
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 30 May 2022
Sec. Neuroinfectious Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.891721
This article is part of the Research TopicAdvance in Diagnostics for Central Nervous System InfectionView all 5 articles
Objective: Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is a severe neurologic complication of febrile infectious diseases in children. At the onset, AESD is clinically manifested as febrile status epilepticus. Subsequent damage to the cerebral cortex is ascribed to neurotoxicity. The incidence of AESD is remarkably high in Japan, suggesting the involvement of genetic factors. The expression of interleukin 1 beta (IL-1β), a member of the cytokine family involved in the inflammatory response, is reportedly associated with rs16944, a polymorphism in the upstream region of the IL-1B gene, being higher in TT genotype. Previous association studies of rs16944 with febrile seizures (FS) have demonstrated a significant excess in the TT vs. CC + CT genotype in the Asian population. Here, we conducted a case-control association study of rs16944 in AESD.
Methods: We genotyped rs16944 by Sanger sequencing on 283 patients with AESD. As controls, we used genotyping data of 104 Japanese individuals obtained from the 1,000 Genomes Project. Then, we performed a case-control association study using the chi-square test.
Results: The ratio of individuals with TT vs. those with CC+CT genotype was significantly lower in AESD than in the controls [p-value 0.021, Odds Ratio (OR) 0.52]. This finding was opposite to that of a previously reported FS.
Conclusion: The AESD has a genetic background distinct from FS and is not a severe type of FS.
Acute encephalopathy with biphasic seizures and late reduced diffusion (AESD) is a rare and intractable neurologic disorder severely affecting the cerebral cortex of infants and young children. The initial or early seizure of AESD is usually a prolonged generalized convulsion with high fever due to common infectious diseases, such as influenza and exanthem subitum. Several days later, the second or late seizure appears as a cluster of focal seizures, followed by signs of cerebral cortical dysfunction and intractable epilepsy. The mechanism of cerebral cortical damage is considered to be excitotoxic neuronal death triggered by febrile status epilepticus (1). The incidence of AESD is remarkably high in East Asia, especially in Japan (2, 3), suggesting the involvement of genetic factors in AESD.
Febrile seizures (FS), on the other hand, are the most common condition of seizures in children. The prevalence of FS is higher in Japan (6–9%) than in European and North American countries (2–5%) (4). Simple FS are clinically benign, whereas complex FS and febrile status epilepticus may produce neurological sequelae and/or predispose to later epilepsies including temporal lobe epilepsy. Moreover, it is often clinically difficult to distinguish between prolonged FS and initial seizures of AESD.
Increasing evidence supports the involvement of inflammatory processes in the pathogenesis of seizures of different etiologies, such as infection, fever, neurotrauma, and stroke (5–7). Several studies have focused on the proinflammatory cytokine interleukin-1β (IL-1β) and its role in FS (7–9). There is an upstream variant, rs16944 (IL-1B−511 T>C, NG_008851.1:g.4490T>C), in the promotor of IL-1B. According to ALFA (Allele Frequency Aggregator), T allele frequency in total, European, and East Asian populations is 0.357, 0.335, and 0.477, respectively (www.ncbi.nlm.nih.gov/snp/docs/gsr/alfa/). Expression of IL-1β is highest in TT genotype and lowest in CC (10). The frequencies of the T allele and TT homozygotes are significantly higher in Japanese children with simple FS than in controls (11). A meta-analysis of association studies of rs16944 with FS reported a significant excess in the TT vs. CC + CT genotype in the Asian population (8). However, no research has explored the association between rs16944 and AESD.
In this study, to explore whether AESD and FS share a common genetic background, we investigated the association between the IL-1B polymorphism rs16944 and AESD.
We recruited Japanese patients with AESD from hospitals in Japan from the year 2008 to 2018, based on the diagnostic criteria consisting of characteristic clinical course, biphasic seizures, typical MRI findings, and delayed appearance of the cerebral subcortical white matter lesions (2, 3). In this study, we adopted the same inclusion criteria as those in our previous study; we included patients with “definite” AESD, fulfilling both the clinical and MRI criteria, and those with “probable” AESD meeting of either of them (12). The patients without late seizures had super refractory or prolonged initial seizures and were mechanically ventilated under high dose barbiturate. Consequently, they had no clinically apparent late seizures. The patients without MRI findings were also critically ill and were intubated, so it was difficult to obtain MR images at the appropriate timing for ADC changes. Therefore, we included “probable” patients with AESD as clinically conceived patients with AESD.
A total of 283 Japanese patients with AESD were enrolled in this study. All patients were Japanese and mutually unrelated. The clinical characteristics of the patients are shown in Table 2.
This study was reviewed and approved by the Institutional Review Board of the University of Tokyo (No. G3504). We obtained written informed consent from the parents of the patients.
As controls, we used genotyping data of Japanese populations (JPT) obtained from the 1000 Genomes Project. JPT individuals consist of 104 healthy Japanese adults (13).
Peripheral blood samples were collected from the patients. Genomic DNA extraction and polymerase chain reaction (PCR) was conducted using a standard protocol. The GRCh37 consensus genome sequence was used as a reference genome (14). A total of 304 base-pair genomic regions, including rs16944, were amplified with primers described in the previous study (Table 1) (15). PCR amplification was performed using AmpliTaq PCR kits (Applied Biosystems). The reaction mixture contained 2 μl buffer, 2 μl of 2 mM dNTP, 1 μl forward and reverse primers (10 pmol), 0.12 μl AmpliTaq, and 1 μl genomic DNA (30 ng). All the PCR products were purified with a PCR product sequencing kit (Amersham Biosciences, Little Chalfont, Buckinghamshire, UK), and were reacted with the Big Dye Terminator FS ready-reaction kit (Applied Biosystems, Foster City, CA, USA). Purified PCR products were sequenced on 310 Genetic Analyzer, 3100 Genetic Analyzer, or 3130xl Genetic Analyzer (Life Technologies, Carlsbad, CA, USA).
We conducted a case-control association study of rs16944 using the chi-square test. The statistical analysis was conducted using R software (version 3.5.1) (16). A p-value <0.05 was considered to indicate a significant difference in the present study.
Clinical features of the patients were similar to those in our previous epidemiological survey (2, 3). At the onset of AESD, many of the children (61%) were younger than 24 months. The most common pathogen of preceding infection was human herpesvirus type 6 or 7. History information was available in 269 patients. Twenty-nine patients had simple febrile seizures, 12 had epilepsy, 8 had some forms of encephalopathy, 9 had intellectual disability, and 2 had acute disseminated encephalomyelitides. There were two patients with tuberous sclerosis complex, and one each with α thalassemia X-linked intellectual disability syndrome, chromosomal abnormality, autism spectrum disorder, periventricular leukomalacia, and spinal muscular atrophy. The duration of initial seizures was longer than 15 min in about 82% of the cases. Late seizures were noted in 73%, and subcortical MRI lesions in 90%. With regard to neurological sequelae, we collected information on motor and intellectual deficits. If the two deficits were different in degree, the more severe was adopted for grading. More than 60% of the patients were left with neurological sequelae. Among the 283 patients with information on outcome, deficits were profound in 34 patients (12%), severe in 47 (16.6%), moderate in 32 (11.3%), mild in 51 (18%), and none in 56 (19.8%) (Table 2).
The ratio of TT genotype in the AESD cohort was 14.1%, whereas that in the controls was 24%. The ratio of TT genotype was significantly lower in AESD cases than in controls (p = 0.021, OR = 0.52, chi-square test, Table 3).
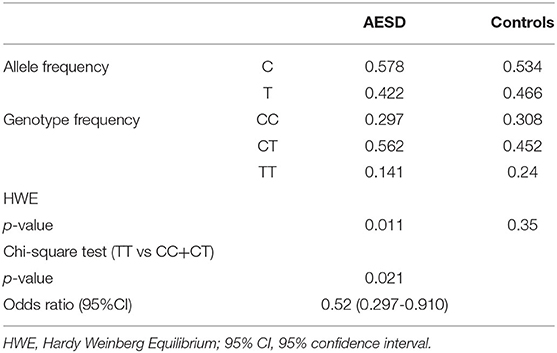
Table 3. Case-control association study of rs16944 in acute encephalopathy with biphasic seizures and late reduced diffusion (AESD).
When we analyzed the association between sex and genotype, TT genotype was significantly higher in females (p = 0.03). There was no association between genotype and type of initial seizures or outcomes.
In this study, the ratio of individuals with TT, a higher producer of IL-1β, was significantly lower in AESD than in the controls. This finding was opposite to that previously reported on FS: a significant excess in the TT vs. CC + CT genotype (8, 11). Although we found that the TT genotype was significantly higher in females, we suppose that it might be due to sampling bias. There was no association between rs16944 and the type of initial seizures or outcome.
Clinical and basic studies have demonstrated that hyperactivation of IL-1β plays important role in the pathogenesis of febrile and epileptic seizures. Intrahippocampal injection of IL-1β before focal application of kainic acid doubled the duration of the seizures induced by kainate in animal models by enhancing glutamatergic neurotransmission (17). According to a systematic review and meta-analysis on cytokines in FS, high levels of cerebrospinal fluid IL-1β and serum IL-6 are associated with an increased risk of FSs in children (18). In drug-resistant epilepsy, the levels of IL-1β+ CD14+ monocytes are reportedly correlated with seizure frequency (19).
Several association studies of rs16944 have previously been conducted in FS and temporal lobe epilepsy. As we mentioned before, a meta-analysis of polymorphisms in FS reported a significant excess in the TT vs. CC + CT genotype in the Asian population (8). TT genotype is also overrepresented in Japanese patients with temporal lobe epilepsy with hippocampal sclerosis (9). Taken together, these genetic studies suggested the detrimental role of IL-lβ in the pathogenesis of FS and temporal lobe epilepsy.
To find biomarkers of AESD, inflammatory cytokines in biological samples of patients with AESD have been vigorously investigated. Several researchers have reported a significant association between AESD and blood cytokines, such as IL-6, IL-10, and TNF-α (20, 21). On the other hand, a study in Japan has reported that serum IL-1β was significantly lower in the patients with AESD with HHV-6 infection than in the controls with exanthem subitem (22), which may be relevant to the finding of our study.
In the field of basic neuroscience, the effects of IL-1β on both excitatory and inhibitory neurotransmitters have been reported. IL-1β can participate in the development of seizures and epileptogenesis through the influence on the calcium influx across the N-methyl- D-aspartate (NMDA) glutamate receptor, the reduction of glutamate uptake by astrocytes, and the increase of glutamate release by glial cells (23, 24). As for inhibitory neurons, fast inhibitory neurotransmission in the brain is principally mediated by the neurotransmitter γ-aminobutyric acid (GABA) and its synaptic target, the type A GABA receptor (GABAA receptor) (25). IL-1β enhances cell-surface expression of GABAA receptors, increases GABAergic tone, and alters synaptic strength at central GABAergic synapses, thereby contributing to cognitive dysfunction in sepsis-associated-encephalopathy (26). The critical role of IL-1β/IL-1 receptor signaling in neuroprotection has been demonstrated by an experimental study using an excitotoxin-damaged mouse retina (27).
Our results suggest that AESD has a genetic background that is partially distinct from FS and temporal lobe epilepsy, and that AESD is not simply a severe form of FS. The differences between our findings on AESD and previous findings on FS may suggest the two faces of IL-1β in fever-induced seizures: a risk factor for seizure and hippocampal sclerosis, and a protective factor against excitotoxic damage to the cerebral cortex. At present, the dual nature of IL-1β in a child's brain remains hypothetical and warrants further investigation.
We did not conduct multivariate logistic regression because statistical power seemed weak due to the small sample size. If we can collect sufficient samples in the future, it would be very informative in considering the relationship between IL-1B and AESD.
In this study, one-fourth of the AESD cases was “symptomatic” with various underlying neurological disorders, and this is in agreement with the findings of a previous study that noted such disorders in one-third of AESD (28). Many of these disorders are caused by variations in genes other than IL-1B. Genetically, AESD is a multifactorial disorder associated with multiple susceptibility genes. Taking these facts into consideration, we decided not to exclude the symptomatic cases from this IL-1B association study.
In our previous genome-wide association study (GWAS) of AESD, rs16944 did not reach genome-wide significance (29). GWAS is a hypothesis-free approach, whereas the present study took a candidate gene approach based on the clinical similarity between AESD and FS, and the association of FS and IL-1β known previously, which we consider can justify the design of this study.
Our results suggest that AESD has a genetic background distinct, at least partially, from FS, and that AESD is not simply a severe form of FS.
The datasets presented in this study can be found in online repositories. The names of the repository and accession number can be found below: [NBDC Human Database, hum0347.v1.freq.v1, https://humandbs.biosciencedbc.jp/en/hum0347-v1].
The studies involving human participants were reviewed and approved by the Institutional Review Board of the University of Tokyo. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AS, AH, and MM contributed to conception and design of the study. AS performed the statistical analysis and wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This research was supported by a Grant-in-Aid for Scientific Research, No. 15H04872, from the Japan Society for the Promotion of Science, and a Grant-in-aid for Policy Research for Intractable Diseases, No. H30-Nanji-Ippan-007/21FC1005, from the National Institute of Public Health, Japan.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to Ms. Aya Shoda for her technical assistance, and to the Collaborative Research Supporting Committee of the Japanese Society of Child Neurology for promoting this study. We wish to thank all the pediatricians for providing patient samples, and the patients and their parents for participating in this study.
1. Takanashi J, Tada H, Terada H, Barkovich AJ. Excitotoxicity in acute encephalopathy with biphasic seizures and late reduced diffusion. Am J Neuroradiol. (2009) 30:132–5. doi: 10.3174/ajnr.A1247
2. Hoshino A, Saitoh M, Oka A, Okumura A, Kubota M, Saito Y, et al. Epidemiology of acute encephalopathy in Japan, with emphasis on the association of viruses and syndromes. Brain Dev. (2012) 34:337–43. doi: 10.1016/j.braindev.2011.07.012
3. Kasai M, Shibata A, Hoshino A, Maegaki Y, Yamanouchi H, Takanashi J ichi, et al. Epidemiological changes of acute encephalopathy in Japan based on national surveillance for 2014–2017. Brain Dev. (2020) 42:508–14. doi: 10.1016/j.braindev.2020.04.006
4. Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. (1994) 35 (Suppl. 2):S1–6. doi: 10.1111/j.1528-1157.1994.tb05932.x
5. Vezzani A, Bartfai T, Bianchi M, Rossetti C, French J. Therapeutic potential of new antiinflammatory drugs. Epilepsia. (2011) 52:67–9. doi: 10.1111/j.1528-1167.2011.03242.x
6. Matsuo M, Sasaki K, Ichimaru T, Nakazato S, Hamasaki Y. Increased IL-1β production from dsRNA-stimulated leukocytes in febrile seizures. Pediatr Neurol. (2006) 35:102–6. doi: 10.1016/j.pediatrneurol.2005.12.005
7. Heida JG, Moshé SL, Pittman QJ. The role of interleukin-1β in febrile seizures. Brain Dev. (2009) 31:388–93. doi: 10.1016/j.braindev.2008.11.013
8. Yu X, Zhang N, Liu S, Xi Z, Zhang Y. Polymorphisms in the interleukin-1β (IL-1B) and interleukin-1α (IL-1A) genes on risk of febrile seizures: a meta-analysis. Neurol Sci. (2018) 39:1529–36. doi: 10.1007/s10072-018-3449-4
9. Kanemoto K, Kawasaki J, Miyamoto T, Obayashi H, Nishimura M. Interleukin (IL)-1β, IL-1α, and IL-1 receptor antagonist gene polymorphisms in patients with temporal lobe epilepsy. Ann Neurol. (2000) 47:571–4. doi: 10.1002/1531-8249(200005)47:5<571::AID-ANA3>3.0.CO;2-A
10. Aguet F, Barbeira A, Bonazzola R, Brown A, Castel S, Jo B, et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. (2020) 369:1318–30. doi: 10.1126/science.aaz1776
11. Kira R, Torisu H, Takemoto M, Nomura A, Sakai Y, Sanefuji M, et al. Genetic susceptibility to simple febrile seizures: interleukin-1β promoter polymorphisms are associated with sporadic cases. Neurosci Lett. (2005) 384:239–44. doi: 10.1016/j.neulet.2005.04.097
12. Shibata A, Kasai M, Terashima H, Hoshino A, Miyagawa T, Kikuchi K, et al. Case-control association study of rare nonsynonymous variants of SCN1A and KCNQ2 in acute encephalopathy with biphasic seizures and late reduced diffusion. J Neurol Sci. (2020) 414:116808. doi: 10.1016/j.jns.2020.116808
13. Auton A, Abecasis GR, Altshuler DM, Durbin RM, Bentley DR, Chakravarti A, et al. A global reference for human genetic variation. Nature. (2015) 526:68–74. doi: 10.1038/nature15393
14. Howe KL, Achuthan P, Allen J, Allen J, Alvarez-Jarreta J, Amode MR, et al. Ensembl 2021. Nucleic Acids Res. (2020) 49:D884–91. doi: 10.1093/nar/gkaa942
15. Chou IC, Lin W De, Wang CH, Tsai CH, Li TC, Tsai FJ. Interleukin (IL)-1β, IL-1 receptor antagonist, IL-6, IL-8, IL-10, and tumor necrosis factor α gene polymorphisms in patients with febrile seizures. J Clin Lab Anal. (2010) 24:154–9. doi: 10.1002/jcla.20374
16. R Core Team. R: A Language Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2018). Available online at: https://www.R-project.org/
17. Vezzani A, Conti M, De Luigi A, Ravizza T, Moneta D, Marchesi F, et al. Interleukin-1β immunoreactivity and microglia are enhanced in the rat hippocampus by focal kainate application: functional evidence for enhancement of electrographic seizures. J Neurosci. (1999) 19:5054–65. doi: 10.1523/JNEUROSCI.19-12-05054.1999
18. Kwon A, Kwak BO, Kim K, Ha J, Kim SJ, Bae SH, et al. Cytokine levels in febrile seizure patients: a systematic review and meta-analysis. Seizure. (2018) 59:5–10. doi: 10.1016/j.seizure.2018.04.023
19. Yamanaka G, Takamatsu T, Morichi S, Yamazaki T, Mizoguchi I, Ohno K, et al. Interleukin-1β in peripheral monocytes is associated with seizure frequency in pediatric drug-resistant epilepsy. J Neuroimmunol. (2021) 352:577475. doi: 10.1016/j.jneuroim.2021.577475
20. Aiba H, Mochizuki M, Kimura M, Hojo H. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology. (2001) 57:295–9. doi: 10.1212/WNL.57.2.295
21. Ichiyama T, Ito Y, Kubota M, Yamazaki T, Nakamura K, Furukawa S. Serum and cerebrospinal fluid levels of cytokines in acute encephalopathy associated with human herpesvirus-6 infection. Brain Dev. (2009) 31:731–8. doi: 10.1016/j.braindev.2008.11.005
22. Kawamura Y, Yamazaki Y, Ohashi M, Ihira M, Yoshikawa T. Cytokine and chemokine responses in the blood and cerebrospinal fluid of patients with human herpesvirus 6B-associated acute encephalopathy with biphasic seizures and late reduced diffusion. J Med Virol. (2014) 86:512–8. doi: 10.1002/jmv.23788
23. Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. J Neurochem. (2007) 102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x
24. Orsini A, Foiadelli T, Costagliola G, Michev A, Consolini R, Vinci F, et al. The role of inflammatory mediators in epilepsy: focus on developmental and epileptic encephalopathies and therapeutic implications. Epilepsy Res. (2021) 172:106588. doi: 10.1016/j.eplepsyres.2021.106588
25. Zhu S, Noviello CM, Teng J, Jr. RMW, Kim JJ, Hibbs RE. Structure of a human synaptic GABA-A receptor. Nature. (2018) 559:67–72. doi: 10.1038/s41586-018-0255-3
26. Serantes R, Arnalich F, Figueroa M, Salinas M, Andrés-Mateos E, Codoceo R, et al. Interleukin-1β enhances GABAA receptor cell-surface expression by a phosphatidylinositol 3-kinase/Akt pathway: relevance to sepsis-associated encephalopathy. J Biol Chem. (2006) 281:14632–43. doi: 10.1074/jbc.M512489200
27. Todd L, Palazzo I, Suarez L, Liu X, Volkov L, Hoang T V., et al. Reactive microglia and IL1β/IL-1R1-signaling mediate neuroprotection in excitotoxin-damaged mouse retina. J Neuroinflammation. (2019) 16:1–19. doi: 10.1186/s12974-019-1505-5
28. Hirayama Y, Saito Y, Maegaki Y, Status Epilepticus Study Group. “Symptomatic” infection-associated acute encephalopathy in children with underlying neurological disorders. Brain Dev. (2017) 39:243–7. doi: 10.1016/j.braindev.2016.09.014
Keywords: IL-1B-511, acute encephalopathy with biphasic seizures and late reduced diffusion (AESD), status epilepticus, association study, rs16944, genetic risk factor
Citation: Shibata A, Kasai M, Hoshino A and Mizuguchi M (2022) Association of IL-1B rs16944 Polymorphism With Acute Encephalopathy With Biphasic Seizures and Late Reduced Diffusion Is Opposite to That of Febrile Seizures. Front. Neurol. 13:891721. doi: 10.3389/fneur.2022.891721
Received: 08 March 2022; Accepted: 19 April 2022;
Published: 30 May 2022.
Edited by:
Peter R. Williamson, National Institutes of Health (NIH), United StatesReviewed by:
Rod C. Scott, University of Vermont, United StatesCopyright © 2022 Shibata, Kasai, Hoshino and Mizuguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akiko Shibata, YXNhc2Fuby10a3lAdW1pbi5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.