
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 24 June 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.893516
This article is part of the Research TopicOutcomes in Subarachnoid HaemorrhageView all 10 articles
 Kexin Yuan1†
Kexin Yuan1† Runting Li1†
Runting Li1† Yahui Zhao1
Yahui Zhao1 Ke Wang1
Ke Wang1 Fa Lin1
Fa Lin1 Junlin Lu1
Junlin Lu1 Yu Chen1
Yu Chen1 Li Ma1
Li Ma1 Heze Han1
Heze Han1 Debin Yan1
Debin Yan1 Ruinan Li1
Ruinan Li1 Jun Yang1
Jun Yang1 Shihao He1
Shihao He1 Zhipeng Li1
Zhipeng Li1 Haibin Zhang1
Haibin Zhang1 Xun Ye1
Xun Ye1 Hao Wang1
Hao Wang1 Hongliang Li2
Hongliang Li2 Linlin Zhang2
Linlin Zhang2 Guangzhi Shi2
Guangzhi Shi2 Jianxin Zhou2
Jianxin Zhou2 Yang Zhao3
Yang Zhao3 Yukun Zhang3
Yukun Zhang3 Youxiang Li4
Youxiang Li4 Shuo Wang1,5,6,7
Shuo Wang1,5,6,7 Xiaolin Chen1,6,7
Xiaolin Chen1,6,7 Yuanli Zhao1,3,5,6,7*
Yuanli Zhao1,3,5,6,7* Qiang Hao1*
Qiang Hao1*Objective: Postoperative pneumonia (POP) is one of the major complications after aneurysmal subarachnoid hemorrhage (aSAH) associated with postoperative mortality, prolonged hospitalization, and increased medical cost. Early recognition of pneumonia and more aggressive management may improve patient outcomes.
Methods: We retrospectively reviewed all patients with aSAH who were admitted to our institution between January 2015 and December 2020. Baseline clinical characteristics, imaging data, and inflammatory biomarkers were reviewed. The risk factors derived from multivariate logistic regression of surgical clipping (SC) and endovascular coiling (EC) were analyzed. The area under the receiver operating characteristic (ROC) curve (AUC) was used to calculate each independent predictor's prediction ability.
Results: A total of 843 patients were enrolled. Compared with patients in the EC group, the incidence of POP was higher in the SC group [143/414 (34.54%) vs. 114/429 (26.57%), p = 0.015]. In the EC group, multivariate analysis revealed that age [p = 0.001; odds ratio (OR) = 1.04, 95% CI = 1.02–1.07], posterior circulation aneurysms (p = 0.021; OR = 2.07, 95% CI = 1.14–3.83), higher neutrophil (NEUT; p < 0.001; OR = 1.13, 95% CI = 1.06–1.21), World Federation of Neurosurgical Societies (WFNS) grade 4 or 5 (p < 0.001; OR = 4.84, 95% CI = 2.67–8.79), modified Fisher Scale (mFS) grade 3 or 4 (p = 0.022; OR = 2.60, 95% CI = 1.15–5.89), and acute hydrocephalus (p = 0.048; OR = 1.74, 95% CI = 1.01–3.00) were independent risk factors for POP. In the SC group, multivariate analysis revealed that age (p = 0.015; OR = 1.03, 95% CI = 1.01–1.05), WFNS grade 4 or 5 (p = 0.037; OR = 1.76, 95% CI = 1.03–3.00), heart disease (p < 0.001; OR = 5.02, 95% CI = 2.03–12.45), higher white blood cell (WBC; p < 0.001; OR = 1.13, 95% CI = 1.07–1.20), and mFS grade 3 or 4 (p = 0.019; OR = 2.34, 95% CI = 1.15–4.77) were independent risk factors for POP.
Conclusion: Patients treated with SC are more likely to develop POP. Comprehensive preoperative evaluation of patients may help physicians to better predict POP and implement preventive measures to improve outcomes.
Aneurysmal subarachnoid hemorrhage (aSAH) is a severe neurosurgical emergency with a high mortality rate of 22–50% (1–3). Even patients receiving surgical clipping (SC) or endovascular coiling (EC) therapy also left approximately one-third of the patients to suffer from being severely disabled and functionally dependent. Early and reliable prediction of the patients' condition after SAH is important in clinical practice for decision-making about treatment options and providing information for the patients and their families (4, 5).
Severe in-hospital complications may affect the prognosis of patients and increase the medical burden on patients' families and countries. Therefore, early identification of complications associated with poor patient prognosis has considerable clinical importance (6, 7). Postoperative pneumonia (POP) is one of the major complications after aSAH surgery and is associated with postoperative mortality, prolonged hospitalization, and increased medical cost (5, 8).
Three large studies have shown that patients with SC had a higher incidence of hospital complications than those with EC, leading to a higher risk of poor discharge outcomes and even long-term disability (9–12). Our team's research showed that POP may have a long-lasting impact on the prognosis of patients (5). Thus, early identification and active prevention of POP become critical. Some studies have tried to identify risk factors associated with POP; however, the two treatment modalities' preoperative indicators related to risk factors for POP have not been reported (13, 14).
This study retrospectively reviewed the preoperative indicators associated with POP in SC and EC groups. Further, we analyzed the potential causes of pneumonia due to these factors to provide clinical evidence for preventing and treating POP.
We retrospectively reviewed the patient data from consecutive patients with aSAH who were admitted to our institution between January 2015 and December 2020. All patient data were from the Long-Term Prognosis of Emergency Aneurysmal Subarachnoid Hemorrhage (LongTEAM) study. The registry is listed at ClinicalTrials.gov (registration no. NCT 04785976).
This study was approved by the Institutional Review Board of Beijing Tiantan Hospital, Capital Medical University (KY 2021-008-01). All participants or their authorized representatives obtained informed consent for clinical analyses. All the analyses were performed by the Declaration of Helsinki and the local ethics policies. Both procedures were performed by specific senior neurosurgeons, with an annual average of more than 250 procedures per neurosurgeon. All patients were managed according to the American Heart Association/American Stroke Association guidelines (15).
All patients had angiographically documented aSAH caused by intracranial aneurysm confirmed by computed tomography (CT) or lumbar puncture. The inclusion criteria were as follows: (1) age ≥18 years; (2) single aneurysm; (3) emergency admission without previous aneurysm rupture; (4) only patients treated by clipping or interventional; (5) complete 90-day follow-up; and (6) no missing data. The exclusion criteria were as follows: (1) admitted over 72 h from rupture to the emergency department; (2) other neurological diseases (tumor, vascular malformation, Parkinson's disease, multiple sclerosis, and primary epilepsy), and functional or neurological deficit of the extremities due to any cause; (3) history of neurosurgery before rupture; and (4) treatments, such as external ventricular drainage, lumbar puncture, angiography, intubation, and mechanical ventilation, at other hospitals before presentation to our hospital.
Baseline clinical characteristics and imaging data were reviewed, such as age, sex, location of the ruptured aneurysm, Graeb score, acute hydrocephalus, and medical and medication history. The severity of aSAH was assessed based on the initial World Federation of Neurosurgical Societies (WFNS) grade and the modified Fisher Scale (mFS) grade. We also collected inflammatory markers, such as white blood cell (WBC), systemic inflammation response index (SIRI), neutrophil (NEUT), monocyte (MONO), monocyte-to-lymphocyte ratio (MLR), platelet-to-white blood cell ratio (PWR), platelet-to-neutrophil ratio (PNR), and neutrophil-to-lymphocyte ratio (NLR).
Postoperative clinical complications, such as rebleeding, delayed cerebral ischemia (DCI), seizures, intracranial infection, stress ulcer bleeding, abnormal hepatic function, urinary tract infection, anemia, hypoproteinemia, POP, and deep vein thrombosis (DVT), during hospitalization were collected. The modified Rankin Scale (mRS) scores were collected at discharge and 90 days after discharge.
The primary outcome was the occurrence of POP. POP was defined as fever, increased WBC and C-reactive protein (CRP) levels, and chest radiograph showed pulmonary infiltrates within 30 days after surgery, which required antibiotic therapy by a surgeon, according to the modified Centers for Disease Control and Prevention criteria (16).
The secondary outcome was the mRS [a stroke outcome scale with scores ranging from 0 (no symptoms) to 6 (dead)] score at discharge and 90 days after discharge (the neurosurgeon followed up with patients via telephone or an outpatient appointment 90 days after discharge). Unfavorable outcomes were defined when the mRS score was ≥3.
Categorical variables were presented as frequency (percentages), and continuous variables were presented as the means ± standard deviations (SD) or median and interquartile range (IQR). In comparing baseline characteristics and outcomes between groups, the Pearson's chi-square test or Fisher's exact test was used to compare categorical variables as appropriate. After testing for normality, continuous variables were analyzed using the independent Student's t-test or Mann-Whitney U rank-sum test (as appropriate). According to the diagnosis of POP, we divided the patients into two groups and performed the univariate regression analysis. Only variables with p < 0.05 in univariate analysis were entered in multivariate logistic regression analysis, with adjustments for other characteristics, a forward stepwise model was used to identify the independent predictors of POP between groups. The odds ratio (OR) and 95% confidence intervals (CIs) of variables were calculated. The sensitivities and specificities of predictive factors were calculated from the receiver operating characteristic (ROC) curve analyses. The area under the ROC curve (AUC) was calculated to measure each independent predictor's prediction ability. p < 0.05 was considered to be statistically significant. Statistical analyses were performed using the R statistical program (R studio; version 3.3.3), SPSS Statistics version 26.0 (IBM Corp.), and GraphPad PRISM 8.3.0 (GraphPad Software Inc.).
A total of 843 patients in the retrospective cohort who had their hospitalization between January 2015 and December 2020 were enrolled in the present study (Figure 1). Compared with the SC group, patients in the EC group had a higher proportion of female patients [265/429 (61.8%) vs. 226/414 (54.6%), p = 0.041] and a higher proportion of posterior circulation aneurysms [77/429 (18.0%) vs. 12/414 (2.9%), p < 0.001; Table 1].
Compared with the EC group, patients in the SC group had higher incidences of DCI [136/414 (32.9%) vs. 90/429 (21.0%), p < 0.001], intracranial infection [83/414 (20.25%) vs. 10/429 (2.33%), p < 0.001], anemia [173/414 (41.79%) vs. 85/429 (19.81%), p < 0.001], hypoproteinemia [194/414 (46.86%) vs. 94/429 (21.91%), p < 0.001], and POP [143/414 (34.54%) vs. 114/429 (26.57%), p = 0.015] and a lower incidence of urinary tract infection [4/414 (0.97%) vs. 19/429 (4.43%), p = 0.004; Table 2]. The SC group had a higher incidence of unfavorable outcome at discharge [201/414 (48.55%) vs. 150/429 (34.97%), p < 0.001] and 90 days after discharge [92/414 (22.22%) vs. 62/429 (14.45%), p < 0.001].
Multivariate analysis showed that age (p < 0.001; OR = 1.03, 95% CI = 1.01–1.04), female patients (p = 0.012; OR = 1.53, 95% CI = 1.10–2.14), WFNS grade 4–5 (p < 0.001; OR = 4.43, 95% CI = 3.09–6.37), mFS grade 3 or 4 (p < 0.001; OR = 3.04, 95% CI = 1.82–5.07), and SC (p = 0.031; OR = 1.43, 95% CI = 1.03–1.98) were independently associated with POP (Table 3).
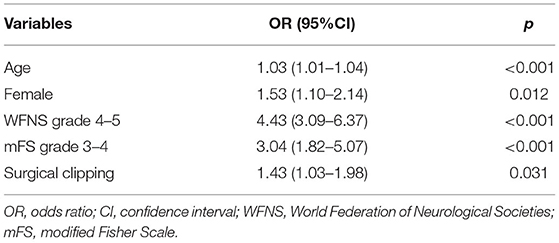
Table 3. Independent risk factors associated with postoperative pneumonia (POP) in all patients (N = 843).
Patients who had POP in the EC group were more likely to have intraventricular hemorrhage on admission [94/114 (82.46%) vs. 173/315 (54.92%), p < 0.001], a higher incidence of WFNS grade 4 or 5 [57/114 (50.00%) vs. 30/315 (9.52%), p < 0.001], a higher incidence of mFS grade 3 or 4 [106/114 (92.98%) vs. 219/315 (69.52%), p < 0.001], a higher incidence of acute hydrocephalus [106/114 (62.28%) vs. 109/315 (34.60%), p < 0.001], a higher incidence of posterior circulation [33/114 (28.95%) vs. 44/315 (13.97%), p < 0.001], a higher incidence of hypertension [88/114 (77.19%) vs. 185/315 (58.73%), p < 0.001], a higher incidence of heart disease [19/114 (16.67%) vs. 21/315 (6.67%), p = 0.003], and higher level of inflammatory biomarkers, such as WBC [14.94 (11.32–17.96) vs. 11.55 (9.32–14.39), p < 0.001], MONO [0.45 (0.30–0.67) vs. 0.36 (0.25–0.54), p < 0.001], NEUT [13.10 (10.02–16.19) vs. 10.20 (7.63–12.91), p < 0.001], SIRI [6.17 (3.58–10.32) vs. 3.56 (2.25–5.77), p < 0.001], MLR [0.47 (0.35–0.67) vs. 0.36 (0.26–0.51), p < 0.001], a lower level of PWR [15.98 (13.17–22.74) vs. 19.58 (15.76–23.47), p = 0.004], and a lower level of PNR [17.95 (14.61–19.65) vs. 22.44 (17.50–28.40), p = 0.002; Table 4).
Patients who had POP in the SC group were more likely to have intraventricular hemorrhage on admission [102/143 (71.33%) vs. 156/271 (57.56%), p = 0.008], a higher incidence of WFNS grade 4 or 5 [52/143 (36.36%) vs. 43/271 (15.87%), p < 0.001], a higher incidence of mFS grade 3 or 4 [131/143 (91.61%) vs. 205/271 (75.65%), p < 0.001], a higher incidence of heart disease 19/143 (13.29%) vs. 8/271 (2.95%), p < 0.001], a higher incidence of current use of anti-platelet aggregation drugs [12/143 (8.39%) vs. 7/271 (2.58%), p = 0.015], and higher level of inflammatory biomarkers, such as WBC [13.59 (11.51–17.37) vs. 11.57 (8.78–14.31), p < 0.001], MONO [0.47 (0.32–0.74) vs. 0.40 (0.27–0.56), p < 0.001], NEUT [11.60 (9.87–16.00) vs. 10.18 (7.52–12.93), p < 0.001], SIRI [5.44 (3.23–10.49) vs. 3.43 (2.34–6.24), p < 0.001], MLR [0.50 (0.32–0.73) vs. 0.38 (0.25–0.55), p < 0.001], PNR [18.29 (13.92–23.39) vs. 22.21 (17.02–28.98), p = 0.005], NLR [12.71 (8.56–20.33) vs. 11.06 (6.32–15.90), p = 0.007], and a lower level of PWR [2.00 (1.37–3.15) vs. 19.37 (14.98–23.83), p < 0.001; Table 5].
Multivariate analysis showed that age (p = 0.001; OR = 1.04, 95% CI = 1.02–1.07), posterior circulation aneurysms (p = 0.021; OR = 2.07, 95% CI = 1.14–3.83), higher NEUT (p < 0.001; OR = 1.13, 95% CI = 1.06–1.21), WFNS grade 4 or 5 (p < 0.001; OR = 4.84, 95% CI = 2.67–8.80), mFS grade 3 or 4 (p = 0.022; OR = 2.60, 95% CI = 1.15–5.89), and acute hydrocephalus (p = 0.048; OR = 1.74, 95% CI = 1.01–3.00) were independently associated with POP in the EC group (Table 6).
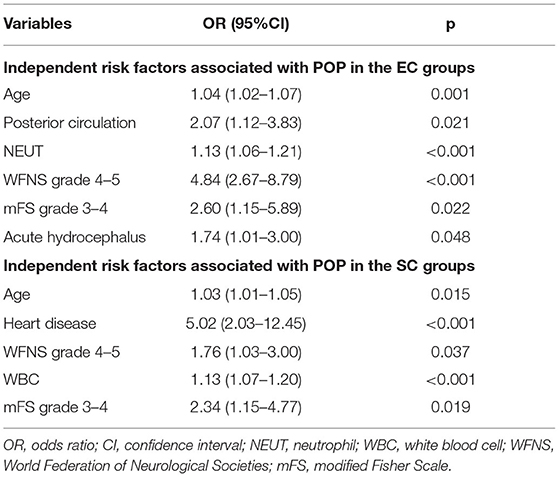
Table 6. Independent risk factors associated with postoperative pneumonia (POP) in the endovascular coiling (EC) and surgical clipping (SC) groups.
Multivariate analysis showed that age (p = 0.015; OR = 1.03, 95% CI = 1.01–1.05), WFNS grade 4 or 5 (p = 0.037; OR 1.76, 95% CI 1.03–3.00), heart disease (p < 0.001; OR = 5.02, 95% CI = 2.03–12.45), higher WBC (p < 0.001; OR = 1.13, 95% CI = 1.07–1.20), and mFS grade 3 or 4 (p = 0.019; OR = 2.34, 95% CI = 1.15–4.77) were independently associated with POP in the SC group (Table 5).
In ROC analysis, when the AUC of a variable was >0.7, the predictor variable was defined as a good predictor. The AUC values for each independent risk factor that predicts POP in the SC group and EC group are shown in Figures 2, 3, respectively.
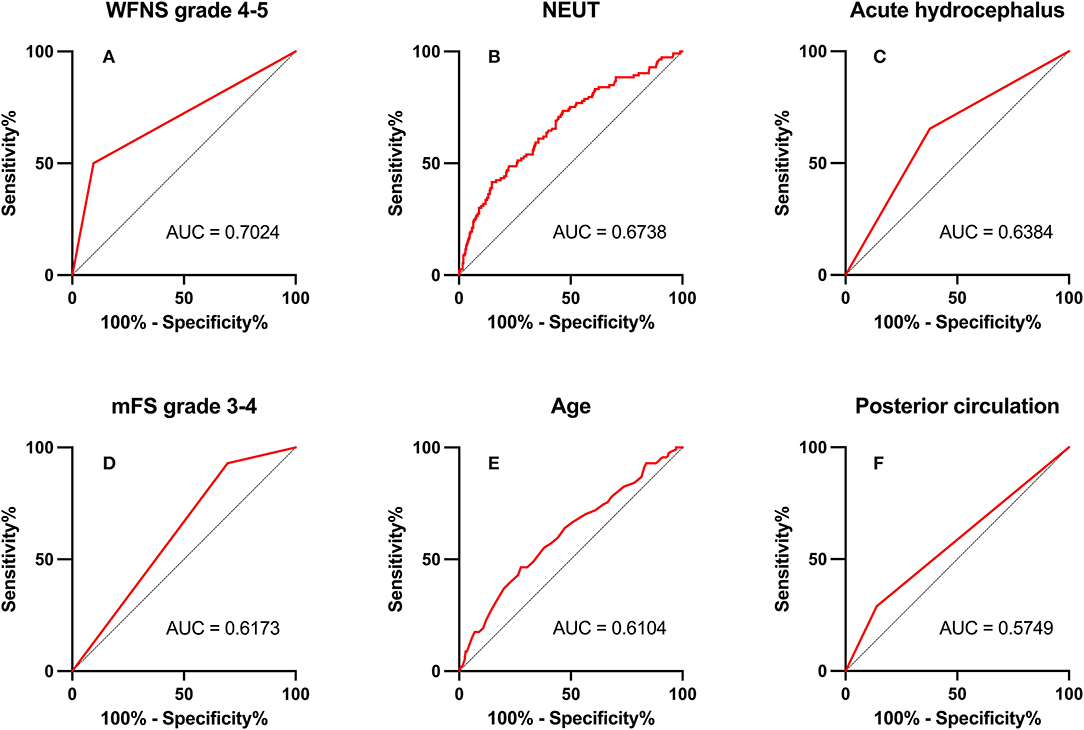
Figure 2. Area under the receiver operating characteristic (AUC) values for preoperative factors that predicted postoperative pneumonia in the endovascular coiling (EC) group. (A) World Federation of Neurosurgical Societies (WFNS) grade 4–5 (AUC = 0.702, 95% CI = 2.67–8.80; p < 0.001). (B) Neutrophil (NEUT; AUC = 0.674, 95% CI = 1.06–1.22; p < 0.001). (C) Acute hydrocephalus (AUC = 0.638, 95% CI = 1.01–3.00; p = 0.048). (D) modified Fisher Scale (mFS) grade 3–4 (AUC = 0.617, 95% CI = 1.15–5.89; p = 0.022). (E) Age (AUC = 0.610, 95% CI = 1.02–1.07; p = 0.001). (F) Posterior circulation (AUC = 0.575, 95% CI = 1.12–3.83; p = 0.021).
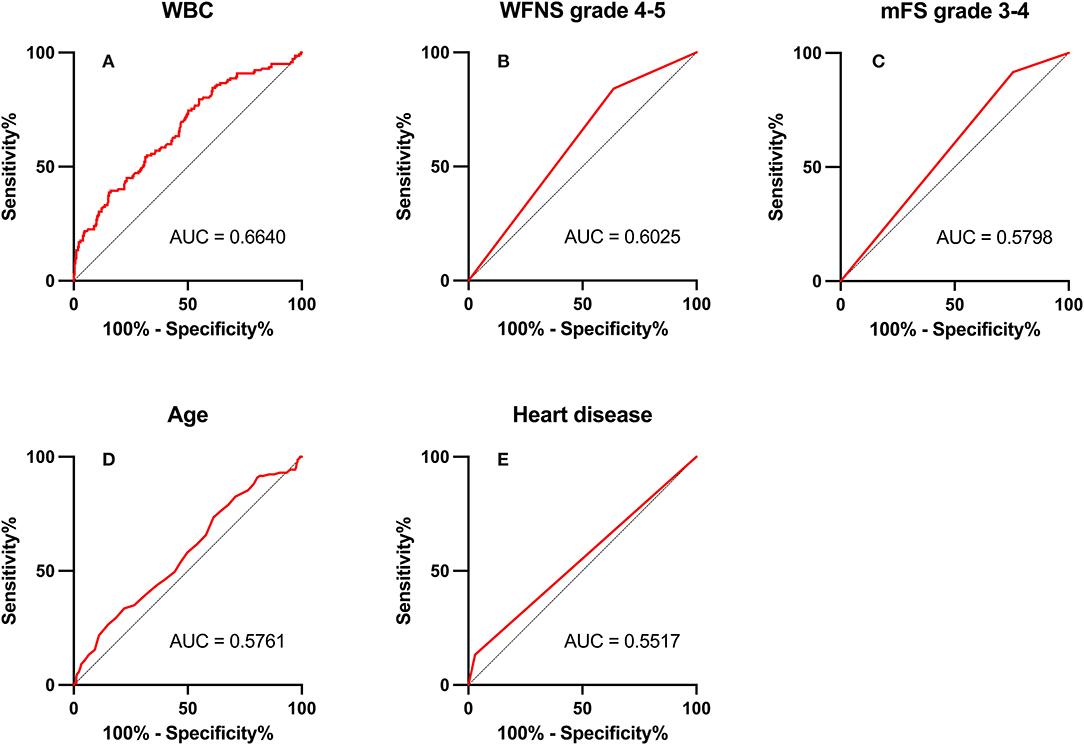
Figure 3. Area under the receiver operating characteristic (AUC) values for preoperative factors that predicted postoperative pneumonia in the surgical clipping (SC) group. (A) White blood count (WBC; AUC = 0.664, 95% CI = 1.07–1.20; p < 0.001). (B) World Federation of Neurosurgical Societies (WFNS) grade 4–5 (AUC = 0.603, 95% CI = 1.03–3.00; p = 0.037). (C) Modified Fisher Scale (mFS) grade 3–4 (AUC = 0.580, 95% CI = 1.15–4.77; p = 0.019). (D) Age (AUC = 0.576, 95% CI = 1.01–1.05; p = 0.015). (E) Heart disease (AUC = 0.552, 95% CI = 2.03–12.45; p < 0.001).
Among preoperative indicators, WFNS grade 4 or 5 showed good predictive ability for POP in the EC group (AUC = 0.702, 2.67–8.80; p < 0.001).
Postoperative pneumonia is one of the major complications after aSAH surgery associated with postoperative mortality, prolonged hospitalization, and increased medical cost (6, 17–19). Our previous study revealed that among in-hospital complications, the occurrence of POP showed good predictive efficacy of 90-day unfavorable outcomes in the SC group and EC group (AUC > 0.7) (5). Therefore, trying to identify the occurrence of POP at an early stage is of great significance to improve patient outcomes.
In previous studies, SC was an independent risk factor for the occurrence of POP after aSAH (13). Therefore, we should pay more attention to the baseline characteristics and laboratory tests before the occurrence of POP after SC and EC to predict the occurrence of POP in advance and take preventive measures in time. Unlike previous studies, this study paid more attention to distinguish between groups with different treatment modalities. Our study showed that age, WFNS grade 4 or 5, and mFS grade 3 or 4 were common independent risk factors associated with POP in both groups.
Aspiration of oropharyngeal fluid containing pathogenic microorganisms is the most common cause of POP (20). In addition, the more bacteria inhaled, the greater the likelihood of aspiration pneumonia (21, 22). Most aSAH operations are emergency operations with inadequate preoperative preparation, and patients often have symptoms of vomiting. Preoperative respiratory exercise and oral care are rarely performed, resulting in more oral bacteria and an increased risk of POP. With the increase in age, the immune function of the human body gradually decreases, the tracheal ciliary protective movement and cough reflex become worse, and the bacterial colonization of the oral and upper respiratory tract is significantly increased, leading to a significant increase in the risk of aspiration. At the same time, the increase in age will also lead to organ function failure, resulting in slower drug metabolism after general anesthesia, prolonged neuromuscular block time after anesthesia, and increased the probability of aspiration, ultimately leading to an increased incidence of POP (23, 24). Patients with a higher initial WFNS grade are more likely to stay in the hospital longer, increasing the probability of aspiration, which in turn increases the potential risk of POP. Previous studies have shown that poor postoperative awareness may be a risk factor for poor prognosis, which may also be partly related to the increased incidence of POP (14). Meanwhile, we analyzed the correlation between WFNS grade and inflammatory indicators and found that WBC and NEUTs were significantly higher in WFNS grade 4–5 patients than in WFNS grade 1–3 patients, that is to say, patients with high WFNS grade may have a more severe inflammatory response.
Studies in mouse models have shown that NEUTs may be important mediators of early cortical hypoperfusion and oxidative stress after aSAH (25). Depletion of NEUTs 3 days after SAH mitigates tissue inflammation and reverses cerebral vasoconstriction in the middle cerebral artery (26); thus, NEUTs are closely associated with oxidative stress and cerebral vasoconstriction. Increased NEUTs or WBCs in the blood before surgery may set the stage for a more intense early systemic inflammatory response in the early stages of brain injury, increasing susceptibility to systemic infections, such as POP. Meanwhile, our data showed that patients with high WFNS grade or high mFS grade had a severe inflammatory response (Figure 4).
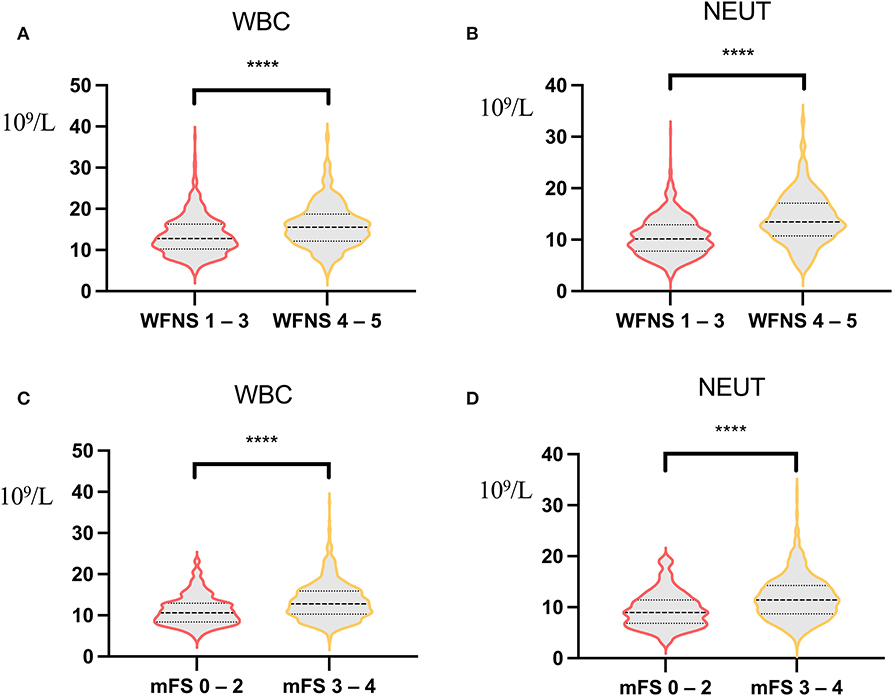
Figure 4. Correlation analysis of World Federation of Neurosurgical Societies (WFNS) score, modified Fisher Scale (mFS) score, and inflammatory indicators. (A) Correlation analysis of white blood count (WBC) between WFNS grade 1–3 and WFNS grade 4–5 [12.14 (3.88) vs. 15.88 (5.19), p < 0.001]. (B) Correlation analysis of neutrophil between WFNS grade 1–3 and WFNS grade 4–5 [10.59 (3.78) vs. 14.03 (4.82), p < 0.001]. (C) Correlation analysis of WBC between mFS grade 0–2 and mFS grade 3–4 [11.18 (3.59) vs. 13.44 (4.57), p < 0.001]. (D) Correlation analysis of neutrophil between mFS grade 0–2 and mFS grade 3–4 [9.50 (3.52) vs. 11.85 (4.31), p < 0.001). ****p < 0.001.
Hydrocephalus caused by aSAH can also manifest as high cranial pressure, which can lead to Cushing's reaction (CR). CR compensates for hypothalamic-mediated acute hyperemic response to intracranial pressure (ICP) increment. In patients with aSAH, CR mainly presents as sympathetic peaks with increased serum epinephrine and/or norepinephrine levels. High levels of these two hormones mediate early lymphocyte activation defects leading to POP (27). The severity of CR at SAH depends on the height of ICP and depends on the amount of blood that ruptured into the subarachnoid space (20, 28). Patients with high mFS grade may develop a persistent and excessive systemic inflammatory response syndrome that leads to immunosuppression and is more prone to POP.
Since posterior circulation aneurysms originate anteriorly in the brain stem, rupture and bleeding of posterior circulation aneurysms may affect the brain stem. Both the International Subarachnoid Aneurysm Trial (ISAT) (9) and International study of Unrupted intracranial Aneurysms (ISUIA) (29) studies confirmed that the complication rate of SC for posterior circulation aneurysms is much higher than that of EC treatment. The posterior circulation aneurysm in our center is also mainly treated by EC. At the same time, posterior circulation aneurysms are more likely than other aneurysms to cause an increase in ICP and hydrocephalus. In this case, the EC treatment itself does not improve the condition of hydrocephalus. All of these aggravated CR and increased the risk of POP. Rupture of posterior circulation aneurysm can also increase the incidence of cerebral vasospasm, which affects the cerebral nerves in the posterior approach group, thereby increasing the risk of vomiting and aspiration, leading to an increased incidence of POP (30). Patients with posterior circulation aneurysms with other risk factors of POP, such as a high WFNS grade and a high mFS grade, can be considered an early routine ICP reduction, such as lumbar cistern drainage, in addition to EC therapy.
For patients with pre-existing heart disease, cardiac dysfunction will lead to decreased lung function, significantly increase the probability of cardiogenic pulmonary edema, seriously impair lung ventilation and ventilation function, and reduce the ability of the lung to fight infection (31). They are more likely to develop POP after a traumatic craniotomy.
Therefore, we should pay more attention to preoperative indicators, especially preoperative laboratory indicators, such as WBC and NEUT, which may be of great significance for the identification of early POP. More prospective trials should be conducted in the future to confirm our results.
For patients who underwent elective coronary artery bypass-grafting surgery, a previous study has proved that taking some preventive, tailored interventions to improve inspiratory muscle function can reduce the incidence of POP by 50%. This raises the possibility of preoperatively identifying patients at high risk for POP and taking preventive measures to reduce the incidence of POP after surgery (32). However, aSAH is mostly emergency surgery, which makes our time even more stressful. Advances in surgical techniques have improved patient outcomes, meanwhile, another challenge is the periprocedural management of patients for anesthesiologists and intensivists (33). At present, there is no recognized effective measure to prevent the occurrence of POP. This is also the goal of our further study, starting with preoperative inflammatory parameters and serum epinephrine and/or norepinephrine levels to reduce the secondary attack of surgery on the lungs. We also plan to collect prospective data and build a scoring model for pneumonia in further studies to make it easier for all newly admitted patients to identify the potential risk of pneumonia as early as possible.
Once associated preoperative risk factors are identified, patients at high risk should take targeted preventive measures, such as quitting smoking as soon as physical examination reveals an unruptured aneurysm, initiating doctor-directed prophylaxis with inhale steroids and bronchodilators at the onset of symptoms, and pay attention to respiratory physical therapy and circulatory management. Strategies to inhibit catecholamine in the hyperacute phase may help to prevent vasospasm and improve patient outcomes (34).
The study has several limitations. First, our data were collected retrospectively. Second, differences in preoperative baseline information between the two groups may lead to different risk factors for POP. Third, our study was a single-center study, lacking multicenter validation.
In summary, this large, single-center retrospective cohort study demonstrated that patients with aSAH treated with SC are more likely to develop POP. Patients with high preoperative inflammatory factors, high WFNS grade, and high mFS grade should be more alert to the occurrence of POP. Comprehensive preoperative evaluation of patients may help physicians to better predict POP and implement preventive measures to improve their outcomes.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Beijing Tiantan Hospital. The patients/participants provided their written informed consent to participate in this study.
QH and YuaZ: conception, design, and reviewed submitted version of manuscript. KY, RunL, FL, YC, JL, HH, DY, RuiL, JY, ZL, HZ, HL, LZ, YanZ, and YukZ: acquisition of data. KY and RunL: drafting the article. KY, RunL, FL, YC, JL, and QH: statistical analysis. YL, SW, GS, and JZ: administrative, technical, and material support. QH, XC, and YuaZ: study supervision. All authors: analysis, interpretation of data, contributed to the article, and approved the submitted version.
This study was supported by the National Key Research and Development Program of China (Grant Nos. 2021YFC2501101 and 2020YFC2004701), the National Natural Science Foundation of China (Grant Nos. 82071296, 81671129, and 81471210), the Beijing Municipal Administration of Hospitals Incubating Program, Beijing, China (Grant No. pX2020023), and the Natural Science Foundation of Beijing, China (Grant No. 7204253).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors acknowledge the contribution of all staff who participated in the present study.
1. Maher M, Schweizer TA, Macdonald RL. Treatment of spontaneous subarachnoid hemorrhage: guidelines and gaps. Stroke. (2020) 51:1326–32. doi: 10.1161/STROKEAHA.119.025997
2. Lovelock CE, Rinkel GJ, Rothwell PM. Time trends in outcome of subarachnoid hemorrhage: population-based study and systematic review. Neurology. (2010) 74:1494–501. doi: 10.1212/WNL.0b013e3181dd42b3
3. Steiner T, Juvela S, Unterberg A, Jung C, Forsting M, Rinkel G. European stroke organization guidelines for the management of intracranial aneurysms and subarachnoid haemorrhage. Cerebrovasc Dis. (2013) 35:93–112. doi: 10.1159/000346087
4. Zhang L-M, Li R, Zhao X-C, Wang M-L. Decreased tidal volume with increased height, but not colloid transfusion, is associated with worse outcomes and postoperative pneumonia after coil embolization of aneurysmal subarachnoid hemorrhage: a retrospective study. Shock. (2018) 50:421–6. doi: 10.1097/SHK.0000000000001095
5. Li R, Lin F, Chen Y, Lu J, Han H, Yan D, et al. In-Hospital complication-related risk factors for discharge and 90-day outcomes in patients with aneurysmal subarachnoid hemorrhage after surgical clipping and endovascular coiling: a propensity score-matched analysis. J Neurosurg. (2021) 1–12. doi: 10.3171/2021.10.JNS211484
6. Mouchtouris N, Lang MJ, Barkley K, Barros G, Turpin J, Sweid A, et al. Predictors of hospital-associated complications prolonging icu stay in patients with low-grade aneurysmal subarachnoid hemorrhage. J Neurosurg. (2019) 132:1829–35. doi: 10.3171/2019.1.JNS182394
7. Wang C, Kou Y, Han Y, Li X. Early serum calprotectin (S100a8/A9) predicts delayed cerebral ischemia and outcomes after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. (2020) 29:104770. doi: 10.1016/j.jstrokecerebrovasdis.2020.104770
8. Ding CY, Peng L, Lin YX Yu LH, Wang DL, Kang DZ. Elevated lactate dehydrogenase level predicts postoperative pneumonia in patients with aneurysmal subarachnoid hemorrhage. World Neurosurg. (2019) 129:e821–30. doi: 10.1016/j.wneu.2019.06.041
9. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (Isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
10. Vergouwen MD, Fang J, Casaubon LK, Stamplecoski M, Robertson A, Kapral MK, et al. Higher incidence of in-hospital complications in patients with clipped versus coiled ruptured intracranial aneurysms. Stroke. (2011) 42:3093–8. doi: 10.1161/STROKEAHA.111.619510
11. Scott RB, Eccles F, Molyneux AJ, Kerr RS, Rothwell PM, Carpenter K. Improved cognitive outcomes with endovascular coiling of ruptured intracranial aneurysms: neuropsychological outcomes from the international subarachnoid aneurysm trial (Isat). Stroke. (2010) 41:1743–7. doi: 10.1161/STROKEAHA.110.585240
12. Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (Isat): long-term follow-up. Lancet Neurol. (2009) 8:427–33. doi: 10.1016/S1474-4422(09)70080-8
13. Chen Y, Lian BQ, Peng L, Ding CY, Lin YX, Yu LH, et al. Neutrophil to lymphocyte ratio is a prognosis factor for post-operative pneumonia in aneurysmal subarachnoid hemorrhage patients. Chin Med J. (2020) 134:682–9. doi: 10.1097/CM9.0000000000001304
14. Sogame LC, Vidotto MC, Jardim JR, Faresin SM. Incidence and risk factors for postoperative pulmonary complications in elective intracranial surgery. J Neurosurg. (2008) 109:222–7. doi: 10.3171/JNS/2008/109/8/0222
15. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
16. Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. doi: 10.1161/STROKEAHA.115.009617
17. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. (2005) 242:326–41; discussion 41–3. doi: 10.1097/01.sla.0000179621.33268.83
18. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the private-sector national surgical quality improvement program. J Am Coll Surg. (2004) 199:531–7. doi: 10.1016/j.jamcollsurg.2004.05.276
19. Zhang D, Zhuo H, Yang G, Huang H, Li C, Wang X, et al. Postoperative pneumonia after craniotomy: incidence, risk factors and prediction with a nomogram. J Hosp Infect. (2020) 105:167–75. doi: 10.1016/j.jhin.2020.03.015
20. Akutsu Y, Matsubara H, Shuto K, Shiratori T, Uesato M, Miyazawa Y, et al. Pre-operative dental brushing can reduce the risk of postoperative pneumonia in esophageal cancer patients. Surgery. (2010) 147:497–502. doi: 10.1016/j.surg.2009.10.048
21. Inglis TJ, Sherratt MJ, Sproat LJ, Gibson JS, Hawkey PM. Gastroduodenal dysfunction and bacterial colonisation of the ventilated lung. Lancet. (1993) 341:911–3. doi: 10.1016/0140-6736(93)91208-4
22. Inglis TJ, Sproat LJ, Sherratt MJ, Hawkey PM, Gibson JS, Shah MV. Gastroduodenal dysfunction as a cause of gastric bacterial overgrowth in patients undergoing mechanical ventilation of the lungs. Br J Anaesth. (1992) 68:499–502. doi: 10.1093/bja/68.5.499
23. Asai T, Isono S. Residual neuromuscular blockade after anesthesia: a possible cause of postoperative aspiration-induced pneumonia. Anesthesiology. (2014) 120:260–2. doi: 10.1097/ALN.0000000000000042
24. Gupta H, Gupta PK, Schuller D, Fang X, Miller WJ, Modrykamien A, et al. Development and Validation of a Risk Calculator for Predicting Postoperative Pneumonia. Mayo Clin Proc. (2013) 88:1241–9. doi: 10.1016/j.mayocp.2013.06.027
25. Neulen A, Pantel T, Kosterhon M, Kramer A, Kunath S, Petermeyer M, et al. Neutrophils mediate early cerebral cortical hypoperfusion in a murine model of subarachnoid haemorrhage. Sci Rep. (2019) 9:8460. doi: 10.1038/s41598-019-44906-9
26. Provencio JJ, Swank V, Lu H, Brunet S, Baltan S, Khapre RV, et al. Neutrophil Depletion after subarachnoid hemorrhage improves memory via Nmda receptors. Brain Behav Immun. (2016) 54:233–42. doi: 10.1016/j.bbi.2016.02.007
27. Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke t helper cell type 1-like immunostimulation. J Exp Med. (2003) 198:725–36. doi: 10.1084/jem.20021098
28. Sarrafzadeh A, Schlenk F, Meisel A, Dreier J, Vajkoczy P, Meisel C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke. (2011) 42:53–8. doi: 10.1161/STROKEAHA.110.594705
29. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
30. Abla AA, Wilson DA, Williamson RW, Nakaji P, McDougall CG, Zabramski JM, et al. The relationship between ruptured aneurysm location, subarachnoid hemorrhage clot thickness, and incidence of radiographic or symptomatic vasospasm in patients enrolled in a prospective randomized controlled trial. J Neurosurg. (2014) 120:391–7. doi: 10.3171/2013.10.JNS13419
31. Yeh JJ, Lin CL, Kao CH. Relationship between pneumonia and cardiovascular diseases: a retrospective cohort study of the general population. Eur J Intern Med. (2019) 59:39–45. doi: 10.1016/j.ejim.2018.08.003
32. Hulzebos EH, Helders PJ, Favié NJ, De Bie RA, Brutel de la Riviere A, Van Meeteren NL. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing cabg surgery: a randomized clinical trial. JAMA. (2006) 296:1851–7. doi: 10.1001/jama.296.15.1851
33. Gritti P, Lorini FL, Lanterna LA, Bilotta F. Periprocedural management of patients with subarachnoid hemorrhage. Curr Opin Anaesthesiol. (2018) 31:511–9. doi: 10.1097/ACO.0000000000000627
Keywords: aneurysmal subarachnoid hemorrhage, post-operative pneumonia, risk factors, endovascular coiling, surgical clipping
Citation: Yuan K, Li R, Zhao Y, Wang K, Lin F, Lu J, Chen Y, Ma L, Han H, Yan D, Li R, Yang J, He S, Li Z, Zhang H, Ye X, Wang H, Li H, Zhang L, Shi G, Zhou J, Zhao Y, Zhang Y, Li Y, Wang S, Chen X, Zhao Y and Hao Q (2022) Pre-Operative Predictors for Post-Operative Pneumonia in Aneurysmal Subarachnoid Hemorrhage After Surgical Clipping and Endovascular Coiling: A Single-Center Retrospective Study. Front. Neurol. 13:893516. doi: 10.3389/fneur.2022.893516
Received: 10 March 2022; Accepted: 30 May 2022;
Published: 24 June 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Luis Rafael Moscote-Salazar, Latinamerican Council of Neurocritical Care (CLaNi), ColombiaCopyright © 2022 Yuan, Li, Zhao, Wang, Lin, Lu, Chen, Ma, Han, Yan, Li, Yang, He, Li, Zhang, Ye, Wang, Li, Zhang, Shi, Zhou, Zhao, Zhang, Li, Wang, Chen, Zhao and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuanli Zhao, emhhb3l1YW5saUBianR0aC5vcmc=; Qiang Hao, aGFvcWlhbmc3MTNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.