- Department of Neurology, CUB Hôpital Erasme, Université libre de Bruxelles (ULB), Brussels, Belgium
Introduction: The cerebellum modulates both motor and cognitive behaviors, and a cerebellar cognitive affective syndrome (CCAS) was described after a cerebellar stroke in 1998. Yet, a CCAS is seldom sought for, due to a lack of practical screening scales. Therefore, we aimed at assessing both the prevalence of CCAS after cerebellar acute vascular lesion and the yield of the CCAS-Scale (CCAS-S) in an acute stroke setting.
Materials and methods: All patients admitted between January 2020 and January 2022 with acute onset of a cerebellar ischemic or haemorrhagic first stroke at the CUB-Hôpital Erasme and who could be evaluated by the CCAS-S within a week of symptom onset were included.
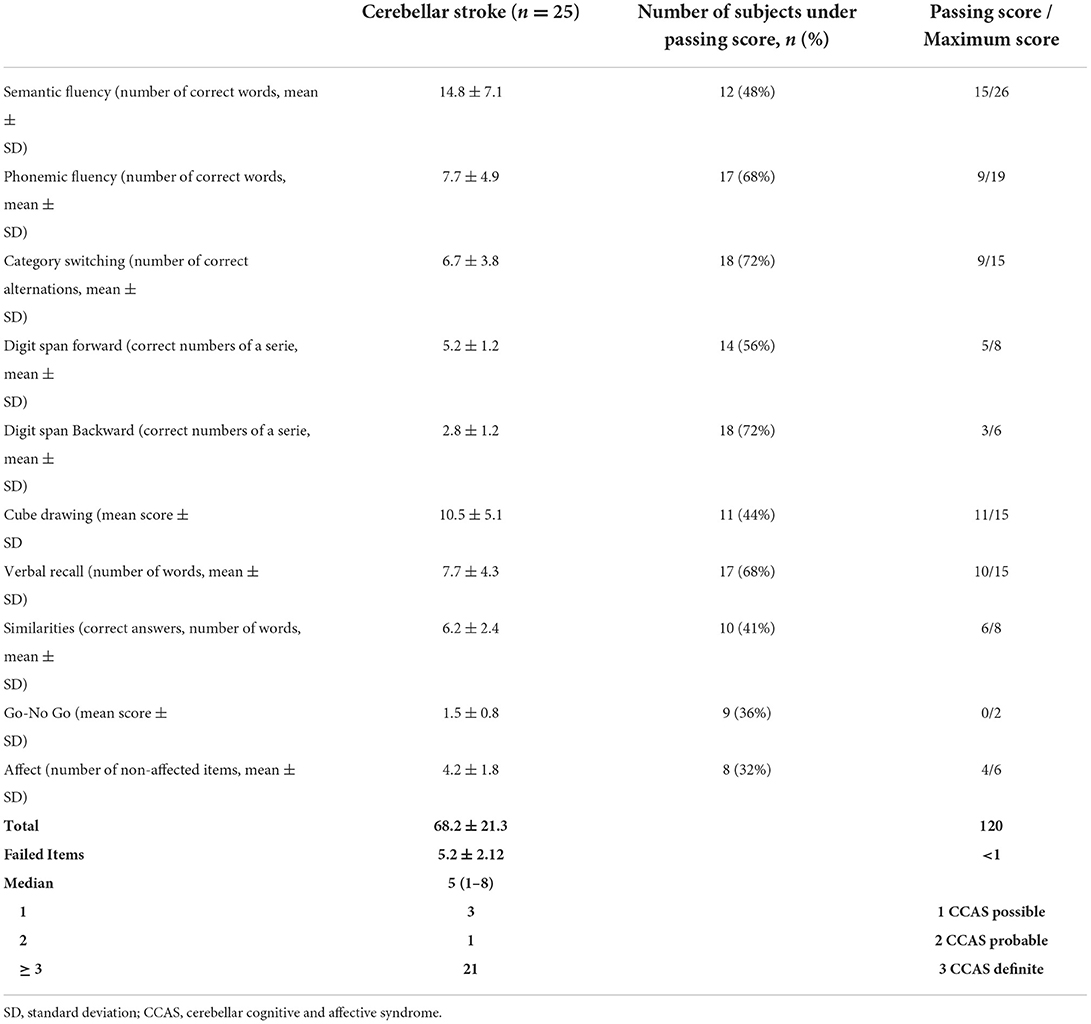
Results: Cerebellar acute vascular lesion occurred in 25/1,580 patients. All patients could complete the CCAS-S. A definite CCAS was evidenced in 21/25 patients. Patients failed 5.2 ± 2.12 items out of 8 and had a mean raw score of 68.2 ± 21.3 (normal values 82–120). Most failed items of the CCAS-S were related to verbal fluency, attention, and working memory.
Conclusion: A definite CCAS is present in almost all patients with acute cerebellar vascular lesions. CCAS is efficiently assessed by CCAS-S at bedside tests in acute stroke settings. The magnitude of CCAS likely reflects a cerebello-cortical diaschisis.
Introduction
Acute vascular cerebellar lesions are proportionally rare and account for 2–3% of all strokes (1, 2). Most cerebellar strokes are considered relatively mild due to low initial National Institutes of Health Stroke Scale (NIHSS) score (3) and mostly favorable outcomes: 70% of patients are deemed fully independent after a cerebellar stroke when consciousness is not impaired at presentation (2, 4, 5). Still, the functional consequences of cerebellar vascular impairment might be underestimated. In the human central nervous system (CNS), the cerebellum hosts four times more neurons than the neocortex and displays with the prefrontal cortex the main relative increase in sapiens' brain neurons compared with other mammals (6, 7). The ratio of cerebellar to neocortical neurons and the cerebellar cortical surface is, furthermore, substantially increased from big apes to humans (6, 8, 9). Historically associated with movement control, the cerebellum is now recognized to play an important role in perceptual and cognitive processes (10, 11). This expansion is one of the explanations for the human brain's higher cognitive performance. The interplay between neocortical areas and the cerebellum occurs thanks to dense reciprocal connections with efferent cerebellar dentato-thalamo-cortical tracts that modulate a wide array of neocortical areas, which in turn are connected back to the cerebellum through cortico-ponto-cerebellar tracts (12–16). The function of the cerebello-cortical loops (CCLs) is to increase the accuracy of both motor and cognitive behaviors (17). Clinically, impairment of the CCL through acute or chronic cerebellar disorders leads to both motor and thought dysmetria (18–20). While motor dysmetria after cerebellar stroke was already well described by Holmes (21), the impact of cerebellar dysfunction on cognitive processes was only outlined two decades ago by Jeremy Schmahmann. In his seminal series, Jeremy Schmahmann described a cohort of twenty patients with cerebellar disorders, thirteen of whom had an acute vascular lesion (22), and various cognitive impairments that included language, emotional regulation, memory, attention, visuospatial, and executive function (22, 23). He coined the cognitive profile associated with cerebellar lesion as the cerebellar cognitive affective syndrome (CCAS). This association between cognitive dysfunction and cerebellar disorders was confirmed in several studies [for a meta-analysis, refer to the example in Ahmadian et al. (24)]. However, the cognitive disorders associated with cerebellar pathology are seldom systematically studied due to the extensive and lengthy neuropsychological test batteries (over 90 min in many of the studies) that were, until recently, required to highlight a CCAS. In 2018, a CCAS screening and follow-up scale (CCAS-S) was developed, based on the paper and pencil neuropsychological tests, that could most efficiently single out individuals with cerebellar cognitive disorders from healthy individuals (23). The CCAS-S allows <10 min to provide evidence for a CCAS in patients with cerebellar disorders (23). To date, the CCAS-S has not been used in patients with acute vascular cerebellar disorders.
The aim of this study was therefore to (i) determine the prevalence of a CCAS in a cohort of patients with acute vascular cerebellar lesion and (ii) assess the practicability of the CCAS-S in the context of acute stroke.
Subjects and methods
Population
The studied population is derived from the stroke registry of the Erasmus Hospital in Brussels (Belgium) where all cases of acute stroke are recorded since January 2015 (25, 26). Our analysis included patients admitted between January 2020 and January 2022 who had an acute onset cerebellar ischaemic or haemorrhagic stroke and for whom a CCAS-S was performed within 1 week of symptom onset.
Acute stroke care and clinical evaluation
Acute stroke management and care followed the European Stroke Organization guidelines and are detailed in Elands et al. (25) and Jodaitis et al. (27). Stroke initial severity was evaluated by the National Institutes of Health Stroke Scale (NIHSS) score at admission. CCAS was evaluated using the CCAS-S. The CCAS-S is composed of 10 items: a semantic fluency task, a phonemic fluency task, a verbal category switching task, a forward digit span, a backward digit span, a cube drawing task, a verbal registration task, a verbal similarities task, a Go No-Go task, and an affect evaluation (23). A raw score is obtained for each task, with a minimum passing score. The number of failed tests determines the likelihood that the subject has CCAS: three or more failed tasks make a definite CCAS, two probable CCAS, and one possible CCAS. The raw score ranges from 82 (sum of minimum passing scores for each item on the scale) to 120 (sum of maximum scores for each item) is not diagnostic but provides quantitative values in each task that can be used for longitudinal follow-up as patients can have definite CCAS (three failed test items) with a total raw score that falls in the 82–120 range. Subjects without CCAS are not expected to fail any task (23). The French translation of the “A” version of the CCAS-S (23) was used in this study.
Results
Population
During the study period, 1,508 patients were admitted to the stroke unit, and 25 of those who presented had an acute vascular cerebellar lesion (1.7%). Patients' characteristics are summarized in Table 1. The mean age was sixty-four, and lesions were ischemic in 18/25 patients and bilateral in 5/25. In ischemic lesions, the posterior inferior cerebellar artery (PICA) territory was most commonly affected, followed by the superior cerebellar artery (SCA) territory. The lesion was located in the posterior cerebellar lobe in all but one patient and predominantly in the right cerebellar hemisphere. Figure 1 illustrates schematically the lesion sizes and locations. The median admission NIHSS score was 1.
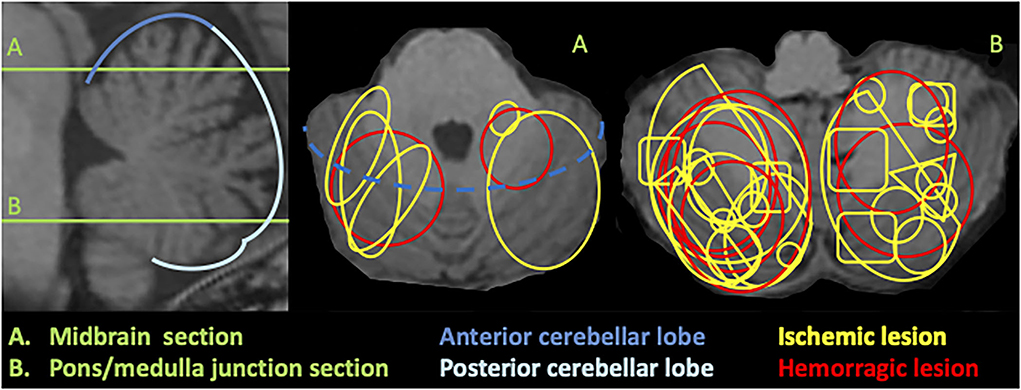
Figure 1. Schematic illustration of cerebellar lesions superimposed on 3DT1 MRI. Sagittal section (left) shows the level of the axial slices (middle and right): (A) corresponds to a midbrain level and (B) to a pons/medulla junction section.
Cerebellar cognitive affective syndrome
CCAS-Scale was possible to realize in all patients. All patients failed at least one CCAS-S item. Twenty-one patients displayed a definite CCAS (84%), 3/25 (12%) a probable CCAS, and 1/25 (4%) a possible CCAS. Semantic category switching and digit span backward tests were the items most patients failed (18/25, 72% of cases), followed by phonemic fluency and verbal registration tests (17/25, 68% of cases). CCAS-S results are detailed in Table 2.
Discussion
The main findings of this study are that a definite cerebellar cognitive affective syndrome is present in most patients with acute cerebellar vascular lesions and that the CCAS-S can be easily completed at bedside tests in acute stroke settings.
The findings of this study, albeit limited by its monocentric nature and the size of the sample, are likely to be generalizable to other populations of acute cerebellar vascular lesions. In fact, our cohort matches the usual proportion of acute vascular cerebellar lesions in stroke units, ranging between 1.5% (2) and 2.3% (1). Similarly, the clinical characteristics of the reported population of an acute vascular cerebellar lesion are considered in terms of age (1, 2, 14), sex (1, 2, 14), admission NIHSS (3, 4, 28, 29), vascular territory involved (30–32), rate of bilateral lesions (1, 31), and predominant involvement of cerebellar posterior lobes (14). However, a selection bias toward less severe cases in our cohort is possible due to the fact that patients with acute cerebellar vascular lesions who need surgery for acute hydrocephalus or acute brainstem compression are not usually hospitalized in the stroke unit but neurosurgery and intensive care units. Such complications occur in 10 to 20% of acute vascular cerebellar lesions and missed inclusion in our cohort (29, 33).
Since the description of the CCAS in 1998 (22), several studies confirmed that almost all patients with acute cerebellar vascular lesion displayed significant cognitive impairments in a wide range of cognitive domains, corresponding to a CCAS (14, 34–36) that mirrored the characteristics of Schmahmann's seminal report (22). However, the identification of a CCAS in those studies required the use of a full neuropsychological test battery, an assessment that requires over an hour in trained hands and is exhausting for acutely ill patients. Those facts limit the application of a full neuropsychological test battery in acute stroke settings. In contrast, in our cohort, the CCAS-S could be performed in all patients and allowed to screen for a CCAS in 10 min, highlighting its yield in the acute cerebellar stroke context. This report, therefore, brings evidence for the validity of the CCAS-S in acute cerebellar disorders and supports previous findings that relied on the CCAS-S to describe cognitive disorders in degenerative cerebellar diseases such as Friedreich Ataxia (20), SCA3 (37), in a mixed cohort of degenerative cerebellar ataxia (38), as well as in patients with chronic cerebellar stroke (39).
The high rate of CCAS in our cohort, with 24/25 subjects displaying lesions in cerebellar posterior lobes, is consistent with lesion-symptom mapping and functional neuroimaging studies that associate CCAS and cerebellar role in cognition to cerebellar posterior lobes (14, 16, 40). The higher rate of cerebellar right-sided posterior lesions in our cohort may also contribute to a more severe cognitive clinical pattern due to the loss of cross-connections between the dominant hemisphere and the right cerebellum (14, 41). Yet, this association between the right cerebellar lesion and worse cognitive outcomes is inconstant and needs further investigation in larger groups of subjects (35). Compared with patients with cerebellar degenerative diseases or chronic cerebellar stroke, patients with acute cerebellar vascular lesions failed more items [5 compared with 3 in Naeije et al. (20), Maas et al. (37) and Chirino-Pérez et al. (39)] and had a worse CCAS-S total raw score [68/120 against 72/120 in Benussi et al. (42) and 88/120 in Chirino-Pérez et al. (39)]. Such poorer performances in patients with acute cerebellar injury are probably related to the CCAS pathophysiology. In fact, the CCAS is thought to build up from the disconnection of neocortical areas involved in cognitive processes and the cerebellum, corresponding to a cerebellocortical diaschisis (CCD) (20, 24). In degenerative disorders, this diaschisis is gradual and allows compensatory mechanisms as highlighted in degenerative cerebellar ataxias (43–46). At the acute vascular cerebellar lesion stage, both cognitive impairments and CCD on functional brain imaging are maximal (35, 47–49), while compensatory strategies through plasticity or recovery have not yet developed. Over time, cognitive impairments related to cerebellar vascular impairment partially improve, suggesting that the acute disconnection from the cerebellum might recover or be compensated (32, 39). Our patients mostly failed the CCAS-S item relating to verbal fluency, attention, and working memory. Neuroanatomically, verbal fluency is considered to rely more on executive than language functions (50, 51) and is dependent on the prefrontal cortex integrity, similarly to attention (52) and working memory (53). Patients with acute cerebellar vascular lesion failed the item that depends on frontal cortex integrity, which parallels the metabolic brain functional imaging studies on CCD that showed that the frontal cortex was the most metabolically impaired cortical area after a cerebellar lesion (48, 49) and supports CCD as the main pathophysiology for CCAS. Only one-third of patients failed the “affect” item of the CCAS when patients with cerebellar disorders display a much higher rate of non-cognitive psychiatric symptoms and social cognition disorders when formally tested (54–57). The self-reported nature of this item may explain its lack of sensitivity in the CCAS-S. This observation is also made in degenerative cerebellar disorders (20) and may warrant the evolution of the “affect” item in further CCAS-S version. The role of the CCD in CCAS related to cerebellar stroke was also further demonstrated by the long-term consequences of cerebellar vascular lesions (58). In fact, a study from 2021 described an atrophy of the neocortical areas functionally connected to the cerebellum in proportion to the acute cerebellar lesion volume (59).
In summary, this study shows that a CCAS is highly prevalent after acute vascular cerebellar injury and likely reflects acute CCD. This study also positions the CCAS-S as a highly sensitive and practical tool to screen for cerebellar cognitive disorders in the stroke context. Further studies are required to assess the relation between CCAS-S scores at acute and chronic stages and the magnitude of the CCD through both functional and structural brain imaging longitudinal studies.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by CUB-Hopital Erasme Ethics Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
NL, AA, and GN: study design, data collection, analysis, and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
GN is a Postdoctorate Clinical Master Specialist at the FRS-FNRS (Brussels, Belgium).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tohgi H, Takahashi S, Chiba K, Hirata Y, Moriyama T, Kanayama S, et al. Cerebellar infarction. Clinical and neuroimaging analysis in 293 patients The Tohoku Cerebellar Infarction Study Group. Stroke. (1993) 24:1697–701. doi: 10.1161/01.STR.24.11.1697
2. Macdonell RAL, Kalnins RM, Donnan GA. Cerebellar infarction: natural history, prognosis, and pathology. Stroke. (1987) 18:849–55. doi: 10.1161/01.STR.18.5.849
3. Martin-Schild S, Albright KC, Tanksley J, Pandav V, Jones EB, Grotta JC, et al. Zero on the NIHSS does not equal the absence of stroke. Ann Emerg Med. (2011) 57:42–5. doi: 10.1016/j.annemergmed.2010.06.564
4. Nickel A, Cheng B, Pinnschmidt H, Arpa E, Ganos C, Gerloff C, et al. Clinical outcome of isolated cerebellar stroke-A prospective observational study. Front Neurol. (2018) 9:580. doi: 10.3389/fneur.2018.00580
5. Malm J, Kristensen B, Carlberg B, Fagerlund M, Olsson T. Clinical features and prognosis in young adults with infratentorial infarcts. Cerebrovasc Dis. (1999) 9:282–9. doi: 10.1159/000015979
6. Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. (2009) 3:31. doi: 10.3389/neuro.09.031.2009
7. Ramnani N. The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci. (2006) 7:511–22. doi: 10.1038/nrn1953
8. Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. (2009) 513:532–41. doi: 10.1002/cne.21974
9. Sereno MI, Diedrichsen J, Tachrount M, Testa-Silva GD, Arceuil H, De Zeeuw C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc Natl Acad Sci U S A. (2020) 117:19538–43. doi: 10.1073/pnas.2002896117
10. Baumann O, Borra RJ, Bower JM, Cullen KE, Habas C, Ivry RB, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. (2015) 14:197–220. doi: 10.1007/s12311-014-0627-7
11. Ivry RB, Spencer RM, Zelaznik HN, Diedrichsen J. The cerebellum and event timing. Ann N Y Acad Sci. (2002) 978:302–17. doi: 10.1111/j.1749-6632.2002.tb07576.x
12. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. (2010) 46:831–44. doi: 10.1016/j.cortex.2009.11.008
13. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. (2009) 44:489–501. doi: 10.1016/j.neuroimage.2008.08.039
14. Stoodley CJ, MacMore JP, Makris N, Sherman JC, Schmahmann JD. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. NeuroImage Clin. (2016) 12:765–75. doi: 10.1016/j.nicl.2016.10.013
15. Guell X, Gabrieli JD E, Schmahmann JD. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. (2018) 172:437–49. doi: 10.1016/j.neuroimage.2018.01.082
16. King M, Hernandez-Castillo CR, Poldrack RA, Ivry RB, Diedrichsen J. Functional boundaries in the human cerebellum revealed by a multi-domain task battery. Nat Neurosci. (2019) 22:1371–8. doi: 10.1038/s41593-019-0436-x
17. Guell X, Gabrieli JDE, Schmahmann JD. Embodied cognition and the cerebellum: Perspectives from the dysmetria of thought and the universal cerebellar transform theories. Cortex. (2018) 100:140–8. doi: 10.1016/j.cortex.2017.07.005
18. Schmahmann JD. The cerebellum and cognition. Neurosci Lett. (2019) 688:62–75. doi: 10.1016/j.neulet.2018.07.005
19. Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. (1998) 2:362–71. doi: 10.1016/S1364-6613(98)01218-2
20. Naeije G, Rai M, Allaerts N, Sjogard M, De Tiège X, Pandolfo M. Cerebellar cognitive disorder parallels cerebellar motor symptoms in Friedreich ataxia. Ann Clin Transl Neurol. (2020) 7:1050–4. doi: 10.1002/acn3.51079
22. Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. (1998) 121:561–79. doi: 10.1093/brain/121.4.561
23. Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. (2018) 141:248–70. doi: 10.1093/brain/awx317
24. Ahmadian N, van Baarsen K, van Zandvoort M, Robe PA. The cerebellar cognitive affective syndrome—a meta-analysis. Cerebellum. (2019) 18:941–50. doi: 10.1007/s12311-019-01060-2
25. Elands S, Casimir P, Bonnet T, Mine B, Lubicz B, Sjøgård M, et al. Early venous filling following thrombectomy: association with hemorrhagic transformation and functional outcome. Front Neurol. (2021) 12:649079. doi: 10.3389/fneur.2021.649079
26. Ligot N, Elands S, Damien C, Jodaitis L, Sadeghi Meibodi N, Mine B, et al. Stroke Core Volume weighs more than recanalization time for predicting outcome in large vessel occlusion recanalized within 6 h of symptoms onset. Front Neurol. (2022) 13:270. doi: 10.3389/fneur.2022.838192
27. Jodaitis L, Ligot N, Chapusette R, Bonnet T, Gaspard N, Naeije G. The hyperdense middle cerebral artery sign in drip-and-ship models of acute stroke management. Cerebrovasc Dis Extra. (2020) 10:36–43. doi: 10.1159/000506971
28. Cano LM, Cardona P, Quesada H, Mora P, Rubio F. Cerebellar infarction: prognosis and complications of vascular territories. Neurol. (2012) 27:330–5. doi: 10.1016/j.nrleng.2012.07.010
29. Edlow JA, Newman-Toker DE, Savitz SI. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. (2008) 7:951–64. doi: 10.1016/S1474-4422(08)70216-3
30. Amarenco P, Hauw JJ. Cerebellar infarction in the territory of the anterior and inferior cerebellar artery. A clinicopathological study of 20 cases. Brain. (1990) 113:139–55. doi: 10.1093/brain/113.1.139
31. Barth A, Bogousslavsky J, Regli F. The clinical and topographic spectrum of cerebellar infarcts: A clinical—magnetic resonance imaging correlation study. Ann Neurol. (1993) 33:451–6. doi: 10.1002/ana.410330507
32. Richter S, Gerwig M, Aslan B, Wilhelm H, Schoch B, Dimitrova A, et al. Cognitive functions in patients with MR-defined chronic focal cerebellar lesions. J Neurol. (2007) 254:1193–203. doi: 10.1007/s00415-006-0500-9
33. Shenkin HA, Zavala M. cerebellar strokes: mortality, surgical indications, and results of ventricular drainage. Lancet. (1982) 320:429–32. doi: 10.1016/S0140-6736(82)90453-6
34. Malm J, Kristensen B, Karlsson T, Carlberg B, Fagerlund M, Olsson T. Cognitive impairment in young adults with infratentorial infarcts. Neurology. (1998) 51:433–40. doi: 10.1212/WNL.51.2.433
35. Erdal Y, Perk S, Keskinkilic C, Bayramoglu B, Soydan Mahmutoglu A, Emre U. The assessment of cognitive functions in patients with isolated cerebellar infarctions: a follow-up study. Neurosci Lett. (2021) 765:136252. doi: 10.1016/j.neulet.2021.136252
36. Taskiran-Sag A, Uzuncakmak Uyanik H, Uyanik SA, Oztekin N. Prospective investigation of cerebellar cognitive affective syndrome in a previously non-demented population of acute cerebellar stroke. J Stroke Cerebrovasc Dis. (2020) 29:104923. doi: 10.1016/j.jstrokecerebrovasdis.2020.104923
37. Maas RPPWM, Killaars S, van de Warrenburg BPC, Schutter DJLG. The cerebellar cognitive affective syndrome scale reveals early neuropsychological deficits in SCA3 patients. J Neurol. (2021) 268:3456–66. doi: 10.1007/s00415-021-10516-7
38. Benussi A, Cantoni V, Manes M, Libri I, Dell'Era V, Datta A, et al. Motor and cognitive outcomes of cerebello-spinal stimulation in neurodegenerative ataxia. Brain. (2021) 144:2310–21. doi: 10.1093/brain/awab157
39. Chirino-Pérez A, Marrufo-Meléndez OR, Muñoz-López JI, Hernandez-Castillo CR, Ramirez-Garcia G, Díaz R, et al. Mapping the cerebellar cognitive affective syndrome in patients with chronic cerebellar. Strokes. (2022) 21:208–18. doi: 10.1007/s12311-021-01290-3
40. Timmann D, Brandauer B, Hermsdörfer J, Ilg W, Konczak J, Gerwig M, et al. Lesion-symptom mapping of the human cerebellum. Cerebellum. (2008) 7:602–6. doi: 10.1007/s12311-008-0066-4
41. Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. (2004) 75:1524–31. doi: 10.1136/jnnp.2003.018093
42. Benussi A, Koch G, Cotelli M, Padovani A, Borroni B. Cerebellar transcranial direct current stimulation in patients with ataxia: a double-blind, randomized, sham-controlled study. Mov Disord. (2015) 30:1701–5. doi: 10.1002/mds.26356
43. Naeije G, Coquelet N, Wens V, Goldman S, Pandolfo M, De Tiège X. Age of onset modulates resting-state brain network dynamics in Friedreich Ataxia. Hum Brain Mapp. (2021) 42:5334–44. doi: 10.1002/hbm.25621
44. Naeije G, Wens V, Coquelet N, Sjøgård M, Goldman S, Pandolfo M, et al. Age of onset determines intrinsic functional brain architecture in Friedreich ataxia. Ann Clin Transl Neurol. (2020) 7:94–104. doi: 10.1002/acn3.50966
45. Gilman S, Junck L, Markel DS, Koeppe RA, Kluin KJ. Cerebral glucose hypermetabolism in Friedreich's ataxia detected with positron emission tomography. Ann Neurol. (1990) 28:750–7. doi: 10.1002/ana.410280605
46. Harding IH, Corben LA, Storey E, Egan GF, Stagnitti MR, Poudel GR, et al. Fronto-cerebellar dysfunction and dysconnectivity underlying cognition in friedreich ataxia: The IMAGE-FRDA study. Hum Brain Mapp. (2016) 37:338–50. doi: 10.1002/hbm.23034
47. Beldarrain G, Garcia-Moncó C, Quintana M, Llorens V, Rodeño E. Diaschisis and neuropsychological performance after cerebellar stroke. Eur Neurol. (1997) 37:82–9. doi: 10.1159/000117415
48. Komaba Y, Osono E, Kitamura S, Katayama Y. Crossed cerebellocerebral diaschisis in patients with cerebellar stroke. Acta Neurol Scand. (2000). doi: 10.1034/j.1600-0404.2000.00002.x
49. Botez-Marquard T. Botez MI. Int Rev Neurobiol. (1997) 41:387–410. doi: 10.1016/S0074-7742(08)60361-X
50. Whiteside D, Kealey T, Semla H, Luu H, Rice L, Basso M, et al. Verbal Fluency: Language or Executive Function Measure? Appl Neuropsychol Adult. (2016) 23:29–34. doi: 10.1080/23279095.2015.1004574
51. Amunts J, Camilleri JA, Eickhoff SB, Heim S, Weis S. Executive functions predict verbal fluency scores in healthy participants. Sci Rep. (2020) 10:11141. doi: 10.1038/s41598-020-65525-9
52. Corbetta M, Shulman GL. Spatial neglect and attention networks. Annu Rev Neurosci. (2011) 34:569–99. doi: 10.1146/annurev-neuro-061010-113731
53. Müller NG, Knight RT. The functional neuroanatomy of working memory: contributions of human brain lesion studies. Neuroscience. (2006) 139:51–8. doi: 10.1016/j.neuroscience.2005.09.018
54. Leroi I, O'Hearn E, Marsh L, Lyketsos CG, Rosenblatt A, Ross CA, et al. Psychopathology in patients with degenerative cerebellar diseases: a comparison to Huntington's disease. Am J Psychiatry. (2002) 159:1306–14. doi: 10.1176/appi.ajp.159.8.1306
55. Beuriat PA, Cohen-Zimerman S, Smith GNL, Krueger F, Gordon B, Grafman J. Evidence of the role of the cerebellum in cognitive theory of mind using voxel-based lesion mapping. Sci Rep. (2022) 12:1–12. doi: 10.1038/s41598-022-09104-0
56. Tamaš O, Kostić M, Kačar A, Stefanova E, Ð*okić BS, Stanisavljević D, et al. Social Cognition in Patients With Cerebellar Neurodegenerative Disorders. Front Syst Neurosci. (2021) 15:664223. doi: 10.3389/fnsys.2021.664223
57. Kronemer SI, Slapik MB, Pietrowski JR, Margron MJ, Morgan OP, Bakker CC, et al. Neuropsychiatric symptoms as a reliable phenomenology of cerebellar ataxia. Cerebellum. (2021) 20:141–50. doi: 10.1007/s12311-020-01195-7
58. van Niftrik CHB, Visser TF, Sebök M, Muscas G, El Amki M, Serra C, et al. Delayed cerebral atrophy after cerebellar stroke: topographical relation and clinical impact. Brain Commun. (2021) 3:fcab279. doi: 10.1093/braincomms/fcab279
Keywords: cerebellar cognitive affective syndrome, cerebellar stroke, crossed cerebellar diaschisis, stroke, cognition, prognosis
Citation: Abderrakib A, Ligot N and Naeije G (2022) Cerebellar cognitive affective syndrome after acute cerebellar stroke. Front. Neurol. 13:906293. doi: 10.3389/fneur.2022.906293
Received: 28 March 2022; Accepted: 06 July 2022;
Published: 11 August 2022.
Edited by:
Nishant K. Mishra, Yale University, United StatesReviewed by:
Lars Evald, Aarhus University, DenmarkJeremy Schmahmann, Massachusetts General Hospital and Harvard Medical School, United States
Louisa Selvadurai, Massachusetts General Hospital and Harvard Medical School, Boston, United States, in Collaboration With Reviewer JS
Copyright © 2022 Abderrakib, Ligot and Naeije. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilles Naeije, R2lsbGVzLk5hZWlqZUBlcmFzbWUudWxiLmFjLmJl
†These authors have contributed equally to this work
 Anissa Abderrakib†
Anissa Abderrakib† Gilles Naeije
Gilles Naeije