
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 30 May 2022
Sec. Experimental Therapeutics
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.891514
Background: Mesenchymal stem cells (MSCs) is an attractive candidate in regenerative research and clinical trials have assessed their therapeutic potential in different neurological conditions with disparate etiologies. In this systematic review, we aimed to assess safety and clinical effect of MSC treatment in traumatic spinal cord injury (TSCI), multiple sclerosis (MS) and ischemic stroke (IS).
Methods: A systematic search was performed 2021-12-10 in MEDLINE, EMBASE, Web of Science and Cochrane where clinical studies assessing MSC treatment in TSCI, MS or IS were included. Studies without control group were excluded for efficacy analysis, but included in the safety analysis. For efficacy, AIS score, EDSS score and mRS were used as clinical endpoints and assessed in a meta-analysis using the random effects model.
Findings: Of 5,548 identified records, 54 studies were included. Twenty-six studies assessed MSC treatment in TSCI, 14 in MS and nine in IS, of which seven, seven and five studies were controlled, respectively. There were seven serious adverse events (SAEs), of which four were related to the surgical procedure and included one death due to complications following the implantation of MSCs. Three SAEs were considered directly related to the MSC treatment and all these had a transient course. In TSCI, a meta-analysis showed no difference in conversion from AIS A to C and a trend toward more patients treated with MSCs improving from AIS A to B as compared to controls (p = 0.05). A subgroup analysis performed per protocol, showed more MSC treated patients improving from AIS A to C in studies including patients within 8 weeks after injury (p = 0.04). In MS and IS, there were no significant differences in clinical outcomes between MSC treated patients and controls as measured by EDSS and mRS, respectively.
Interpretation: MSC-treatment is safe in patients with TSCI, MS and IS, although surgical implantation of MSC led to one fatal outcome in TSCI. There was no clear clinical benefit of MSC treatment, but this is not necessarily a proof of inefficacy due to the low number of controlled studies. Future studies assessing efficacy of MSC treatment should aim to do this in randomized, controlled studies.
For neurological diseases affecting the central nervous system (CNS), there are no available therapies that may repair and thereby reverse neurological disability. So far, this has been the common denominator in CNS injury, regardless the cause.
Mesenchymal stem cells (MSCs), also known as mesenchymal stromal cells, are heterogeneous cells with self-renewal potential and multipotent properties that can be found in all postnatal tissues (1). MSCs do not have a unique cell marker, but are defined according to international guidelines by the presence and absence of different cell surface proteins and tri-lineage differentiation potential in vitro (2).
Recent studies have highlighted the systemic role of MSCs in tissue repair (3–5). In this setting, MSCs have been shown to possess regenerative capabilities, also for conditions affecting the CNS. Animal studies have revealed that MSCs can migrate toward sites of injury (6) and promote repair of myelin and neurons, thus leading to improved functional outcomes in models of central nervous diseases (7, 8). This effect is likely mediated through different mechanisms, such as the paracrine stimulation of endogenous progenitor- and stem cells through the MSC secretome (9), mitochondria donations (10), immunomodulation (11) and transdifferentiation toward neural cell lines (12).
MSCs can be obtained from different tissues, such as bone marrow (BM), adipose tissue and umbilical cord, and expanded ex vivo. The use of autologous or allogeneic MSCs represent no ethical concerns as compared to other stem cell therapies based on embryonal or fetal stem cells. This, along with the promising results from animal studies, have made MSCs an attractive candidate for regenerative human studies.
Numerous studies have been performed the last years assessing MSC treatment in neurological conditions. As injury to the human CNS may be caused by different mechanisms, an important question is whether MSC treatment is safe and whether it possesses a neuroregenerative effect across separate etiologies. In this systematic review and meta-analysis, we aimed to assess safety and clinical effect of MSC treatment in traumatic spinal cord injury (TSCI), multiple sclerosis (MS) and ischemic stroke (IS).
This systematic review and meta-analysis was performed in accordance with the PRISMA guidelines (13). The protocol was completed before the search and registered at The National Institute for Health Research with ID CRD42021285638.
Clinical studies including patients with TSCI, MS or IS treated with MSCs were included. Follow-up studies, case reports and studies without defined inclusion and exclusion criteria were excluded. Studies without control group were excluded for the efficacy analysis, but included in the safety analysis. Details concerning eligibility criteria are listed in the Supplementary Material 1. In the protocol, inclusion criteria were originally restricted to papers using the English language. This criteria was subsequently removed as a number of eligible papers were published in Chinese, and not including these could represent a bias. Therefore, papers in all languages could be included in the analysis.
Studies were identified by searching the electronic databases MEDLINE (Ovid), EMBASE (Ovid), Web of Science and Cochrane Library. Variants of subject headings and free-text terms of “Mesenchymal stem cell transplantation” were applied in combination with different terms of traumatic spinal cord injury, multiple sclerosis and ischemic stroke. The complete search strings are shown in the Supplementary Material 2. The searches were performed on December 10th, 2021.
Eligibility assessment was performed in a two-step screening process. After removal of duplicates, the first screening was conducted by assessment of title and abstract. The cause for exclusion was recorded. The first screening was performed by one reviewer (CEK) in a standardized manner. The remaining studies were read in full text in a second screening. This step was performed non-independently by two unblinded reviewers (CEK/LB). Disagreements between reviewers were resolved by consensus. Data extraction was performed by using a pre-developed data extraction sheet. The following information was extracted: (1) study identity; (2) condition and its characteristics; (3) study design; (4) number of patients in treatment and control groups; (5) details concerning mesenchymal stem cell treatment, including origin of cells, timing of treatment, way of administration and cell dose; (6) safety data with adverse events (AEs)/serious adverse events (SAEs); (7) efficacy data as specified in the protocol.
For safety analysis, the AEs and SAEs considered by the authors to be related to the MSC treatment or MSC administration (possibly, likely or definitely) were registered in each study. If the authors did not state the relationship between the AE/SAE and MSC treatment/administration, all AEs/SAEs in the treatment arm were included in the analysis. For efficacy outcome analysis, American Spinal Injury Association Impairment Scale (AIS) (conversion AIS type A to B-C, and mean AIS scores) were extracted for TSCI, Expanded Disability Status Scale (EDSS) scores (patients improving, remaining stable and worsening, and mean difference in EDSS score) for MS and modified Rankin Scale (mRS) (patients with mRS 0–2 and mean mRS) for IS. Data were extracted from tables and/or graphs published in either the main paper or Supplementary Material. We contacted nine authors due to missing outcome data, and received reply from one.
Risk of bias within the controlled studies were evaluated by using “The Revised Cochrane risk-of-bias tool for randomized trials (RoB 2)” and “The Risk Of Bias In Non-randomized Studies – of Interventions (ROBINS-I) assessment tool” for randomized and non-randomized studies, respectively. One reviewer (CEK) performed the assessments and results were reviewed a second time by another reviewer (LB) before completion. The reviews were not performed in an independent manner. Disagreements were resolved by consensus.
Safety data was registered by type and severity, and reported in frequency per procedure. Meta-analyses for dichotomous efficacy outcomes were performed by computing relative risks and risk differences with corresponding 95% confidence intervals using the Mantel-Haenszel method in a random effects model. For ordinal data, differences in mean were calculated with corresponding 95% confidence intervals using the inverse variance method in a random effects model. The random effects model was applied based on the assumption that the different studies were estimating different, yet related, intervention effects. Intention-to-treat data from the studies were used. If studies had multiple treatment arms with different doses of MSCs and only one control arm, the arm with the highest dose showing safety, was used in the meta-analysis of efficacy as comparison to the control group. Likewise, if studies used both intravenous and intrathecal administration modes, the arm with the intrathecal administration was used in the meta-analysis as comparison to the control group. Heterogeneity was assessed by using the inconsistency index (I2). Risk of bias across studies was not assessed due to the low number of studies available for each outcome analysis. No additional analyses were performed apart from subgroup analyses as specified in the protocol. Revman 5.4.1 software (Cochrane Collaboration, Oxford, UK) was used for the analyses.
The search identified 5,548 records, of which 3,802 remained after duplication removal (Figure 1). After the exclusion of 3,688 records in the first screening, 114 records were assessed in full text. The second screening discarded 60 additional records due to fulfillment of various exclusion criteria. A total of 35 studies remained for safety analysis (14–48) and 19 studies for the combined efficacy and safety analysis (49–67). A summary of the risk of bias for the studies included in the efficacy and safety analysis is shown in Figure 2.
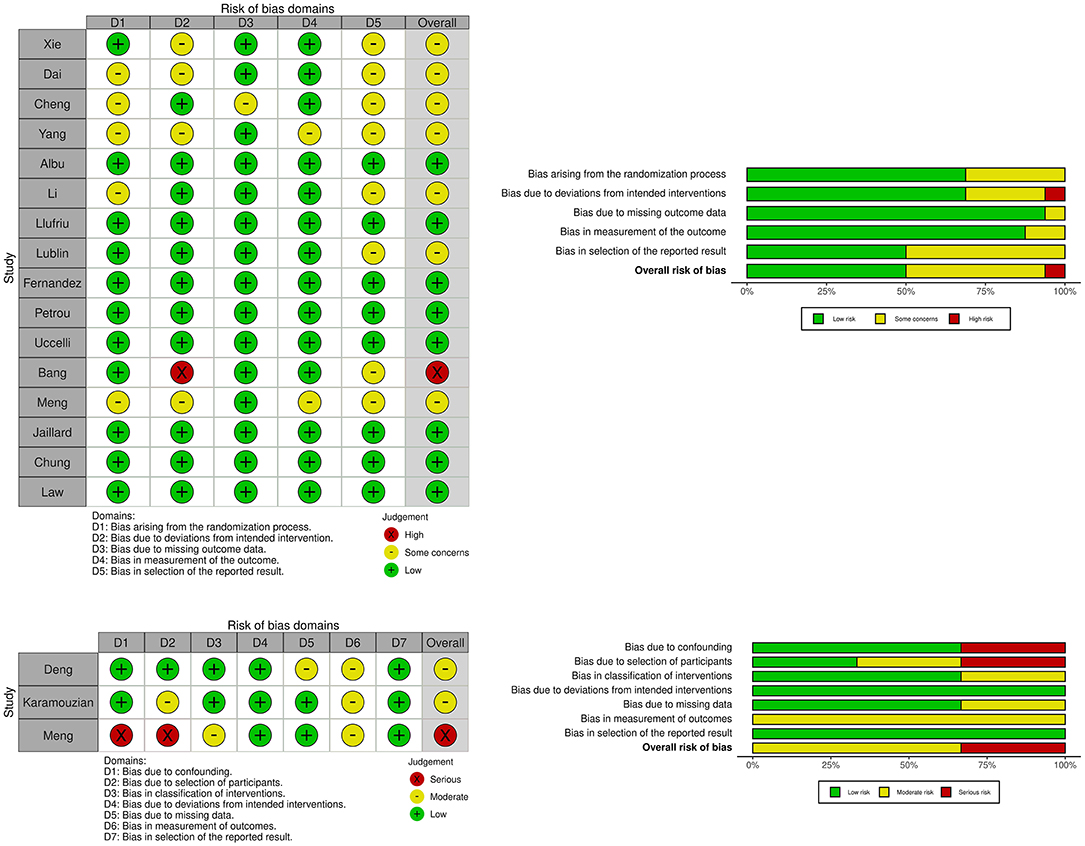
Figure 2. Plots showing risk of bias for controlled studies assessing efficacy of mesenchymal stem cell treatment in traumatic spinal cord injury, multiple sclerosis and ischemic stroke. Risk of bias for randomized studies (16 studies). Risk of bias for non-randomized studies (3 studies).
In total, 26 of the included studies assessed TSCI (14–16, 18–32, 49–55), 19 MS (33–44, 56–62) and 9 IS (45–48, 63–67). Study characteristics are shown in Table 1.
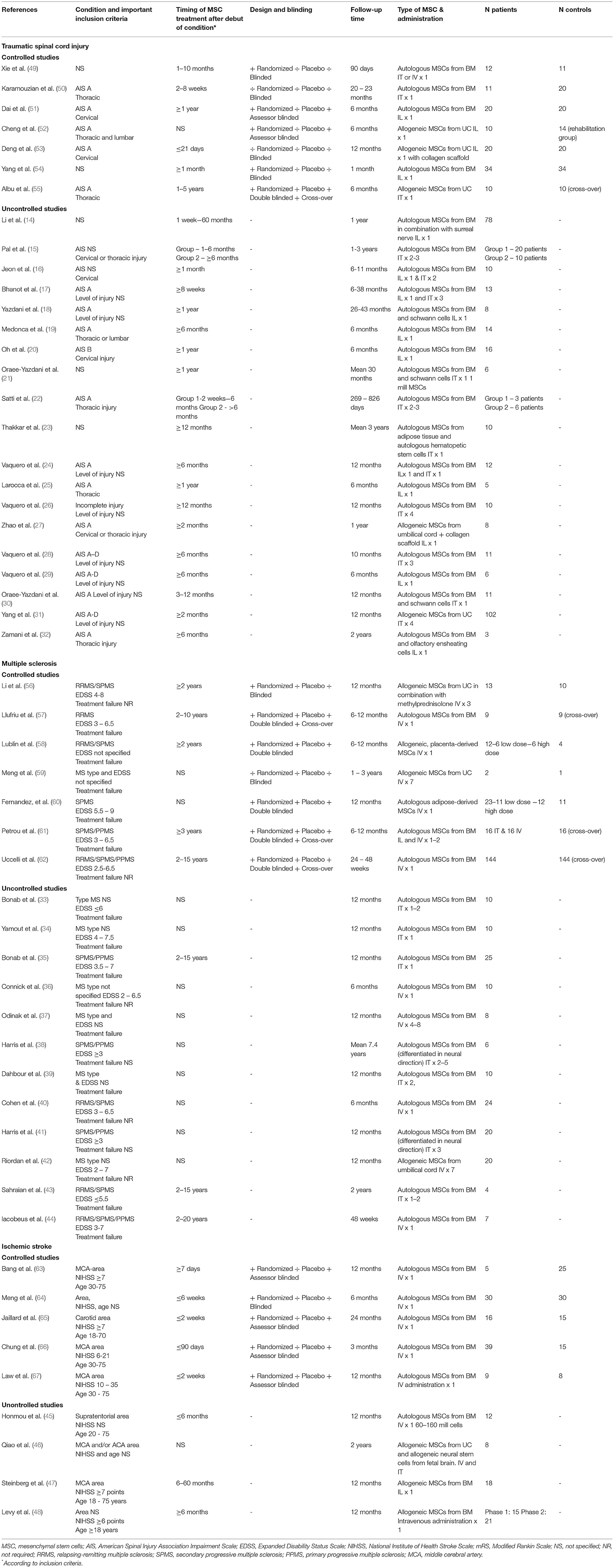
Table 1. Overview over studies of mesenchymal stem cell treatment in traumatic spinal cord injury, multiple sclerosis and ischemic stroke.
Of the 26 included studies, seven were controlled (49–55), of which five were randomized (49, 51, 52, 54, 55), one double-blinded (55) and two assessor-blinded (51, 52). Fourteen studies included patients with isolated AIS A (17–19, 22, 24, 25, 27, 30, 32, 50–53, 55), one isolated AIS B (20) whereas 11 studies included all AIS classifications or did not specify this in the inclusion criteria (14–16, 21, 23, 26, 28, 29, 31, 49, 54). Four studies included only patients with cervical injury (16, 20, 51, 53) and five included only patients with thoracic injury (22, 25, 32, 50, 55). The remaining studies included injuries in several segments of the spinal cord or did not specify this (14, 15, 17–19, 21, 23, 24, 27–31, 49, 52, 54). According to inclusion criteria, MSC treatment was administered within the first 3 weeks after injury in one study (53), within 2–8 weeks in one study (50) and after 6 months or more in 13 studies (18–21, 24–26, 28, 29, 32, 51, 55). Twelve studies used intralesional administration via surgery or guided injections (14, 18–20, 25, 27, 29, 32, 51–54) and ten studies administered the cells intrathecally via lumbar puncture (15, 21–23, 26, 28, 30, 31, 50, 55), whereas four used combinations of different administration methods (16, 17, 24, 49).
A total of 19 studies were included (33–44, 56–62), of which six were randomized (56–58, 60–62) and five double-blinded (57, 58, 60–62). Five studies only included patients with progressive MS (35, 38, 41, 60, 61) and 12 studies had failure to standard disease modifying treatment as an inclusion criteria (33–35, 37, 39, 43, 44, 57–61). In 11 studies, the stem cells were given intravenously (36, 37, 40, 42, 44, 56–60, 62), in seven intrathecally (33–35, 38, 39, 41, 43) and in one study both intravenously and intrathecally (61). The follow-up period varied between 6 months and 7 years.
Nine studies were included (45–48, 63–67), of which five were controlled (63–67) and five assessor-blinded (63, 65–67). Six studies included only patients with moderate or severe stroke (47, 48, 63, 65–67), whereas this was not specified in three studies (45, 46, 64). The stem cells were administered intravenously in all studies except for one where the stem cells were also were injected intrathecally (46) and one study that used local administration (47). Patients were treated within 2 weeks after stroke onset in two studies (65, 67), whereas two studies only included patients with chronic stroke, surpassing 6 months after onset (47, 48). The follow-up time varied between 6 and 24 months.
In 1,044 patients receiving 1,810 transplantations via either intravenous, intrathecal or intralesional administration routes, a total of 845 AEs were reported. There were 429 (70.8%) AEs for patients treated intravenously, 248 (30.7%) intrathecally, 85 (39.7%) intralesionally and 72 (39.8%) with different combinations of administration routes (in 11 patients route of administration was not specified). Of the seven reported SAEs, two received MSCs intravenously, two intrathecally and three intralesionally. Safety data are shown in Supplementary Table 3.
In TSCI, 479 patients received 713 intrathecal and 231 intralesional treatments. One SAE was reported and this was a patient who died due to complications after surgery where the MSCs were implanted (14). Fever (8%) and headache (3%) were the most common AE, irrespective of administration mode.
In patients treated with intrathecal administration, the most frequent AE per procedure was fever (9%) and headache (4%) whereas paresthesia (4%) and neuropathic pain (3%) were among the most frequent reported events in patients treated with intralesional administration.
In MS, 394 patients were treated with in total 491 intravenous and 186 intrathecal injections. Three SAEs were considered related to treatment; one anaphylactic reaction (58), one infection (62) and one transient encephalopathy with epileptic seizures (34). All these reactions had a transient course. Of the specific AEs, headache (14%) and injection site symptoms (4%) were most frequent. In patients receiving the MSC intravenously, headache (6%) and fatigue (5%) were the most commonly reported AE, and headache (32%) and fever (12%) when injected intrathecally.
A total of 171 patients with ischemic stroke were treated, of which 159 received MSC intravenously, 12 intrathecally and 18 via intralesional implantation. There were three serious adverse events; one epileptic seizure, one subdural hematoma, one pneumonia (47). All occurred in a study where MSCs were implanted in the lesion site and all were considered related to the surgical procedure. In total, headache (10%) and fever (6%) were most frequently reported as AE, irrespective of administration mode. For patients receiving only intravenous injections, fever (3%) and urinary infection (3%) were most common.
A total of 679 patients (236 SCI, 261 MS and 182 IS) were included in the combined efficacy and safety analyses (Table 1), of which 472 patients (154 SCI, 217 MS and 101 IS) reported clinical data that enabled them to enter one or more of the pre-specified meta-analyses. The forest plots are shown in Figures 3A–I. Due to the low number of included studies, a majority of subgroup analyses specified in the protocol could not be performed. As an adjustment from protocol, worsening in EDSS was also included in the analysis.
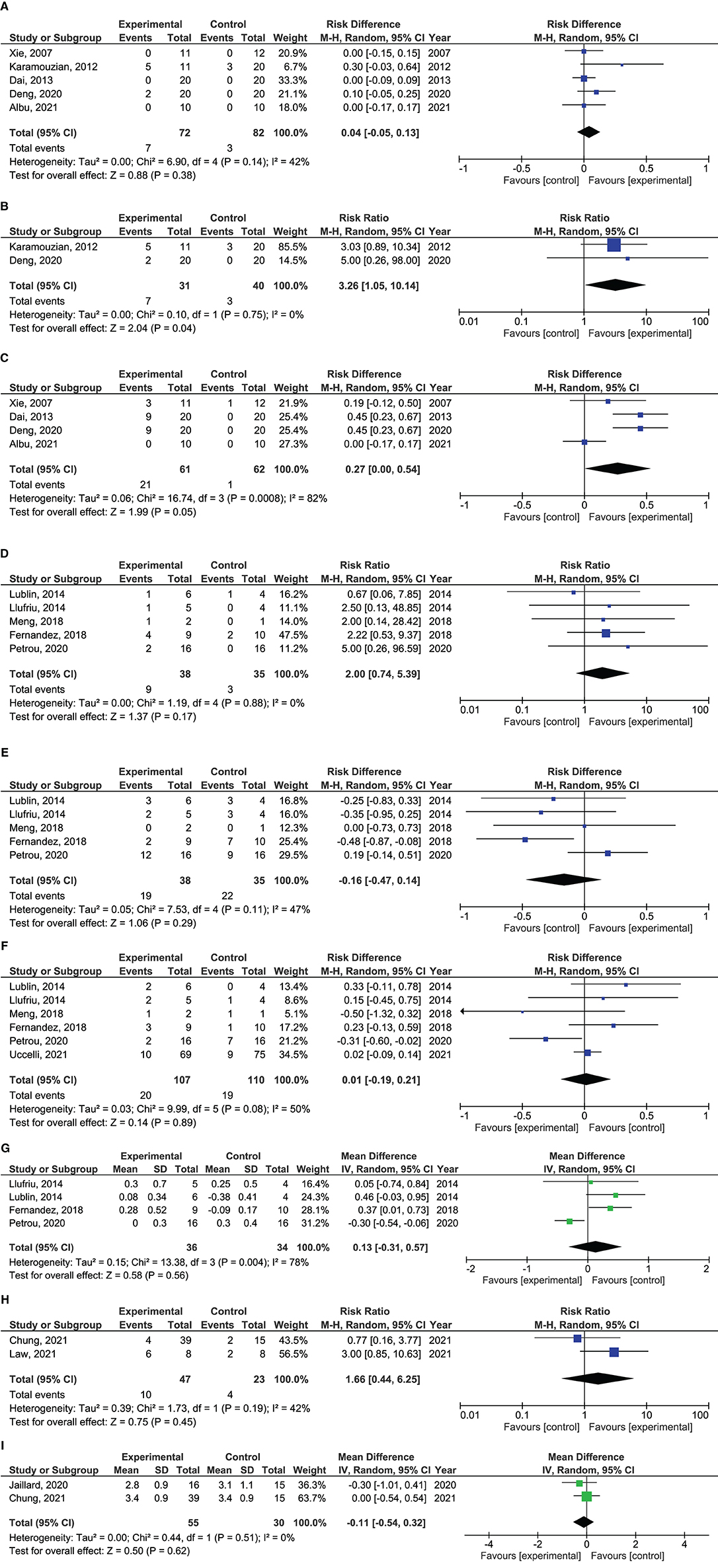
Figure 3. Forrest plots showing effect size of different outcomes. (A) Risk difference of improvement from ASIA A to ASIA C in patients with traumatic spinal cord injury treated with mesenchymal stem cells. (B) Risk difference of improvement from ASIA A to ASIA C in patients with traumatic spinal cord injury treated with mesenchymal stem cells within 8 weeks after injury. (C) Risk difference in improvement from ASIA A to ASIA B in patients with traumatic spinal cord injury treated with mesenchymal stem cells. (D) Risk ratio of EDSS improvement in patients with multiple sclerosis treated with mesenchymal stem cells. (E) Risk difference in remaining stable in EDSS in patients with multiple sclerosis treated with mesenchymal stem cells. (F) Risk difference in EDSS worsening in patients with multiple sclerosis treated with mesenchymal stem cells. (G) Mean difference in ΔEDSS scores in patients with multiple sclerosis treated with mesenchymal stem cells. (H) Risk ratio of patients with mRS 0-2 in patients with ischemic stroke treated with mesenchymal stem cells. (I) Mean difference in mRS scores in patients with ischemic stroke treated with mesenchymal stem cells.
Five studies reported proportion of patients with improvement from AIS classification A to C (49–51, 53, 55) and four from A to B (49, 51, 53, 55). Total AIS scores were not assessed as only one of the studies reported this (51). In the pooled analysis, there were no differences in proportion of patients converting from AIS A to C (risk diff: 0.04; 95% CI −0.05–0.13; p = 0.38) between MSC treated and controls. There was a trend toward more patients treated with MSCs improving from AIS A to B (risk diff: 0.27; 95% CI 0.00–0.54; p = 0.05). This analysis had a high heterogeneity with I2 = 82%. Exploration of heterogeneity identified one study that, in contrast to the other studies, did not report any improvement from AIS A to B in any patients (55). This was the only randomized, placebo-controlled study in the analyses, and the study was therefore not removed. A subgroup analysis performed per protocol, showed more MSC treated patients improving from AIS A to C as compared to controls when only studies including patients within 8 weeks after injury were analyzed (RR: 3.26; 95% CI 1.05–10.14; p = 0.04) (50, 53).
Five studies reported proportion of patients with improvement and stabilization in EDSS (57–61), and six reported worsening (57, 58, 60–62). Four studies reported mean difference in EDSS after treatment (57, 58, 60, 61). There were no significant differences in rates of EDSS improvement (RR 2.00; 95% CI 0.74–5.39; p = 0.17), stabilization (Risk diff: −0.16; 95% CI −0.47–0.14; p = 0.29) or worsening (risk diff: 0.01; 95% CI −0.19–0.21; p = 0.89) between patients receiving MSCs and controls. Also, no difference was found in ΔEDSS scores between treatment groups (mean diff: 0.13; 95% CI −0.31–0.57; p = 0.56). This analysis had a high heterogeneity with I2 = 78%, which was caused by one study (61). This was the only study where MSCs were administered intrathecally and the study was not removed.
Two studies reported on proportion of patients with mRS 0–2 (66, 67) and total mRS scores (65, 66). There were no differences between MSC treatment vs. controls in rates of mRS 0–2 (RR 1.66; 95% CI 0.44–6.25; p = 0.45), or mean differences in total mRS (mean diff: −0.11; 95% CI −0.54–0.32; p = 0.62) after treatment.
Our systematic review showed that MSC treatment is reasonably safe and well-tolerated in TSCI, MS and IS. Low grade fever and headache were the most frequent reported adverse events. In the largest study, adverse events were registered in organ classes and no differences in safety parameters were reported between treatment and control group (62).
There were seven serious adverse events, of which three were considered directly related to the stem cell treatment; one anaphylactic reaction, one infection and one transient encephalopathy. A Chinese study reported a complication after the surgical procedure that led to the death of one patient with TSCI where MSCs in combination with sural nerve tissue was implanted into the injured spinal cord (14). This was the only serious adverse event that resulted in death. Of the events considered directly related to the treatment with MSCs, the transient encephalopathy may be regarded as the most serious. This patient had MS and received a high number of cells (100 million MSCs) intrathecally 50/50% via lumbar and intracisternal puncture (34). The patient developed epileptic seizures few days after transplantation, which required hospitalization and intravenous valproate. Reportedly, the patient recovered without significant sequelae. Of notice, another study reported two cases of iatrogenic meningitis as adverse events in patients with MS after intrathecal injection of MSCs (35). There were no abnormalities in CSF and microbiological studies were negative. They received antibiotics for 14 days and were discharged without sequelae. Our findings concerning safety of MSC treatment are in harmony with another review that assessed MSC treatment in a spectrum of different medical conditions and found no risk of serious complications such as tumorigenicity or toxicity (68).
In the meta-analysis of clinical efficacy, there was no overall motoric effect of MSC treatment that enabled the re-classification of AIS A to C. In our view, an ability to regain motoric function below the level of injury in patients with complete injuries would not only be the most important clinical benefit from a patient point of view, but also a clear indication of clinical efficacy. A possible effect was, however, noted in a subgroup analysis where only patients treated within 8 weeks after injury were included. This may suggest that the optimal timepoint of MSC treatment is within the first weeks after TSCI, which seems biologically reasonable in the sense that the MSCs in this time period may have better access to the injured nervous tissue in the absence of scar tissue and mature gliosis. The finding must, however, be interpreted with caution as only two studies with 71 patients in total were included in the analysis (50, 53). Also, more patients transformed from AIS A to AIS B in the MSC arm. This result should also be interpreted cautiously due to a considerable heterogeneity that was caused by the neutral results of the only double-blinded, placebo-controlled study in the analysis. Skin sensation is a more subjective parameter than motor abilities, and may thus be more prone to bias in studies where the patients are unblinded. With this backdrop, it is noteworthy that the only patient blinded trial in this analysis did not show any effect of MSC-treatment on transformation from AIS A to B (55). This trial included only patients with chronic TSCI.
In the meta-analysis of clinical efficacy in MS patients, there were no apparent clinical benefits of MSC treatment as compared to controls in either improving, stabilizing or preventing worsening in EDSS. The neutral findings are in concordance with a recently published large, randomized study that included 148 patients and failed to show an effect of intravenous MSC treatment in disease activity (62). A meta-analysis including both controlled and uncontrolled MS-studies have suggested that intrathecal administration of MSCs may be more efficacious than the intravenous route (69). This may also seem biological plausible as animal studies have shown that intravenously injected MSCs are trapped in the lungs and deposed out of the organism after short time (70, 71). These findings is also in harmony with our findings, as a majority of the included MS studies used an intravenous administration form (56–60, 62). Also the four controlled studies assessing efficacy of MSC treatment in ischemic stroke used intravenous administration (63, 65–67). Only three studies assessed mRS in a way that permitted comparison in a meta-analysis (65–67). The synthesis of these results did not show any clear clinical benefit of MSC treatment compared to the controls. These results are in concordance with a large, randomized trial that investigated safety and efficacy of intravenously administered BM-derived adult multipotent progenitor cells with similar properties as MSCs (72). The treatment was safe and well-tolerated, but could not demonstrate significant neurological improvements at 90 days after treatment.
The lack of effect in our meta-analysis is in contrast to a number of uncontrolled studies, which have reported promising results with MSC treatment in the same conditions using similar clinical endpoints (19, 23, 24, 34–39, 41, 43, 45). It is likely that there is a considerable placebo effect in studies where patients are treated with advanced medicinal treatment, such as mesenchymal stem cells. Perhaps especially in neurological conditions where no curative treatment is available. This may also provide a basis for publication bias, where positive case reports and case series are more often published than studies where no effect can be shown. Our findings, in combination with these speculations, highlight the need for future MSC studies being randomized, and if possible, blinded for the patients and assessors. Such a design has already been proposed, as the “International Mesenchymal Stem Cells Transplantation Study Group” in 2010 recommended the use of double-blinded, randomized, controlled cross-over studies for the assessment of MSC treatment in MS (73). According to clinicaltrials.gov, three MSC trials are per now recruiting patients with TSCI, four trials are recruiting MS patients and eight trials IS patients, of which two, two and six are randomized, respectively (Supplementary Table 4).
Also, in addition to applying clinical scales such as ASIA, EDSS and mRS, future trials should consider to assess more sensitive efficacy parameters in order to be able to demonstrate “proof-of-concept.”
Our systematic review has limitations. We aimed to assess the clinical effect because this is the most relevant outcome from a patient point of view. Clinical efficacy is also a parameter that is measurable and comparable across different neurological conditions. Outcomes with AIS, EDSS and mRS may however be a crude effect estimate when the number of included patients are low, such as in our analyses. In TSCI, we also only reported shifts in AIS classification due to the low number of studies reporting total AIS scores. The lack of benefit of MSC treatment in our main analysis should therefore not be interpreted as a proof of inefficacy.
Another limitation is the low number of controlled studies that entered the meta-analysis. This is mainly because only few controlled trials have been published so far and these were also slightly inconsistent in the reporting of clinical outcomes. In addition, the included studies used different administration methods and treated the patients in different time windows after the debut of the conditions. We still found it reasonable to synthetize the results in a meta-analysis, as all studies investigated the clinical effect of MSC treatment as compared to control groups.
In conclusion, our systematic review showed that MSC treatment is safe in patients with TSCI, MS and IS, although surgical implantation of MSC led to one fatal outcome in TSCI. There was no clear clinical benefit of MSC treatment in the main analyses, but this is not necessarily a proof of inefficacy due to a low number of controlled studies. Future studies assessing efficacy of MSC treatment should aim to do this in randomized, controlled studies, if possible.
The raw data supporting the conclusions of this article are available from the corresponding author upon reasonable request.
CK and TK designed the study. CK and LB conducted the search and extraction of data. All authors contributed to the statistical analysis plan and revised the final version of the manuscript.
This work was funded by Helse Vest and the National Programme for Clinical Therapy Research (KLINBEFORSK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank librarian Randi Bolstad at the library of the University of Bergen for her assistance in performing the systematic search.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.891514/full#supplementary-material
1. Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. (2019) 4:22. doi: 10.1038/s41536-019-0083-6
2. Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy. (2006) 8:315–7. doi: 10.1080/14653240600855905
3. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal stem cell migration and tissue repair. Cells. (2019) 8:784. doi: 10.3390/cells8080784
4. Xia HM, Li X, Gao WW, Fu X, Fang RH, Zhang LF, et al. Tissue repair and regeneration with endogenous stem cells. Nat Rev Mater. (2018) 3:174–93. doi: 10.1038/s41578-018-0027-6
5. Gjerde C, Mustafa K, Hellem S, Rojewski M, Gjengedal H, Yassin MA, et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res Ther. (2018) 9:213. doi: 10.1186/s13287-018-0951-9
6. Lee SH, Jin KS, Bang OY, Kim BJ, Park SJ, Lee NH, et al. Differential migration of mesenchymal stem cells to ischemic regions after middle cerebral artery occlusion in rats. PLoS ONE. (2015) 10:e0134920. doi: 10.1371/journal.pone.0134920
7. He J, Huang Y, Liu J, Lan Z, Tang X, Hu Z. The efficacy of mesenchymal stem cell therapies in rodent models of multiple sclerosis: an updated systematic review and meta-analysis. Front Immunol. (2021) 12:711362. doi: 10.3389/fimmu.2021.711362
8. Maeda Y, Otsuka T, Takeda M, Okazaki T, Shimizu K, Kuwabara M, et al. Transplantation of rat cranial bone-derived mesenchymal stem cells promotes functional recovery in rats with spinal cord injury. Sci Rep. (2021) 11:21907. doi: 10.1038/s41598-021-01490-1
9. Salgado AJ, Sousa JC, Costa BM, Pires AO, Mateus-Pinheiro A, Teixeira FG, et al. Mesenchymal stem cells secretome as a modulator of the neurogenic niche: basic insights and therapeutic opportunities. Front Cell Neurosci. (2015) 9:249. doi: 10.3389/fncel.2015.00249
10. Mohammadalipour A, Dumbali SP, Wenzel PL. Mitochondrial transfer and regulators of mesenchymal stromal cell function and therapeutic efficacy. Front Cell Dev Biol. (2020) 8:603292. doi: 10.3389/fcell.2020.603292
11. Song N, Scholtemeijer M, Shah K. Mesenchymal stem cell immunomodulation: mechanisms and therapeutic potential. Trends Pharmacol Sci. (2020) 41:653–64. doi: 10.1016/j.tips.2020.06.009
12. Yan L, Jiang B, Niu Y, Wang H, Li E, Yan Y, et al. Intrathecal delivery of human ESC-derived mesenchymal stem cell spheres promotes recovery of a primate multiple sclerosis model. Cell Death Discov. (2018) 4:28. doi: 10.1038/s41420-018-0091-0
13. Tetzlaff J, Page M, Moher D. The prisma 2020 statement: development of and key changes in an updated guideline for reporting systematic reviews and meta-analyses. Value Health. (2020) 23:S312–3. doi: 10.1016/j.jval.2020.04.1154
14. Li ZY, Bu XY, Zhang SX, Liang QH Li TP, Chen SL, et al. Autologous bone marrow mesenchymal stem cells in combination with peripheral nerve transplantation for treating spinal cord injury. J Clin Rehabil Tissue Eng Res. (2008) 12:3041–4.
15. Pal R, Venkataramana NK, Bansal A, Balaraju S, Jan M, Chandra R, et al. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: a pilot clinical study. Cytotherapy. (2009) 11:897–911. doi: 10.3109/14653240903253857
16. Jeon SR, Park JH, Lee JH, Kim DY, Kim HS, Sung IY, et al. Treatment of spinal cord injury with bone marrow-derived, cultured autologous mesenchymal stem cells. Tissue Eng Regen Med. (2010) 7:316–22.
17. Bhanot Y, Rao S, Ghosh D, Balaraju S, Radhika CR, Satish Kumar KV. Autologous mesenchymal stem cells in chronic spinal cord injury. Br J Neurosurg. (2011) 25:516–22. doi: 10.3109/02688697.2010.550658
18. Yazdani SO, Hafizi M, Zali AR, Atashi A, Ashrafi F, Seddighi AS, et al. Safety and possible outcome assessment of autologous Schwann cell and bone marrow mesenchymal stromal cell co-transplantation for treatment of patients with chronic spinal cord injury. Cytotherapy. (2013) 15:782–91. doi: 10.1016/j.jcyt.2013.03.012
19. Mendonca MVP, Larocca TF, Souza BSD, Villarreal CF, Silva LFM, Matos AC, et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. (2014) 5:126. doi: 10.1186/scrt516
20. Oh SK, Choi KH, Yoo JY, Kim DY, Kim SJ, Jeon SR. A phase III clinical trial showing limited efficacy of autologous mesenchymal stem cell therapy for spinal cord injury. Neurosurgery. (2016) 78:436-47; discussion 47. doi: 10.1227/NEU.0000000000001056
21. Oraee-Yazdani S, Hafizi M, Atashi A, Ashrafi F, Seddighi AS, Hashemi SM, et al. Co-transplantation of autologous bone marrow mesenchymal stem cells and Schwann cells through cerebral spinal fluid for the treatment of patients with chronic spinal cord injury: safety and possible outcome. Spinal Cord. (2016) 54:102–9. doi: 10.1038/sc.2015.142
22. Satti HS, Waheed A, Ahmed P, Ahmed K, Akram Z, Aziz T, et al. Autologous mesenchymal stromal cell transplantation for spinal cord injury: a phase I pilot study. Cytotherapy. (2016) 18:518–22. doi: 10.1016/j.jcyt.2016.01.004
23. Thakkar UG, Vanikar AV, Trivedi HL, Shah VR, Dave SD, Dixit SB, et al. Infusion of autologous adipose tissue derived neuronal differentiated mesenchymal stem cells and hematopoietic stem cells in post-traumatic paraplegia offers a viable therapeutic approach. Adv Biomed Res. (2016) 5:51. doi: 10.4103/2277-9175.178792
24. Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, et al. An approach to personalized cell therapy in chronic complete paraplegia: the Puerta de Hierro phase I/II clinical trial. Cytotherapy. (2016) 18:1025–36. doi: 10.1016/j.jcyt.2016.05.003
25. Larocca TF, Macedo CT, Souza BSD, Andrade-Souza YM, Villarreal CF, Matos AC, et al. Image-guided percutaneous intralesional administration of mesenchymal stromal cells in subjects with chronic complete spinal cord injury: a pilot study. Cytotherapy. (2017) 19:1189–96. doi: 10.1016/j.jcyt.2017.06.006
26. Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Fernandez C, et al. Repeated subarachnoid administrations of autologous mesenchymal stromal cells supported in autologous plasma improve quality of life in patients suffering incomplete spinal cord injury. Cytotherapy. (2017) 19:349–59. doi: 10.1016/j.jcyt.2016.12.002
27. Zhao Y, Tang F, Xiao Z, Han G, Wang N, Yin N, et al. Clinical study of neuroregen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. (2017) 26:891–900. doi: 10.3727/096368917X695038
28. Vaquero J, Zurita M, Rico MA, Aguayo C, Bonilla C, Marin E, et al. Intrathecal administration of autologous mesenchymal stromal cells for spinal cord injury: safety and efficacy of the 100/3 guideline. Cytotherapy. (2018) 20:806–19. doi: 10.1016/j.jcyt.2018.03.032
29. Vaquero J, Zurita M, Rico MA, Aguayo C, Fernandez C, Rodriguez-Boto G, et al. Cell therapy with autologous mesenchymal stromal cells in post-traumatic syringomyelia. Cytotherapy. (2018) 20:796–805. doi: 10.1016/j.jcyt.2018.04.006
30. Oraee-Yazdani S, Akhlaghpasand M, Golmohammadi M, Hafizi M, Zomorrod MS, Kabir NM, et al. Combining cell therapy with human autologous Schwann cell and bone marrow-derived mesenchymal stem cell in patients with subacute complete spinal cord injury: safety considerations and possible outcomes. Stem Cell Res Ther. (2021) 12:445. doi: 10.1186/s13287-021-02515-2
31. Yang Y, Pang M, Du C, Liu ZY, Chen ZH, Wang NX, et al. Repeated subarachnoid administrations of allogeneic human umbilical cord mesenchymal stem cells for spinal cord injury: a phase 1/2 pilot study. Cytotherapy. (2021) 23:57–64. doi: 10.1016/j.jcyt.2020.09.012
32. Zamani H, Soufizomorrod M, Oraee-Yazdani S, Naviafar D, Akhlaghpasand M, Seddighi A, et al. Safety and feasibility of autologous olfactory ensheathing cell and bone marrow mesenchymal stem cell co-transplantation in chronic human spinal cord injury: a clinical trial. Spinal Cord. (2022) 60:63–70. doi: 10.21203/rs.3.rs-435425/v1
33. Bonab MM, Yazdanbakhsh S, Lotfi J, Alimoghaddom K, Talebian F, Hooshmand F, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. (2007) 4:50–7.
34. Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. (2010) 227:185–9. doi: 10.1016/j.jneuroim.2010.07.013
35. Bonab MM, Sahraian MA, Aghsaie A, Karvigh SA, Hosseinian SM, Nikbin B, et al. Autologous mesenchymal stem cell therapy in progressive multiple sclerosis: an open label study. Curr Stem Cell Res Ther. (2012) 7:407–14. doi: 10.2174/157488812804484648
36. Connick P, Kolappan M, Crawley C, Webber DJ, Patani R, Michell AW, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: an open-label phase 2a proof-of-concept study. Lancet Neurol. (2012) 11:150–6. doi: 10.1016/S1474-4422(11)70305-2
37. Odinak MM, Bisaga GN, Novitskii AV, Tyrenko VV, Fominykh MS, Bilibina AA, et al. Transplantation of mesenchymal stem cells in multiple sclerosis. Neurosci Behav Physiol. (2012) 42:516–20. doi: 10.1007/s11055-012-9593-z
38. Harris VK, Vyshkina T, Sadiq SA. Clinical safety of intrathecal administration of mesenchymal stromal cell-derived neural progenitors in multiple sclerosis. Cytotherapy. (2016) 18:1476–82. doi: 10.1016/j.jcyt.2016.08.007
39. Dahbour S, Jamali F, Alhattab D, Al-Radaideh A, Ababneh O, Al-Ryalat N, et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther. (2017) 23:866–74. doi: 10.1111/cns.12759
40. Cohen JA, Imrey PB, Planchon SM, Bermel RA, Fisher E, Fox RJ, et al. Pilot trial of intravenous autologous culture-expanded mesenchymal stem cell transplantation in multiple sclerosis. Mult Scler. (2018) 24:501–11. doi: 10.1177/1352458517703802
41. Harris VK, Stark J, Vyshkina T, Blackshear L, Joo G, Stefanova V, et al. Phase I trial of intrathecal mesenchymal stem cell-derived neural progenitors in progressive multiple sclerosis. EBioMedicine. (2018) 29:23–30. doi: 10.1016/j.ebiom.2018.02.002
42. Riordan NH, Morales I, Fernandez G, Allen N, Fearnot NE, Leckrone ME, et al. Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med. (2018) 16:57. doi: 10.1186/s12967-018-1433-7
43. Sahraian MA, Mohyeddin Bonab M, Baghbanian SM, Owji M, Naser Moghadasi A. Therapeutic use of intrathecal mesenchymal stem cells in patients with multiple sclerosis: a pilot study with booster injection. Immunol Invest. (2019) 48:160–8. doi: 10.1080/08820139.2018.1504301
44. Iacobaeus E, Kadri N, Lefsihane K, Boberg E, Gavin C, Andren AT, et al. Short and long term clinical and immunologic follow up after bone marrow mesenchymal stromal cell therapy in progressive multiple sclerosis-a phase I study. J Clin Med. (2019) 8:2102. doi: 10.3390/jcm8122102
45. Honmou O, Houkin K, Matsunaga T, Niitsu Y, Ishiai S, Onodera R, et al. Intravenous administration of auto serum-expanded autologous mesenchymal stem cells in stroke. Brain. (2011) 134:1790–807. doi: 10.1093/brain/awr063
46. Qiao L, Huang F, Zhao M, Xie J, Shi J, Wang J, et al. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. (2014) 23(Supplement 1):S65–72. doi: 10.3727/096368914X684961
47. Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. (2016) 47:1817–24. doi: 10.1161/STROKEAHA.116.012995
48. Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II study of safety and preliminary efficacy of intravenous allogeneic mesenchymal stem cells in chronic stroke. Stroke. (2019) 50:2835–41. doi: 10.1161/STROKEAHA.119.026318
49. Xie ZW, Cui GX, Li YZ, Li BW, Zhu SW, Song CZ, et al. Curative effect of autologous mesenchymal stem cell transplantation on spinal cord injury. J Clin Rehabil Tissue Eng Res. (2007) 11:1277–9.
50. Karamouzian S, Nematollahi-Mahani SN, Nakhaee N, Eskandary H. Clinical safety and primary efficacy of bone marrow mesenchymal cell transplantation in subacute spinal cord injured patients. Clin Neurol Neurosurg. (2012) 114:935–9. doi: 10.1016/j.clineuro.2012.02.003
51. Dai G, Liu XB, Zhang Z, Wang XD, Li M, Cheng HB, et al. Comparative analysis of curative effect of CT-guided stem cell transplantation and open surgical transplantation for sequelae of spinal cord injury. J Transl Med. (2013) 11:315. doi: 10.1186/1479-5876-11-315
52. Cheng H, Liu X, Hua R, Dai G, Wang X, Gao J, et al. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J Transl Med. (2014) 12:253. doi: 10.1186/s12967-014-0253-7
53. Deng WS, Ma K, Liang B, Liu XY, Xu HY, Zhang J, et al. Collagen scaffold combined with human umbilical cord-mesenchymal stem cells transplantation for acute complete spinal cord injury. Neural Regen Res. (2020) 15:1686–700. doi: 10.4103/1673-5374.276340
54. Yang Y, Zhang L, Sun W, Li W, Wang K. Therapeutic effect of mesenchymal stem cell in spinal cord injury. Int J Clin Exp Med. (2020) 13:1979–86.
55. Albu S, Kumru H, Coll R, Vives J, Valles M, Benito-Penalva J, et al. Clinical effects of intrathecal administration of expanded Wharton jelly mesenchymal stromal cells in patients with chronic complete spinal cord injury: a randomized controlled study. Cytotherapy. (2021) 23:146–56. doi: 10.1016/j.jcyt.2020.08.008
56. Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, et al. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. (2014) 23 (Suppl. 1):S113–22. doi: 10.3727/096368914X685005
57. Llufriu S, Sepulveda M, Blanco Y, Marin P, Moreno B, Berenguer J, et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE. (2014) 9:e113936. doi: 10.1371/journal.pone.0113936
58. Lublin FD, Bowen JD, Huddlestone J, Kremenchutzky M, Carpenter A, Corboy JR, et al. Human placenta-derived cells (PDA-001) for the treatment of adults with multiple sclerosis: a randomized, placebo-controlled, multiple-dose study. Mult Scler Relat Disord. (2014) 3:696–704. doi: 10.1016/j.msard.2014.08.002
59. Meng M, Liu Y, Wang W, Wei C, Liu F, Du Z, et al. Umbilical cord mesenchymal stem cell transplantation in the treatment of multiple sclerosis. Am J Transl Res. (2018) 10:212–23.
60. Fernandez O, Izquierdo G, Fernandez V, Leyva L, Reyes V, Guerrero M, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: a triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. (2018) 13:e0195891. doi: 10.1371/journal.pone.0195891
61. Petrou P, Kassis I, Levin N, Paul F, Backner Y, Benoliel T, et al. Beneficial effects of autologous mesenchymal stem cell transplantation in active progressive multiple sclerosis. Brain. (2020) 143:3574–88. doi: 10.1093/brain/awaa333
62. Uccelli A, Laroni A, Ali R, Battaglia MA, Blinkenberg M, Brundin L, et al. Safety, tolerability, and activity of mesenchymal stem cells versus placebo in multiple sclerosis (MESEMS): a phase 2, randomised, double-blind crossover trial. Lancet Neurol. (2021) 20:917–29. doi: 10.1016/S1474-4422(21)00301-X
63. Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. (2005) 57:874–82. doi: 10.1002/ana.20501
64. Meng XG, Zhu SW, Gao H, Li YZ, Shi Q, Hou HS, et al. Treatment of cerebral infarction using autologous marrow mesenchymal stem cells transplantation: a six-month follow-up. J Clin Rehabil. Tissue Eng Res. (2009) 13:6374–8.
65. Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, et al. Autologous mesenchymal stem cells improve motor recovery in subacute ischemic stroke: a randomized clinical trial. Transl Stroke Res. (2020) 11:910–23. doi: 10.1007/s12975-020-00787-z
66. Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK, et al. Efficacy and safety of intravenous mesenchymal stem cells for ischemic stroke. Neurology. (2021) 96:e1012–23. doi: 10.1212/WNL.0000000000011440
67. Law ZK, Tan HJ, Chin SP, Wong CY, Wan Yahya WNN, Muda AS, et al. The effects of intravenous infusion of autologous mesenchymal stromal cells in patients with subacute middle cerebral artery infarct: a phase 2 randomized controlled trial on safety, tolerability and efficacy. Cytotherapy. (2021) 23:833–40. doi: 10.1016/j.jcyt.2021.03.005
68. Wang Y, Yi H, Song Y. The safety of MSC therapy over the past 15 years: a meta-analysis. Stem Cell Res Ther. (2021) 12:545. doi: 10.1186/s13287-021-02609-x
69. Zhou Y, Zhang X, Xue H, Liu L, Zhu J, Jin T. Autologous mesenchymal stem cell transplantation in multiple sclerosis: a meta-analysis. Stem Cells Int. (2019) 2019:8536785. doi: 10.1155/2019/8536785
70. Eggenhofer E, Benseler V, Kroemer A, Popp FC, Geissler EK, Schlitt HJ, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. (2012) 3:297. doi: 10.3389/fimmu.2012.00297
71. Levy O, Kuai R, Siren EMJ, Bhere D, Milton Y, Nissar N, et al. Shattering barriers toward clinically meaningful MSC therapies. Sci Adv. (2020) 6:eaba6884. doi: 10.1126/sciadv.aba6884
72. Hess DC, Wechsler LR, Clark WM, Savitz SI, Ford GA, Chiu D, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. (2017) 16:360–8. doi: 10.1016/S1474-4422(17)30046-7
73. Freedman MS, Bar-Or A, Atkins HL, Karussis D, Frassoni F, Lazarus H, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: consensus report of the International MSCT Study Group. Mult Scler. (2010) 16:503–10. doi: 10.1177/1352458509359727
Keywords: ischemic stroke, mesenchymal stem cells, multiple sclerosis, regenerative medicine, traumatic spinal cord injuries
Citation: Kvistad CE, Kråkenes T, Gjerde C, Mustafa K, Rekand T and Bø L (2022) Safety and Clinical Efficacy of Mesenchymal Stem Cell Treatment in Traumatic Spinal Cord Injury, Multiple Sclerosis and Ischemic Stroke – A Systematic Review and Meta-Analysis. Front. Neurol. 13:891514. doi: 10.3389/fneur.2022.891514
Received: 07 March 2022; Accepted: 22 April 2022;
Published: 30 May 2022.
Edited by:
Blanca Fuentes, University Hospital La Paz, SpainReviewed by:
Francisco Moniche, Virgen del Rocío University Hospital, SpainCopyright © 2022 Kvistad, Kråkenes, Gjerde, Mustafa, Rekand and Bø. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Elnan Kvistad, ZWNockBoZWxzZS1iZXJnZW4ubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.