- 1Department of Neurology, Wroclaw Medical University, Wroclaw, Poland
- 2Department of Trauma and Orthopedic Surgery, Regional Specialist Hospital, Wroclaw, Poland
- 3Faculty of Earth Sciences and Environmental Management, University of Wroclaw, Wroclaw, Poland
Background: The gold standards for the diagnosis and treatment of carpal tunnel syndrome (CTS) and its outcome are undecided. Using clinical and electrophysiological methods, we tried to establish which fibers achieved full postoperative recovery, and the possibility of using non-standard electrophysiological tests as outcome predictors.
Methods: The study group consisted of 35 patients and controls. The Historical–Objective Scale, standard neurography, conduction velocity distribution tests (CVD), and quantitative sensory testing (QST) were performed before and after CTS surgery.
Results: Clinical improvement was observed on average in 54.3% of the patients, higher in less advanced CTS. All parameters improved significantly after surgery, except for CVD; most remained worse than in the controls. Only QST parameters fully returned to normal limits. Patient age and CTS severity were important in the estimation of the risk of no improvement.
Conclusions: The efficiency of minimally invasive CTS surgery is higher in younger patients with less advanced CTS. Complete recovery was present only in small fibers; larger fibers could most likely be responsible for residual signs. We did not notice any benefits in CTS diagnosis using methods of small fiber assessment. QST seemed to be useful in the diagnosis of residual signs, and in deciding upon possible reoperation.
Introduction
The most common entrapment neuropathy is carpal tunnel syndrome (CTS). Surgical intervention is the most reliable method to treat CTS. This has been demonstrated in several publications. For conservative treatment, night-time splinting has proven to be effective for mild CTS symptoms. Spontaneous improvement without any intervention is also possible (1–3).
The results of CTS diagnoses and therapeutic methods and outcomes may be difficult to interpret. This is because the results of diagnostic procedures, mainly electrophysiological tests (standard nerve conduction studies – NCS), are sometimes border line or could present the pathology without any clinical symptoms. Alternatively, patients with severe clinical CTS symptoms could have no significant NCS abnormalities or very little pathology (4–7). Mondelli et al. (8) proved that the correlation between CTS clinical symptoms and NCS is highly significant but weak. There are also no clear tools for the prediction and estimation of post-operative results. Neither NCS, ultrasonography, nor MRI are fully unequivocal (9–11). The lack of a gold standard in CTS diagnostics and hard scientific evidence for new diagnostic and therapeutic guidelines, together with limited prognostic biomarkers, demonstrate the need for extensive further research (12, 13). Such research should help in understanding CTS pathological mechanisms, and correlations between clinical, neurophysiological and imaging findings, and in the prediction of surgical outcomes.
In our study, we analyzed the function of the different median nerve fibers in patients with CTS before and after surgical intervention. The study was based on clinical scales, standard NCS, and non-standard tests used for small fiber function estimation. We tried to establish which fibers achieved full or partial improvement after surgery, the correlation between their neurophysiological recovery and the clinical status, and the possibility of using these tests as outcome predictors. We undertook the research in order to expand our knowledge of CTS, and to enable us to predict outcomes after surgical intervention.
Materials and Methods
The study was approved by the Ethics Committee of Wroclaw Medical University, Poland. All patients and volunteers gave their informed consent to participate in the study.
Ultimately, the study group consisted of 35 patients, 3 patients dropped out from the study. CTS recognition was based on clinical and electrophysiological criteria (4, 5, 12, 14, 15), e.g., clinical symptoms were present, and NCS allowed the recognition of CTS in accordance with American Association of Electrodiagnostic Medicine guidelines (12), and with use of the Padua neurophysiological classification (7). None of the patients had coexisting medical problems influencing the peripheral nerve function. We excluded pregnant women, patients after wrist injury or after previous surgical intervention, and those with diabetes mellitus, chronic renal disease, gout, hormonal dysfunction (including thyroid function), vitamin deficiencies, neoplasms, rheumatological disorders, polyneuropathies, and plexopathies. 18 of our patients were smokers. We excluded patients with positive clinical symptoms of CTS but without neurophysiological confirmation or with clinically silent CTS seen only in neurophysiological tests.
The control group consisted of 35 sex-matched healthy volunteers, who were recruited from among physicians, nurses, hospital assistants, and family members. None of the volunteers had risk factors for CTS as above, and standard NCS tests were within normal limits.
All patients and volunteers were right-handed. All of them underwent neurological and neurophysiological examinations. In the patient group, the examination was performed twice: before and after the surgical intervention, average 14.6 weeks (4–18 weeks) after the operation.
Subjective and objective neurological examinations, together with the Historical–Objective Scale (Hi-Ob) after Mondelli et al. with modifications (8, 14), were performed. The scale consists of 6 points from a score of 0–lack of clinical symptoms, to 5–severe atrophy and paralysis of thenar muscles.
The electrophysiological studies were carried out using the following medical equipment: Viking Quest version 10.0 device connected to a Thermal Sensory Analyzer II 2001 (TSA II), and a VSA – 3000 Vibratory Sensory Analyzer (Medoc, Israel), Viking Select version 7.1.1c., Nicolet Biomedical device with Multi Mode Program (MMP Plus) software. We used standard neurographic methods (12, 16). The room temperature was between 21 and 23 °C. Hand temperature was equal or higher than 32°C. Standard motor and sensory conduction studies were performed in the median nerve in the patient and control groups. Additionally we analyzed standard conduction parameters in the ulnar nerve only in order to exclude coexisting pathology, e.g., radiculopathy or plexopathy. We estimated the distal latency (L) of motor (Compound Motor Action Potential – CMAP), and onset latency (L) of sensory potentials (Sensory Nerve Action Potential – SNAP) in milliseconds – ms, amplitude (A) (in millivolts (mV) for motor conduction, and in microvolts (μV) for sensory conduction), and motor and sensory conduction velocities (V) (in meters per second – m/s) in the median nerve.
The adductor pollicis brevis muscle was used to receive motor potentials from the median nerve. For sensory conduction velocity estimation, we used an antidromic technique, using ring recording electrodes, fixed on the second finger. A standard distance between electrodes and points of motor fiber stimulations at the wrist was preserved, i.e., 5.5 cm. In sensory test, the distance between stimulating and ring recording electrodes was 13 cm. Current stimulation option was applied, and the duration of a single stimulus was 0.2 ms.
A conduction velocity distribution test (CVD), using the collision technique, was also performed in the median nerve (16, 17). We used supramaximal stimulations at two points of stimulation on wrist and elbow levels. The interstimulus interval (ISI) was changed according to the distance between the two points of the stimulations and this was extended gradually and automatically by 0.1 ms. The method can show the lower (10%) and upper (90%) quartiles of conduction velocities, and median (50%) value. We additionally calculated the spread of conduction velocities, i.e., the difference between lower and upper quartiles (90–10%).
Quantitative sensory testing (QST) was used to assess the sensation and pain thresholds for low and high temperatures (18–20). Furthermore, we estimated the vibration threshold using a special device. The threshold assessment was based on limit methods. We calculated cold sensation (CS), warm sensation (WS), cold pain (CP), heat pain (HP), and vibration sensation (VS) thresholds. Additionally, we calculated the dispersion of the temperature, i.e., the temperature differences between low temperatures (CS and CP), and high temperatures (WS and HP). A thermode was attached to the skin of the thenar, corresponding to the innervation of the median nerve. The thermode active area was 30 x30 mm; the temperature changed by 1 °C/s for temperature threshold estimation, and 2 °C/s for the pain threshold; the temperature range was 0–50°C, and the adaptation temperature - 32°C. For temperature and pain assessment, the procedures were repeated 4 times and 3 times, respectively.
We analyzed thresholds for vibratory stimuli using a vibratory sensation analyzer. The sensation of vibration was assessed using a vibrating button located on the index finger. We used 6 repetitions of the vibrating stimulation. The vibration threshold represents the amplitude of vibration (in microns - μ). The stimulation rate was 100 Hz, the amplitude changed with a rate of 0.3 microns per second (μ/s), the range of the amplitude was 0–130 μ, and the stimulating area was 1.22 cm2. Stimulation for temperature, pain and vibration was stopped by the patients pressing a button (18, 19).
Surgical treatment was performed on all patients using seed anesthesia of Wide-awake local anesthesia, no tourniquet – Walant type (Lidocaine+Adrenalin+Bicarbonate). The surgical incision was made proximal to the flexor cord or on the level of the metacarpus (minimally invasive method). The essence of the procedure is the cutting of the flexor cord and the release of the median nerve. The type of anesthesia described above allows the procedure to be performed without the use of a tourniquet. The wound is closed only with skin sutures after the wound has soaked in. After the surgical intervention, the patient starts immediate motor improvement rehabilitation (neuromobilization, fitness exercises).
Statistical analyses included a distribution analysis and descriptive statistics, a comparison of a group of patients with a control group, and comparison of patient parameters before and after surgery. To test the normality of distribution, the Shapiro–Wilk test was used. Due to the lack of a normal distribution of the parameters calculated for both patients and the control group, the Mann–Whitney U-test with the Bonferroni correction was used for these comparisons. The Wilcoxon signed-rank test was used to compare the patients' results before and after surgery. In order to identify the factor determining the success of the surgery, logistic regression modeling was performed. Statistical analysis was performed using STATISTICA 13.0 software. All tests were conducted at the significance level of α = 0.05.
Results
We investigated 35 CTS patients, mean age was 50.83 years (SD = 13.14 years), 30 women (mean age – 51.00 ± 13.83 years) and 5 men (mean age – 49.80 ± 8.84 years). The control group consisted of 35 sex-matched healthy volunteers, mean age was 47.75 years (SD = 15.3 years). A BMI above 25 kg/m2 was observed in 3 CTS patients, in a further 3 BMI was above 30 kg/m2. None of the CTS patients achieved stage 5 on the Hi-Ob scale at baseline. Also, none of them was in stage 0 before the surgery. Only one patient was classified in stage 1, 16 in stage 2, 5 in stage 3, and 13 in stage 4. After the surgical intervention, most of the patients with output stages 2 and 3 were classified as stage 1, none as stage 0. None of the patients with an initial score of 4 changed their classification after the surgery. Improvement was seen in 19 patients (54.3%). In stage 2, there was improvement in 94%, in stage 3 - in 80% of the patients. The exact data are shown in Figure 1. In the presented visual material, stage 0 is not included, because we did not analyze patients without clinical symptoms of CTS, and none of the patients presented stage 0 after the operation.
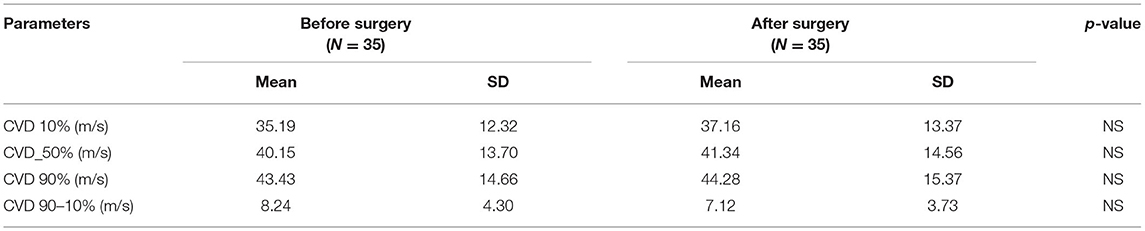
Mean values for the electrophysiological tests (standard, CVD and QST before and after surgery) with p-values are included in Tables 1A, 2A, 3A, respectively. A comment is needed concerning the evaluation of sensory nerve action potential latency (SNAP L) in the median nerve. Before the CTS operation, SNAPs were absent in 12 patients, after the operation – in 6. Therefore, we calculated the mean value of SNAP latency only for those patients in whom the response was present in order to avoid the use of very high values or symbol of infinity. The p-value was 0.065 (non-significant) for the study groups before and after treatment (Table 1A). In standard neurography, CMAP amplitude (CMAP A) did not differ between groups before and after surgery. The rest of the parameters changed significantly, and the differences were still very significant (p < 0.0000) when we compared the study group after the operation and the control group (Table 1B). Based on the Padua neurophysiological classification we were able to distinct the following classes of CTS in our patients: severe in 9 (25, 7%) of them, moderate – in 21 (60%), and mild – in 5 (14, 3%). None of the patients fullfield the criteria for extreme or minimal CTS. After the operation 6 (17.1%) patients still had severe CTS, in 19 (54, 3%) – moderate CTS was diagnosed, in 7 (20%) – mild, and in 3 (8.6%) – minimal.
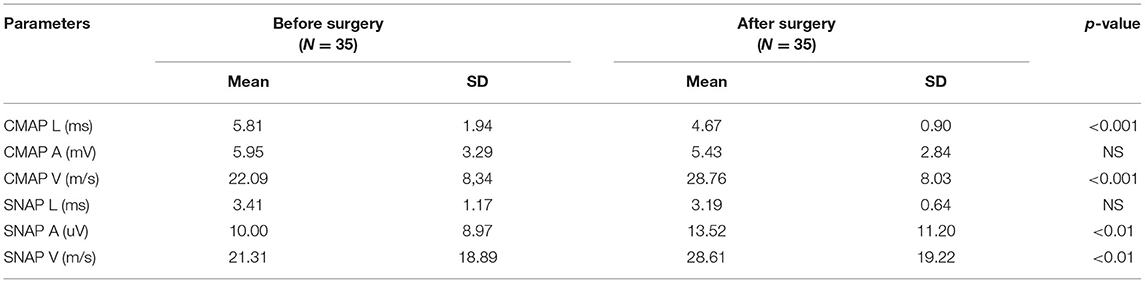
Table 1A. Mean values of standard motor and sensory conduction tests before and after surgery in CTS patients.
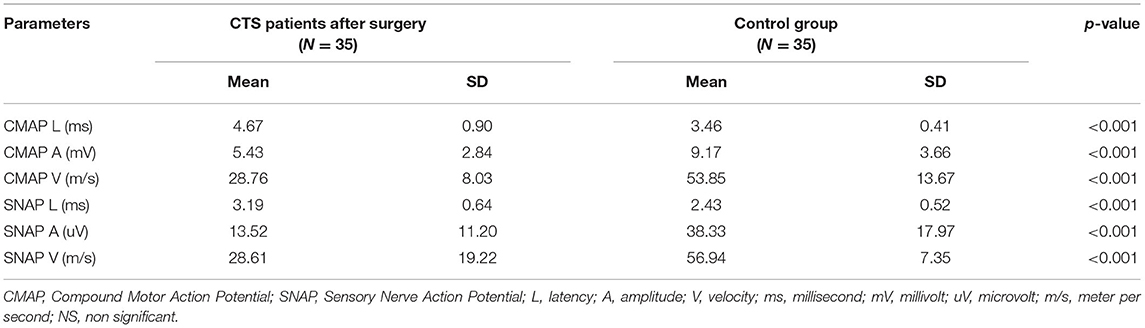
Table 1B. Comparison of standard motor and sensory parameters in CTS patients after the surgery and in the control group.
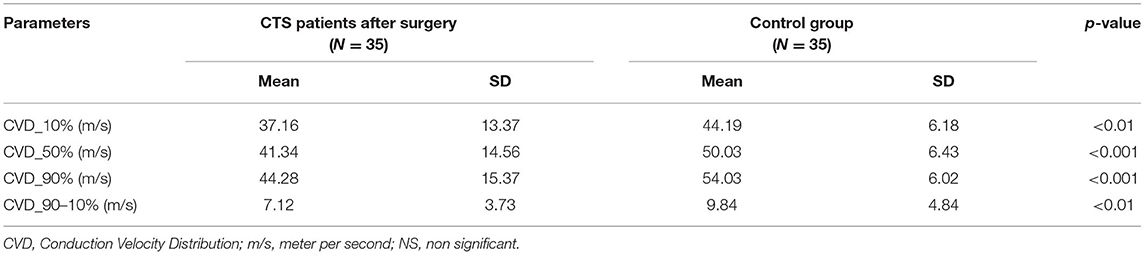
CVD analysis did not reveal important statistical differences between the study groups for all assessed quartiles of conduction velocities. In all quartiles, the conduction velocities were faster after the surgery than before it, but in the treatment group standard deviation (SD) values were very high (Table 2A). This indicated a large variation of parameters. Mean CVD parameters after the surgery did not reach the normal values, i.e., those seen in the control group, and the statistical differences between study and control groups remained significant. The spread of conduction velocities before and after surgery as well as in comparison to controls did not differ significantly (Table 2B).
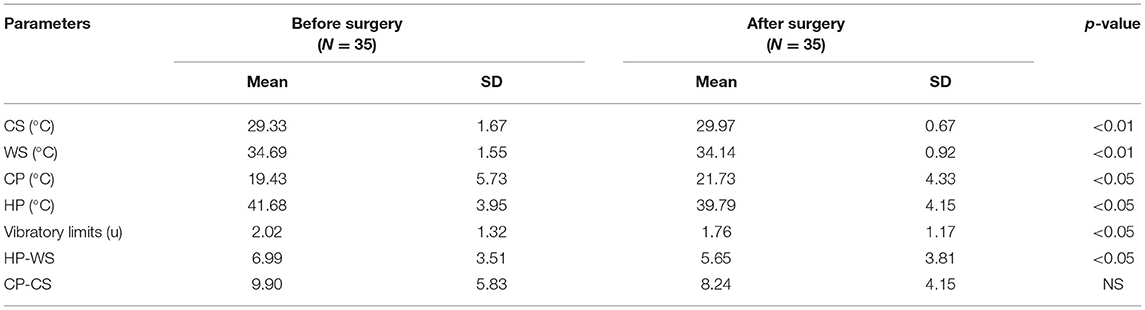
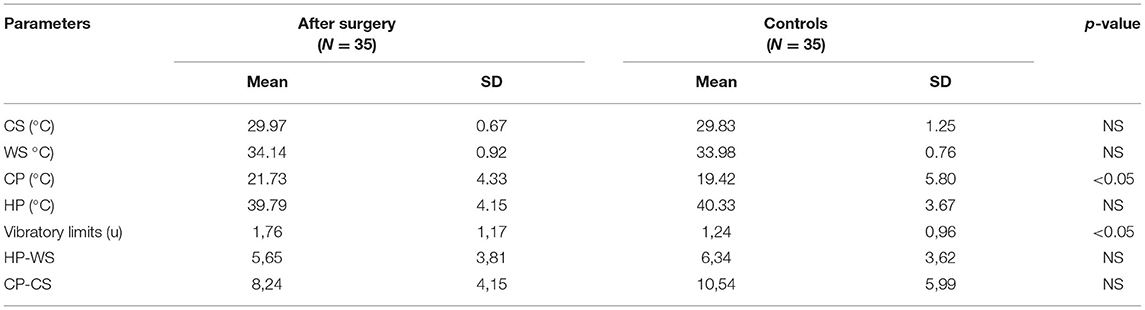
QST results in all modalities (temperature, pain, vibration) differed significantly before and after CTS surgery, and all of these improved (Table 3A). Additionally, the dispersion of the high temperature was significantly greater before surgery (6.99 ± 3.51 °C) than after surgery (5.65 ± 3.81 °C, p < 0.05). The dispersion of low temperature did not achieve statistical significance, and also tended to be smaller (9.90 ± 5.83°C vs. 8.24 ± 4.15°C) after the surgery. When we compared the QST results after surgery with the control group (Table 3B), most of the parameters, among them temperature dispersion values, did not differ between groups. We still noticed significant differences only for CP values (p < 0.05) and vibratory limits (1.76 ± 1.17 v. 1.24 ± 0.96, p < 0.05).
Logistic regression modeling revealed statistical importance only for patients' age and CTS severity on the Hi-Ob scale. The older a patient is, the lower the effectiveness of the treatment. The results of logistic regression modeling for age are presented in Table 4. Logistic regression modeling for CTS severity allowed an estimation of the risk of no improvement: 3% for stage 2 on the Hi-Ob scale, 43% for stage 3, and nearly no improvement (96%) for stage 4 (Table 5).
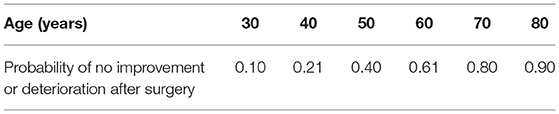
Table 4. The risk of no improvement in CTS after surgery based on the logistic regression modeling of patients' age.

Table 5. The risk of no improvement in CTS after surgery based on the logistic regression modeling of CTS severity on the Hi-Ob scale.
Discussion
Surgical intervention is a well-known and effective method of CTS treatment. Different surgical methods are considered: open, mini-open, and endoscopic decompression of the median nerve at the wrist (1, 21, 22). In all our patients, an open, minimally invasive method was performed, with a surgical incision above the flexor cord or on the level of the metacarpus with immediate neuromobilization and fitness exercises after the operation. Surgical treatment of CTS, regardless of the method used, is thought to be a safe therapy (21–23). None of our patients reported any complications, and none of them required reoperation within the period of observation. A cohort analysis by Lane et al. (24) conducted in an English population (855 832 initial surgeries) showed a very small rate of serious complications requiring hospitalization or further surgery equalling <0.1% (incidence rate: 1 per 1,000 per year).
The postoperative results and long-term effects of CTS surgical treatment seem not to differ between open and endoscopic methods (3, 25). However, endoscopic surgery potentially increases the risk of damage to the motor branch of the median nerve, and therefore most hand surgeons choose the open method. According to van den Broeke et al. (22), the mini-open method probably needs a longer time for a good outcome than the standard period of 3 to 6 months and is normally characterized by persistent post-intervention complaints. Some reports consider the endoscopic technique to be better in terms of pain relief and patient satisfaction in the early postoperative period. In the long term, the differences between open and endoscopic methods disappear. In van den Broeke et al.'s meta-analysis (22), the advantage of functional status after endoscopic treatment compared to open methods was not as clear as it was for pain relief 3. In our study, most of the patients (54.3%) improved, but none of them reached stage 0 on the Hi-Ob scale. The function of sensory and motor fibers, assessed in standard electrophysiological tests, significantly differed between groups before and after surgery, and also when compared to controls. The improvement was clear, but the electrophysiological results did not achieve normal values after the operation. We can conclude that all of our patients had residual, mainly sensory, symptoms in the observation period up to 18 weeks. Residual signs, clinical and electrophysiological, have been seen in many previous studies (9, 25–29).
The analysis of different populations of motor fibers in the CVD test also confirmed the presence of postoperative residual signs. CVD results after the operation had very large variations, and did not achieve statistical significance, although the rough data in all cases were noticeably better. They remained worse in comparison to the controls. CVD results did not satisfy the conditions for logistic regression modeling for CTS severity. The method did not turn out to be a useful tool in predicting the results of CTS surgery. This method was used by Sundar et al. (17) in CTS patients. They estimated the velocity ranges and concluded that severity of CTS connected with fiber diameter could be established more precisely using CVD tests. In our study, we compared the spread of conduction velocities in the study group before and after surgery, and in the controls. The diversity of the fiber types was similar in all groups, but in CTS patients conduction velocities were generally once shifted to slower, a fact which has been noticed previously, i.a. by Nishimura et al. (30).
Using standard electrophysiological tests, we were able to conclude that all parameters were significantly worse before surgery, and improved after the operation. However, they never achieved the correct values obtained in the control group. The results supported the knowledge of residual signs after CTS surgery (9, 25, 26, 31). When we compared QST values, most were within normal limits after the surgery, and similar to controls. We could only find differences for CP values and vibratory limits both after surgery and in the controls; these parameters remained higher in CTS patients. The logistic regression modeling for CP and vibratory limits did not reveal any statistical importance. Cold sensitivity has previously been described as a possible predictor of CTS outcome (32). The higher the cold sensitivity found, the higher the preoperative and postoperative disability, and the more severe the symptoms that can be expected. Thermal perception depends on the intensity, duration and rate of changes in a thermal stimulus. Responsiveness is different in different anatomical locations, and for cold and warm temperature. It is probably linked to the more diffuse sense of warmth than of cold, with greater spatial summation for warming stimuli with a lower number of receptors for high temperatures (33–35). Nevertheless, QST estimation did not provide more information than standard electrophysiological sensory tests. Based on our study and the literature, QST data do not seem to be useful as a therapy outcome predictor in CTS (1, 36).
In our study, improvement based on the Hi-Ob scale was seen in 54.3% of the patients in comparison to other studies showing an improvement in about or sometimes above 80% of patients (22, 23, 26, 36, 37). The observation time was not very long; therefore, we might anticipate further improvement after the end of our study. The results were not satisfactory for patients qualified to the most severe CTS stage, i.e., stage 4 on the Hi-Ob scale. Similar to most literature reports, the percentage of improvement in our study was very high in less severe CTS (94% and 80% in stage 2 and 3, respectively). The risk of no improvement was very small (3%) for stage 2 on the Hi-Ob scale, and nearly 96% in stage 4 in the logistic regression modeling for CTS severity. Some other studies have shown similar results for post-operative predictors (9, 13, 38–40). In contrast, van der Broeke et al. (22) did not confirm the dependence of treatment results on the initial severity and duration of CTS. Age seems to be an important CTS outcome predictor. The probability of no improvement or deterioration after surgery was 0.1 for the age of 30, while this reached 0.9 for the age of 80 in our study. Similarly, poorer surgical results in older patients have been shown in many studies (36, 39, 41, 42). Others have not found any relationship between postoperative results and older age (22, 37, 43). Contrasting results were shown in Townshend et al.'s 26 study. They achieved better results in older patients with lower symptom scores and higher levels of satisfaction after CTS surgery.
Schmidt et al. (44) found the affection of myelinated and unmyelinated nerve fiber populations in CTS. Based on our study, large fibers of the median nerve seem to be damaged more and earlier in CTS. Analysis of standard motor and sensory conduction showed persistent and significant differences in postoperative electrophysiological values when compared to the control group. Standard electrophysiology allows an assessment of only the biggest and fastest nerve fibers (15, 16). In CVD, which allows assessment of the function of motor fibers of different diameter, we also found much worse results after the operation than in the controls. The same situation was noticed when we analyzed vibratory limits depending on the function of large A-beta fibers. We considered that the lack of complete improvement within large fibers could be responsible for the residual signs in postoperative CTS patients. In contrast, we noticed complete recovery of QST parameters after the operation. QST is used to analyze the function of small sensory fibers: A-delta and C (18, 19). These fibers are severely damaged in the course of CTS. We noted higher thermal pain thresholds for high temperatures, and lower thermal thresholds for low temperatures with greater temperature dispersion for high temperatures in CTS patients than in healthy subjects. These values returned to normal values after the operation. Thermal sensation, innocuous and painful, is a complicated process, which depends on the integration of data from nociceptive and non-nociceptive channels, and is modulated by several mechanisms (45, 46). Additionally, significant variability in heat pain thresholds has been reported in the literature, which probably depends on the different experimental conditions (47, 48). Some studies have indicated elevated thresholds for both low and high temperatures in CTS (49). For high temperatures we observed “hyposensitivity;” in particular, the threshold for heat pain in CTS patients was much higher than in controls. The hypersensitivity to low temperature thresholds noticed in our study has been described in many pathological conditions, often in chronic pain. Hyperalgesic response could be explained by central sensitization in the course of long-lasting median nerve damage (50, 51).
We are aware of the study's limitations. Firstly, we assessed the onset latency of sensory potentials. In the literature (16) the “peak latency” is thought to be more reliable, but in our laboratory the reference values are based on the onset latency measurement. QST is a psychophysical method, partially subjective, because the response is a patient's subjective report. Therefore, advanced techniques, contact-heat-evoked potentials (CHEPS) and functional MRI could improve diagnostics, simplify the interpretatFirstlyion of results, and exclude subjectivity. We are aware that the control group consisted of hospital workers (physicians, nurses), who in the majority have healthier lifestyles than the general population, and as a consequence fewer CTS risk factors. Our study group was not very big and consisted of patients at different CTS stage at baseline. The period of observation should be longer for all patients with repeated tests, and these should be repeated at least 3 times. This will allow more precise assessment of residual signs. Therefore, we are planning a third part of our project.
In conclusion, the study confirmed the high level of efficiency of the minimally invasive surgical method in CTS with immediate neuromobilization, and fitness exercises. This efficiency is higher in younger patients with less advanced CTS. The improvement affected all fibers in the median nerve, but complete recovery was present only in small fibers. The rest of the fibers (motor, large sensory fibers) improved partially, which is most likely the cause of residual signs occurring a few months after CTS surgery. We did not notice any additional benefits in CTS diagnosis from the use of non-standard methods, such as CVD and QST, for assessment of fibers of different diameters. Our study clearly showed that QST can be used for diagnosis of real residual signs in postoperative CTS. Incorrect QST in postoperative CTS coexisting with clinical symptoms strongly points to unsatisfactory treatment results, while normal QST could help to avoid unnecessary reoperation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Wroclaw Medical University, Poland. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
MK made substantial contributions to the conception and study design, data interpretation, and manuscript preparation. MS substantial contributions to acquisition of data. JG did patient validation and carried out surgery. MW carried out statistical analysis. KS prepared the data for calculation, and tables and figures. SB made substantial contributions to the conception and design, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The work was supported by Wroclaw Medical University SUBZ.220.22.102.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. (2016) 15:1273–84. doi: 10.1016/S1474-4422(16)30231-9
2. Gupta S, Tewari AK, Nair V, Gupta A. Reliability of motor parameters for follow-up after local steroid injection in carpal tunnel syndrome. J Neurosci Rural Pract. (2013) 4:392–6. doi: 10.4103/0976-3147.120233
3. Orhurhu V, Orman S, Peck J, Urits I, Orhurhu MS, Jones MR, et al. Carpal tunnel release surgery- a systematic review of open and endoscopic approaches. Anesth Pain Med. (2020) 10:e112291. doi: 10.5812/aapm.112291
4. Kasius KM, Claes F, Verhagen WIM, Meulstee J. Motor nerve conduction tests in carpal tunnel syndrome. Front Neurol. (2019) 10:149. doi: 10.3389/fneur.2019.00149
5. Olde Dubbelink TBG, De Kleermaeker FGCM, Meulstee J, Bartels RHMA, Claes F, Verhagen WIM. Augmented diagnostic accuracy of ultrasonography for diagnosing carpal tunnel syndrome using an optimised wrist circumference-dependent cross-sectional area equation. Front Neurol. (2020) 11:577052. doi: 10.3389/fneur.2020.577052
6. Kerasnoudis A, Barmpalios G, Ntasiou P, Lakkos T, Venouziou A. Ultrasound, clinical, and electrophysiological findings in persistent carpal tunnel syndrome. J Neuroimaging. (2019) 29:218–22. doi: 10.1111/jon.12585
7. Padua L, Lo Monaco M, Padua R, Gregori B, Tonali P. Neurophysiological classification of carpal tunnel syndrome: assessment of 600 symptomatic hands. Ital J Neurol Sci. (1997) 18:145–50. doi: 10.1007/BF02048482
8. Mondelli M, Reale F, Sicurelli F, Padua L. Relationship between the self-administered Boston questionnaire and electrophysiological findings in follow-up of surgically-treated carpal tunnel syndrome. J Hand Surg Br. (2000) 25:128–34. doi: 10.1054/jhsb.2000.0361
9. Aksekili MA, Biçici V, Işik Ç, Aksekili H, Ugurlu M, Dogan M. Comparison of early postoperative period electrophysiological and clinical findings following carpal tunnel syndrome: is EMG necessary? Int J Clin Exp Med. (2015) 8:6267–71.
10. Hiltunen J, Kirveskari E, Numminen J, Lindfors N, Göransson H, Hari R. Pre- and post-operative diffusion tensor imaging of the median nerve in carpal tunnel syndrome. Eur Radiol. (2012) 22:1310–9. doi: 10.1007/s00330-012-2381-x
11. Crnković T, Trkulja V, Bilić R, Gašpar D, KolundŽić R. Carpal tunnel and median nerve volume changes after tunnel release in patients with the carpal tunnel syndrome: a magnetic resonance imaging (MRI) study. Int Orthop. (2016) 40:981–7. doi: 10.1007/s00264-015-3052-8
12. American American Association of Electrodiagnostic Medicine American American Academy of Neurology and and American Academy of Physical Medicine and Rehabilitation. Practice parameter for electrodiagnostic studies in carpal tunnel syndrome: summary statement. Muscle Nerve. (2002) 25:918–22. doi: 10.1002/mus.10185
13. Galasso O, Mariconda M, Donato G, Di Mizio G, Padua L, Brando A, et al. Histopathological, clinical, and electrophysiological features influencing postoperative outcomes in carpal tunnel syndrome. J Orthop Res. (2011) 29:1298–304. doi: 10.1002/jor.21356
14. Giannini F, Cioni R, Mondelli M, Padua R, Gregori B, D'Amico P, et al. new clinical scale of carpal tunnel syndrome: validation of the measurement and clinical-neurophysiological assessment. Clin Neurophysiol. (2002) 113:71–7. doi: 10.1016/S1388-2457(01)00704-0
15. Osiak K, Mazurek A, Pekala P, Koziej M, Walocha JA, Pasternak A. Electrodiagnostic studies in the surgical treatment of carpal tunnel syndrome-a systematic review. J Clin Med. (2021) 10:2691. doi: 10.3390/jcm10122691
16. Oh SJ. Clinical Electromyography: Nerve Conduction Studies. Philadelphia: Lippincott, Williams and Wilkins. (2003).
17. Sundar S, Gonzalez-Cueto JA, Gilbert ChS. Conduction velocity distribution estimation using the collision technique- theory and simulation study. Biomed Signal Process Control. (2008) 3:94–101. doi: 10.1016/j.bspc.2007.11.004
18. Siao P, Cros DP. Quantitative sensory testing. Phys Med Rehabil Clin N Am. (2003) 14:261–86. doi: 10.1016/S1047-9651(02)00122-5
19. Shy ME, Frohman EM, So YT, Arezzo JC, Cornblath DR, Giuliani MJ, et al. Quantitative sensory testing. Report of the therapeutics and technology assessment subcommittee of the American Academy of Neurology. Neurology. (2003) 60:898–904. doi: 10.1212/01.WNL.0000058546.16985.11
20. Rolke R, Baron R, Maier C, Tölle TR, Treede -DR, Beyer A, et al. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values [published correction appears in Pain. 2006 125:197]. Pain. (2006) 123:231–43. doi: 10.1016/j.pain.2006.01.041
21. Kilinc F, Behmanesh B, Seifert V, Marquardt G. Does recurrence of carpal tunnel syndrome (CTS) after complete division of the transverse ligament really exist? J Clin Med. (2021) 10:4208. doi: 10.3390/jcm10184208
22. van den Broeke LR, Theuvenet WJ, van Wingerden JJ. Effectiveness of mini-open carpal tunnel release: an outcome study. Arch Plast Surg. (2019) 46:350–8. doi: 10.5999/aps.2018.00535
23. Tamaru Y, Yanagawa A, Matsugi A. Sensory nerve conduction velocity predicts improvement of hand function with nerve gliding exercise following carpal tunnel release surgery. J Clin Med. (2021) 10:4121. doi: 10.3390/jcm10184121
24. Lane JCE, Craig RS, Rees JL, Gardiner MD, Green J, Prieto-Alhambra D, et al. Serious postoperative complications and reoperation after carpal tunnel decompression surgery in England: a nationwide cohort analysis. Lancet Rheumatol. (2020) 3:e49–57. doi: 10.1016/S2665-9913(20)30238-1
25. Chung KC. Current status of outcomes research in carpal tunnel surgery. Hand (N Y). (2006) 1:9–13. doi: 10.1007/s11552-006-0002-3
26. Townshend DN, Taylor PK, Gwynne-Jones DP. The outcome of carpal tunnel decompression in elderly patients. J Hand Surg Am. (2005) 30:500–5. doi: 10.1016/j.jhsa.2004.11.006
27. Tran TA, Williams LM, Bui D, Anthonisen C, Poltavskiy E, Szabo RM. Prospective Pilot Study Comparing Pre- and Postsurgical CTSAQ and Neuro-QoL Questionnaire with Median Nerve High-Resolution Ultrasound Cross-Sectional Areas. J Hand Surg Am. (2018) 43:184.e1–184.e9. doi: 10.1016/j.jhsa.2017.08.015
28. Kim JY, Yoon JS, Kim SJ, Won SJ, Jeong JS. Carpal tunnel syndrome: clinical, electrophysiological, and ultrasonographic ratio after surgery. Muscle Nerve. (2012) 45:183–8. doi: 10.1002/mus.22264
29. Merolli A, Luigetti M, Modoni A, Masciullo M, Lucia Mereu M, Lo Monaco M. Persistence of abnormal electrophysiological findings after carpal tunnel release. J Reconstr Microsurg. (2013) 29:511–6. doi: 10.1055/s-0033-1348038
30. Nishimura A, Ogura T, Hase H, Makinodan A, Hojo T, Katsumi Y, et al. A correlative electrophysiologic study of nerve fiber involvement in carpal tunnel syndrome using current perception thresholds. Clin Neurophysiol. (2004) 115:1921–4. doi: 10.1016/j.clinph.2004.03.022
31. Orak MM, Gümüştaş SA, Onay T, Uludag S, Bulut G, Börü ÜT. Comparison of postoperative pain after open and endoscopic carpal tunnel release: a randomized controlled study. Indian J Orthop. (2016) 50:65–9. doi: 10.4103/0019-5413.173509
32. Zimmerman M, Nyman E, Dahlin LB. Occurrence of cold sensitivity in carpal tunnel syndrome and its effects on surgical outcome following open carpal tunnel release. Sci Rep. (2020) 10:13472. doi: 10.1038/s41598-020-70543-8
33. Green BG. Temperature perception on the hand during static versus dynamic contact with a surface. Atten Percept Psychophys. (2009) 71:1185–96. doi: 10.3758/APP.71.5.1185
34. Yang GH, Kwon DS, Jones LA. Spatial acuity and summation on the hand: the role of thermal cues in material discrimination. Atten Percept Psychophys. (2009) 71:156–63. doi: 10.3758/APP.71.1.156
35. Ho HN, Watanabe J, Ando H, Kashino M. Mechanisms underlying referral of thermal sensations to sites of tactile stimulation. J Neurosci. (2011) 31:208–13. doi: 10.1523/JNEUROSCI.2640-10.2011
36. Coggon D, Ntani G, Harris EC, Linaker C, Van der Star R, Cooper C, et al. Impact of carpal tunnel surgery according to pre-operative abnormality of sensory conduction in median nerve: a longitudinal study. BMC Musculoskelet Disord. (2013) 14:241. doi: 10.1186/1471-2474-14-241
37. Porter P, Venkateswaran B, Stephenson H, Wray CC. The influence of age on outcome for carpal tunnel syndrome. a prospective study. J Bone Joint Surg. (2002) 84B:688–91. doi: 10.1302/0301-620X.84B5.0840688
38. Jørgsholm P, Flondell M, Björkman A, Thomsen NOB. Outcome of carpal tunnel release in patients with normal nerve conduction studies. J Orthop Sci. (2020) 23.
39. Alimohammadi E, Bagheri SR, Hadidi H, Rizevandi P, Abdi A. Carpal tunnel surgery: predictors of clinical outcomes and patients' satisfaction. BMC Musculoskelet Disord. (2020) 21:51. doi: 10.1186/s12891-020-3082-2
40. Sasaki Y, Terao T, Saito E, Ohara K, Michishita S, Kato N, et al. Clinical predictors of surgical outcomes of severe carpal tunnel syndrome patients: utility of palmar stimulation in a nerve conduction study. BMC Musculoskelet Disord. (2020) 21:725. doi: 10.1186/s12891-020-03750-z
41. Bland JD. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve. (2001) 24:935–40. doi: 10.1002/mus.1091
42. Mondelli M, Padua L, Reale F. Carpal tunnel syndrome in elderly patients: results of surgical decompression. J Peripher Nerv Syst. (2004) 9:168–76. doi: 10.1111/j.1085-9489.2004.09309.x
43. Park TS, Park JS, Moon JG, Park YS. Changes of the six-item carpal tunnel syndrome (CTS) symptoms scale and the nerve electrophysiological findings after surgery for CTS with abnormal nerve electrophysiological findings. Arthrosc Orthop Sports Med. (2014) 1:40–5. doi: 10.14517/aosm13017
44. Schmid AB, Bland JD, Bhat MA, Bennett DL. The relationship of nerve fibre pathology to sensory function in entrapment neuropathy. Brain. (2014) 137(Pt 12):3186–99. doi: 10.1093/brain/awu288
45. Defrin R, Ohry A, Blumen N, Urca G. Sensory determinants of thermal pain. Brain. (2002) 125(Pt 3):501–10. doi: 10.1093/brain/awf055
46. Sorensen L, Molyneaux L, Yue DK. The relationship among pain, sensory loss, and small nerve fibers in diabetes. Diabetes Care. (2006) 29:883–7. doi: 10.2337/diacare.29.04.06.dc05-2180
47. Park S, Roh SH, Lee JY. Body regional heat pain thresholds using the method of limit and level: a comparative study. Eur J Appl Physiol. (2019) 119:771–80. doi: 10.1007/s00421-018-04068-4
48. Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O'Brien PC. Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology. (1993) 43:1500–8. doi: 10.1212/WNL.43.8.1500
49. Goadsby PJ, Burke D. Deficits in the function of small and large afferent fibers in confirmed cases of carpal tunnel syndrome. Muscle Nerve. (1994) 17:614–22. doi: 10.1002/mus.880170608
50. Woolf CJ. Central sensitization: Implications for the diagnosis and treatment of pain. Pain. (2011) 3:S2–S15. doi: 10.1016/j.pain.2010.09.030
Keywords: carpal tunnel syndrome, carpal tunnel release, nerve conduction study, quantitative sensation testing, conduction velocity distribution
Citation: Koszewicz M, Szydlo M, Gosk J, Wieczorek M, Slotwinski K and Budrewicz S (2022) The Relevance of Collision Tests and Quantitative Sensory Testing in Diagnostics and Postoperative Outcome Prediction in Carpal Tunnel Syndrome. Front. Neurol. 13:900562. doi: 10.3389/fneur.2022.900562
Received: 20 March 2022; Accepted: 06 May 2022;
Published: 13 June 2022.
Edited by:
Ghazala Hayat, Saint Louis University, United StatesReviewed by:
Anna Potulska-Chromik, Warszawski Uniwersytet Medyczny, PolandPeyman Roomizadeh, Iran University of Medical Sciences, Iran
Copyright © 2022 Koszewicz, Szydlo, Gosk, Wieczorek, Slotwinski and Budrewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Koszewicz, bWFnZGEua29zemV3aWN6QG9uZXQucGw=
†ORCID: Mariusz Szydlo orcid.org/0000-0003-3527-053X
 Magdalena Koszewicz
Magdalena Koszewicz Mariusz Szydlo1†
Mariusz Szydlo1† Jerzy Gosk
Jerzy Gosk Malgorzata Wieczorek
Malgorzata Wieczorek Krzysztof Slotwinski
Krzysztof Slotwinski Slawomir Budrewicz
Slawomir Budrewicz