
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 04 July 2022
Sec. Dementia and Neurodegenerative Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.897867
This article is part of the Research TopicNon-Motor Dysfunction in Parkinson's DiseaseView all 5 articles
Backgrounds: Bile acid (BA) plays a crucial role in various neurodegenerative diseases, including Parkinson's disease (PD). However, no clinical evidence supports BA's potential role in patients with PD with mild cognitive impairment (PD-MCI).
Objectives: This study aimed at investigating the differential BA profile between patients with PD-MCI and those with normal cognitive function (PD-NC).
Methods: Ultra-high performance liquid chromatography-MS/MS was applied for BA quantitation. After between-group differences of the BA profile were addressed, orthogonal projections to latent structures—discriminant analysis (OPLS-DA) and the area under the receiver-operating-characteristic curve (AUC-ROC) were implemented for further verification.
Results: Lower levels of chenodeoxycholic acid (CDCA), cholic acid (CA), and ursodeoxycholic acid (UDCA) were significantly associated with PD-MCI (p < 0.01 for both; VIP ≈ 2.67, 1.66, and 1.26, respectively). AUC-ROC were 78.1, 74.2, and 74.5% for CDCA, CA, and UDCA, respectively.
Conclusion: CA, CDCA, and UDCA might be distinct BA signatures for patients with PD-MCI.
Cognitive impairment is considered one of the common non-motor symptoms (NMS) of Parkinson's disease (PD). There has been increasing awareness of the high incidence and damaging effects of cognitive impairment in PD in recent years. At the time when a PD diagnosis is established, almost all patients with PD suffer from some level of cognitive impairment (1). However, when the severity of cognitive impairment is not enough to significantly impair patients' daily life and functional independence, it is often ignored by patients or physicians in clinical practice (2). Although severe cognitive decline is not inevitable in all cases, patients with PD are six times more likely to develop dementia than the general population (3), significantly affecting patient functioning. Caring for someone with Parkinson's disease dementia (PDD) poses profound emotional, physical, and financial challenges for families and communities. Although there are some palliative treatments for the symptoms of dementia, there are no known disease-modifying treatments for PDD (1).
Mild cognitive impairment in PD (PD-MCI) is defined as an intermediate cognitive stage between PD with normal cognition (PD-NC) and PDD. Compared with patients with PD-NC, patients with PD-MCI have a higher risk of developing into PDD (4). Longitudinal studies have shown that the annual probability of patients with PD-MCI progressing to PDD is 6–15%, and the conversion rate of progressing to PDD within 5 years is about 39% (5). Within 3 years of follow-up, 28% of patients with PD-MCI returned to a state with normal cognitive function (6). When follow-up time was longer than 3 years, the reversion rate decreased, while the conversion rates to MCI and dementia increased (6). Therefore, PD-MCI is a critical period for early intervention of PDD. Accurate identification of PD-MCI and early intervention can help reduce the probability of PD-MCI converting to PDD.
The Movement Disorder Society (MDS) Task Force on MCI in PD has established an operative definition of PD-MCI to homogenize clinical practice and research (7). According to this definition, the formal diagnosis of PD-MCI requires the presence of four key features and the absence of exclusion criteria, mainly based on a comprehensive neuropsychological assessment. However, this has some limitations: first, it is time-consuming. In addition, the neuropsychological assessment results are subjective and affected by many factors, such as the patient's mental state and the severity of motor symptoms, so it is difficult to guarantee the homogeneity of results. Therefore, there is an urgent need to develop reliable diagnostic markers for PD-MCI, including new biochemical markers.
Bile acids (BA) are amphoteric molecules that mainly act as a solvent for dietary lipids and fat-soluble vitamins. The liver synthesizes primary bile acids, including cholic acid (CA), chenodeoxycholic acid (CDCA), and their conjugated form with glycine or taurine (8). Under the action of intestinal microorganisms, bile acids entering the intestine will undergo a multi-step metabolic reaction to generate secondary bile acids, such as deoxycholic acid (DCA), lithocholic acid (LCA), and ursodeoxycholic acid (UDCA) (9). Both primary and secondary BAs are present in the brains of mice with the ability to cross the blood-brain barrier (10–13). In recent years, BA signaling has been found to play a role in various neurodegenerative diseases (9, 14). Changes in BA metabolism and potential BA markers were observed in PD studies using rodent models (15–19). Li et al. found the upregulation of gut bacteria responsible for secondary bile acid synthesis and increased secondary bile acid DCA and LCA in patients with PD (20). Previous studies have also found that bile acids are associated with changes in cognitive impairment. Gut microbiota affect cognitive function by regulating primary bile acid metabolism in patients with Alzheimer's disease (AD) (21). However, bile acid has not been studied in PD cognitive impairment, and its specificity and sensitivity as biomarkers for early diagnosis are still unclear.
Plasma is an ideal sample for biomarker discovery and clinical diagnosis because it is simple to collect, relatively noninvasive, and easy to analyze. To test our hypothesis that the patients with PD-MCI showed changes in the bile acid profile, we performed a metabonomics analysis of plasma bile acids in the patients with PD-MCI and the patients with PD-NC.
This study was conducted in the Department of Neurology, Guangdong Provincial People's Hospital, Guangzhou, People's Republic of China. A diagnosis of idiopathic PD was assigned according to the MDS-Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS) (7) by 2 neurologists with experience in movement disorders. Exclusion criteria included: (1) patients with secondary Parkinsonism or Parkinson-plus syndrome; (2) patients with hyperlipidemia or diabetes mellitus; (3) patients with a tumor, heart failure, chronic obstructive pulmonary disease, nephritis, infectious disease, or other severe chronic diseases; (4) a history of cerebrovascular disease, intracranial surgery, head trauma, AD, epilepsy, mental illness, or other conditions that may affect cognitive function; (5) patients failing to cooperate or complete the trial.
Demographic information was collected from each subject, including sex, gender, age, education level, age of the onset, duration, medications, smoking, and drinking history. A detailed medical history and physical examination were collected from all the participants. Unified Parkinson's Disease Rating Scale Part III (UPDRS III) and Hoehn-Yahr Scale (H-Y) were used to assess motor function. Mental status was assessed by Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD).
Our neuropsychological protocol included Mini Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA) to assess general cognitive functioning, memory impairment, and executive dysfunction. The attention/working memory domain was tested by Symbol Digit Modalities Test (SDMT) and Digit Span Test (DST). Executive functions were evaluated by the Verbal Fluency Test (VFT) and Clock Drawing Test. Memory was assessed by visual reproduction (VR) and logical memory. Language function was tested by the Similarities Test and Category Fluency Task. Visuo-spatial and visuo-perceptive functions were assessed by the Block design Test. The patients were diagnosed with PD-MCI based on Movement Disorders Society Task Force Level II 20 using a cut-off of 1.5 standard deviations below standard scores in one or more cognitive domains in neuropsychological tests as previously described (7).
A fasting venous blood sample was collected with a lithium heparin tube from all the subjects in the morning. The serum was separated and stored in the refrigerator at −80°C until experimental analysis. Thirty BA metabolites in serum were detected by ultra-high performance liquid chromatography-MS/MS (UHPLC-MS/MS). After quality-control checks, the data set included 19 BAs (11 were too few for statistical analysis) for a total of 67 subjects.
Statistical analyses were performed using SPSS25 software. Between-group (PD-MCI vs. PD-NC) differences in terms of demographics, clinical data, and BA characteristics were addressed by independent-sample t-tests or Mann–Whitney U-test for continuous variables and χ2 for categorical variables. False discovery rate (FDR)-based multiple comparison adjustment with the Benjamini-Hochberg procedure was used for BAs comparison to increase the significance of the findings.
To simplify the complexity of the high-dimensional metabolome data, orthogonal projections to latent structures—discriminant analysis (OPLS-DA) was implemented to identify the differential expression of patients with BAs in PD with or without cognitive impairment. Two iterations of the permutation test verified the model. Through OPLS-DA, a variable importance in projection (VIP) value could be obtained for each metabolite. The larger the VIP is, the more significant the contribution of the metabolite to distinguish the two groups. Differential metabolites were defined as VIP >1.0 and FDR-corrected p-values < 0.05. Then, according to the HMDB database, these differentially expressed metabolites were chemically classified. The area under the receiver-operating-characteristic curve (AUC-ROC) was also calculated.
This study was performed following the principles outlined in the Declaration of Helsinki. Informed consent was obtained from all the individual participants included in the study.
A total of 33 patients with PD-NC and 34 patients with PD-MCI were enrolled in this study. Demographic and clinical characteristics for all the subjects are shown in Table 1. Categorical data are displayed as absolute numbers and continuous data as mean ± standard error. The demographic characteristics of the two groups were compared, including gender, age, and education level, and the differences were not statistically significant (p > 0.05), indicating comparability (Table 1).
Mean and standard errors of 19 detected BAs are presented in Table 2. After controlling for multiple testing using FDR, lower levels of cholic acid (CA), chenodeoxycholic acid (CDCA), ursodeoxycholic acid (UDCA), and 7-Ketolithocholic acid were significantly associated with PD-MCI compared to patients with PD-NC (corrected p < 0.01). The OPLS-DA model was also constructed to show statistical differences in CDCA (VIP ≈ 2.67), Glycochenodeoxycholic acid (GCDCA, VIP ≈ 1.846), CA (VIP ≈ 1.658), glycoursodeoxycholic acid (GUDCA, VIP ≈ 1.298), and UDCA (VIP ≈ 1.26) levels between the PD-MCI and PD-NC groups. We identified CA, CDCA, and UDCA with VIP > 1 and corrected p-value < 0.05 as the differential BA metabolites in patients with PD-MCI (Figure 1; Table 2). The data distribution characteristics of these 3 BAs are shown in Figures 2A–C. AUC-ROC for CA, CDCA, and UDCA were 74.2, 78.1, and 74.5%, respectively (Figures 2D–F; Table 2).
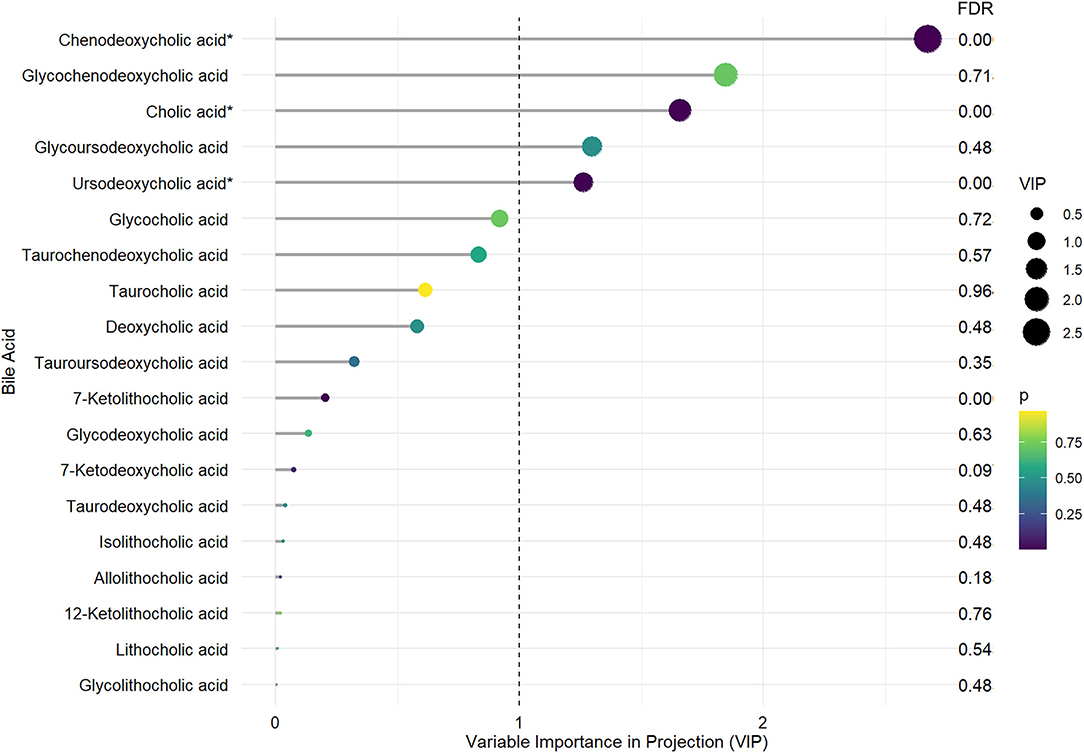
Figure 1. The distinct bile acid profile in PD-MCI compared to patients with PD-NC. The length of the lines and the size of the dots represent the value of variable importance in projection (VIP); the longer the lines and larger the dots, the larger the VIP value. The color of the dots represents the FDR-corrected p-value; the purple the color, the lower the p-value, and the yellower the color, the larger the p-value. *Differential BA metabolites with VIP >1. and FDR-corrected p-values < 0.05.
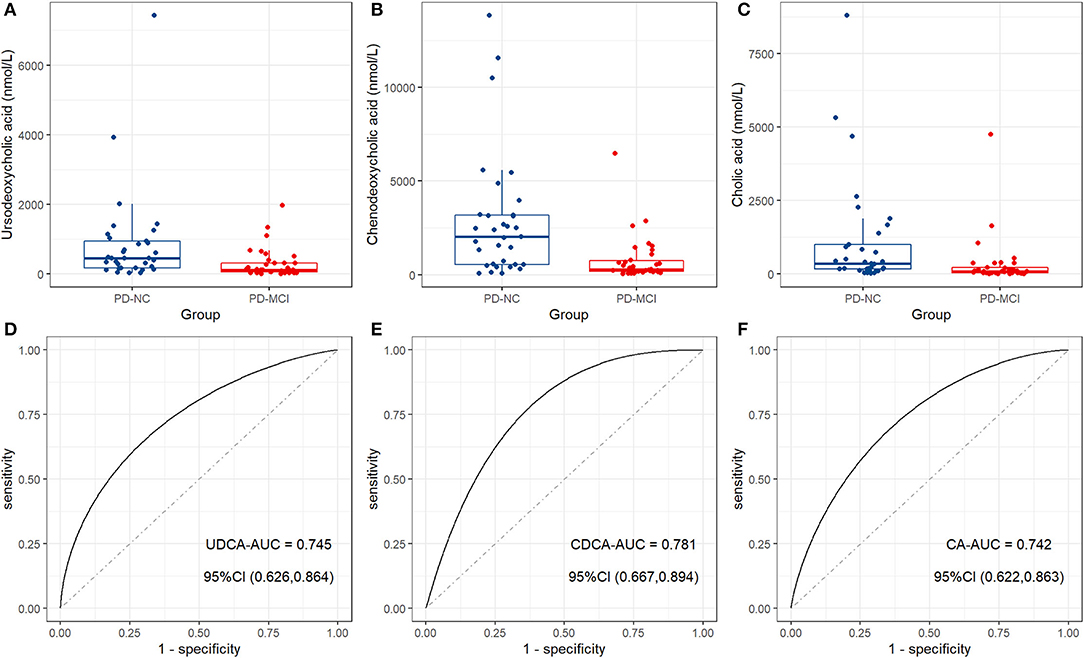
Figure 2. The distinct bile acid profile in patients with PD-MCI compared to patients with PD-NC. Box plots show the median (the horizontal line), 25–75 percentile (the box), and 5–95 percentile (the whisker) of the three BAs with VIP > 1 and FDR-corrected p-value < 0.05: (A) Ursodeoxycholic acid (UDCA); (B) chenodeoxycholic acid (CDCA); (C) cholic acid (CA). ROC curve analysis shows the diagnostic efficacy of CA (D), CDCA (E), and UDCA (F) for distinguishing between patients with PD-MCI and patients with PD-NC.
After determining the differential bile acids between the PD-MCI and PD-NC groups, we conducted correlation analysis between these bile acid levels and the scores of various cognitive scales to find the specific cognitive domains affected by bile acids. Unfortunately, we found no significant correlation between bile acid levels and specific cognitive domains.
Currently, the mechanisms of cognitive impairment in Parkinson's disease are not fully understood. There is a lack of effective therapeutic strategies to alleviate or delay cognitive deterioration in Parkinson's disease (1). Evidence shows that PD-MCI is an independent risk factor in the occurrence of PDD (4) and is a critical period for early intervention of PDD. Accurate identification of PD-MCI and early intervention can help reduce the probability of cognitive deterioration to PDD. Identifying biomarkers for PD-MCI is an essential step toward improving current diagnostic criteria. In this study, we, for the first time, explored changes in bile acid profiles in patients with PD-MCI, which may provide clues to understand the disease mechanism of PD-MCI and be used to identify abnormal biochemical pathways and therapeutic targets for new effective drugs.
Metabolomics data are high dimensional and massive. There might be highly relevant characteristics between certain variables. Univariate analysis, such as t-test, cannot quickly, thoroughly, and accurately mine the potential information in the metabolomics data. To reduce the risk of false-positive errors and model overfitting, we combined univariate analysis and OLPS-DA, one of the multivariate analysis methods, to explore the distinct BA profile in the patients with PD-MCI. We found that CA, CDCA, and UDCA were significantly decreased in the patients with PD-MCI compared with those with normal cognition.
We also found that certain types of BAs were statistically significant only in univariate analysis or multivariate analysis. For instance, although the corrected p-value for 7-ketolithocholic acid was <0.001, the VIP value was small. On the one hand, that is probably because of the different analytic perspectives that the two types of statistical methods offer. Univariate analysis pays more attention to the independent change of the metabolite levels, while multivariate analysis concentrates more on the influence of the metabolites in the metabolic process. On the other hand, the negative results might be explained by the small statistical power related to the small sample size of this study. In other words, there are significant changes in these BAs, but the statistical methods showed false-negative results.
The existence of BA is an emerging research topic in neurodegenerative disease (9). CA and CDCA are the only primary bile acids synthesized in humans, impacting fat digestion and energy metabolism signaling (22, 23). A previous study has shown that CBJC, a combination of Chinese herb active components, including cholic acid, can ameliorate the IBO-induced dementia in rats (24). CDCA has been shown to play a significant neuroprotective role in cerebrotendinous xanthomatosis (25, 26). Besides, CDCA significantly attenuates the cognitive dysfunction and spatial deficits induced by AlCl3 in the rat model (27). UDCA, another critical bile acid we discovered, is converted from CDCA catalyzed by 7α-hydroxysteroid dehydrogenase (7α-HSDH) and 7β-HSDH (28, 29). UDCA has been widely used in treating primary biliary cirrhosis and proved to have good safety and tolerability (30). It crosses the blood-brain barrier in a dose-dependent manner and improves mitochondrial function with anti-apoptotic effects in AD and PD models.
At the micro-level, BAs might be associated with cognitive functions in PD through the theory of neuroinflammation and apoptosis. CDCA could bind with Takeda G-protein-coupled receptor 5 (TGR5), a membrane receptor that alleviates neuroinflammation and inhibits cell apoptosis (31, 32). Besides, daily intraperitoneal injection of UDCA can significantly reduce the levels of nuclear factor-κB (NF-κB), Bcl2-related X apoptotic regulator (Bax), and caspase-9 mRNA, thus exerting anti-apoptotic effects (32). The interaction of BAs with anti-apoptotic substances may explain the relationships between BA and PD-MCI in some ways.
At a macro-level, abnormalities in BA metabolism may affect disease progression by altering structural networks in specific brain regions. Researchers have found that changes in BA of AD and patients with MCI were associated with changes in particular areas in brain imaging (21), which may also happen in patients with PD-MCI. We would combine metabolomics and radiomics to explore the association between BA, specifically CA, CDCA, and UDCA, and brain imaging changes in future studies.
Bidirectional biochemical communication between the brain and the gut is associated with various neurodegenerative diseases, including Parkinson's disease. α-synuclein, a pathological marker of PD, was found to accumulate in the gastrointestinal tract of patients with early PD (33). After intestinal flora obtained from feces of patients with PD was transplanted into the intestinal tract of germ-free rats, all the previously disease-free rats developed PD symptoms (34). This suggests that the gut microbiome might be a vital contributor to Parkinson's disease. In terms of cognitive impairment, our previous studies have found that patients with PD-MCI have unique gut microbial characteristics, suggesting that intestinal flora may be related to the pathogenic mechanism of PD-MCI.
Intestinal flora is an integral part of bile acid metabolism. In rats with PD symptoms that overexpress α- synuclein, intestinal microbial diversity was changed, and the levels of related primary bile acids were significantly increased and thus affecting motor symptoms (16). Another study has found that intestinal bacteria adjust the primary bile acid metabolism in patients with AD and, in turn, affect cognitive function (21). Increased levels of metabolites of intestinal microbiota, including deoxycholic acid and glycocholic acid, were significantly associated with thinning of cortical regions in bilateral frontal, parietal, and temporal lobes (21). This suggests that bile acids may serve as a “bridge” between gut microbiome disorder and the occurrence and development of cognitive impairment in PD.
Because the inclusion criteria were relatively strict, the total number of samples included was relatively small, which might decrease statistical power and cause some false-negative results. In addition, only the patients with PD-MCI and the patients with PD-NC were included in this study to explore the bile acid metabolites in PD cognitive impairment preliminarily. We will recruit more patients with PD with cognitive impairment in future studies, including those with dementia.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Research Ethics Committee, Guangdong Provincial People's Hospital, Guangdong Academy of Medical Sciences. The patients/participants provided their written informed consent to participate in this study.
KN, YL, and JZ contributed to conception and design of the study. KN and JZ organized the database. JZ performed the statistical analysis. YL wrote the first draft of the manuscript. KN, JZ, YQ, RG, and YG wrote sections of the manuscript. All the authors contributed to manuscript revision and read and approved the submitted version.
This work was supported by Science and Technology Planning Project of Guangzhou (202102080054); Guangdong Medical Research Foundation (A2021308); Science and Technology Planning Project of Guangdong Province (2020A0505140006).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, Weintraub D, et al. Cognitive decline in Parkinson disease. Nat Rev Neurol. (2017) 13:217–31. doi: 10.1038/nrneurol.2017.27
2. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
3. Martinez-Horta S, Kulisevsky J. Mild cognitive impairment in Parkinson's disease. J Neural Transm. (2019) 126:897–904. doi: 10.1007/s00702-019-02003-1
4. Goldman JG, Holden S, Bernard B, Ouyang B, Goetz CG, Stebbins GT. Defining optimal cutoff scores for cognitive impairment using Movement Disorder Society Task Force criteria for mild cognitive impairment in Parkinson's disease. Mov Disord. (2013) 28:1972–9. doi: 10.1002/mds.25655
5. Pedersen KF, Larsen JP, Tysnes OB, Alves G. Natural course of mild cognitive impairment in Parkinson disease: a 5-year population-based study. Neurology. (2017) 88:767–74. doi: 10.1212/WNL.0000000000003634
6. Domellöf ME, Ekman U, Forsgren L, Elgh E. Cognitive function in the early phase of Parkinson's disease, a five-year follow-up. Acta Neurol Scand. (2015) 132:79–88. doi: 10.1111/ane.12375
7. Litvan I, Goldman JG, Tröster AI, Schmand BA, Weintraub D, Petersen RC, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: movement Disorder Society Task Force guidelines. Mov Disord. (2012) 27:349–56. doi: 10.1002/mds.24893
8. Di Ciaula A, Garruti G, Lunardi Baccetto R, Molina-Molina E, Bonfrate L, Wang DQ, et al. Bile acid physiology. Ann Hepatol. (2017) 16:s4–s14. doi: 10.5604/01.3001.0010.5493
9. Grant SM, DeMorrow S. Bile acid signaling in neurodegenerative and neurological disorders. Int J Mol Sci. (2020) 21:982. doi: 10.3390/ijms21175982
10. Quinn M, McMillin M, Galindo C, Frampton G, Pae HY, DeMorrow S. Bile acids permeabilize the blood brain barrier after bile duct ligation in rats via Rac1-dependent mechanisms. Dig Liver Dis. (2014) 46:527–34. doi: 10.1016/j.dld.2014.01.159
11. Zheng X, Chen T, Zhao A, Wang X, Xie G, Huang F, et al. The brain metabolome of male rats across the lifespan. Sci Rep. (2016) 6:24125. doi: 10.1038/srep24125
12. Pan X, Elliott CT, McGuinness B, Passmore P, Kehoe PG, Hölscher C, et al. Metabolomic profiling of bile acids in clinical and experimental samples of Alzheimer's Disease. Metabolites. (2017) 7:28. doi: 10.3390/metabo7020028
13. Higashi T, Watanabe S, Tomaru K, Yamazaki W, Yoshizawa K, Ogawa S, et al. Unconjugated bile acids in rat brain: analytical method based on LC/ESI-MS/MS with chemical derivatization and estimation of their origin by comparison to serum levels. Steroids. (2017) 125:107–13. doi: 10.1016/j.steroids.2017.07.001
14. Ackerman HD, Gerhard GS. Bile acids in neurodegenerative disorders. Front Aging Neurosci. (2016) 8:263. doi: 10.3389/fnagi.2016.00263
15. Graham SF, Rey NL, Ugur Z, Yilmaz A, Sherman E, Maddens M, et al. Metabolomic profiling of bile acids in an experimental model of prodromal Parkinson's Disease. Metabolites. (2018) 8:71. doi: 10.3390/metabo8040071
16. O'Donovan SM, Crowley EK, Brown JR, O'Sullivan O, O'Leary OF, Timmons S, et al. Nigral overexpression of α-synuclein in a rat Parkinson's disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol Motil. (2020) 32:e13726. doi: 10.1111/nmo.13726
17. Moreira S, Fonseca I, Nunes MJ, Rosa A, Lemos L, Rodrigues E, et al. Nrf2 activation by tauroursodeoxycholic acid in experimental models of Parkinson's disease. Exp Neurol. (2017) 295:77–87. doi: 10.1016/j.expneurol.2017.05.009
18. Rosa AI, Duarte-Silva S, Silva-Fernandes A, Nunes MJ, Carvalho AN, Rodrigues E, et al. Tauroursodeoxycholic acid improves motor symptoms in a mouse model of Parkinson's Disease. Mol Neurobiol. (2018) 55:9139–55. doi: 10.1007/s12035-018-1062-4
19. Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic acid ameliorates apoptotic cascade in the rotenone model of parkinson's disease: modulation of mitochondrial perturbations. Mol Neurobiol. (2016) 53:810–7. doi: 10.1007/s12035-014-9043-8
20. Li P, Killinger BA, Ensink E, Beddows I, Yilmaz A, Lubben N, et al. Gut microbiota dysbiosis is associated with elevated bile acids in Parkinson's Disease. Metabolites. (2021) 11:29. doi: 10.3390/metabo11010029
21. Nho K, Kueider-Paisley A, MahmoudianDehkordi S, Arnold M, Risacher SL, Louie G, et al. Altered bile acid profile in mild cognitive impairment and Alzheimer's disease: relationship to neuroimaging and CSF biomarkers. Alzheimers Dement. (2019) 15:232–44. doi: 10.1016/j.jalz.2018.08.012
22. Needham BD, Kaddurah-Daouk R, Mazmanian SK. Gut microbial molecules in behavioural and neurodegenerative conditions. Nat Rev Neurosci. (2020) 21:717–31. doi: 10.1038/s41583-020-00381-0
23. Russell DW. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. (2003) 72:137–74. doi: 10.1146/annurev.biochem.72.121801.161712
24. Zhang J, Li P, Wang Y, Liu J, Zhang Z, Cheng W, et al. Ameliorative effects of a combination of baicalin, jasminoidin and cholic acid on ibotenic acid-induced dementia model in rats. PLoS ONE. (2013) 8:e56658. doi: 10.1371/journal.pone.0056658
25. Nie S, Chen G, Cao X, Zhang Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet J Rare Dis. (2014) 9:179. doi: 10.1186/s13023-014-0179-4
26. Amador MDM, Masingue M, Debs R, Lamari F, Perlbarg V, Roze E, et al. Treatment with chenodeoxycholic acid in cerebrotendinous xanthomatosis: clinical, neurophysiological, and quantitative brain structural outcomes. J Inherit Metab Dis. (2018) 41:799–807. doi: 10.1007/s10545-018-0162-7
27. Bazzari FH, Abdallah DM, El-Abhar HS. Chenodeoxycholic acid ameliorates alcl(3)-induced Alzheimer's disease neurotoxicity and cognitive deterioration via enhanced insulin signaling in rats. Molecules. (2019) 24:992. doi: 10.3390/molecules24101992
28. Kiriyama Y, Nochi H. The biosynthesis, signaling, and neurological functions of bile acids. Biomolecules. (2019) 9:232. doi: 10.3390/biom9060232
29. Lepercq P, Gérard P, Béguet F, Raibaud P, Grill JP, Relano P, et al. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by Clostridium baratii isolated from human feces. FEMS Microbiol Lett. (2004) 235:65–72. doi: 10.1111/j.1574-6968.2004.tb09568.x
30. Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet. (2015) 386:1565–75. doi: 10.1016/S0140-6736(15)00154-3
31. Nakhi A, McDermott CM, Stoltz KL, John K, Hawkinson JE, Ambrose EA, et al. 7-Methylation of chenodeoxycholic acid derivatives yields a substantial increase in TGR5 receptor potency. J Med Chem. (2019) 62:6824–30. doi: 10.1021/acs.jmedchem.9b00770
32. McMillin M, Frampton G, Tobin R, Dusio G, Smith J, Shin H, et al. TGR5 signaling reduces neuroinflammation during hepatic encephalopathy. J Neurochem. (2015) 135:565–76. doi: 10.1111/jnc.13243
33. Chandra R, Hiniker A, Kuo YM, Nussbaum RL, Liddle RA. α-Synuclein in gut endocrine cells and its implications for Parkinson's disease. JCI Insight. (2017) 2:e92295. doi: 10.1172/jci.insight.92295
Keywords: Parkinson's disease, mild cognitive impairment, bile acid metabolites, chenodeoxycholic acid, ursodeoxycholic acid, cholic acid
Citation: Nie K, Li Y, Zhang J, Gao Y, Qiu Y, Gan R, Zhang Y and Wang L (2022) Distinct Bile Acid Signature in Parkinson's Disease With Mild Cognitive Impairment. Front. Neurol. 13:897867. doi: 10.3389/fneur.2022.897867
Received: 16 March 2022; Accepted: 13 June 2022;
Published: 04 July 2022.
Edited by:
Jinyoung Youn, Sungkyunkwan University School of Medicine, South KoreaReviewed by:
Antonio Emanuele Elia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyCopyright © 2022 Nie, Li, Zhang, Gao, Qiu, Gan, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Wang, d2xqZ2Q2OEAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.