Dysfunction is associated with an increased risk of cognitive impairment, dementia, and Alzheimer's disease. Vestibular loss–particularly impairment of the saccule–also predicts poorer spatial cognition in a subset of patients with Alzheimer's disease (57, 58). Cross-sectional analysis of 3 prospective cohort studies on aging populations demonstrated a link between vestibular decline and cognitive decline: the Baltimore Longitudinal Study on Aging (BLSA) (6), the National Health Interview Survey (8), and National Health and Nutrition Examination Survey (60). Within a cross-sectional analysis of the BLSA, an association between olfaction and motor function was identified (61).
Subjects with baseline olfactory impairment are more likely to develop cognitive impairment during longitudinal follow-up. Pooled analysis of 8 cohort studies (2, 62–68) encompassing 13,165 participants demonstrated a relative risk of 2.37 (95% CI = 1.91–2.94) for accelerated cognitive decline when subjects with olfactory impairment were followed longitudinally (69). Odor detection is associated with word recall and orientation scores on the Alzheimer's Disease Assessment Scale (70). Odor detection and identification is also correlated with blood flow to the left temporal lobe, entorhinal cortex, and frontal lobes; and activation of the right anterior piriform cortex on fMRI.
“Vestibular Cognition” as a concept is evolving (71) to encompass the peripheral end organs as well as projections through the brainstem to a widespread distribution in higher cortical centers. Low-level reflexes such as the vestibulo-ocular reflex (72) and vestibulospinal reflex (73) stabilize gaze and posture, respectively. These reflexes, along with proprioceptive and visual input interface with higher-order projections to the cerebellum and somatosensory cortex to provide a “sense” of balance. This complex interplay between lower brainstem reflexes and higher-order cortical processing makes contextualizing balance and cognition challenging. For this reason, the Timed-Up-and-Go test was felt to be a simple, easily interpretable proxy for gait and balance function. Due to the need for visual, proprioceptive, and vestibular coordination, this single test was utilized in the present study (13). No studies to date have looked at balance in the context of multisensory impairment, and none of the previously mentioned prospective cohorts have included balance and vestibular dysfunction alongside hearing and olfaction during analyses. Our results show that individual sensory deficits are significantly correlated to co-incident cognitive impairment. More interesting, the effects of multiple sensory impairments appear to be additive in the odds of having CI.
Hearing, olfactory, and vestibular impairments are critically important to recognize due to their potentially modifiable nature. Each of the sensory impairments represents a unique opportunity to intervene and improve outcomes. Our data demonstrate an OR for cognitive impairment of 15.7 in subjects with deficits in three sensory modalities vs. an OR of 5.32 for those with deficits in only two. This indicates that referring subjects with multisensory impairment for hearing restoration via hearing aids, vestibular therapy, and/or olfactory training may meaningfully modify the risk of neurocognitive decline. Hearing aids have been shown to potentially mitigate the risk for cognitive decline (74–76). Mertens et al. (77) showed that cochlear implantation in cognitively impaired patients could slow, and even reverse, cognitive changes associated with aging. Olfactory training has been shown to improve olfaction and increase neural connectivity within and between brain regions. When comparing performance on assessments of cognition, depression, overall brain health, and olfaction of non-cognitively impaired community-dwelling senior citizens, 6 months of olfactory training was superior to 6 months of sudokus (78). Olfactory stimulation with scent-impregnated patches placed on the sternum has also been shown to improve vestibular performance and decrease fall risk (79). Taken together, this evidence further highlights the importance of assessing and rehabilitating multisensory dysfunction.
This study is not without limitations. Due to its cross-sectional nature, we are unable to comment on the long-term outcomes of the included subjects. There is an inherent risk of bias during subject recruitment as not all eligible subjects were willing to participate in the study. A degree of selection bias is present due to the exclusion of a large portion of eligible subjects; approximately 31% of initially screened subjects were included in the final cohort. Olfactory assessment utilized AROMA, a relatively novel objective test of olfactory performance, which could be viewed as a limitation. However, prior studies using AROMA have demonstrated high test-retest reliability and a significant correlation of AROMA results with more commonly used tests like the UPSIT (11, 12). Additionally, the study was underpowered to stratify hearing contribution by severity (Normal, Mild, Moderate, Moderately Severe, Severe, Profound), due to a low number of patients with hearing loss worse than “mild.” Recent literature has suggested that central, rather than auditory processing may be more strongly tied to cognitive status (80), rather than PTA as measured here. Secondary analysis of the Adult Changes in Thought (ACT) study (81) showed that decreased performance status on two dichotic central auditory processing tests predicted a higher likelihood of dementia and Alzheimer's disease.
To assess cognitive status, the MoCA was utilized. The MoCA is commonly deployed in a screening capacity and is not capable of differentiating between etiologies of cognitive impairment. While a more robust neuropsychological battery would be preferable, adding a 1–2-h evaluation to each subject's clinical visit with the psychometricians needed to perform them was not logistically possible. The MoCA is supported in the literature as superior to other common tests like the Mini-Mental Status Exam because it includes measures of executive function (14). Educational status is known to confound MoCA performance with higher education levels positively correlated with MoCA score. Higher education status was found to be a contributing protective factor for the identification of CI in our study, with an OR of 0.22, 95% CI [0.07, 0.58], p < 0.001, and was controlled for during multivariate modeling. Future studies will include longitudinal data as well as the impact of sensory deficit-specific rehabilitation of cognitive outcomes. Patients with severe or profound hearing loss were planned to undergo the modified MoCA for the hearing impaired (MoCA-HI) that has previously been validated in this population (82). However, there were no patients with hearing levels worse than “Moderately Severe” recruited, when using the best hearing ear for classification purposes. On retrospective review of the study's recruitment practices, patients with more severe hearing were more commonly funneled into visits for cochlear implant evaluations and so were not as easily captured during enrollment. These subjects are now recruited in a more targeted fashion and are the topic of future study.
Multisensory impairment is common and associated with cognitive impairment. Deficits in hearing, balance, and olfaction significantly increase the odds of co-incident cognitive impairment vs. those with deficits in fewer domains. When considering single sensory deficits, olfactory dysfunction was the strongest predictor of cognitive impairment. The significance of these findings is in their immediate clinical applicability. These sensory impairments are testable in a point of care fashion and are amenable to rehabilitation. Assessment of multisensory impairment in patients presenting with subjective loss in any of these domains should be considered to facilitate subsequent therapeutic intervention to improve sensory impairments and potentially prevent cognitive decline.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB), University of Kansas Medical Center. The patients/participants provided their written informed consent to participate in this study.
JL conceived the study and coordinated study design, performed statistical analysis, wrote, and critically revised the manuscript. ZA designed data acquisition protocols and critically revised the manuscript. AA and KY critically revised the manuscript. NF performed statistical analysis and critically revised the manuscript. LD'S, HS, and JV conceived the study, participated in study design and data acquisition protocols, and critically revised the manuscript. JV conceived the study, participated in study design and data acquisition protocols, performed statistical analysis, wrote, and critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Fees for open access publication are provided at the expense of the Department of Otolaryngology-Head and Neck Surgery at the University of Kansas Medical Center.
JV discloses intellectual property and a filed patent (17/281121–“Olfactory Diagnostic and Training Kits and Methods”) related to the objective olfactory testing methods used in this research.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank Scott Fassas, Emily Cummings, and Shaan Somani for their efforts in recruitment and testing of subjects. Special thank to Mark Villwock for consultation on statistical modeling.
1. Claes AJ, Mertens G, Gilles A, Hofkens-Van den Brandt A, Fransen E, Van Rompaey V, et al. The repeatable battery for the assessment of neuropsychological status for hearing impaired individuals (RBANS-H) before and after cochlear implantation: a protocol for a prospective, longitudinal cohort study. Front Neurosci. (2016) 10:512. doi: 10.3389/fnins.2016.00512
2. Fischer ME, Cruickshanks KJ, Schubert CR, Pinto AA, Carlsson CM, Klein BEK, et al. Age-related sensory impairments and risk of cognitive impairment. J Am Geriatr Soc. (2016) 64:1981–7. doi: 10.1111/jgs.14308
3. Schubert CR, Cruickshanks KJ, Fischer ME, Pinto AA, Chen Y, Huang GH, et al. Sensorineural impairments, cardiovascular risk factors, and 10-year incidence of cognitive impairment and decline in midlife: the beaver dam offspring study. J Gerontol A Biol Sci Med Sci. (2019) 74:1786–92. doi: 10.1093/gerona/glz011
4. Brenowitz WD, Kaup AR, Lin FR, Yaffe K. Multiple sensory impairment is associated with increased risk of dementia among black and white older adults. J Gerontol A Biol Sci Med Sci. (2019) 74:890–6. doi: 10.1093/gerona/gly264
5. Correia C, Lopez KJ, Wroblewski KE, Huisingh-Scheetz M, Kern DW, Chen RC, et al. Global sensory impairment in older adults in the United States. J Am Geriatr Soc. (2016) 64:306–13. doi: 10.1111/jgs.13955
6. Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, et al. Association between visuospatial ability and vestibular function in the baltimore longitudinal study of aging. J Am Geriatr Soc. (2015) 63:1837–44. doi: 10.1111/jgs.13609
7. Gopinath B, Schneider J, McMahon CM, Burlutsky G, Leeder SR, Mitchell P. Dual sensory impairment in older adults increases the risk of mortality: a population-based study. PLoS ONE. (2013) 8:e55054. doi: 10.1371/journal.pone.0055054
8. Bigelow RT, Semenov YR, du Lac S, Hoffman HJ, Agrawal Y. Vestibular vertigo and comorbid cognitive and psychiatric impairment: the 2008 national health interview survey. J Neurol Neurosurg Psychiatry. (2016) 87:367–72. doi: 10.1136/jnnp-2015-310319
9. Harun A, Oh ES, Bigelow RT, Studenski S, Agrawal Y. Vestibular impairment in dementia. Otol Neurotol. (2016) 37:1137–42. doi: 10.1097/MAO.0000000000001157
10. Lee HW, Lim YH, Kim SH. Dizziness in patients with cognitive impairment. J Vestib Res Equilib Orientat. (2020) 30:17–23. doi: 10.3233/VES-190686
11. Villwock JA, Li J, Moore C, Chiu AG, Sykes KJ. Affordable rapid olfaction measurement array: a novel, essential oil-based test strongly correlated with upsit and subjective outcome measures. Ann Otol Rhinol Laryngol. (2020) 129:39–45. doi: 10.1177/0003489419870833
12. Li J, Bur AM, Villwock MR, Shankar S, Palmer G, Sykes KJ, et al. Olfactory phenotypes differentiate cognitively unimpaired seniors from alzheimer's disease and mild cognitive impairment: a combined machine learning and traditional statistical approach. J Alzheimers Dis JAD. (2021) 81:641–50. doi: 10.3233/JAD-210175
13. Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. (1991) 39:142–8. doi: 10.1111/j.1532-5415.1991.tb01616.x
14. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
16. Campbell J, Graley J, Meinke D, Vaughan L, Aungst R, Madison T. Guidelines for manual pure-tone threshold audiometry. ASHA. (1978) 20:297–301. Available online at: https://www.asha.org/policy/gl2005-00014/
17. Gurgel RK, Jackler RK, Dobie RA, Popelka GR. A new standardized format for reporting hearing outcome in clinical trials. Otolaryngol Head Neck Surg. (2012) 147:803–7. doi: 10.1177/0194599812458401
18. Guide for the evaluation of hearing handicap. JAMA. (1979) 241:2055–9. doi: 10.1001/jama.241.19.2055
19. Gurgel RK, Popelka GR, Oghalai JS, Blevins NH, Chang KW, Jackler RK. Is it valid to calculate the 3-kilohertz threshold by averaging 2 and 4 kilohertz? Otolaryngol Head Neck Surg. (2012) 147:102–4. doi: 10.1177/0194599812437156
20. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. (1986) 67:387–9.
21. Christopher A, Kraft E, Olenick H, Kiesling R, Doty A. The reliability and validity of the timed up and go as a clinical tool in individuals with and without disabilities across a lifespan: a systematic review. Disabil Rehabil. (2019) 26:1–15. doi: 10.1080/09638288.2019.1682066
22. Whitney SL, Marchetti GF, Schade A, Wrisley DM. The sensitivity and specificity of the Timed “Up & Go” and the dynamic gait index for self-reported falls in persons with vestibular disorders. J Vestib Res Equilib Orientat. (2004) 14:397–409. doi: 10.3233/VES-2004-14506
23. Pinto TCC, Machado L, Bulgacov TM, Rodrigues-Júnior AL, Costa MLG, Ximenes RCC, et al. Is the montreal cognitive assessment (MoCA) screening superior to the mini-mental state examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer's disease (AD) in the elderly? Int Psychogeriatr. (2019) 31:491–504. doi: 10.1017/S1041610218001370
24. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kedziora-Kornatowska K. Is the montreal cognitive assessment (MoCA) test better suited than the mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis Psychiatr Pol. (2016) 50:1039–52. doi: 10.12740/PP/45368
25. Uhlmann RF, Larson EB, Rees TS, Koepsell TD, Duckert LG. Relationship of hearing impairment to dementia and cognitive dysfunction in older adults. JAMA. (1989) 261:1916–9. doi: 10.1001/jama.261.13.1916
26. Bergen G. Falls and fall injuries among adults aged ≥65 Years — United States, 2014. MMWR Morb Mortal Wkly Rep. (2016) 65:993–8. doi: 10.15585/mmwr.mm6537a2
27. Cuevas-Trisan R. Balance problems and fall risks in the elderly. Phys Med Rehabil Clin N Am. (2017) 28:727–37. doi: 10.1016/j.pmr.2017.06.006
28. Ward A, Arrighi HM, Michels S, Cedarbaum JM. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement J Alzheimers Assoc. (2012) 8:14–21. doi: 10.1016/j.jalz.2011.01.002
29. R Core Team. R: A Language Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2021). Available online at: https://www.R-project.org/
30. Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, et al. Welcome to the tidyverse. J Open Source Softw. (2019) 4:1686. doi: 10.21105/joss.01686
32. Lehnert B. Audiometry: Standard Conform Pure Tone Audiometry (PTA) Plots (2020). Available online at: https://CRAN.R-project.org/package=audiometry
33. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
34. Tun PA, McCoy S, Wingfield A. Aging, hearing acuity, and the attentional costs of effortful listening. Psychol Aging. (2009) 24:761–6. doi: 10.1037/a0014802
35. Rabbitt P. Mild hearing loss can cause apparent memory failures which increase with age and reduce with IQ. Acta Oto Laryngol Suppl. (1990) 476:167–75. doi: 10.3109/00016489109127274
36. Strawbridge WJ, Wallhagen MI, Shema SJ, Kaplan GA. Negative consequences of hearing impairment in old age: a longitudinal analysis. Gerontologist. (2000) 40:320–6. doi: 10.1093/geront/40.3.320
37. Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, et al. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain J Neurol. (2005) 128:2732–41. doi: 10.1093/brain/awh617
38. Göttlich M, Jandl NM, Sprenger A, Wojak JF, Münte TF, Krämer UM, et al. Hippocampal gray matter volume in bilateral vestibular failure. Hum Brain Mapp. (2016) 37:1998–2006. doi: 10.1002/hbm.23152
39. Schmitt AL, Livingston RB, Reese EM, Davis KM. The relationship between the repeatable battery for the assessment of neuropsychological status (RBANS) and olfaction in patients referred for a dementia evaluation. Appl Neuropsychol. (2010) 17:163–71. doi: 10.1080/09084281003715667
40. Suzuki Y, Yamamoto S, Umegaki H, Onishi J, Mogi N, Fujishiro H, et al. Smell identification test as an indicator for cognitive impairment in Alzheimer's disease. Int J Geriatr Psychiatry. (2004) 19:727–33. doi: 10.1002/gps.1161
41. Rezek DL. Olfactory deficits as a neurologic sign in dementia of the Alzheimer type. Arch Neurol. (1987) 44:1030–2. doi: 10.1001/archneur.1987.00520220036012
42. Hummel T, Landis BN, Hüttenbrink KB. Smell and taste disorders. GMS Curr Top Otorhinolaryngol Head Neck Surg. (2011) 10:Doc04. doi: 10.3205/cto000077
43. Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. (1992) 42:631–9. doi: 10.1212/WNL.42.3.631
44. Sánchez SM, Duarte-Abritta B, Abulafia C, De Pino G, Bocaccio H, Castro MN, et al. White matter fiber density abnormalities in cognitively normal adults at risk for late-onset Alzheimer's disease. J Psychiatr Res. (2020) 122:79–87. doi: 10.1016/j.jpsychires.2019.12.019
45. Gili T, Cercignani M, Serra L, Perri R, Giove F, Maraviglia B, et al. Regional brain atrophy and functional disconnection across Alzheimer's disease evolution. J Neurol Neurosurg Psychiatry. (2011) 82:58–66. doi: 10.1136/jnnp.2009.199935
46. Zelano C, Bensafi M, Porter J, Mainland J, Johnson B, Bremner E, et al. Attentional modulation in human primary olfactory cortex. Nat Neurosci. (2005) 8:114–20. doi: 10.1038/nn1368
47. Sela L, Sobel N. Human olfaction: a constant state of change-blindness. Exp Brain Res. (2010) 205:13–29. doi: 10.1007/s00221-010-2348-6
48. Pessoa L. On the relationship between emotion and cognition. Nat Rev Neurosci. (2008) 9:148–58. doi: 10.1038/nrn2317
49. Tabert MH, Steffener J, Albers MW, Kern DW, Michael M, Tang H, et al. Validation and optimization of statistical approaches for modeling odorant-induced fMRI signal changes in olfactory-related brain areas. Neuroimage. (2007) 34:1375–90. doi: 10.1016/j.neuroimage.2006.11.020
50. Nelson L, Tabet N. Slowing the progression of Alzheimer's disease; what works? Ageing Res Rev. (2015) 23:193–209. doi: 10.1016/j.arr.2015.07.002
51. Knipper M, Singer W, Schwabe K, Hagberg GE, Li Hegner Y, Rüttiger L, et al. Disturbed balance of inhibitory signaling links hearing loss and cognition. Front Neural Circuits. (2022) 15:785603. doi: 10.3389/fncir.2021.785603
52. Steinbach S, Hundt W, Vaitl A, Heinrich P, Förster S, Bürger K, et al. Taste in mild cognitive impairment and Alzheimer's disease. J Neurol. (2010) 257:238–46. doi: 10.1007/s00415-009-5300-6
53. Hinz A, Luck T, Riedel-Heller SG, Herzberg PY, Rolffs C, Wirkner K, et al. Olfactory dysfunction: properties of the Sniffin' sticks screening 12 test and associations with quality of life. Eur Arch Otorhinolaryngol. (2019) 276:389–95. doi: 10.1007/s00405-018-5210-2
54. Schubert CR, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. Olfactory impairment in older adults: five-year incidence and risk factors. Laryngoscope. (2011) 121:873–8. doi: 10.1002/lary.21416
55. Talamo BR, Rudel R, Kosik KS, Lee VM, Neff S, Adelman L, et al. Pathological changes in olfactory neurons in patients with Alzheimer's disease. Nature. (1989) 337:736–9. doi: 10.1038/337736a0
56. Tian Q, Chastan N, Thambisetty M, Resnick SM, Ferrucci L, Studenski SA. Bimanual gesture imitation links to cognition and olfaction. J Am Geriatr Soc. (2019) 67:2581–6. doi: 10.1111/jgs.16151
57. Wei EX, Oh ES, Harun A, Ehrenburg M, Agrawal Y. saccular impairment in Alzheimer's disease is associated with driving difficulty. Dement Geriatr Cogn Disord. (2017) 44:294–302. doi: 10.1159/000485123
58. Wei EX, Oh ES, Harun A, Ehrenburg M, Agrawal Y. vestibular loss predicts poorer spatial cognition in patients with Alzheimer's disease. J Alzheimers Dis JAD. (2018) 61:995–1003. doi: 10.3233/JAD-170751
59. Agrawal Y, Smith PF, Rosenberg PB. Vestibular impairment, cognitive decline and Alzheimer's disease: balancing the evidence. Aging Ment Health. (2019) 29:1–4. doi: 10.1080/13607863.2019.1566813
60. Semenov YR, Bigelow RT, Xue QL, du Lac S, Agrawal Y. Association between vestibular and cognitive function in US adults: data from the national health and nutrition examination survey. J Gerontol A Biol Sci Med Sci. (2016) 71:243–50. doi: 10.1093/gerona/glv069
61. Tian Q, Resnick SM, Studenski SA. Olfaction is related to motor function in older adults. J Gerontol A Biol Sci Med Sci. (2017) 72:1067–71. doi: 10.1093/gerona/glw222
62. Graves AB, Bowen JD, Rajaram L, McCormick WC, McCurry SM, Schellenberg GD, et al. Impaired olfaction as a marker for cognitive decline: interaction with apolipoprotein E epsilon4 status. Neurology. (1999) 53:1480–7. doi: 10.1212/WNL.53.7.1480
63. Adams DR, Kern DW, Wroblewski KE, McClintock MK, Dale W, Pinto JM. Olfactory dysfunction predicts subsequent dementia in older U. S adults. J Am Geriatr Soc. (2018) 66:140–4. doi: 10.1111/jgs.15048
64. Devanand DP, Lee S, Luchsinger JA, Andrews H, Goldberg T, Huey ED, et al. Intact Global Cognitive Olfactory Ability Predicts Lack of Transition to Dementia. Alzheimers Dement. (2019) Available online at: https://www.sciencedirect.com/science/article/pii/S1552526019353749 (accessed May 19, 2022).
65. Yaffe K, Freimer D, Chen H, Asao K, Rosso A, Rubin S, et al. Olfaction and risk of dementia in a biracial cohort of older adults. Neurology. (2017) 88:456–62. doi: 10.1212/WNL.0000000000003558
66. Lipnicki DM, Sachdev PS, Crawford J, Reppermund S, Kochan NA, Trollor JN, et al. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney memory and ageing study. PLoS ONE. (2013) 8:e65841. doi: 10.1371/journal.pone.0065841
67. Roberts RO, Christianson TJH, Kremers WK, Mielke MM, Machulda MM, Vassilaki M, et al. Association between olfactory dysfunction and amnestic mild cognitive impairment and Alzheimer disease dementia. JAMA Neurol. (2016) 73:93–101. doi: 10.1001/jamaneurol.2015.2952
68. Stanciu I, Larsson M, Nordin S, Adolfsson R, Nilsson LG, Olofsson JK. Olfactory impairment and subjective olfactory complaints independently predict conversion to dementia: a longitudinal, population-based study. J Int Neuropsychol Soc JINS. (2014) 20:209–17. doi: 10.1017/S1355617713001409
69. Chen Z, Xie H, Yao L, Wei Y. Olfactory impairment and the risk of cognitive decline and dementia in older adults: a meta-analysis. Braz J Otorhinolaryngol. (2021) 87:94–102. doi: 10.1016/j.bjorl.2020.07.009
70. Kashibayashi T, Takahashi R, Fujita J, Fujito R, Kamimura N, Okutani F, et al. Correlation between cerebral blood flow and olfactory function in mild cognitive impairment and Alzheimer's disease. Int J Geriatr Psychiatry. (2021) 36:1103–9. doi: 10.1002/gps.5527
71. Mast FW, Ellis AW. Internal models, vestibular cognition, and mental imagery: conceptual considerations. Multisensory Res. (2015) 28:443–60. doi: 10.1163/22134808-00002503
72. Raphan T, Cohen B. The vestibulo-ocular reflex in three dimensions. Exp Brain Res. (2002) 145:1–27. doi: 10.1007/s00221-002-1067-z
73. Dichgans J, Diener HC. The contribution of vestibulo-spinal mechanisms to the maintenance of human upright posture. Acta Otolaryngol. (1989) 107:338–45. doi: 10.3109/00016488909127518
74. Maharani A, Dawes P, Nazroo J, Tampubolon G, Pendleton N, SENSE-Cog WP1 group. Longitudinal relationship between hearing aid use and cognitive function in older Americans. J Am Geriatr Soc. (2018) 66:1130–6. doi: 10.1111/jgs.15363
75. Jafari Z, Kolb BE, Mohajerani MH. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. (2019) 56:100963. doi: 10.1016/j.arr.2019.100963
76. Sarant J, Harris D, Busby P, Maruff P, Schembri A, Lemke U, et al. The effect of hearing aid use on cognition in older adults: can we delay decline or even improve cognitive function? J Clin Med. (2020) 9:E254. doi: 10.3390/jcm9010254
77. Mertens G, Andries E, Claes AJ, Topsakal V, Van de Heyning P, Van Rompaey V, et al. Cognitive improvement after cochlear implantation in older adults with severe or profound hearing impairment: a prospective, longitudinal, controlled, multicenter study. Ear Hear. (2020) 42:606-14. doi: 10.1097/AUD.0000000000000962
78. Birte-Antina W, Ilona C, Antje H, Thomas H. Olfactory training with older people. Int J Geriatr Psychiatry. (2018) 33:212–20. doi: 10.1002/gps.4725
79. Sakamoto Y, Ebihara S, Ebihara T, Tomita N, Toba K, Freeman S, et al. Fall prevention using olfactory stimulation with lavender odor in elderly nursing home residents: a randomized controlled trial. J Am Geriatr Soc. (2012) 60:1005–11. doi: 10.1111/j.1532-5415.2012.03977.x
80. Mohammed A, Gibbons LE, Gates G, Anderson ML, McCurry SM, McCormick W, et al. Association of performance on dichotic auditory tests with risk for incident dementia and Alzheimer dementia. JAMA Otolaryngol Neck Surg. (2022) 148:20–7. doi: 10.1001/jamaoto.2021.2716
81. Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, et al. Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol. (2002) 59:1737–46. doi: 10.1001/archneur.59.11.1737
Keywords: multisensory impairment, age-related hearing impairment, vestibular impairment, olfactory impairment, cognitive impairment, AROMA, Montreal Cognitive Assessment (MoCA)
Citation: Lucas JC, Arambula Z, Arambula AM, Yu K, Farrokhian N, D'Silva L, Staecker H and Villwock JA (2022) Olfactory, Auditory, and Vestibular Performance: Multisensory Impairment Is Significantly Associated With Incident Cognitive Impairment. Front. Neurol. 13:910062. doi: 10.3389/fneur.2022.910062
Received: 31 March 2022; Accepted: 21 June 2022;
Published: 11 July 2022.
Edited by:
Michael Strupp, Ludwig Maximilian University of Munich, GermanyReviewed by:
Juan Carlos Amor-Dorado, Hospital Can Misses, SpainCopyright © 2022 Lucas, Arambula, Arambula, Yu, Farrokhian, D'Silva, Staecker and Villwock. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jacob C. Lucas, amFrZS5sdWNhcy5lbnRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.