
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 04 July 2022
Sec. Headache and Neurogenic Pain
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.900436
The role of GABAergic cell transplantation in improving neuropathic pain is controversial. We comprehensively searched the relevant literature to identify animal studies of GABAergic cell transplantation that recorded pain behaviors as an outcome according to the Cochrane Handbook 5.0.2. Controlled studies assessing the administration of GABAergic neurons or GABAergic neuronal progenitor cells to rat or mouse neuropathic pain animal models were included. Basic design information and mechanical allodynia thresholds and heat hyperalgesia thresholds data were collected. The risk of bias for the animal experiments was assessed according to the SYRCLE's tool. This study included 10 full-text articles. GABAergic cells transplantation leads to a statistically significant improvement of allodynia (SMD = 5.26; 95% confidence interval: 3.02–7.51; P < 0.001) and hyperalgesia (SMD: 4.10; 95% confidence interval: 1.84–6.35; P < 0.001). Differentiated GABAergic cells and without antibiotics using may have a better effect for improving neuropathic pain. GABAergic cell transplantation is a promising treatment for improving neuropathic pain. This systematic review and meta-analysis evaluated the effects of GABAergic cell transplantation on neuropathic pain, which can guide future clinical trials and possible clinical treatments, and better attenuate neuropathic pain caused by abnormal circuit hyperexcitability.
Chronic pain can be divided into two categories, inflammatory pain and neuropathic pain. The second is the pain caused by a lesion or disease of the somatosensory nervous system. The prevalence of neuropathic pain is 6.9–10% around the world (1). Drugs such as gabapentin, pregabalin, serotonin/noradrenaline reuptake inhibitors, and tricyclic antidepressants are usually recommended (2), while the long-term effect and tolerance may limit its clinical application. A report on chronic pain in Europe showed that 64% of those taking prescription medicine reported that their pain medication was sometimes inadequate, and 14% had stopped due to side effects (3). In addition to pharmacological treatments, a variety of other interventions are available for neuropathic pain, including intrathecal baclofen bolus, physical and psychological therapies, spinal cord stimulation, surgery, and transcranial magnetic stimulation (4–9). However, due to the complex etiologies of neuropathic pain, current treatments are often inadequate and/or produce severe side effects (10). Scientists must therefore find more efficient and safer solutions to relieve neuropathic pain.
Neuropathic pain is closely related to central sensitization of the spinal dorsal horn, a reduction in the thresholds of cutaneous nociceptors or an increase in the excitability of the central nervous system (CNS) caused by dysfunction of gamma-aminobutyric acid-ergic (GABAergic) neurons in the CNS (11, 12). The central sensitization is closely related to the plasticity of synaptic transmission, and the mechanism may be neuronal sensitization mediated by Glutamate/NMDA receptors (13), glia crosstalk induced by activation of microglia and astrocytes (14, 15), and changes in the (pro-inflammatory) cytokine microenvironment (16). Spinal cord injury (SCI) or peripheral nerve injury (PNI) may negatively affect the function of GABAergic neurons in dorsal horn of the spinal cord, resulting in mechanical allodynia and heat hyperalgesia (17, 18). The recovery of GABAergic neuron function therefore plays an important role in the treatment of pain. It was reported that stem cell transplantation could supplement the inhibitory interneurons in the CNS, restore the function of GABA neurons, and inhibit the mechanical hypersensitivity reaction (19). GABAergic neurons or neural progenitor cells (NPCs) may have similar potential to improve neuropathic pain in SCI or PNI models (20–24). After transplantation into the spinal cord, GABAergic cells can migrate and mainly differentiate into GABAergic intermediate neurons, integrate with the spinal circuit in the deep dorsal horn of the spinal cord (laminae III-V), and may normalize the mechanical threshold and alleviate neuropathic pain (22).
Although some studies analyzed the therapeutic effects of these cells on neuropathic pain in animal models, there was no consistent conclusion or no quantitative data on animal experiments and clinical trials of GABAergic cell therapy for neuropathic pain. Therefore, we comprehensively searched the literature and systematically analyzed animal experimental studies on GABAergic cell transplantation for neuropathic pain, and evaluated the effects of cell transplantation in order to assess the efficacy of GABAergic cell transplantation.
This meta-analysis was under the guide of PRISMA Checklist (eMethod 1) and comprehensively retrieved the relevant literature to identify animal experiments of GABAergic cell transplantations and evaluate the effect of these cells on neuropathic pain. The PICO statements are as follows:
1) Population: Neuropathic pain animal models involved rats or mice suffered from SCI or PNI.
2) Intervention: GABAergic cell transplantation.
3) Comparisons: placebo (saline, culture medium, or inactive cells) or no treatment.
4) Outcomes: mechanical allodynia thresholds and heat hyperalgesia thresholds.
A comprehensive search was conducted to identify all animal experimental studies of GABAergic cell transplantation into models that recorded pain threshold as an outcome according to the Cochrane Handbook 5.0.2 (25). We searched PubMed, Cochrane Library, Web of Science, China Academic Journals Full-text Database, and Wanfang databases for original studies and reviews published with these keywords (“neuropathic pain” OR “neuralgia” OR “allodynia” OR “hyperalgesias” OR “hypersensitivity”) AND (“GABAergic neurons” OR “GABAergic cell” or “neural progenitor cell”) up to March 1, 2021 without language restriction. All titles were independently examined by two reviewers, and any report potentially related was initially included. In addition, we conducted an individual search in the reference lists of relevant articles to find additional studies. We also attempted to contact the authors of the studies with insufficient data to request additional relevant unpublished data. See eMethod 2 for search strategy.
This meta-analysis included studies of transplantation of GABAergic neurons or NPCs into SCI or PNI induced neuropathic pain models. Measured outcomes were the evaluation of allodynia and hyperalgesia. Original research studies regarding the influence of transplantation, regardless of donor species or tissue origin, were included.
Any coculture concomitant injection with other cell types, or use of adjuvant products (e.g., matrices, scaffolding), Chemotherapy models, and diabetic neuropathy lead to exclusion. In addition, review articles, commentaries, editorials, letters and experiments without detailed process were excluded.
Two reviewers independently appraised all potentially included studies. Any disagreement was resolved after the third reviewer read through the experiment completely.
The data were extracted independently by two reviewers and rechecked after extraction. Any disagreement during the extraction was discussed and resolved. The content included animal characteristics (species, strain, sex, weight or week age), interventions (allogeneic or xenogeneic, delivery route, total number of transplanted cells, degree of cell differentiation, randomization, antibiotic and immunosuppressive usage), effect after the transplantation, and observation (follow-up) time. The data of peak allodynic/hyperalgesic effects after transplantation compared with the control group was extracted as the outcome measures in order to avoid spontaneous recovery over time. The authors were contacted if mean values and standard deviations were not reported. If the information was reported as graphs, we used the method recommended by Sistrom and Mergo to convert raw value of scanned images to original data values from the full text (26).
The risk of bias (RoB) for the included experiments was assessed according to the Systematic Review Center for Laboratory Animal Experimentation's tool (SYRCLE's tool) (27). Each study was assessed by two reviewers independently and each domain was judged as “low” RoB, “unclear” RoB, or “high” RoB, respectively. Any disagreement was discussed and resolved under the guidance of the third reviewers. According to the recommendation of the SYRCLE's tool, the summary score for each individual study was not calculated for its difficulty to justify the weights assigned (27). Therefore, the sensitivity analysis was not performed.
The subgroup analyses were based on the following items:
1) Animal species: Rat models or mice models.
2) Sex: Male animals or female animals.
3) Type of neuropathy: CNS injury (hemisection SCI; contusion SCI, excitotoxic SCI, and compression SCI) or PNI [chronic constriction injury (CCI), spared nerve injury (SNI), spinal nerve ligation (SNL)].
4) Randomization: Yes or no.
5) Transplantation time: Within 2 or 2 weeks after the injury.
6) Delivery route: Intraspinal transplantation or intrathecal transplantation.
7) Graft type: Allogeneic or xenogeneic.
8) Use of antibiotic: Yes or no.
9) Use of immunosuppressive: Yes or no.
10) Number of transplanted cells: <1.5 × 106 cell dose/kg or ≥1.5 × 106 cell dose/kg.
11) Degree of cell differentiation: GABAergic neurons or GABAergic NPCs.
We used the Review Manager Software package (version 5.3.0; https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman) and STATA 16.0 (https://www.stata.com/) to conduct the meta-analysis. Effect sizes were computed and the standardized mean difference (SMD) with a 95% confidence interval (CI) was entered in all analyses. By calculating the effect size, and pooling the findings, modifying the bias caused by small sample size was possible.
Statistical heterogeneity among studies and subgroups was evaluated with I2 test and chi-square tests. If P > 0.1 and I2 < 25%, there was no significant heterogeneity between studies, and a fixed-effect model was used. If P was < 0.1 and I2 was > 25%, there was likely substantial heterogeneity, and a random effect model was used. The subgroup analyses were adopted to analyze the source of heterogeneity. Due to few included studies, Publication bias has not been evaluated in the subgroup analysis. A two-sided P-value < 0.05 was considered statistically significant.
We found 2,034 unduplicated articles using the search strategies described earlier. Of these, 49 potentially eligible studies were selected for in-depth reading. Excluding irrelevant, republished research (1, 28) and reports that ultimately failed to obtain exact data from papers or authors (22, 24, 29), 10 full-text articles were included for the meta-analysis and were studied in detail as shown in Table 1 (20, 21, 23, 30–36). One studies had raw data (35). The data of other 9 studies were extracted from the pictures by the method recommended by Sistrom and Mergo's (26) and the results of the included reports in the different time intervals was shown in eResult 1. The flow of information from identification to inclusion of studies is summarized in Figure 1. These studies contained a total of 295 rats/mice including 140 GABAergic cell-treated animals and 155 controls. Six studies reported only the impact of GABAergic cell transplantation on mechanical allodynia and four assessed its effects both on mechanical allodynia and heat hyperalgesia.
According to the results of the therapeutic effects of GABAergic cells, a significant statistical heterogeneity was found with allodynia (I2: 95%; P < 0.001) and hyperalgesia (I2: 85%; P < 0.001). Therefore, in these cases a random-effect model was used. In addition, we were not able to calculate the pooled-effect size in mice, female animals or experiments without immunosuppression because there were only two eligible studies.
The main outcome measure was the assessment of mechanical allodynia and heat hyperalgesia thresholds. According to our analyses, using the random-effects model, GABAergic cell transplantation leads to a statistically significant improvement of mechanical allodynia (SMD: 5.26; 95% CI: 3.02–7.51) and a significant effect on heat hyperalgesia (SMD: 4.10; 95% CI: 1.84–6.35). Forest plots of the effects of GABAergic cells on mechanical allodynia and heat hyperalgesia are shown in Figure 2.
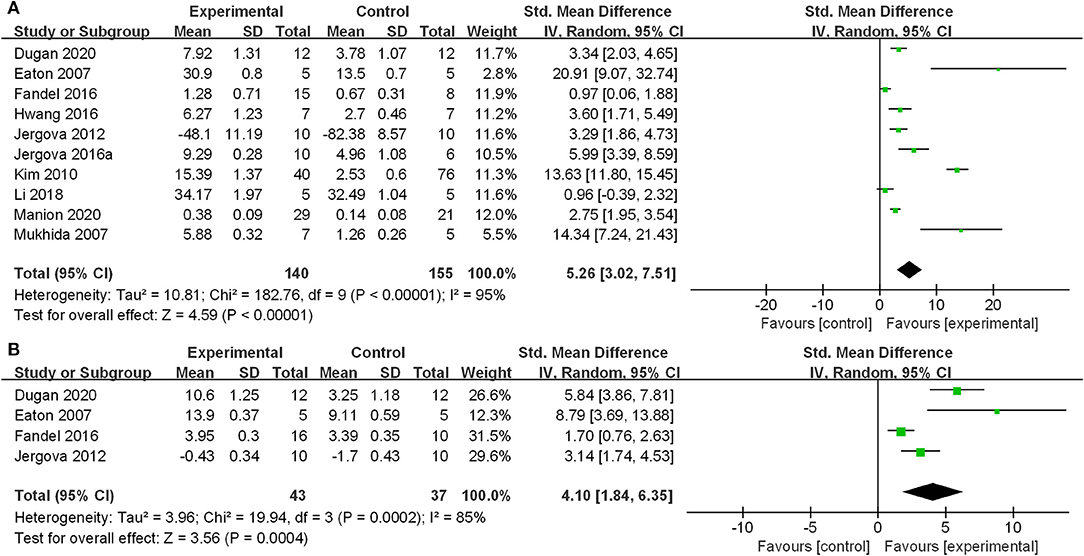
Figure 2. Effect of GABAergic cells on mechanical allodynia and heat hyperalgesia. (A) Mechanical allodynia; (B) Heat hyperalgesia. GABAergic cell transplantation attenuated the mechanical allodynia and heat hyperalgesia of the animal models. The standardized mean differences were 5.26 (95% CI: 3.02–7.51) and 4.10 (95% CI: 1.84–6.35), respectively.
Subgroup analyses of mechanical allodynia thresholds were performed based on the above items. Due to the small number of studies included (four studies), subgroup analyses of heat hyperalgesia were not done.
Table 2 shows the subgroup analysis of allodynia thresholds. Multivariate meta-regression analysis showed that randomization (P = 0.04), antibiotic (P = 0.01), and the cell type (P = 0.03) influenced the improvement of mechanical allodynia after GABAergic cell transplantation. As shown in the Table 2, no differences were found in the effect of GABAergic cells transplantation on allodynia in PNI model or CNS injury model (P = 0.46), delivery route (P = 0.06), varying the transplantation time (P = 0.17) and the number of transplanted cells (P = 0.54).
All domains of these studies were marked as low or unclear RoB according to the SYRCLE's tool. Because some circumstances or randomization sequences were not clearly described, and could not be judged based on the information, 56.0% of the entries were rated as “unclear RoB.” See Table 3 (and eResult 2 for support information for judgement) for details.
The cell repair research in recent years mainly focuses on the restoration of motor function (37–40), but rarely involves in improving neuropathic pain. Most preclinical studies were conducted on rodents and involve direct nerve injury, often to the sciatic nerve or spinal cord. These models bear poor resemblance to the case history of most neuropathic pain patients, but they do enable disease processes to be investigated, and some treatments are able to reverse the pain behaviors (41). Although these models had their limitations, and translation was currently far from perfect, such models have identified changes at all levels in the pain pathway in neuropathic pain, including alterations in sensory neuron protein expression, changes in spinal cord synaptic function and descending control of pain from the brain (42).
We found that although both GABAergic neurons and GABAergic NPCs can improve neuropathic pain, direct transplantation with GABAergic neurons was more effective than cell therapy with GABAergic NPCs. This may be due to the fact that NPCs can only function if they differentiate into GABAergic cells, but will not be effective when they differentiate into glial cells (21). And transplanted cell had different fates in differentiation, which was related to the type of cells transplanted. In Braz's and Hwang's studies, transplanted GABAergic neuron precursors mainly differentiated into neurons rather than microglia and astrocytes (22, 32). In Fandel's and Kim's study, transplanted hESC-MGEs migrate within the injured spinal cord and mainly differentiating into GABAergic neuron subtypes and glial cells (31, 34). However, different transplanted cells mainly integrated with the spinal circuit in the deep dorsal horn of the spinal cord (laminae III-V) (22, 23). Transplanted GABAergic cells can survive in the spinal cord, and alleviate mechanical allodynia and heat hyperalgesia in neuropathic pain models.
According to existing cell transplantation clinical trials to treat SCI, we found that the cell doses were approximately 1.5 × 106 cell dose/kg (43), 1.8 × 106 cell dose/kg (44), and 4.5 × 106 cell dose/kg (45), assuming that all patients weighed 67 kg. So we evaluated the effect of cell dose on the neuropathic pain model using 1.5 x 106 cell dose/kg as the cut-off value. However, after dividing by this value, both high and low doses have effectively alleviated neuropathic pain and no statistical difference were found.
Our results indicated that with or without antibiotics, both treatments could relieve neuropathic pain, and animals that did not use antibiotics received better mechanical allodynia relief. The five articles reported the use of antibiotics, and all were injected after injury. Because the number was too small, effective analysis was difficult. Another meta-analysis showed that olfactory ensheathing cell transplantation had the same effect with or without antibiotics (46). This result could stimulate the interest of other scientists and promote further studies regarding the use of antibiotics in animals after transplantation. Xenogeneic cell transplantation may improve neuropathic pain more effectively than allogeneic cell transplantation. This was caused by the difference of animal species in this meta-analysis for animal models were mainly rats while the transplanted cells were mainly from humans and mice.
Some studies did not report or analyze the measured outcomes at the same time points, which led to the design of this meta-analysis only analyzed the peak effects of cell transplantation, rather than the results at the same time point after transplantation. The meta-analysis of some cells such as bone marrow-derived mesenchymal stem cells and olfactory ensheathing cells had proved the long-term effect of alleviating neuropathic pain after cell transplantation (46, 47). The time of nociceptive testing post-transplantation varied greatly, such as time intervals (for example, weekly, bi-weekly), and the longest follow-up time is also inconsistent (see Table 1; eResult 1). The responsiveness to mechanical and thermal stimuli may alter over time, perhaps due to the changes in cell viability, spontaneous recovery following injury, or dynamic reorganization, which would also limit the promotion of this meta-analysis (31, 48).
The SYRCLE's tool, based on the Cochrane Collaboration RoB Tool, aims to assess methodological quality and has been adapted to aspects of bias and plays a role in animal experiments (27). However, several previous meta-analyses of cell transplantation to alleviate neuropathic pain have not analyzed RoB based on the SYRCLE's tool (19, 46, 47). Another systematic review evaluated the therapeutic potential of regulatory T lymphocytes in periodontitis animal models and rated 42.11% of projects as unclear RoB (49). In future studies, it is strongly recommended that animal experiments of alleviating neuropathic pain should refer to some checklist to improve the quality control, for example, the guidelines of Planning Research and Experimental Procedures on Animals: Recommendations for Excellence (48).
Drew et al. found that mechanical allodynia in rats after SCI was related to the loss of GABAergic inhibition in the dorsal horn (17). Kim et al. (34) confirmed the major cause of pain was the loss of the spinal GABAergic system following SCI. This provided a theoretical basis for treatment of neuralgia using GABAergic neuron transplantation. Thus, transplantation of GABAergic neurons or GABAergic NPCs may have effects on mechanical allodynia and heat hyperalgesia. Llewellyn-Smith et al. (48) transplanted GFP-expressing MGE derived neuronal precursors into mice with SCI and found that cells directly integrated into the intact dorsal horn circuitry and the transplanted MGE neurons retained their GABAergic phenotypes and integrated dynamically into host-transplant synaptic circuits. These transplanted cells developed into mature neurons, and exhibited a specific discharge pattern of cortical and spinal cord inhibitory interneurons, and integrated with the host circuit (24, 32). Integrated cells alleviated neuropathic pain through synaptic release of GABA in the spinal cord and affected the intrathecal spinal environment for sensory system modulation (25, 50). These results illustrate the remarkable plasticity of spinal cord and the potential of cell-based therapy in human.
After PNI, the proportion of GABA-immunoreactive neurons in the spinal dorsal horn on the nerve-injured side was reduced (51), which may disrupt GABAergic inhibition in spinal dorsal horn and cause some forms of neuropathic pain (23, 52). These included studies did not show a difference in effects comparing with SCI models, indicating that GABAergic cells are equally effective in neuropathic pain caused by PNI. Manion et al. observed that the transplanted GABAergic neurons also survived for a long time in the uninjured spinal cord and integrate with the original synapses (36). These transplanted cells may restore GABAergic function of the spinal cord dorsal horn to reduce the hyperexcitability and exaggerate dorsal horn neuronal firings that develop in dorsal horn projection neurons (21, 23).
Although, similar study had been published (53), our meta-analysis is not redundant. We set stricter inclusion and exclusion criteria to ensure the consistency of studies, the included studies were not completely consistent, and sensitivity and publication bias could not be assessed. It should be noted that the meta-analysis conducted by Askarian-Amiri S et al. appears to be loose on the risk of bias assessment, and two studies were not included (30, 35). The evaluation basis can be inquired in the attachment. Finally, there is no doubt about the benefit of GABAergic neuron transplantation in improving neuropathic pain in animals. Therefore, we focused on the mechanism of cell transplantations in improving neuropathic pain and the limitations of these studies, and also conducted the subgroup analysis for the included experiments.
In the literature we searched, there was no such clinical trial on GABAergic cell transplantation to treat human neuropathic pain, even after we specifically searched the literature on human trials. Therefore, it is difficult to summarize the potential risks of intraspinal GABAergic cell transplantation. These animals involved were rats or mice, and no experiments were found in other mammals. The study by Fandel et al. (31) showed that transplantation of MGE-like precursor cells in the normal mouse spinal cord did not alter spinal cord function. Another study showed that transplantation of well-differentiated GABAergic cell into the central nervous system did not form tumors in rats (20). Among the included studies, 56.0% were rated as unclear RoB, which posed a challenge for the further trials of these cells. The publication bias results indicated that more research was needed to supplement the evidence for the effect of GABAergic neurons on neuropathic pain relief. Although there are no clinical trials that specifically applied GABAergic cells to improve neuropathic pain behaviors, this meta-analysis suggests that a combination of multiple cell transplantations may be necessary to achieve maximum benefits.
Although the methodological quality of the 10 included trials had low risk bias, the number of trials examined was small because of different animal species, type of neuropathy, cell types/origins, cell quantities transfused, and transplantation times after SCI; thus, the heterogeneity between each study was high and may lead to a far greater effect size. This meta-analysis therefore had a potential RoB and only four studies involved to thermal reflex hypersensitivity, which prompts the need to avoid the risk for over-speculation. In addition, some of the included studies did not list how many animals were excluded from testing. Consequently, additional studies with larger sample sizes, longer-term outcome measurements, and more detailed observations and explanations are needed to validate the findings described in this meta-analysis. Few included reports, limited reporting of treatment effect estimates and unclear RoB suggest using caution when assessing the effects of GABAergic cells transplantation.
GABAergic cell transplantation is a very promising treatment for attenuating neuropathic pain. Our meta-analysis showed that GABAergic cells can partially alleviate mechanical allodynia and heat hyperalgesia after SCI or PNI. Intrathecal transplantation, a xenogeneic cell source, differentiated GABAergic cells, and no antibiotics may be better for improving neuropathic pain. It is necessary to reduce the RoB and improve the quality of animal experiments. This will guide future clinical trials and possible clinical treatments, and better alleviate neuropathic pain caused by abnormal circuit hyperexcitability.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Material preparation, data collection and analysis were performed by Z-RZ, F-YW, and YW. The first draft of the manuscript was written by Z-RZ and all authors commented on previous versions of the manuscript. All authors contributed to the study conception and design, read, and approved the final manuscript.
This work was funded by National Key R&D Program of China (Project No. 2021YFF0501600 and Subject No. 2021YFF0501604) and Capital's Funds for Health Improvement and Research (2022-2-6013).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.900436/full#supplementary-material
eMethod 1. PRISMA Checklist.
eMethod 2. Search strategy of every database.
eResult 1. Data set of the included reports.
eResult 2. Support for judgement the SYRCLE's risk of bias tool.
CCI, chronic constriction injury; CI, confidence interval; CNS, central nervous system; GABA, gamma-aminobutyric acid; hESC, human embryonic stem cell; mESC, mouse embryonic stem cell; mMGE, mouse medial ganglion eminence; NPC, neural progenitor cell; PNI, peripheral nerve injury; RoB, risk of bias; SCI, spinal cord injury; SD rats, Sprague–Dawley rats; SMD, standardized mean difference; SNI, spared nerve injury; SNL, spinal nerve ligation; SYRCLE's tool, Systematic Review Center for Laboratory Animal Experimentation's tool.
1. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. (2014) 155:654–62. doi: 10.1016/j.pain.2013.11.013
2. Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14:162–73. doi: 10.1016/S1474-4422(14)70251-0
3. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. (2006) 10:287–333. doi: 10.1016/j.ejpain.2005.06.009
4. Baron R, Binder A, Wasner G. Neuropathic pain: diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. (2010) 9:807–19. doi: 10.1016/S1474-4422(10)70143-5
5. Dobson JL, McMillan J, Li L. Benefits of exercise intervention in reducing neuropathic pain. Front Cell Neurosci. (2014) 8:102. doi: 10.3389/fncel.2014.00102
6. Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, et al. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Arch Phys Med Rehabil. (2015) 96:S156–172. doi: 10.1016/j.apmr.2014.11.010
7. Kumru H, Benito-Penalva J, Kofler M, Vidal J. Analgesic effect of intrathecal baclofen bolus on neuropathic pain in spinal cord injury patients. Brain Res Bull. (2018) 140:205–11. doi: 10.1016/j.brainresbull.2018.05.013
8. Sdrulla AD, Guan Y, Raja SN. Spinal cord stimulation: clinical efficacy and potential mechanisms. Pain Pract. (2018) 18:1048–67. doi: 10.1111/papr.12692
9. Joshi HP, Jo HJ, Kim YH, An SB, Park CK, Han I. Stem cell therapy for modulating neuroinflammation in neuropathic pain. Int J Mol Sci. (2021) 22:4853. doi: 10.3390/ijms22094853
10. St John Smith E. Advances in understanding nociception and neuropathic pain. J Neurol. (2018) 265:231–8. doi: 10.1007/s00415-017-8641-6
11. Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. (2001) 429:23–37. doi: 10.1016/S0014-2999(01)01303-6
12. Cohen SP, Mao J. Neuropathic pain: mechanisms and their clinical implications. BMJ. (2014) 348:f7656. doi: 10.1136/bmj.f7656
13. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. (2009) 139:267–84. doi: 10.1016/j.cell.2009.09.028
14. Tiwari V, Guan Y, Raja SN. Modulating the delicate glial-neuronal interactions in neuropathic pain: promises and potential caveats. Neurosci Biobehav Rev. (2014) 45:19–27. doi: 10.1016/j.neubiorev.2014.05.002
15. Old EA, Clark AK, Malcangio M. The role of glia in the spinal cord in neuropathic and inflammatory pain. Handb Exp Pharmacol. (2015) 227:145–70. doi: 10.1007/978-3-662-46450-2_8
16. Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. (2001) 8:1–10. doi: 10.1006/nbdi.2000.0360
17. Drew GM, Siddall PJ, Duggan AW. Mechanical allodynia following contusion injury of the rat spinal cord is associated with loss of GABAergic inhibition in the dorsal horn. Pain. (2004) 109:379–88. doi: 10.1016/j.pain.2004.02.007
18. Yin Y, Yi MH, Kim DW. Impaired autophagy of GABAergic interneurons in neuropathic pain. Pain Res Manag. (2018) 2018:9185368. doi: 10.1155/2018/9185368
19. Chen X, Xue B, Li Y, Song C, Jia P, Ren X, et al. Meta-analysis of stem cell transplantation for reflex hypersensitivity after spinal cord injury. Neuroscience. (2017) 363:66–75. doi: 10.1016/j.neuroscience.2017.06.027
20. Eaton MJ, Wolfe SQ, Martinez M, Hernandez M, Furst C, Huang J, et al. Subarachnoid transplant of a human neuronal cell line attenuates chronic allodynia and hyperalgesia after excitotoxic spinal cord injury in the rat. J Pain. (2007) 8:33–50. doi: 10.1016/j.jpain.2006.05.013
21. Mukhida K, Mendez I, McLeod M, Kobayashi N, Haughn C, Milne B, et al. Spinal GABAergic transplants attenuate mechanical allodynia in a rat model of neuropathic pain. Stem Cells. (2007) 25:2874–85. doi: 10.1634/stemcells.2007-0326
22. Braz JM, Sharif-Naeini R, Vogt D, Kriegstein A, Alvarez-Buylla A, Rubenstein JL, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. (2012) 74:663–75. doi: 10.1016/j.neuron.2012.02.033
23. Jergova S, Hentall ID, Gajavelli S, Varghese MS, Sagen J. Intraspinal transplantation of GABAergic neural progenitors attenuates neuropathic pain in rats: a pharmacologic and neurophysiological evaluation. Exp Neurol. (2012) 234:39–49. doi: 10.1016/j.expneurol.2011.12.005
24. Etlin A, Braz JM, Kuhn JA, Wang X, Hamel KA, Llewellyn-Smith IJ, et al. Functional synaptic integration of forebrain GABAergic precursors into the adult spinal cord. J Neurosci. (2016) 36:11634–45. doi: 10.1523/JNEUROSCI.2301-16.2016
25. Lefebvre C, Manheimer E, Glanville J. Searching for studies. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. Chichester: The Cochrane Collaboration (2009).
26. Sistrom CL, Mergo PJ. A simple method for obtaining original data from published graphs and plots. AJR Am J Roentgenol. (2000) 174:1241–4. doi: 10.2214/ajr.174.5.1741241
27. Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE's risk of bias tool for animal studies. BMC Med Res Methodol. (2014) 14:43. doi: 10.1186/1471-2288-14-43
28. Jergova S, Gajavelli S, Varghese MS, Shekane P, Sagen J. Analgesic effect of recombinant GABAergic cells in a model of peripheral neuropathic pain. Cell Transplant. (2016) 25:629–43. doi: 10.3727/096368916X690782
29. Vaysse L, Sol JC, Lazorthes Y, Courtade-Saidi M, Eaton MJ, Jozan S. GABAergic pathway in a rat model of chronic neuropathic pain: modulation after intrathecal transplantation of a human neuronal cell line. Neurosci Res. (2011) 69:111–20. doi: 10.1016/j.neures.2010.10.006
30. Dugan EA, Jergova S, Sagen J. Mutually beneficial effects of intensive exercise and GABAergic neural progenitor cell transplants in reducing neuropathic pain and spinal pathology in rats with spinal cord injury. Exp Neurol. (2020) 327:113208. doi: 10.1016/j.expneurol.2020.113208
31. Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, et al. Transplanted human stem cell-derived interneuron precursors mitigate mouse bladder dysfunction and central neuropathic pain after spinal cord injury. Cell Stem Cell. (2016) 19:544–57. doi: 10.1016/j.stem.2016.08.020
32. Hwang I, Hahm SC, Choi KA, Park SH, Jeong H, Yea JH, et al. Intrathecal transplantation of embryonic stem cell-derived spinal GABAergic neural precursor cells attenuates neuropathic pain in a spinal cord injury rat model. Cell Transplant. (2016) 25:593–607. doi: 10.3727/096368915X689460
33. Jergova S, Gajavelli S, Pathak N, Sagen J. Recombinant neural progenitor transplants in the spinal dorsal horn alleviate chronic central neuropathic pain. Pain. (2016) 157:977–89. doi: 10.1097/j.pain.0000000000000471
34. Kim DS, Jung SJ, Nam TS, Jeon YH, Lee DR, Lee JS, et al. Transplantation of GABAergic neurons from ESCs attenuates tactile hypersensitivity following spinal cord injury. Stem Cells. (2010) 28:2099–108. doi: 10.1002/stem.526
35. Li X. Human embryonic stem cell derived spinal GABAergic neural progenitor cells for neuropathic pain and spasticity after spinal cord injury in rats (Master thesis). Huazhong University of Science and Technology, Wuhan, China (2018). doi: 10.7666/d.D01546702
36. Manion J, Khuong T, Harney D, Littleboy JB, Ruan T, Loo L, et al. Human induced pluripotent stem cell-derived GABAergic interneuron transplants attenuate neuropathic pain. Pain. (2020) 161:379–87. doi: 10.1097/j.pain.0000000000001733
37. Huang H, Sharma HS, Chen L, Saberi H, Mao G. 2018 yearbook of neurorestoratology. J Neurorestoratol. (2019) 7:8–17. doi: 10.26599/JNR.2019.9040003
38. Huang H, Chen L, Mao G, Bach J, Xue Q, Han F, et al. The 2019 yearbook of neurorestoratology. J Neurorestoratol. (2020) 08:1–11. doi: 10.26599/JNR.2020.9040004
39. Huang H, Chen L, Mao G, Sharma HS. Clinical neurorestorative cell therapies: Developmental process, current state and future prospective. J Neurorestoratol. (2020) 8:61–82. doi: 10.26599/JNR.2020.9040009
40. Guo X, Feng Y, Sun T, Feng S, Tang J, Chen L, et al. Clinical guidelines for neurorestorative therapies in spinal cord injury (2021 China version). J Neurorestoratol. (2021) 9:31–49. doi: 10.26599/JNR.2021.9040003
41. Burma NE, Leduc-Pessah H, Fan CY, Trang T. Animal models of chronic pain: advances and challenges for clinical translation. J Neurosci Res. (2017) 95:1242–56. doi: 10.1002/jnr.23768
42. Kumar A, Kaur H, Singh A. Neuropathic pain models caused by damage to central or peripheral nervous system. Pharmacol Rep. (2018) 70:206–16. doi: 10.1016/j.pharep.2017.09.009
43. Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, et al. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast. (2015) 2015:630932. doi: 10.1155/2015/630932
44. Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernandez C, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. (2017) 19:349–59. doi: 10.1016/j.jcyt.2016.12.002
45. Vaquero J, Zurita M, Rico MA, Aguayo C, Fernandez C, Rodriguez-Boto G, et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. (2018) 20:796–805. doi: 10.1016/j.jcyt.2018.04.006
46. Nakhjavan-Shahraki B, Yousefifard M, Rahimi-Movaghar V, Baikpour M, Nasirinezhad F, Safari S, et al. Transplantation of olfactory ensheathing cells on functional recovery and neuropathic pain after spinal cord injury; systematic review and meta-analysis. Sci Rep. (2018) 8:325. doi: 10.1038/s41598-017-18754-4
47. Hosseini M, Yousefifard M, Aziznejad H, Nasirinezhad F. The effect of bone marrow-derived mesenchymal stem cell transplantation on allodynia and hyperalgesia in neuropathic animals: a systematic review with meta-analysis. Biol Blood Marrow Transplant. (2015) 21:1537–44. doi: 10.1016/j.bbmt.2015.05.008
48. Llewellyn-Smith IJ, Basbaum AI, Braz JM. Long-term, dynamic synaptic reorganization after GABAergic precursor cell transplantation into adult mouse spinal cord. J Comp Neurol. (2018) 526:480–95. doi: 10.1002/cne.24346
49. Cafferata EA, Jerez A, Vernal R, Monasterio G, Pandis N, Faggion CMJr. The therapeutic potential of regulatory T lymphocytes in periodontitis: a systematic review. J Periodontal Res. (2019) 54:207–17. doi: 10.1111/jre.12629
50. Eaton MJ, Berrocal Y, Wolfe SQ. Potential for cell-transplant therapy with human neuronal precursors to treat neuropathic pain in models of PNS and CNS injury: comparison of hNT2.17 and hNT2.19 cell lines. Pain Res Treat. (2012) 2012:356412. doi: 10.1155/2012/356412
51. Liu H, Li W, Xu B, Jiang J, Zhang Y, Yang F. Stereological study of changes of GABA-immunoreactive neurons in spinal dorsal horn of SNI rats. Biomed Res Int. (2021) 2021:6633834. doi: 10.1155/2021/6633834
52. Schoffnegger D, Heinke B, Sommer C, Sandkuhler J. Physiological properties of spinal lamina II GABAergic neurons in mice following peripheral nerve injury. J Physiol. (2006) 577:869–78. doi: 10.1113/jphysiol.2006.118034
Keywords: GABAergic neurons, cell transplantation, neuropathic pain, mechanical allodynia, heat hyperalgesia
Citation: Zhang Z-R, Wu Y, Wang W-J and Wang F-Y (2022) The Effect of GABAergic Cells Transplantation on Allodynia and Hyperalgesia in Neuropathic Animals: A Systematic Review With Meta-Analysis. Front. Neurol. 13:900436. doi: 10.3389/fneur.2022.900436
Received: 20 March 2022; Accepted: 13 June 2022;
Published: 04 July 2022.
Edited by:
Massimiliano Valeriani, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Andrew M. Tan, Yale University, United StatesCopyright © 2022 Zhang, Wu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Yong Wang, d2Z5YmVpamluZ0AxNjMuY29t; orcid.org/0000-0002-9499-8654
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.