- 1Institute of Basic Research in Clinical Medicine, China Academy of Chinese Medical Sciences, Beijing, China
- 2Center for Evidence-Based Chinese Medicine, Beijing University of Chinese Medicine, Beijing, China
- 3College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 4State Key Laboratory of Cognitive Neuroscience and Learning, Beijing Normal University, Beijing, China
Objectives: This study aims to explore the benefits and harms of Chinese Herbal Medicine (CHM) for mild cognitive impairment (MCI).
Methods: Electronic searching was conducted in two English and four Chinese databases till 2021 December. Randomized clinical trials on CHM compared to no intervention, placebo or other therapies for MCI were included.
Results: Forty-nine RCTs (48 finished trials and 1 protocol) were identified. The overall methodological quality of included trials was relatively low. This review found that compared to no intervention or placebo, CHM can significantly decrease the number of patients who progressed to dementia (RR 0.36, 95% CI 0.22–0.58) and increase the cognitive function assessed by MMSE (MD 1.96, 95% CI 1.41–2.50) and MoCA (MD 2.44, 95% CI 1.57–3.31). The subgroup analysis of different CHM showed that Ginko leaf tablets can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 2.03, 95% CI 1.18–2.88) and MoCA (MD 3.11, 95% CI 1.90–4.33). Compared to western medicine, CHM can significantly increase the score of MMSE (MD 0.88 95% CI 0.46–1.30) and MoCA (MD 0.87, 95% CI 0.33–1.41), but there was no significant difference on the score of ADL (SMD −0.61, 95% CI −1.49 to 0.27). None of the RCTs reported on the quality of life. Of 22 RCTs that reported adverse events, there was no statistical difference between the CHM and the control group.
Conclusions: CHM, Ginko leaf extracts in particular, could help to prevent progression into dementia and to improve cognitive function and ability of daily living activities. More qualified RCTs were needed to confirm the conclusion due to the low quality of current trials.
Systematic Review Registration: Unique Identifier: CRD42020157148.
Introduction
Mild cognitive impairment (MCI) represents a transitional state between normal aging and dementia (1). It is diagnosed by the presence of one or more domains of cognitive impairment without fulfilling the diagnostic criteria for dementia (2). If impairment involves memory, it is known as amnestic MCI, and if not, non-amnestic MCI (2). The population-based studies showed that the frequency of MCI is estimated to be 15–20% in persons 60 years and older (3). The annual rate of development of dementia in people with MCI varied between 5 and 15% (3). People with MCI are at higher risk of progression to dementia than age-matched general people, therefore, it is critical to find treatments that may improve symptoms or prevent or delay progression to dementia (2).
Currently, no treatments, pharmacologic or non-pharmacologic, are approved specifically for MCI by the Food and Drug Administration (2). Doctors sometimes prescribe approved drugs that were for Alzheimer's disease to patients with MCI, such as cholinesterase inhibitors, but according to the practice guideline by the American Academy of Neurology, doctors may not choose cholinesterase inhibitors and must first discuss lack of evidence when offering it to people with MCI (2). The guideline found evidence on 12 pharmacologic treatments (e.g., Donepezil, Galantamine, Rivastigmine), but none was recommended to be used for MCI as no high-quality evidence exists (2). For non-pharmacologic treatments, exercise training and cognitive training may help improve cognitive measures (2).
In recent years, Chinese herbal extracts and preparations have received considerable research attention for the management of cognition impairment. Previously published systematic reviews of Chinese herbal medicine (CHM) for MCI have been limited by enrolling people with mixed cognitive impairment (including MCI and other memory impairment), or focusing only on Montreal Cognitive Assessment (MoCA) (4), Mini-mental state examination (MMSE) (5), or Alzheimer's Disease Assessment Scale-Cognitive subscale (ADAS-Cog) (5), but not on suggested clinically meaningful patient outcomes such as development into dementia or quality of life (5). These reviews were likely not to provide dependable results because of possible flaws in methodology, such as lack of protocol (4–6), the inadequate technique for risk of bias assessment (6), or synthesis of data regardless of significant clinical heterogeneity (5). An update of evidence is also warranted as these systematic reviews were based on evidence up till December 2017 (4–6). Therefore, this study was conducted to summarize the up-to-date evidence and to explore the benefits and harms of CHM for MCI in a systematic review of randomized clinical trials (RCTs) with rigorous and reasonable methodology.
Methods
The protocol of this review was registered on PROSPERO (registration number: CRD42020157148). We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Interventions (7).
Inclusion Criteria
Types of Studies
We searched for RCTs regardless of language, publication year, publication format, and publication status.
Types of Participants
We included participants of any gender and at any age diagnosed as MCI by trialists or according to guidelines.
Types of Interventions
We included CHM at any dose and form, compared with placebo, no intervention or other commonly used therapies (e.g., western medication, cognitive training, or lifestyle change) for MCI. CHM with fixed production process and controllable quality, approved by national medical products administration and state administration for market regulation (SAMR) were included. Comparison between two different CHM was excluded. We allowed co-intervention when it was administered equally to the intervention and the control group.
Types of Outcome Measures
Primary outcomes were the number of participants progressing into dementia, cognitive function assessed by authority scales such as MoCA and MMSE, and the number of participants with adverse events. Secondary outcomes were quality of life and assessment of behavior or psychiatric symptoms.
Search Strategy
We searched MEDLINE, Cochrane CENTRAL, China National Knowledge Infrastructure (CNKI), Chongqing VIP (CQVIP), Wanfang Data, and Sinomed from their inception to 31 December 2021. We also searched the reference lists of the meta-analyses on this topic and the references of the included trials. The search strategy (see Supplementary Table 1 for details) included the following key medical keywords: “mild cognitive impairment”, “Chinese herbal medicine”, “Chinese patent medicine”, “random”, “randomization”, and “randomized clinical trials”.
Data Selection and Extraction
Reviewers in pairs (SH Yang, CH Liang, LD Gao, S Wang) independently screened titles and abstracts to retrieve the potentially eligible trials, and read full-text papers to identify the trials that should be included. Reviewers (YX Chen, SH Yang) extracted the information from the included trials through a pre-piloted table created in Microsoft Excel. Another author (N Liang) rechecked the extracted information. The extracted information included publication data (e.g., publication year, authors); study characteristics and design; characteristics of the participants (e.g., age, gender, diagnostic and inclusion criteria); interventions and controls (e.g., dose, form, duration); and outcomes. We extracted data at maximum follow-up.
Assessment of Risk of Bias in Included Trials
Reviewers in pair (SH Yang, CH Liang, LD Gao, S Wang) independently assessed the risk of bias according to the Cochrane “risk of bias” tool (7). We evaluated the following items: allocation sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and other bias.
Statistical Analysis
RevMan 5.3 was used to perform statistical analyses. We used the risk ratio (RR) for measuring dichotomous outcomes and mean difference (MD) for continuous data, with 95% confidence intervals (CIs). When studies used different instruments to measure the same continuous outcome, we calculated the standard mean difference (SMD), with 95% CI. We assessed clinical and methodological heterogeneity by carefully examining trial participant and intervention characteristics and the design of the included trials. We assessed our intervention effects with both fixed-effect model and random-effects model, and we reported both results when results differed (e.g., one giving a significant intervention effect, while the other no significant intervention effect); otherwise, we reported the estimate closest to the zero effect (the highest P-value). We started by looking at the forest plots for signs of statistical heterogeneity. Next, we used the Chi2 test with a significance threshold set as P < 0.10 and measured the amount of heterogeneity with the I2 statistic to assess to what extent variation is from heterogeneity rather than chance. We interpreted the I2 statistic as suggested in Cochrane Handbook: 0–40%: might not be important; 30–60%: might represent moderate heterogeneity; 50–90%: might represent substantial heterogeneity; 75–100%: considerable heterogeneity (7). In case of available data, we performed the subgroup analyses in terms of different interventions and controls, and different causes of MCI. We used sensitivity analyses whenever we wanted to test the robustness of our findings. We planned to assess reporting bias using funnel plots if we obtained data from at least 10 trials per comparison. To assess the risk of publication bias, we intended to look for symmetry or asymmetry of each funnel plot.
Results
Description of the Search
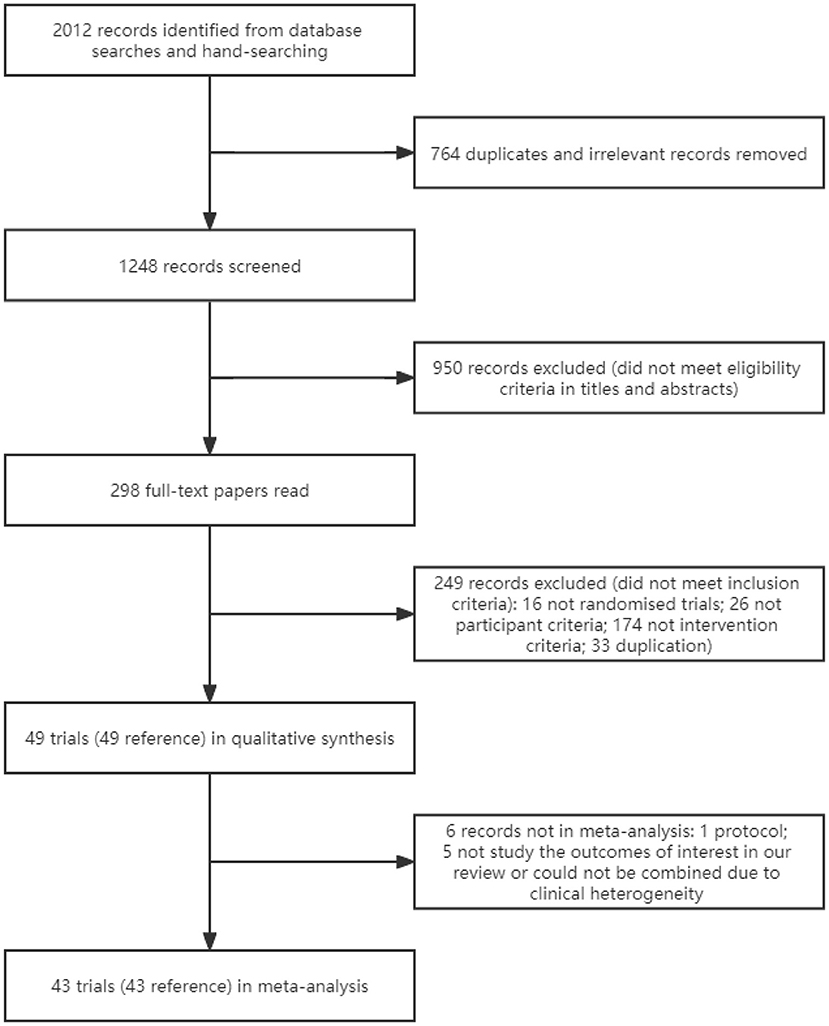
We identified 2,012 references through searching the databases and the reference lists of meta-analyses on this topic and included trials (Figure 1). After excluding duplicates and irrelevant studies, titles and abstracts of 1,248 references were screened; of these, 298 full-text papers were read. Totally, 49 references of 49 trials were included (8–56).
Basic Characteristics of Included Studies
We included 48 finished trials and one protocol (30). Of the 48 finished trials, 43 trials provided data for meta-analyses. The remaining five trials either did not study the outcomes of interest in our review or could not be combined due to clinical heterogeneity (19, 34, 38, 53, 55); hence we used the provided information only in a narrative way. Fifteen trials received funding from government or academic institution; four trials were funded by pharmaceutical companies, and high risk of financial supports was suspected even though no conflict of interest was declared (33, 42, 48, 53); the remaining 30 trials did not provide information on funding. Undisclosed funding may influence trial results and may lead to poor trial design.
Sample size ranged from 18 to 245 participants, and a total of 4,656 participants were randomized. For 42 trials that reported the gender of participants, the total ratio of female to male was 1,897:1,892. Age of participants was reported in 44 trials, and mean age of participants in these trials was 67.82 years old (Table 1).
Twenty-seven CHM were explored in the included trials (see Supplementary Table 2 for details). The dosage form included capsules (n = 13), pills (n = 4), tablets (n = 4), granules (n = 3), oral liquids (n = 2), and injection (n = 2). The duration of CHM treatment ranged from 14 to 24 months.
Risk of Bias in Included Studies
We carried out the risk of bias assessment for 48 finished trials. We judged many trials to have unclear risk of bias in certain domains because we could not obtain additional information from the trial authors after we contacted them (Figures 2, 3).
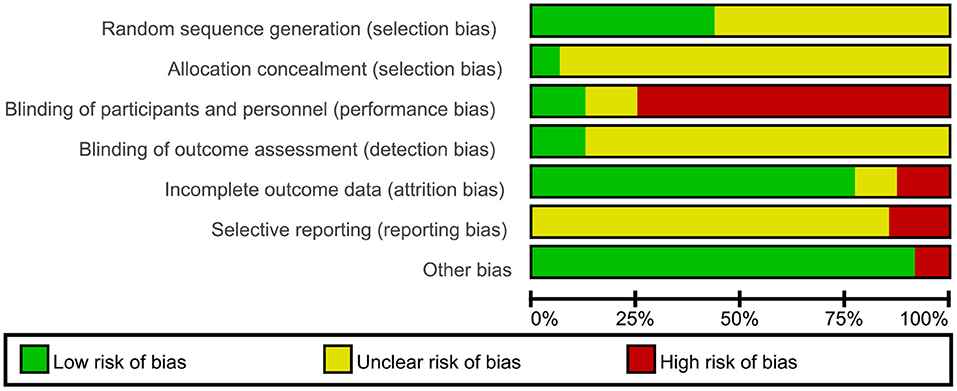
Figure 2. Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
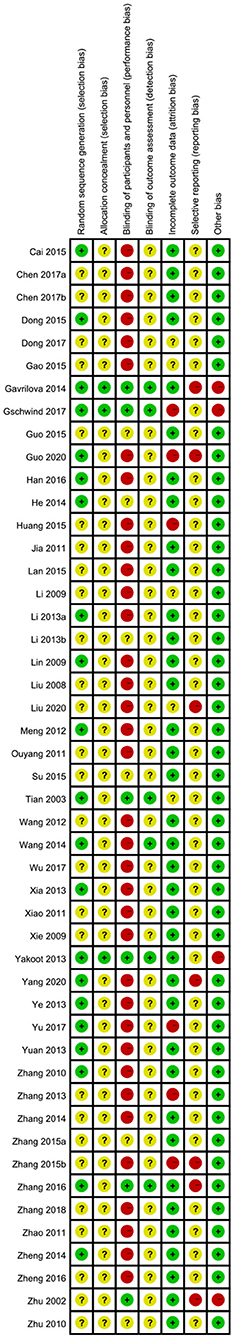
Figure 3. Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
For selection bias, 21 trials (9, 14, 19, 21, 27, 29, 33, 35, 37, 41–45, 47, 48, 54, 56) which either used SAS software or the randomization table to generate a randomization sequence were assessed at low risk of bias; the remaining 27 trials did not specify the randomization method, and therefore we assessed at unclear risk of bias. The method used to conceal allocation was not reported in most of the included trials, except for three trials (33, 42, 48) assessed at low risk of bias regarding allocation concealment. Six trials (31, 33, 35, 42, 48, 53) adequately performed blinding of participants and personnel; the remaining 42 trials did not adequately describe any blinding methods of participants and personnel; moreover, 36 trials of them were assessed at high risk of bias as CHM in addition to a co-intervention was compared to a co-intervention or the form of CHM was quite different from that of control intervention. Blinding of outcome assessors was adequately performed in six trials (20, 31, 33, 35, 42, 48); the remaining 42 trials did not describe the method used for blinding of outcome assessors. 38 trials reported having no missing data and included all participants in data analyses, showing low risk of attribution bias; six trials (9, 17, 33, 38, 46, 54) had high dropout rates and excluded dropouts from the analyses, so we assessed them at high risk of bias as they did not properly deal with the missing data; four trials (11, 18, 31, 52) did not report the information of dropouts, and therefore were assessed at unclear risk of bias. The risk of selective reporting of one trial was low as the published paper was consistent with the protocol; 40 trials was unclear because of lack of pre-published trial protocols though one primary outcome was reported; seven trials (35, 38, 42, 53–56) was assessed to be of high risk as no pre-published protocols and no primary outcome was reported. Except four trials (33, 42, 48, 53) which was funded by pharmaceutical companies, and resulted our assessments of high risk of bias, the remaining 44 trials appeared to be free of other factors that could put them at risk of bias.
Effect Estimates
All 48 finished trials employed parallel design, 33 of which compared CHM with no intervention/placebo allowing for co-intervention; the remaining 15 trials (9, 12, 13, 22, 24, 26–28, 33, 35, 37, 38, 42, 47, 48) compared CHM with western drugs allowing for co-intervention.
CHM vs. No Intervention/Placebo (Co-intervention Was Allowed)
Number of Participants Progressed to Dementia
Seven RCTs (9, 14, 23, 25, 44–46) reported this outcome, and the meta-analysis showed that CHM significantly decreased the number of patients who progressed to dementia compared to no intervention or placebo (RR 0.36, 95% CI 0.22–0.58, 7 trials, 771 participants, I2 = 0%) (Figure 4).
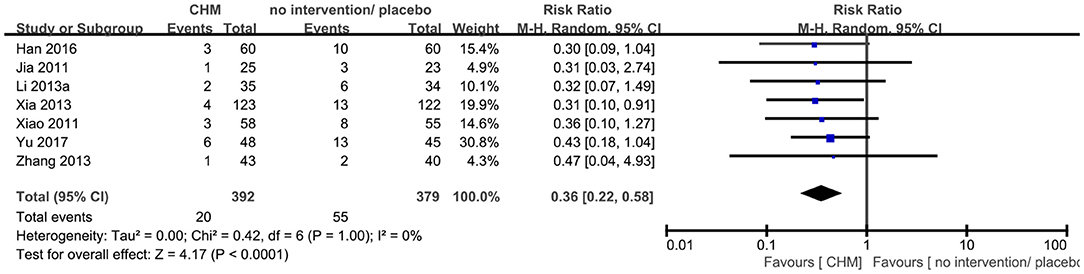
Figure 4. Forest plot of comparison: CHM vs. no intervention/placebo, outcome: number of participants progressed to dementia.
Cognitive Function
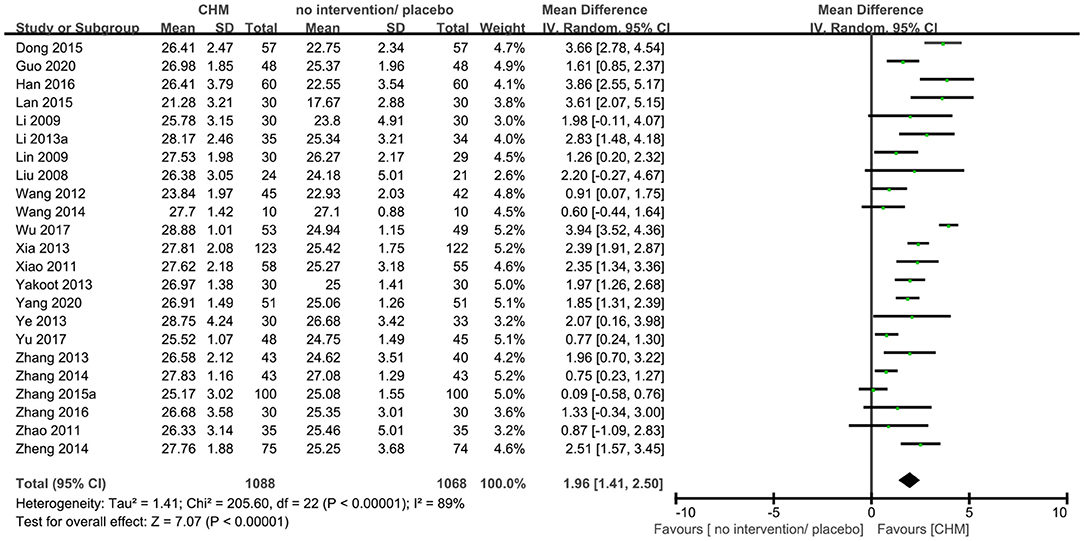
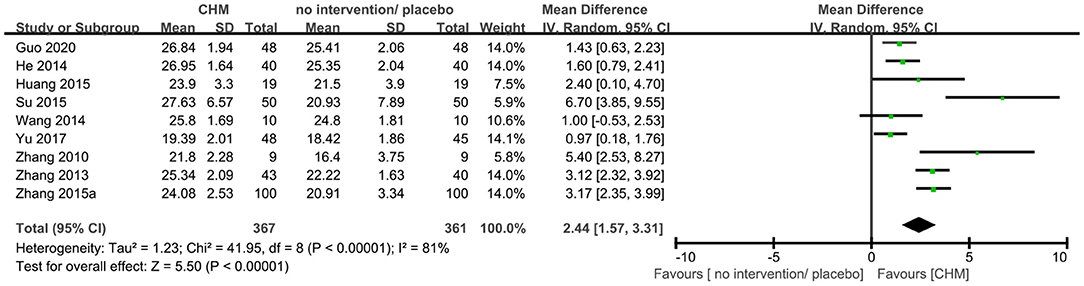
Twenty-three trials (9, 10, 14, 16, 19, 20, 23, 25, 29, 30, 35, 36, 40, 41, 44–46, 48, 50–52, 54, 56) reported MMSE outcome, and the meta-analysis showed that that CHM can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 1.96, 95% CI 1.41–2.50, 23 trials, 2156 participants, I2 = 89%, Figure 5). Nine trials (9, 15, 17, 20, 27, 36, 43, 46, 54) reported MoCA outcome, and the meta-analysis showed that CHM can significantly improve the cognitive function compared to no intervention or placebo when assessed by MoCA (MD 2.44, 95% CI 1.57–3.31, 728 participants, I2 = 81%, Figure 6). The heterogeneity was high. Therefore, we tried to conduct subgroup analyses in attempts to identify the difference in the effect. The results showed statistically significant differences when comparing trials with different CHM (test for subgroup difference: MMSE: P < 0.00001, I2 = 89.6%; MoCA: P = 0.05, I2 = 57.8%, Supplementary Figures 1, 2), trials with different causes of MCI (test for subgroup difference: MMSE: P < 0.00001, I2 = 89.1%; MoCA: P = 0.0002, I2 = 84.4%, Supplementary Figures 3, 4), and trials with different treatment duration (test for subgroup difference: MMSE: P = 0.06, I2 = 72.4%; MoCA: P < 0.00004, I2 = 92.1%, Supplementary Figures 5, 6). The subgroup analysis of different CHM showed that Ginko leaf tablets can significantly improve the cognitive function compared to no intervention or placebo when assessed by MMSE (MD 2.03, 95% CI 1.18–2.88, 1117 participants, I2 = 90%, Supplementary Figure 1) and MoCA (MD 3.11, 95% CI 1.90–4.33, 463 participants, I2 = 82%, Supplementary Figure 2). The sensitivity analysis by including only trials with relatively lower risk of bias found similar results (MD 1.87, 95% CI 1.22–2.52, 2 trials, 120 participants) with that including all trials (MD 1.96, 95% CI 1.41–2.50, 23 trials, 2,156 participants) showing robustness of the results on MMSE (Supplementary Figure 13).
ADL
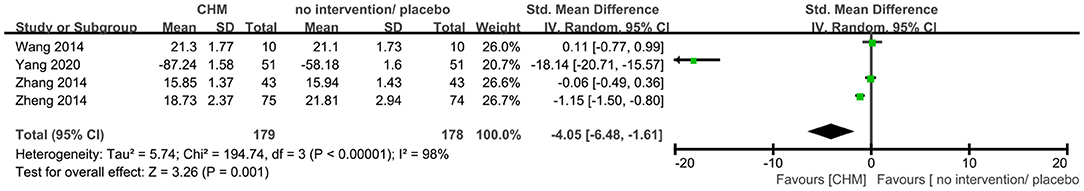
Four trials (20, 40, 41, 56) reported ADL outcome, of which one trial (56) used 0–100 ADL scale with higher score showing better results while the other three trials used the ADL scale with lower score showing better results. As ADL scales with differences in the direction have been used, we first multiplied the mean value of the trial set (56) by −1 to ensure the scales in the same direction, and then used SMD to synthesize data as recommended by Cochrane handbook. The meta-analysis showed that CHM significantly decreased the ADL score compared to no intervention and placebo (SMD −4.05, 95% CI −6.48 to −1.61, 357 participants, I2 = 100%, Figure 7).
CHM vs. Western Medicine (Co-intervention Was Allowed)
Cognitive Function
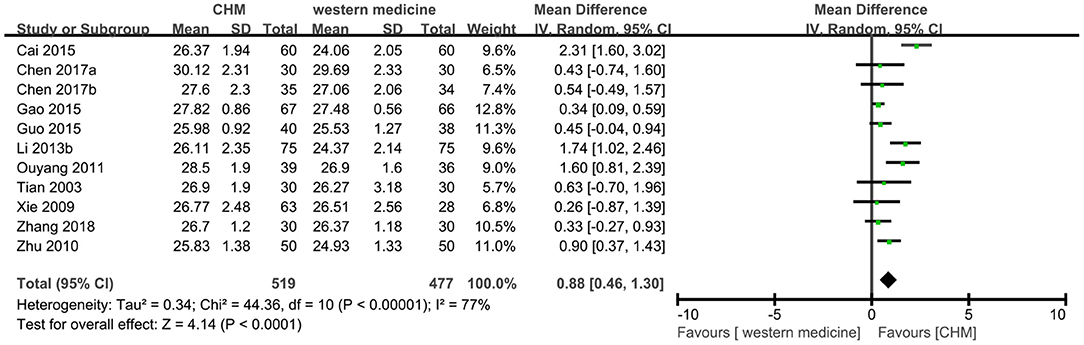
Eleven trials (8, 12, 13, 18, 24, 26, 28, 31, 37, 39, 49) reported MMSE outcome, and the meta-analysis showed that TCM had a significant increase on the score of MMSE compared to western medicine (MD 0.88, 95% CI 0.46–1.30, 996 participants, I2 = 77%, Figure 8). Because of the high heterogeneity, the subgroup analyses were conducted according to different interventions and causes of MCI. The statistically significant subgroup difference was found when comparing different interventions (test for subgroup difference: P < 0.0001, I2 = 77.5%, Supplementary Figure 7), and when comparing different causes of MCI (test for subgroup difference: P < 0.0001, I2 = 82.9%, Supplementary Figure 8).
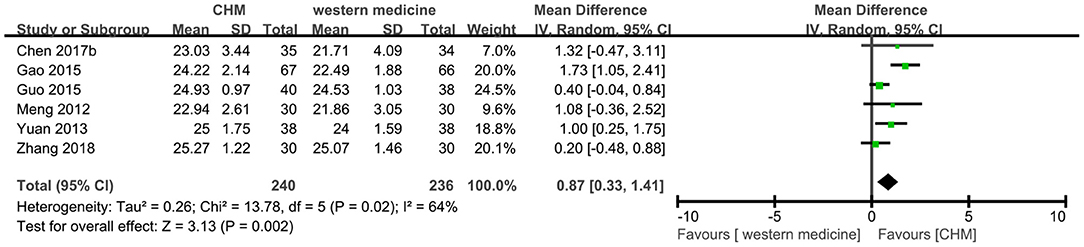
Six trials (8, 13, 18, 22, 39, 47) reported the MoCA score, and the meta-analysis showed that CHM had a significant increase on the score of MoCA compared to western medicine (MD 0.87, 95% CI 0.33–1.41, I2 = 64%, Figure 9). Because of the high heterogeneity, the subgroup analyses were conducted according to different interventions and causes of MCI. The statistically significant subgroup difference was found when comparing different interventions (test for subgroup difference: P = 0.01, I2 = 69.6%, Supplementary Figure 9), and when comparing different causes of MCI (test for subgroup difference: P = 0.17, I2 = 42.8%, Supplementary Figure 10).
ADL
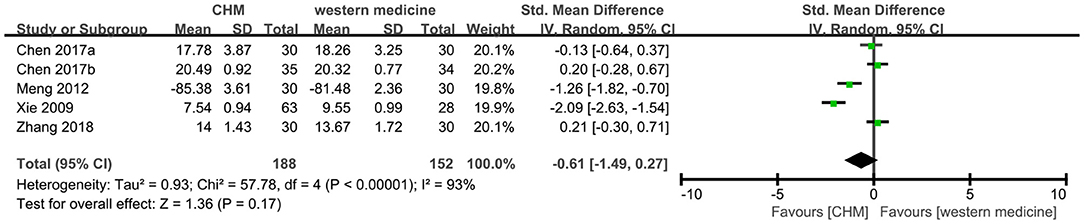
Five trials (8, 12, 13, 22, 28) reported this outcome, of which one trial (22) used ADL scale (higher score indicates better results) in the different direction with other scales (lower score indicates better results). We multiplied the mean value of 22 and used SMD model to synthesize the data. And the meta-analysis showed that CHM had no significant difference in the score of ADL compared to western medicine (SMD −0.61, 95% CI −1.49 to 0.27, I2 = 93%, Figure 10).
Adverse Events
Twenty-four trials reported adverse events, of which 13 trials reported that no adverse events ever occurred in both the CHM group and the control group. The remaining 11 trials found no significant difference between two groups on adverse events.
Discussion
This review summarized current evidence on the comparative effectiveness of CHM, either individually tested or in combined remedies, for treatment of MCI, and finally identified 49 trials (48 finished trials and 1 protocol). This study found that CHM was better than no intervention or placebo (when co-intervention was allowed) in improving the number of participants who progressed to dementia and the score of MMSE, MoCA, ADL. And CHM was better than western medicine (when co-intervention was allowed) on improving the score of MMSE and MoCA but the significant difference was not found on the score of ADL. Considering the high heterogeneity, the subgroup analysis was conducted according to intervention, cause of MCI, and treatment duration.
In the subgroup analysis of intervention, we found that CHM Gingko leaf extracts were explored most. The combined effect from meta-analyses showed that Gingko leaf extracts as adjuvant therapy could slow down the progression to dementia, improve the MMSE score and MoCA score. Previous study showed that Gingko leaf extracts have been widely used in treating neuropsychiatric disorders (57). A previous systematic review published in 2016 (58) evaluated Ginkgo biloba in MCI and Alzheimer's diseases, and provided a similar result with our review stating that Ginkgo biloba in combination with conventional medicine is superior to conventional medicine alone in improving MMSE scores for people with MCI. The results of our study may provide support that Ginkgo leaf extracts can be considered for use in patients with MCI. However, still more qualified RCTs were needed to confirm the effect and optimize the use of Gingko leaf extracts. In the subgroup analysis of the cause of MCI, we found that CHM can improve the number of participants who progressed to dementia and the score of MMSE and MoCA in Parkinson related MCI patients compared to no intervention or placebo; CHM can improve the score of MMSE and MoCA of post-stroke and vascular MCI patients compared to no intervention or placebo; CHM can improve the score of MMSE, MoCA and ADL of post-stroke MCI patients compared to western medicine. However, more high-quality RCTs with large samples were needed to further confirm the effectiveness of CHM for the specific type of MCI. In the subgroup analysis of treatment duration, we found that CHM can improve the score of MMSE and MoCA in both subgroups, more than 6 months group and ≤ 6 months group.
Our review identified that the recommended outcomes by the guideline, including progression to dementia, reduction of ability to undertake daily activities, and quality of life (59), were infrequently assessed in the included trials than scales/ tools for cognition. Seven out of the included 45 trials have used a long-term endpoint progression to dementia as an outcome; eight trials have used scales of daily life activities; none of the trials has used quality of life. Oppositely, many different scales/ tools for cognitive function and global function have been used. The various scales/tools being used within the included trials limited the synthesis of data from different clinical trials. Moreover, the superiority of one scale/tool over another one has not been proved and should be explored in future (59). Besides, preservation of the patient's personality or the accessibility of disease information and health services should also be focused on future MCI studies from the voices of stakeholders (60). Outcome recommendations for dementia or cognitive function have been reported though (59–61), core outcome set specifically for MCI is still needed and should be developed.
As for methodological quality, future trials are recommended to be reported according to the CONSORT statement and its extension for herbal medicinal interventions (62, 63). Placebo-controlled design should be introduced. For trials comparing TCM with other western medicine, the double-dummy technique should be adopted to compare drugs with very different appearances to reduce observer and patients bias (64). The high risk of other bias was mainly shown in the funding and personnel team of the included trials. Six trials had only one author and we could not obtain any information of acknowledgment. Although the quality of RCTs cannot be decided by the number of the authors, for RCTs if conducted by only one author, the blinding was impossible. Four trials were suspected of sponsorship bias either because the authors were from the pharmaceutical companies or the companies fully or partially funded the trials. The previous review has suggested that industry-sponsored studies were biased in favor of the sponsor's products (65). The small sample size effect on publication bias should be cautioned as the included studies seemed to be not large, ranging from 48 to 245 participants. Sample size estimation is not available in most of the included studies, thereby whether the statistical power is enough seems to be unclear. Large sample size trials with strong power is warranted in future CHM studies for MCI.
There were similar systematic reviews previously published. One systematic review published in 2009 (6) explored CHM for MCI and age-associated memory impairment, and found the effects of the CHM was at least equivalent to piracetam on MMSE scores. Two systematic reviews by Lin et al. (4, 5) explored the effects of CHM for MCI by focusing either on MMSE and Alzheimer's disease assessment scale-cognitive subscale (4) or on MoCA (5). Our review was registered on PROSPERO and conducted a comprehensive up-to-date search of Chinese and English language databases to 2021 December. In this review, we focused on CHM, which is characterized by refined dosage forms and relative standardization and is approved by the State Administration for Market Regulation (66). To explore the benefits and harms of CHM, in this review, we focused on primary outcomes including the number of participants who progressed into dementia, cognitive function measured by accepted scales/tools, and adverse events.
There were some limitations in this review. Firstly, many included trials were judged to have the unclear risk of bias because we could not obtain enough information from the trial authors. Furthermore, there was certain clinical heterogeneity in the included RCTs because of causes of MCI, treatment duration.
Conclusion
CHM, Ginko leaf extracts in particular, could help to prevent progression into dementia and to improve cognitive function and ability of daily living activities. However, due to the low quality of current trials, more qualified large-sample randomized controlled trials were needed to confirm the conclusion.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
NS was the project leader and initiated the study. YW and ZZ contributed to the conception of the study. NL provided the methodological guidance, trained the reviewers, and drafted the manuscript. SY, CL, LG, and SW searched the literature, collected the data, and evaluated the quality of the included studies. YC performed the meta-analysis and drafted the manuscript. All authors read and approved the final manuscript.
Funding
The study was supported by National Key R&D Plan (2019YFC1712000) and National Science and Technology Major Project (2018ZX10101001-005).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.903224/full#supplementary-material
Abbreviations
AchE, Acetylcholinesterase; ADAS-Cog, Alzheimer's Disease Assessment Scale-Cognitive subscale; ADL, Activities of daily living scale; AE, Adverse events; AVLT, Auditory verbal learning test; BI, Barthel index; BNT, Boston naming test; C, Control; CACMS, China Academy of Chinese Medical Sciences; CDT, Clock drawing task; CGI- EI, Clinical global impression-efficacy index; CHD, Coronary heart disease; CHM, Chinese herbal medicine; CIs, confidence intervals; CNKI, China National Knowledge Infrastructure; CQVIP, Chongqing VIP; CRP, C-reactive protein; CVFT, Category verbal fluency test; DASS-21, 21-item Depression; Anxiety, Stress scale; D-KEFS, Delis-Keplan executive function system; DS, Digit span; DSR, Delayed story recall; EEG, Electroencephalogram; ERP P300, Event-related potentials P300; GDS, Geriatric depression scale; HAMD, Hamilton Depression Scale; HDL-C, High density liptein-cholesterol; I, Intervention; IADL, Instrumental activities of daily living scale; IBRCM, Institute of Basic Research in Clinical Medicine; LDL-C, Low density lipoprotein-cholesterol; MCI, mild cognitive impairment; MD, mean difference; MDA, Maloudialdehyde; MMSE, Mini-mental state examination; MoCA, Montreal Cognitive Assessment; MQ, Memory quotient; MRI, Magnetic resonance imaging; NHFPC, National Health and Family Planning Commission; NR, Not reported; RCFS, Rey complex figure test; RCTs, randomized clinical trials; ROCF, Rey-Osterrieth complex figure test; RR, risk ratio; RVR, Rapid verbal retrieve; SAMR, State Administration for Market Regulation; SAS, Self-rating anxiety scale; SCWT, Stroop color and word test; SDMT, Symbol digit modalities test; SDS, Self-rating depression scale; SMD, standard mean difference; STAI-X1, State-Trait anxiety inventory; SVD, Cerebral small vessel disease; TC, Total cholesterol; TCM, Traditional Chinese medicine; TG, Triglyceride; TMT, Trail-Making test; WMS, Wechsler memory scale.
References
1. Petersen RC. Clinical practice. Mild cognitive impairment. N Eng J Med. (2011) 364:2227–34. doi: 10.1056/NEJMcp0910237
2. Petersen RC, Lopez O, Armstrong MJ, Getchius TSD, Ganguli M, Gloss D, et al. Practice guideline update summary: mild cognitive impairment: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. (2018) 90:126–35. doi: 10.1212/WNL.0000000000004826
3. Petersen RC. Mild cognitive impairment. Continuum. (2016) 22:404–18. doi: 10.1212/CON.0000000000000313
4. Lin D, Hyde AJ, Zhang AL, Xue CC, May BH. Chinese herbal medicine for mild cognitive impairment using montreal cognitive assessment: a systematic review. J Altern Complement Med. (2019) 25:578–92. doi: 10.1089/acm.2018.0346
5. Lin D, May BH, Feng M, Hyde AJ, Tan HY, Guo X, et al. Chinese herbal medicine for mild cognitive impairment: a systematic review and meta-analysis of cognitive outcomes. Phytother Res. (2016) 30:1592–604. doi: 10.1002/ptr.5679
6. May BH, Yang AW, Zhang AL, Owens MD, Bennett L, Head R, et al. Chinese herbal medicine for mild cognitive impairment and age associated memory impairment: a review of randomised controlled trials. Biogerontology. (2009) 10:109–23. doi: 10.1007/s10522-008-9163-5
7. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
8. Zhang J, Wang C, Li J. Therapeutic effect of compound huo nao shu on mild cognitive impairment of insufficiency Parkinson's disease. Chin J Clinical Rational Drug Use. (2018) 1:68–9. doi: 10.15887/j.cnki.13-1389/r.2018.01.035
9. Yu E, Liao Z, Tan Y, Qiu Y, Zhu J, Shi M, et al. Preventive effects of Haishe capsule on the conversion of amnestic mild cognitive impairment to Alzheimer's disease. Chin J Geriatrics. (2017) 36:278–81. doi: 10.3760/cma.j.issn.0254-9026.2017.03.013
10. Wu D, Guo R, Wang Y. Clinical observation of sweet dream liquid curing deficiency of spleen and kidney style MCI. Chin Traditional Herb Drugs. (2017) 23:4958–62. doi: 10.7501/j.issn.0253-2670.2017.23.022
11. Dong C. The clinical effect of cognitive function training combined with Bushenyang Chinese patent medicine on prevention and treatment of cognitive impairment in old people. Chin. Control Endemic Dis. (2017) 32:568–71. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2017&filename=DYBF201705051&uniplatform=NZKPT&v=8NNOONuj0KBgFI-V19YY2SIS-rLEsvlN2xoBeupj1NX0vMaIn0xuJiLcAS6JvBCj
12. Chen K, Chen L, Hu W. Clinical efficacy observation on Bushen Huatan Quyu method in the treatment of mild cognitive impairment of kidney deficiency and phlegm type. China Mod Dr. (2017) 55:97–100. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhZ6d2tqemxtbC15eXdzMjAxNzI1MDI4GggydmE5NHJubA%3D%3D
13. Chen J, Sun S, Yu Z, Sheng F, Zhou L. Clinical observation on Shenzhiling oral liquid in the treatment of mild cognitive impairment. Chin J Integr Med Cardiocerebrovasc Dis. (2017) 15:365–8. doi: 10.3969/j.issn.1672-1349.2017.03.032
14. Han Z. Clinical effect of Ginkgo Biloba tablet on mild cognitive impairment. Practical J Card Cereb Pneumal Vasc Dis. (2016) 24:91–4. doi: 10.3969/j.issn.1008-5971.2016.01.027
15. Su W, Liu B, Su Y. Ginkgo biloba preparation combined with donepezil hydrochloride in the treatment of old people cognitive impairment and its effect on EEG and ERPP300. Shaanxi J TCM. (2015) 36:542–4. doi: 10.3969/j.issn.1000-7369.2015.05.012
16. Lan P, Pan F, Su J. Clinical study on Qizhi Tongluo capsule intervention for mild cognitive impairment. Med Inf. (2015) 28:59–60. doi: 10.13192/j.issn.1000-1719.2016.04.029
17. Huang Y, Chen C, Qin X, Lan H. Modified Liuwei Dihuang Pills to treat mild cognitive dysfunction with kidney essence deficiency syndrome. J Guangxi University of Chin Med. (2015) 18:8–10. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhFneHp5eHl4YjIwMTUwMzAwMxoIcWl2cnM2dGs%3D
18. Gao L, Zhang X, Liu X, Xu Y, Wang M, Chen L, et al. Clinical research of mild cognitive impairment after stroke treated with Fufang Congrong Yizhi capsules. World J Integr Traditional and Western Med. (2015) 10:65–8. doi: 10.13935/j.cnki.sjzx.150120
19. Dong J. Effects of Ginkgo biloba combined with donepezil hydrochloride on cognitive function, mental state and negative emotions in elderly patients with mild cognitive dysfunction. Shaanxi J TCM. (2015) 36:820–2. doi: 10.3969/j.issn.1000-7369.2015.07.026
20. Wang Y. Kidney Phlegm and Activating Blood Method in the Treatment of Mild Cognitive Impairment After Stroke. Shijiazhuang: Hebei Medical University (2014).
21. Ye H. Study on memantine combined with tongxinluo in treatment for amnestic mild cognitive impairment. Int Med Health Guidance News. (2013) 19:1158–61. doi: 10.3760/cma.J.issn.1007-1245.2013.08.040
22. Meng C. The Tonifying Kidney and Promoting Blood Circulation and Resolving Phlegm Method in Treatment of Stroke After Mild Cognitive Impairment Clinical Study. Changchun: Changchun University of Chinese Medicine (2012).
23. Xiao S. The Clinical Research of Ginkgo Biloba Extract on Mild Cognitive Impairment and the Conversion Rate to Dementia. Shanghai: Fudan University (2011).
24. Ouyang S, Ouyang Z. Therapeutic effects of Jiannao Bushen pills for mild cognitive dysfunction after ischemic stroke in 39 cases. Guiding J TCM. (2011) 17:41–2. doi: 10.3969/j.issn.1672-951X.2011.05.019
25. Jia X, Kang X, Yan Z. Research on treatment of mild cognitive impairment by Tongxinluo combined with oxiracetam. Chin J Difficult and Complicated Cases. (2011) 10:495–7. doi: 10.3969/j.issn.1671-6450.2011.07.006
26. Zhu L. Clinical effect of Xuanyunning tablet in treating senile patients with mild cognitive impairment of turbid-phlegm blocking orifice syndrome. Chin J Med Guide. (2010) 12:2107–9.
27. Zhang Y. Clinical Study on TCM Intervention for Mild Vascular-Derived Cognitive Impairment With Kidney Deficiency, Phlegm Turbidity and Blood Stasis Syndrome Based on the Theory of Brain Marrow. Changchun: Changchun University of Chinese Medicine (2010).
28. Xie Z, Xie N, Feng Y. Clinical study on Tianzhi granules in treating amnesia mild cognitive impairment with liver yang hyperactivity syndrome. Chin J Exp Trad Med Formulae. (2009) 15:95–6. doi: 10.3969/j.issn.1005-9903.2009.09.033
29. Lin L. The Clinical Study of Mongolian Milkvetch Root Injection for Improving the Congnitive Disorder of Mild Vascular Cognitive Impairment Patients. Nanning: Guangxi University of Chinese Medicine (2009).
30. Liu N, Deng Y, Zhou B, Tang Z. Clinical study on the effect of Yangxueqingnao Granules on patients with mild vascular cognitive dysfunction. Chin J Integr Med Cardiocerebrovasc Dis. (2008) 6:407–9. doi: 10.3969/j.issn.1672-1349.2008.04.018
31. Tian J, Zhu A, Zhong J. A follow-up study on a randomized, single-blind control of King's Brain Pills in treatment of memory disorder in elderly people with MCI in a Beijing community. Zhongguo Zhong Yao Za Zhi. (2003) 28:987–91. doi: 10.3321/j.issn:1001-5302.2003.10.031
32. Steiner G, Bensoussan A, Liu J, Hohenberg M, Chang D. Study protocol for a randomised, double-blind, placebo-controlled 12-week pilot phase II trial of Sailuotong (SLT) for cognitive function in older adults with mild cognitive impairment. Trials. (2018) 19:510–22. doi: 10.1186/s13063-018-2912-0
33. Gschwind Y, Bridenbaugh S, Reinhard S, Granacher U, Monsch A, Kressig R. Ginkgo biloba special extract LI 1370 improves dual-task walking in patients with MCI: a randomised, double-blind, placebo-controlled exploratory study. Aging Clin Exp Res. (2017) 29:609–19. doi: 10.1007/s40520-016-0699-y
34. Zheng W, Zheng W. Effects of Ginkgo biloba leaves on cognitive function in patients with mild cognitive impairment. World Latest Med Inf. (2016) 16:127. doi: 10.3969/j.issn.1671-3141.2016.12.087
35. Zhang J., Liu Z., Zhang H., Yang C., Heli, Li X., Chen K., et al. (2016). A two-year treatment of amnestic mild cognitive impairment using a compound Chinese medicine: a placebo controlled randomized trial. Sci. Rep. 6, 28982. doi: 10.1038/srep28982
36. Zhang L, Wu Q, Chang L, Huang G. The effect of Ginkgo biloba leaves injection on elderly patients with mild cognitive impairment and influence onplasmahs-CRPandIL-6. Chin J Clin Healthc. (2015) 18:167–9. doi: 10.3969/J.issn.1672-6790.2015.02.018
37. Cai Z, Mai J, Wang F. Therapeutic effect of Shuganjieyu capsules on mild cognitive impairment associated with depression in elderly patients. Chin J Difficult Comp Cases. (2015) 14:458–61. doi: 10.3969/j.issn.1671-6450.2015.05.08
38. Zhang X, Gao L, Jiao J, Sui X, Song W, Mou S, et al. Impacts of Fufang Congrong Yizhi capsules on cerebral blood flow and vascular endothelial function in the patients of post-stroke mild cognitive impairment. World J Integr Trad Western Med. (2015) 10:533–6. doi: 10.13935/j.cnki.sjzx.150426
39. Guo M, Xue Q, An Y. Curative effect of Xinnaoning capsule-on the treatment of mild vascular cognitive impairment no dementia. Beijing Med J. (2015) 37:97–9.
40. Zhang Q, Chen A, Chen Y, Liu W. The effect of Ginkgo ketone dispersible tablets on mild cognitive impairment caused by cerebrovascular disease. Chin J Integr Med Cardiocerebrovasc Dis. (2014) 12:1231–2. doi: 10.3969/j.issn.16721349.2014.10.034
41. Zheng E, Wang X, Zhou S. Clinical study of Yindanxinnaotong soft capsule combined with Aniracetam on elder mild cognitive impairment with phlegm and blood stasis into resistance syndrome. Guiding J TCM and Pharm. (2014) 20:46–9. doi: 10.13862/j.cnki.cn43-1446/r.2014.06.016
42. Gavrilova SI, Preuss UW, Wong JWM, Hoerr R, Kaschel R, Bachinskaya N, et al. Efficacy and safety of Ginkgo biloba extract EGb 761® in mild cognitive impairment with neuropsychiatric symptoms: a randomized, placebo-controlled, double-blind, multi-center trial. Int J Geriatr Psychiatry. (2014) 29:1087–95. doi: 10.1002/gps.4103
43. He H. Clinical Observation Yinxingdamo Injection Treatment of Ischemic Stroke With Mild Cognitive Impairment. Nanning: Guangxi University of Chinese Medicine (2014).
44. Li W, Tian D, Yan W, Liu X, Zhu Y. Therapeutic observation on Ginkgo Biloba tablet for cognitive impairment in hyperuricemia patients. World Chin Med. (2013) 8:48–50. doi: 10.3969/j.issn.1673-7202.2013.01.018
45. Xia X. Effect of Ginkgo Biloba extract on mild cognitive impairment. Zhejiang Zhejiang J Integ Trad Chin West Med. (2013) 23:876–8. doi: 10.3969/j.issn.1005-4561.2013.11.004
46. Zhang C, Feng J, Li H, Wang J, Ye J, Wang X. Efficacy observation on Ginkgo biloba extract in the treatment of Parkinson's disease with mild cognitive impairment. China Mod Dr. (2013) 51:109–11. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDHIS2&filename=ZDYS201331047&uniplatform=NZKPT&v=23OcE9a5MOgRhwmRPFzcXnL62ZZoB1X8ALyCZ2ajz7KMtAobIbEtxbUQW8NcWpuK
47. Yuan J, Shao Y, Xie W, Ou S. Huan Shao capsule in adjuvants for treating mild cognitive impairment. J Guangxi Univ Chin Med. (2013) 16:14–5. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDHIS2&filename=GSZB201304007&uniplatform=NZKPT&v=yc3Xoba7a0GGWE-yZFKGj4dU–w2x9KEhm-oLvRYV1e7xjHyJvsVjo3YkaEYBZO3
48. Yakoot M, Salem A, Helmy S. Effect of Memo®, a natural formula combination, on mini-mental state examination scores in patients with mild cognitive impairment. Clin Interv Aging. (2013) 8:975–81. doi: 10.2147/CIA.S44777
49. Li B, Lai H, Li G. The clinical efficacy of Niergoline tablets in the treatment of cognitive impairment of elder people. Natl Med Front China. (2013) 8:66–7.
50. Wang B, Zhong Y, Yan H. Ginkgo biloba in treating elderly people with cognitive dysfunction and its clinical efficacy and effects on change of event-related potential P300. Chin J Gerontol. (2012) 32:2495–6.
51. Zhao L, Xiang K. The effects of Yangxueqingnao granules on mild cognitive dysfunction in elderly diabetic people from the perspective of oxidative stress. J China TCM Inf. (2011) 3:20–1. Available online at: https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjIwNDE1EhB6Z3p5eXp4MjAxMTIyMDE0Ggh3YWIzNmRhYg%3D%3D
52. Li P. The therapeutic effect of Quantianma capsule on mild cognitive dysfunction. Chin J Prim Med and Pharm. (2009) 16:728–9. doi: 10.3760/cma.j.issn.1008-6706.2009.04.114
53. Zhu X, Zhang B. 41 cases of mild senile cognitive impairment of liver and kidney Yin deficiency syndrome treated by Naolibao pills. J TCM. (2002) 43:204–5. doi: 10.3321/j.issn:1001-1668.2002.03.032
54. Guo W, Peng C, Li W, Xie H. Clinical Research on sitagliptin combined with jinlida granules in the treatment of elderly type 2 diabetic patients with mild cognitive impairment. Chin J Pharmacoepidemiol. (2020) 29:657–61. Available online at: https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CJFD&dbname=CJFDLAST2020&filename=YWLX202010001&uniplatform=NZKPT&v=YREcSwk7ikKFijbenO9WanUAqykoNd9hIioBiSuw9cc0peWJBuq3IZMmz796VP8O
55. Liu H. Effect of Zhongfeng Huichun Tablet combined with Butylphthalide and Sodium Chloride Injection on mild cognitive dysfunction after acute cerebral infarction. Pract Clin J Integ Trad Chin West Med. (2020) 20:95–6. doi: 10.13638/j.issn.1671-4040.2020.17.048
56. Yang X, Fan X, Yang X. Clinical study on Yinxing Tongzhi tablets combined with Butylphthalide Soft capsules in treatment of vascular mild cognitive impairment. Drugs Clinic. (2020) 35:1089–92. doi: 10.7501/j.issn.1674-5515.2020.06.007
57. Kandiah N, Ong PA, Yuda T, Ng L-L, Mamun K, Merchant RA, et al. Treatment of dementia and mild cognitive impairment without cerebrovascular disease: expert consensus of Ginkgo biloba extract, EGb 761. CNS Neurosci Ther. (2019) 25:288–98. doi: 10.1111/cns.13095
58. Yang G, Wang Y, Sun J, Zhang K, Liu J. Ginkgo biloba for mild cognitive impairment and Alzheimer's disease: a systematic review and meta-analysis of randomized controlled trials. Curr Top Med Chem. (2016) 16:520–8. doi: 10.2174/1568026615666150813143520
59. Tochel C, Smith M, Baldwin H, Gustavsson A, Ly A, Bexelius C, et al. What outcomes are important to patients with mild cognitive impairment or Alzheimer's disease, their caregivers, and health-care professionals? A systematic review. Alzheimers Dementia. (2019) 11:231–47. doi: 10.1016/j.dadm.2018.12.003
60. Bossers W, Woude LVD, Boersma F, Scherder E, Heuvelen MV. Recommended measures for the assessment of cognitive and physical performance in older patients with dementia: a systematic review. Dement Geriatr Cogn Dis Extra. (2012) 2:589–609. doi: 10.1159/000345038
61. Lees R, Fearon P, Harrison J, Broomfield N, Quinn T. Cognitive and mood assessment in stroke research. Stroke. (2012) 43:1678–80. doi: 10.1161/STROKEAHA.112.653303
62. Schulz K, Altman D, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. (2010) 152:726–32. doi: 10.7326/0003-4819-152-11-201006010-00232
63. Gagnier JJ, Boon H, Rochon P, Moher D, Barnes J, Bombardier C, et al. Recommendations for reporting randomized controlled trials of herbal interventions: explanation and elaboration. J Clin Epidemiol. (2006) 59:1134–49. doi: 10.1016/j.jclinepi.2005.12.020
64. Marušić A, Ferenčić S. Adoption of the double dummy trial design to reduce observer bias in testing treatments. J R Soc Med. (2013) 106:196–8. doi: 10.1177/0141076813485350
65. Lundh A, Lexchin J, Mintzes B, Schroll J, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. (2017) 2:MR000033. doi: 10.1002/14651858.MR000033.pub3
Keywords: mild cognitive impairment (MCI), Chinese herbal medicine, systematic review, meta-analysis, traditional Chinese medicine
Citation: Liang N, Chen YX, Yang S-H, Liang C-H, Gao L-D, Wang S, Wang Y-P, Zhang Z-J and Shi N-N (2022) Chinese Herbal Medicine for Mild Cognitive Impairment: A Systematic Review of Randomized Controlled Trials. Front. Neurol. 13:903224. doi: 10.3389/fneur.2022.903224
Received: 05 April 2022; Accepted: 10 June 2022;
Published: 30 June 2022.
Edited by:
Marialuisa Gandolfi, University of Verona, ItalyReviewed by:
Guido Santiago Dorman, Instituto de Neurología Cognitiva, ArgentinaShaonan Liu, Guangdong Provincial Hospital of Chinese Medicine, China
Copyright © 2022 Liang, Chen, Yang, Liang, Gao, Wang, Wang, Zhang and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanping Wang, d2FuZ3lhbnBpbmc0ODE2QDE2My5jb20=; Zhanjun Zhang, emhhbmdfcnpzQGJudS5lZHUuY24=; Nannan Shi, MTM4MTE4MzkxNjRAdmlwLjEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
 Ning Liang
Ning Liang Yaxin Chen
Yaxin Chen Sihong Yang1
Sihong Yang1 Lidong Gao
Lidong Gao Shang Wang
Shang Wang Zhanjun Zhang
Zhanjun Zhang