
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 24 June 2022
Sec. Neuro-Otology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.890256
This article is part of the Research Topic Autoinflammation of the Inner Ear View all 8 articles
 Yehree Kim1†
Yehree Kim1† Sang-Yeon Lee2†
Sang-Yeon Lee2† Min Young Kim1
Min Young Kim1 Kyusun Park1
Kyusun Park1 Jin Hee Han1
Jin Hee Han1 Jung Ho Kim3
Jung Ho Kim3 Bong Jik Kim4*
Bong Jik Kim4* Byung Yoon Choi1*
Byung Yoon Choi1*Objective: The pathogenesis of hearing loss in autoinflammatory disorders due to activation of the inflammasome remains incompletely understood. Previously no animals expressing mutant Nlrp3 (NOD-, LRR- and pyrin domain-containing protein 3) survived to an age when hearing evaluation was possible due to embryonic lethality. We aimed to establish a novel mouse model that manifests quantifiable hearing loss with other syndromic features due to alteration of Nlrp3 and investigate the audiologic and histopathologic phenotype in the cochlea to clarify how the genetic alterations of NLRP3 could induce autoinflammatory hearing loss.
Methods: To induce inner ear expression of the mutant Nlrp3, Nlrp3D301NneoR mice were bred with Gfi1Cre knock-in mice for conditional mutant Nlrp3 activation in the cochlea and hematopoietic cells. Hearing thresholds were measured. Hematoxylin-eosin sections of the cochlea, brain, kidney, and liver were examined under light microscopy. Immunohistochemical analyses using polyclonal anti-NLRP3 antibodies on cochlear whole-mount preparations and frozen sections were performed.
Results: We, for the first time in the literature, established a mouse model that manifests quantifiable hearing loss due to Nlrp3 alteration. ABR recordings of Nlrp3D301NneoR/+; Gfi1Cre/+ mice, albeit with limited life expectancy, exhibited severe to profound hearing loss at postnatal day 20 (P20). There was overall overexpression of mutant Nlrp3, and mutant Nlrp3 expression was noted in the spiral prominence, the outer sulcus region (Claudius cells and outer sulcus cells), the organ of Corti, the inner sulcus, and the spiral ganglion neurons in the cochlea. The hematoxylin-eosin sections of Nlrp3D301NneoR/+; Gfi1Cre/+ mice cochleae at P12 exhibited a disorganized organ of Corti between the outer hair cells/supporting Deiters' cells and basilar membrane compared with the normal phenotype mice, leading to a collapsed Nuel's space. This morphologic feature gradually returned to normal by P15. Varying degrees of inflammation with lymphocytic infiltrations were observed in the brain, kidney, and liver.
Conclusion: We report the first mutant Nlrp3 overexpression mouse model (Nlrp3D301NneoR/+; Gfi1Cre/+) that shows obvious overexpression of Nlrp3 in the cochlea, a transient developmental lag of the cochlea, and severe to profound hearing loss. We expect that this mouse line, which models human autoinflammatory hearing loss, could provide a valuable tool to elucidate the underlying pathogenic mechanism of inflammasome activation-mediated hearing loss.
The NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) gene encodes the NLRP3 protein, the central component of the NLRP3 inflammasome (1). The NLRP3 inflammasome is an innate immune sensor expressed in immune cells (2, 3), that leads to the secretion of interleukin-1β when activated. Activation of the NLRP3 inflammasome transforms the inactive procaspase-1 to active caspase-1 which can process pro–IL-1β to mature IL-1β (4).
Alterations in the NLRP3 gene are associated with several autoinflammatory disorders affecting several organs, referred to as cryopyrin-associated periodic syndromes (CAPS), which include a spectrum of disorders—chronic infantile neurological cutaneous and articular syndrome (CINCA or NOMID, neonatal onset multisystem inflammatory disease), Muckle-Wells syndrome and familial cold autoinflammatory syndrome (FCAS) (5, 6).
Hearing loss is one of the presenting symptoms of these autoinflammatory disorders. About 76, 86, 33, and 25% of NOMID, NOMID/MWS, MWS, and FCAS subjects exhibit hearing loss (7). Recent reports have suggested that hearing loss could be the sole symptom in a subset of autoinflammatory diseases (DFNA34) (8, 9).
Activation of the NLRP3 inflammasome requires at least two signals—the initial priming signal and the second activation signal (10). However, in patients with pathogenic variants of NLRP3 gene, this process only requires the initial priming signal to induce IL-1β secretion(11). Anakinra, a non-glycosylated recombinant version of the endogenous human IL-1 receptor antagonist, was reported to control systemic inflammation (12). Intriguingly, it also showed partial effectiveness in the treatment of autoinflammatory hearing loss (9).
Cochlear implantation in patients with this disease entity demonstrated successful auditory rehabilitation outcomes (13). Cochlear implantation could work as a rescue measure in cases of advanced, profound NLRP3-related hearing loss, and an anti-IL-1β agent could be beneficial for early diagnosed, mild hearing loss. However, it has not been clearly elucidated how hearing loss develops in this disease entity; therefore, prevention or timely intervention remains a challenging issue in the management of hearing loss as a presenting symptom of autoinflammatory disorders.
A prior study identified Nlrp3 expression in normal mouse cochlear macrophage-like cells expressing Cx3cr1 (8). The authors found that the NLRP3 inflammasome could be activated with the priming and the activating signals, thus indicating that macrophage/monocyte-like cells in the cochlea can cause hearing loss through innate immune response.
To study the pathogenesis of hearing loss in autoinflammatory disorders due to activation of the inflammasome from developmental and immunological perspectives, a mouse model recapitulating human autoinflammatory disorders with quantifiable hearing loss is mandatory. However, in a previous model, severe inflammation due to Nlrp3 mutation resulted in an extremely short life expectancy, which made an objective hearing assessment impossible.
We generated a double knock-in mouse model by breeding Nlrp3D301NneoR knock-in mice with Gfi1Cre (growth factor independent 1 transcriptional repressor) knock-in mice, a known tool for inducing inner ear hair cell-specific expression of a target gene (14–16) thereby generating the first-ever mouse model in the literature manifesting with both hearing loss and overexpression of Nlrp3. In this study, first, we conducted detailed audiologic and histopathologic phenotyping of gain of function of mutant Nlrp3 in the cochlea. By doing this, we aimed to elucidate how alterations of NLRP3 in the cochlea could play a role in the development of autoinflammatory hearing loss.
To generate the Nlrp3 gain-of-function model, we bred congenital-onset, multisystemic inflammatory disease mice (Nlrp3D301NneoR, obtained from The Jackson Laboratory, Jax #017971) with heterozygous Gfi1Cre knock-in mice (15) to generate mice mutant (Nlrp3 D301NneoR /+; Gfi1Cre/+) and normal phenotype littermate controls (Nlrp3 D301NneoR/+; Gfi1+/+) on a mixed background (Nlrp3D301NneoR was generated on a C57BL/6J background and Gfi1Cre knock-in mice on 129S6 and C57BL/6J mixed background) expressing mutant Nlrp3 in the inner ear and the hematopoietic cells.
For hearing threshold measurement and histopathologic analyses, in which haploinsufficiency of Gfi1 could affect the outcomes (15), the heterozygous Gfi1Cre knock-in mice (Nlrp3+/+; Gfi1Cre/+) were also tested out as controls.
All animals were kept on a 12-h light-dark cycle in a specific-pathogen-free facility with controlled temperature and humidity and had free access to food and water. All experimental procedures were conducted according to protocols approved by the Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital (Seongnam, Republic of Korea, IACUC No.: BA-2004-294-038-09).
To induce inner ear expression of the mutant Nlrp3, Nlrp3D301NneoR mice bearing a loxP-flanked neomycin-resistant cassette (reverse orientation) in intron 2 and a point mutation in exon 3 of Nlrp3 were bred with Gfi1Cre knock-in mice, generating Nlrp3 D301NneoR /+; Gfi1Cre/+ mice for conditional mutant Nlrp3 activation only in the cochlea and hematopoietic cells.
Genotyping was performed by polymerase chain reaction (PCR) on genomic DNA extracted from ear punches. The primers used for Gfi1Cre genotyping were as follows: Gfi1 wild-type forward (5′-GGG ATA ACG GAC CAG TTG-3′), Gfi1 wild-type reverse (5′-CCG AGG GGC GTT AGG ATA-3′), and Gfi1Cre reverse (5′-GCC CAA ATG TTG CTG GAT AGT-3′). The primers used for Nlrp3 genotyping were as follows: Nlrp3 wild-type forward (5′- CAC CCT GCA TTT TGT TG−3′), Nlrp3 mutant forward (5′- GCT ACT TCC ATT TGT CAC GTC C−3′), and common reverse (5′- CGT GTA GCG ACT GTT GAG GT -3′).
The hearing threshold of 10 Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (seven males and three females), six Nlrp3 D301NneoR /+; Gfi1+/+ mice (four males and two females), four Nlrp3+/+; Gfi1Cre/+ mice (two males and two females) and four Nlrp3 +/+; Gfi1+/+ mice (two males and two females) were measured at postnatal day 20 (P20). The hearing threshold of the right ear of each animal was used for analysis. For auditory brainstem response (ABR) testing, mice were anesthetized with an intraperitoneal injection of a mixture of zolazepam/tiletamine (50 mg/kg, Zoletil 50; Virbac, Carros, France) and 10 mg/kg xylazine (Rompun; Bayer Health Care, Leverkusen, Germany). Reference electrodes were attached posterior to the right pinna, a non-inverting electrode was attached at the vertex of the skull, and a ground electrode was attached to the contralateral ear (17). Hearing thresholds were determined at 4, 8, 16, and 32 kHz using a SmartEP device (Intelligent Hearing Systems, Miami, FL, USA). Acoustic stimuli were presented directly to the entrance of the ear canal. Tone burst sound stimuli were presented from 100 dB SPL and decreased 10 dB decrements until the lowest level at which a distinct ABR wave pattern could be recognized by two of the investigators. The ABR threshold was determined as this lowest recognizable ABR response. For mice with no ABR responses, the hearing threshold was calculated as 110 dB SPL. All recordings were performed in a soundproof box, and ABR was tested at postnatal day 20 (P20).
To check for cochlear inflammation, pre- and post-contrast enhanced magnetic resonance imaging (MRI) was performed (n = 2, one Nlrp3 D301NneoR /+; Gfi1Cre/+ and one Nlrp3 D301NneoR /+; Gfi+/+, both male) at P15. All MR scans were performed on a MRS*DRYMAG 7 Tesla (T) MRI system (MR solutions, Guilford, UK) using gradients with a maximum gradient strength of 420 mT/m. Mice were anesthetized as described in the ABR section and body temperature was maintained at 37°C by circulating warm air through the magnet bore. T1-weighted images were acquired first. The mice were scanned using a two-dimensional (2D) spoiled gradient echo sequence (acquisition matrix = 256 × 256; field of view = 20 mm × 20 mm; slice thickness = 3 mm; flip angle = 90°).
Immediately after T1 measurement, the mouse was removed from the scanner, injected with a single bolus of gadolinium meglumine (Dotarem; Guerbet, Villepinte, France) at a dose of 0.2 mmol/kg through the tail vein, and then re-positioned in the scanner. Proper delivery of contrast agent was confirmed first by scanning the abdomen and checking for contrast material in the mouse bladder. Thirty minutes after the injection of contrast agent, contrast enhanced images were acquired using the same acquisition matrix as the precontrast images.
To examine the presence of any cochlear structural abnormalities, hematoxylin-eosin stain was performed at P12, P15, and P20 (n = 4 cochleae of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice, and n = 4 cochleae of Nlrp3 D301NneoR /+; Gfi+/+ for each timepoint). Mice were anesthetized as described before in the ABR section and decapitated. Both cochleae were extracted and fixed in ice-cold 4% paraformaldehyde overnight. The fixed cochleae were washed, dehydrated, and embedded in paraffin. Serial sections were made at 5-μm thickness, attached to the slide glass, and air-dried. The tissue sections were deparaffinized, hydrated, stained with HE, dehydrated, cleared, and sealed with a cover glass. The histologic characteristics were examined under a light microscope.
Immunohistochemistry was performed on Nlrp3 D301NneoR /+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi+/+ mice at P17 (n = 4 cochleae for each type). Cochlear turns were carefully excised, and whole-mount preparations were made and incubated in blocking/permeabilizing buffer (phosphate-buffered saline [PBS] with 5% goat serum and 0.25% Triton X-100). Cochlear frozen sections were also prepared and incubated in blocking/permeabilizing buffer. The preparations were incubated overnight at 4°C with 1:100 rabbit anti-NLRP3 (PA5-88709, Invitrogen, Waltham, MA, USA) primary polyclonal antibodies diluted in the blocking/permeabilizing buffer. After 3 washes, the cochlear turns were reacted with fluorescence-labeled 1:400 goat anti-rabbit (ab150077, Abcam, Cambridge, UK) secondary antibodies diluted in blocking/permeabilizing buffer for 2 h at room temperature. After 3 washes with PBS, the cochlear turns were reacted with 1:100 rhodamine phalloidin (R415, Invitrogen, Waltham, MA, USA) diluted in blocking/permeabilizing buffer for 1 h at room temperature. The samples were then rinsed 3 times with PBS. High-resolution images were obtained using a confocal laser scanning microscope (LSM710, Zeiss). Each image stack was reduced to a two-dimensional maximum intensity Z-projection image.
Prior to the immunohistochemistry procedures on cochlear tissues, the anti-NLRP3 antibody was validated on human embryonic kidney (HEK) 293 cells transfected with NLRP3 (Myc-DDK-tagged) cDNA (catalog no. RC220952) (Supplementary Figure 1).
The number of inner and outer hair cells were counted from the confocal images of the whole-mount preparations described above (Immunohistochemistry of the cochlear tissue). Serial confocal images of hair cells labeled with phalloidin were obtained from each cochlea using laser scanning confocal microscope (LSM710, Zeiss). The entire length of the organ of Corti was reconstructed by overlapping the common cells at the edges of the individual images. The reconstructed image was then divided into segments, each spanning 1% of the total length. The number of IHCs and OHCs in each segment vs. the relative distance from the apex were plotted (18).
Histologic evaluation with HE staining was performed on two Nlrp3 D301NneoR /+; Gfi1Cre/+ mice and one Nlrp3 D301NneoR /+; Gfi+/+ mouse at P20 to check for histopathologic signs of systemic inflammation in other organs. HE-stained tissue slides of the brain, eyes, liver, spleen, stomach, kidney and legs were prepared and the findings were assessed by an experienced pathologist (JHK) with more than 15 years of diagnostic experience. The type of immune cells infiltrating each tissue was determined by the microscopic morphology.
Data analysis was carried out using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). For comparisons between two samples, the significance of data was assessed by the Student t-test when samples showed a normal distribution or by the Mann-Whitney test when samples did not pass the normality test. Comparisons of more than 2 samples were conducted using one-way analysis of variance with the Tukey post-hoc test when data passed the normality test or the Kruskal-Wallis test with the Dunn's post-hoc test when data did show a normal distribution. A P-value of < 0.05 was considered to indicate statistical significance.
The hearing phenotype was assessed at P20 by ABR recordings, which revealed that Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (n = 10 mice) exhibited severe to profound hearing loss. For comparison, the ABR thresholds of Nlrp3+/+; Gfi1Cre/+ mice (n = 4), Nlrp3 D301NneoR /+; Gfi1+/+ mice (n = 6) and Nlrp3+/+; Gfi+/+ mice (n = 4) were recorded at P20.
When compared to the ABR thresholds for Nlrp3+/+; Gfi1Cre/+ mice (four frequency average 37.8 ± 13.9 dB), Nlrp3 D301NneoR /+; Gfi1+/+ mice (37.1 ± 7.5) and Nlrp3+/+; Gfi+/+ mice (39.4 ± 13.8 dB), the ABR thresholds of the Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (96.4 ± 20.4 dB) were significantly higher across the four tested frequencies (Figure 1, P≤0.05, Kruskal–Wallis test with the Dunn post-hoc test). The difference between the results of the Nlrp3 D301NneoR /+; Gfi1Cre/+ mice and the Nlrp3+/+; Gfi1Cre/+ mice means that the hearing loss observed in Nlrp3 D301NneoR /+; Gfi1Cre/+ mice was entirely due to the p.D301N variant and not due to Gfi1 haploinsufficiency.
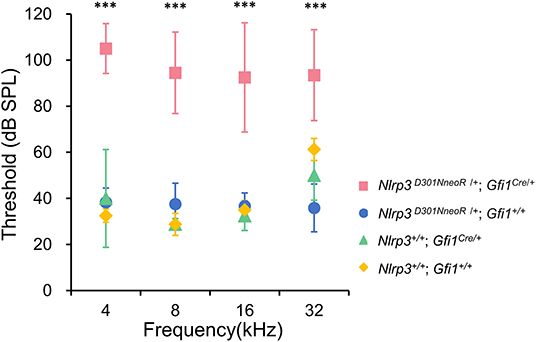
Figure 1. ABR thresholds in Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (pink, n = 10 mice) were elevated compared to Nlrp3 D301NneoR /+; Gfi1+/+ mice (blue, n = 6 mice), Nlrp3 +/+; Gfi1Cre/+ mice (green, n = 4 mice), and Nlrp3 +/+; Gfi1+/+ mice (yellow, n = 4 mice) at P20. At each of the four tested frequencies, the threshold increases for Nlrp3 D301NneoR /+; Gfi1Cre/+ vs. Nlrp3 D301NneoR /+; Gfi1+/+, Nlrp3 D301NneoR /+; Gfi1Cre/+ vs. Nlrp3+/+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1Cre/+ vs. Nlrp3+/+; Gfi1+/+, were significant (Kruskal–Wallis test with the Dunn multiple-comparison test between all four groups). ABR, auditory brainstem response; P20, postnatal day 20. *** denotes P ≤ 0.001.
The pre-contrast T1-weighted images of both the Nlrp3D301NneoR/+; Gfi1+/+ and the Nlrp3D301NneoR/+; Gfi1Cre/+ mouse showed similar signal intensity of the cochlea (Figures 2A,C). The same images were acquired after injection of contrast material into the tail vein which showed enhancement of the fluid compartment of the cochlea of the Nlrp3D301NneoR/+; Gfi1Cre/+ (Figure 2D). As for the Nlrp3D301NneoR/+; Gfi1+/+ mouse, there was no enhancement of the cochlea (Figure 2B).
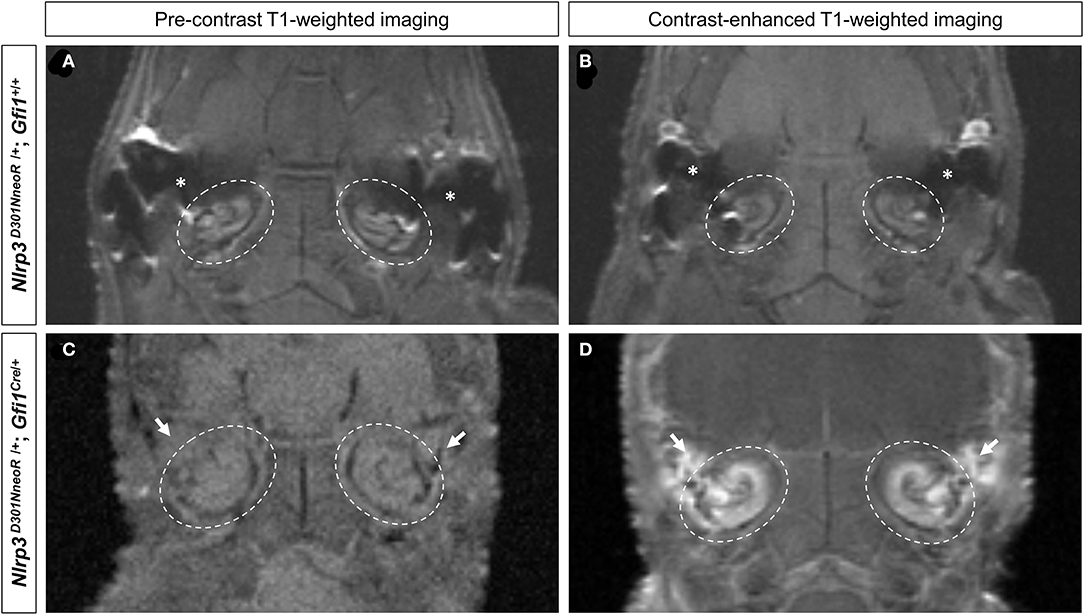
Figure 2. Pre- and post-contrast-enhanced T1-weighted images of the mouse head. Location of the cochlea is shown in dashed circles. The pre-contrast images of both the Nlrp3 D301NneoR /+; Gfi1+/+ (A) and the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse (C) show similar signal intensity of the cochlea to its surrounding tissue. Images taken 30 min after gadolinium injection, show enhancement of the cochlea in the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse (D) but not in the cochlea of the Nlrp3 D301NneoR /+; Gfi1+/+ mouse (B). The Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse also has inflammation in the middle ear (white arrows) where in the healthy condition should contain air and appear black as can be seen in the middle ear of the Nlrp3 D301NneoR /+; Gfi1+/+ mouse (asterisks).
Of note, middle ear inflammation was also visible on the pre- and post-contrast enhanced images of the Nlrp3D301NneoR/+; Gfi1Cre/+mouse (Figures 2C,D).
To specify Nlrp3 expression in the cochlear tissue, we performed immunohistochemical staining using anti-NLRP3 antibody. The expression of Nlrp3 protein was evaluated in the cochleae of Nlrp3 D301NneoR /+; Gfi1+/+ and Nlrp3 D301NneoR /+; Gfi1Cre/+ mice at P15 (Figures 3, 4). In the cochlea of Nlrp3 D301NneoR /+; Gfi1+/+ mouse, which was expected to show wild-type expression of Nlrp3, Nlrp3 immunoreactivity was mainly concentrated in the spiral prominence (Figures 3A,D), the organ of Corti (Figures 3A,C), and the spiral ganglion neurons (Figures 3A,B). In the cochlea of Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse, there appeared an overall overexpression of mutant Nlrp3, and the immunostaining was noted in the spiral prominence, the outer sulcus region (Claudius cells and outer sulcus cells) (Figures 3E,H), the organ of Corti, the inner sulcus (Figures 3E,G), and the spiral ganglion neurons (Figures 3E,H). Similar immunostaining patterns of Nlrp3 were seen throughout the whole cochlea along the tonotopic axis (Figures 3I,J). Noticeably, the Nlrp3 expression pattern was shown to differ the most in the outer sulcus region and the inner sulcus between the two types of mice.

Figure 3. Inner ear section immunohistochemistry from P15 Nlrp3 D301NneoR /+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1+/+ mice stained with an anti-NLRP3 antibody. Nlrp3 was more widely overexpressed in the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse (E–H) than in the Nlrp3 D301NneoR /+; Gfi1+/+ mouse (A–D). In both types of mice, Nlrp3 was expressed in the spiral prominence (D,H), the organ of Corti (C,G), and the spiral ganglion neurons (B,F). A difference in the expression of Nlrp3 was noted in the inner sulcus cells (arrows), Hensen's cells (asterisks), and the outer sulcus region (arrowheads). Similar expression pattern of Nlrp3 was observed in two other cochlear turns of Nlrp3 D301NneoR /+; Gfi1+/+ (I) and Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (J). (n = 6 cochleae for Nlrp3 D301NneoR /+; Gfi1Cre/+ and n = 6 cochleae for Nlrp3 D301NneoR /+; Gfi1+/+) Nlrp3 in green, DAPI in blue, the scale bars represent 100 μm for (A,E,I,J) and 20 μm for (B–D,F–H). P15, postnatal day 15.
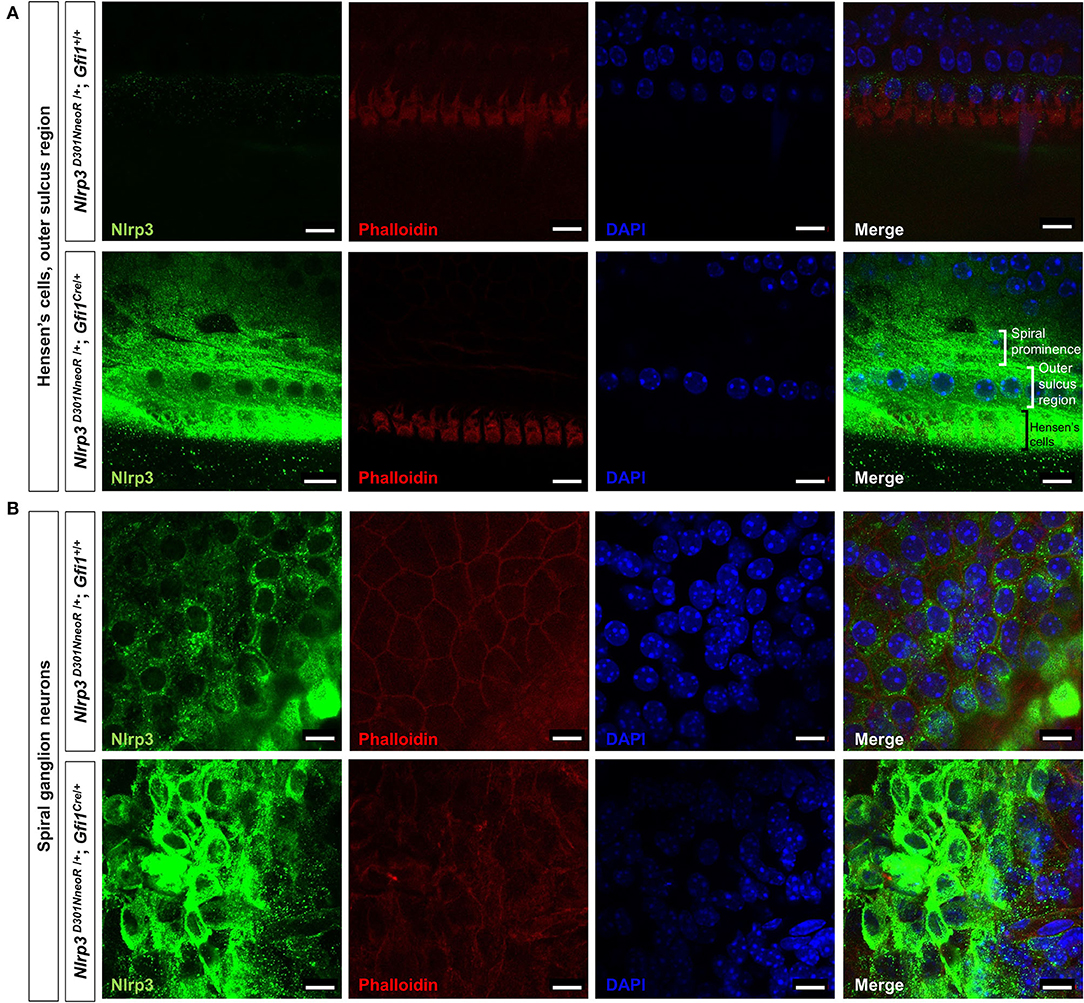
Figure 4. Whole-mount immunofluorescence of a P15 Nlrp3 D301NneoR /+/+; Gfi1Cre/+ mouse cochlea and a Nlrp3 D301NneoR /+; Gfi1+/+ mouse cochlea. Nlrp3 was overexpressed in the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse [bottom panels of (A,B)] compared to the Nlrp3 D301NneoR /+; Gfi1+/+ mouse [top panels of (A,B)] in Hensen's cells and the outer sulcus region (A) and the spiral ganglion neurons (B). (n = 6 cochleae for Nlrp3 D301NneoR /+; Gfi1Cre/+ and n = 6 cochleae for Nlrp3 D301NneoR /+; Gfi1+/+) Nlrp3 in green, phalloidin in red, DAPI in blue, the scale bars represent 10 μm. P15, postnatal day 15.
Meanwhile, immunohistochemical staining of the whole mount preparations revealed similar but rather restricted expression pattern compared with those observed in the frozen section. The overexpression of mutant Nlrp3 compared to the pattern of wild-type Nlrp3 expression was most prominent at the Hensen's cells and the outer sulcus region (Figure 4A), as well as the spiral ganglion neurons (Figure 4B).
The number of inner and outer hair cells were quantified and compared in Nlrp3 D301NneoR /+; Gfi1+/+ mice (n = 6 cochleae) and Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (n = 6 cochleae) at P15. Neither IHC nor OHC number showed significant difference in these two groups (Supplementary Figure 2). All inner and outer hair cells looked morphologically intact in Nlrp3 D301NneoR /+; Gfi1Cre/+ mice while the ABR results showed profound hearing loss in this group.
A close evaluation of the organ of Corti demonstrated a difference in histopathology between Nlrp3 D301NneoR /+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1+/+ (normal phenotype) mice at P12 (Figure 5). At the middle and apical turns of the cochlea, hair and supporting cells were present in mice of both genotypes. However, Nlrp3 D301NneoR /+; Gfi1Cre/+ mice consistently exhibited a severely collapsed or disorganized organ of Corti between outer hair cells/supporting dieter's cell and the basilar membrane compared with Nlrp3 D301NneoR /+; Gfi1+/+mice, leading to an apparently collapsed Nuel's space (space between outer pillar cells and outer hair cells/dieter's cell). Additionally, nuclei of the greater epithelial ridge and other supporting cells close to the spiral ligament in Nlrp3 D301NneoR /+; Gfi1Cre/+ mice tended to be more condensed than in Nlrp3 D301NneoR /+; Gfi1+/+ mice. Meanwhile, no meaningful differences were found in histopathology between Nlrp3+/+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1+/+ mice, implying that the collapsed morphology of the organ of Corti was also not attributable to Gfi1 haploinsufficiency (Supplementary Figure 3). Interestingly, the sections of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice tended to gradually restore normal morphology of the organ of Corti at P15, as compared to P12, ultimately revealing normal cochlear morphology in most Nlrp3D301N/+; Gfi1Cre/+ mice at P20 (Figure 6A). The proportion of collapsed organ of Corti at P12 was 100%, which gradually decreased to 21.1% at the apex, 16.7% at the middle and 0% at the base at P20 (Figure 6B). Together, the data indicate that Nlrp3 gain-of-function might predispose mice to a developmental delay of the organ of Corti, especially at the early postnatal stage.

Figure 5. Cochlear morphology of Nlrp3 D301NneoR /+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1+/+ (normal phenotype) mice at P12. Representative hematoxylin and eosin-stained paraffin sections of Nlrp3 D301NneoR /+; Gfi1+/+ mouse [(A), apex/(C), middle] and Nlrp3 D301NneoR /+; Gfi1Cre/+ mice [(B), apex/(D), middle]. Close-ups of the organ of Corti (dotted box) from Nlrp3 D301NneoR /+; Gfi1+/+ mice (E,G) and Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (F,H) are illustrated. Notice the apparent collapse of organ of Corti in Nlrp3D301N/+ mice (P12). P12, postnatal day 12; OHCs, outer hair cells; IHCs, inner hair cells; DCs, Dieter's cells; BM, basilar membrane; OP, outer pillar cells; IP, inner pillar cells; GER, greater epithelial ridge. The scale bars represent 500 mm.
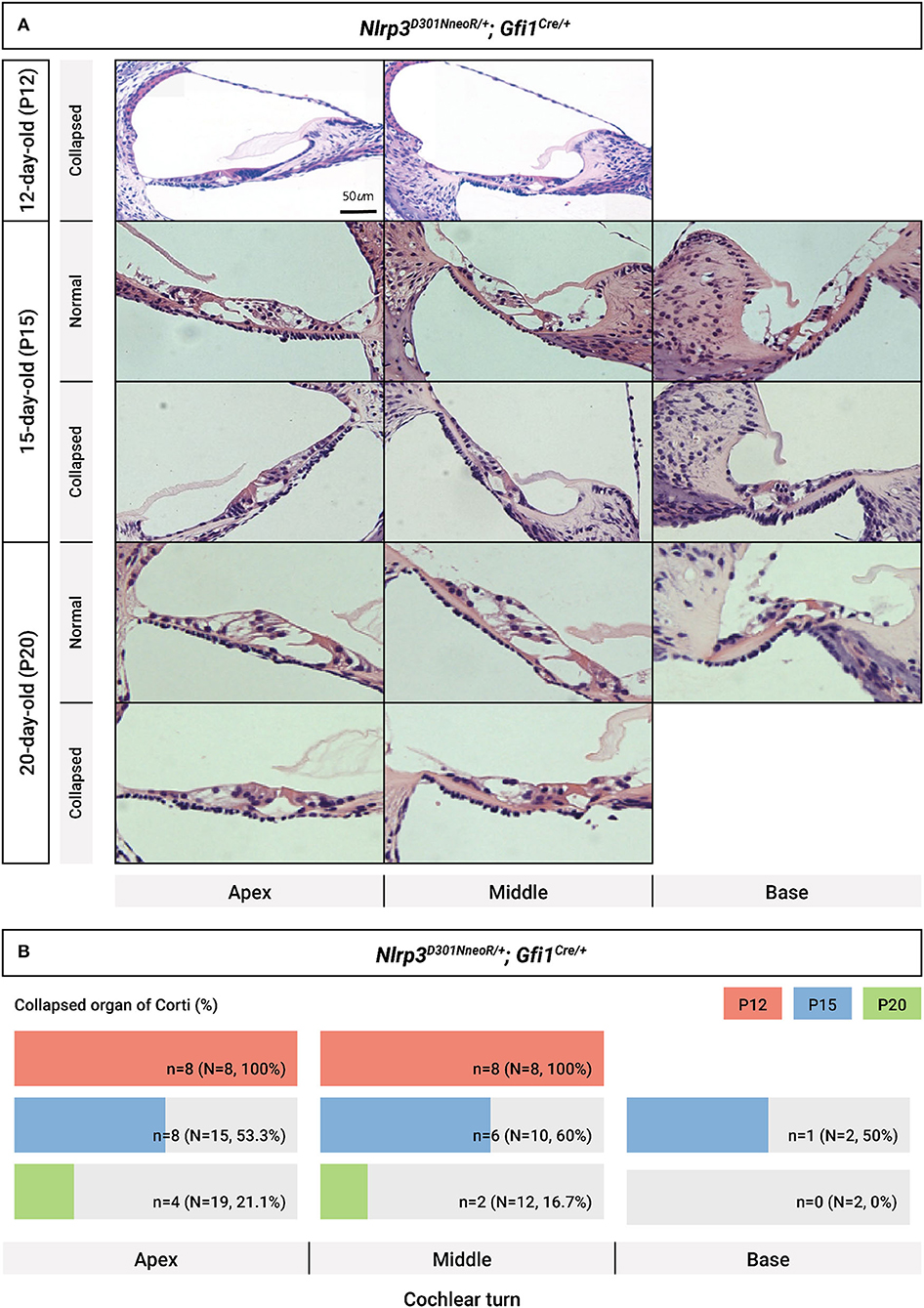
Figure 6. Chronological evaluation of cochlear morphology in Nlrp3 D301NneoR /+; Gfi1Cre/+ mice. (A) Representative sections from the cochlear turns obtained at P12, P15, and P20, respectively, are depicted (normal cochlear morphology vs. collapsed morphology of organ of Corti). (B) Proportion of collapsed organs of Corti at each time point, depending on cochlear turns. The scale bars represent 500 mm (P12) and 1,000 mm (P15 and P20). P, postnatal day.
Various degrees of inflammation were observed on H&E-stained tissue slides of the brain, kidney, and liver (Figure 7). In detail, mild to moderate perivascular lymphocytic infiltration was observed in the brain parenchyma (representative image of the thalamus is shown in Figure 7), and increased inflammatory infiltrates were observed in the renal parenchyma. There was also moderate lymphocytic infiltration in the portal and lobular areas of the hepatic parenchyma. In the spleen, the lymphoid structure seems to be effaced, but definite inflammation was not seen. Definite inflammation was not observed in sections of the eye, stomach, arm, and leg.
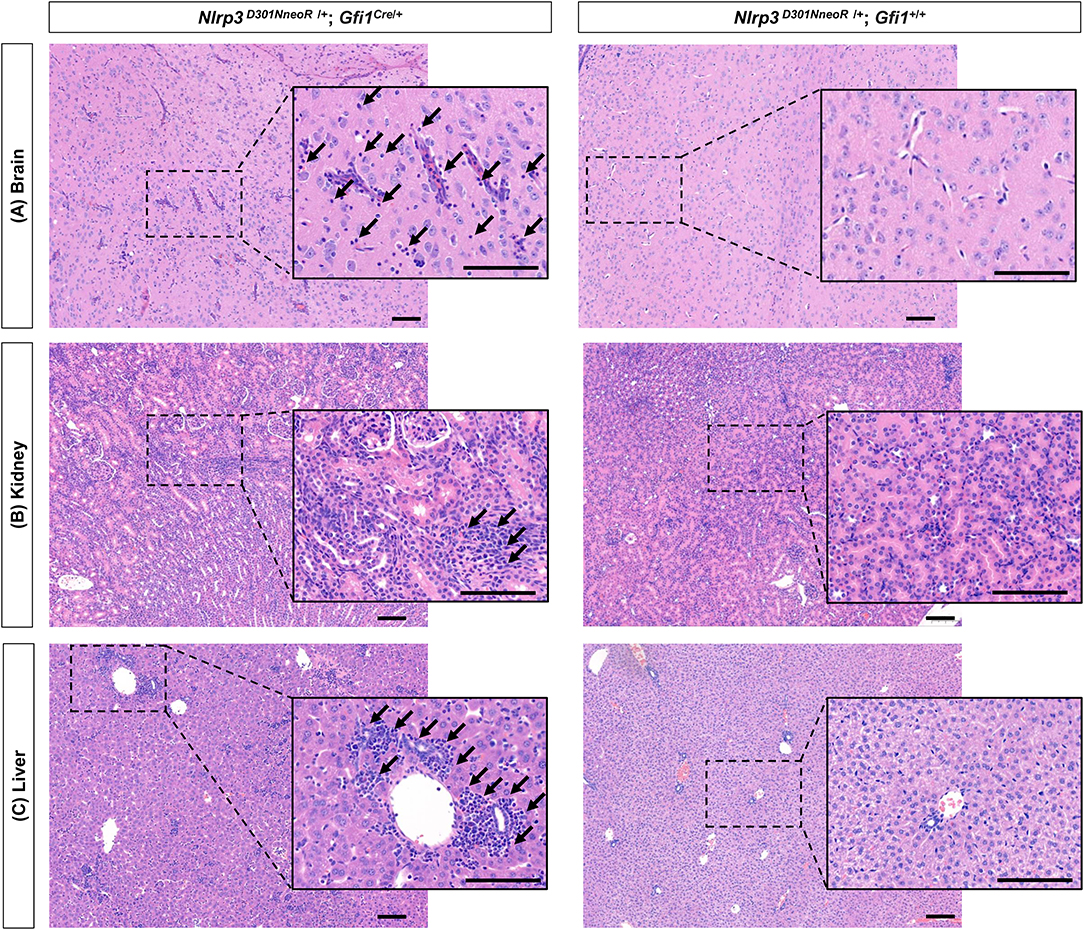
Figure 7. Generalized inflammation observed in several organs of Nlrp3D301NneoR/+; Gfi1Cre/+ mice compared to Nlrp3 D301NneoR /+; Gfi1+/+ mice. Representative image of inflammation at P20 in (A) brain (thalamus), (B) kidney, and (C) liver sections of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice (left panels) compared to normal findings in Nlrp3 D301NneoR/+; Gfi1+/+ mice (right panels). Diffuse lymphocytic infiltration can be seen throughout the whole section. Insets show enlarged views of the dotted areas. Black arrows denote examples of areas with lymphocytic infiltration. P20, postnatal day 20. The scale bars represent 100 μm.
We, for the first time, report the detailed quantifiable auditory and cochlear histopathologic phenotype of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice. In this animal model, Nlrp3 expression was driven by the Gfi1 promoter, which might be different from the endogenous Nlrp3 expression. Nonetheless, the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse is the first mouse model to show overexpression of Nlrp3 in the cochlea and potentially associated hearing loss, which could also provide a valuable tool to investigate the link between mutant Nlrp3 overexpression and hearing loss, further revealing the underlying mechanism of immune-related pathophysiology of autoinflammatory hearing loss.
Originally, we intended to generate inner ear-specific expression of Nlrp3 (D301N) by crossing Nlrp3 D301NneoR /+ onto Gfi1-Cre knock-in mice, which was reported as a useful tool for inducing inner ear hair cell-specific expression of a target gene (15). However, Gfi1 encodes a zinc-finger transcription factor that is also important for the development and maintenance of hematopoiesis, which could interfere with maturation of the immune system in the neonatal period and early infancy in mice. Accordingly, Nlrp3 D301NneoR /+; Gfi1Cre/+ mice in our study grew poorly (Supplementary Figure 4) and did not survive longer than 3 weeks (mean survival: 17.5 ± 2.7 days, n = 24), which might have been partly due to the intrinsic effect of systemic Nlrp3 alterations corresponding to the phenotypes observed in human NLRP3-related autoinflammatory disorders, such as CINCA, which presents with fever, rash, joint symptoms, and central nervous system symptoms (19). However, its effects might be potentiated by systemic effects on several organs due to the abnormal development of the immune system, as was observed in comparisons of HE staining of several organs between Nlrp3 D301NneoR /+; Gfi1Cre/+ and Nlrp3 D301NneoR /+; Gfi1+/+ mice.
The widespread inflammation and very short life expectancy observed in the animal model may limit the impact of this study, which only yielded insights into the early changes of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice in terms of systemic effects and developmental dysregulation of the cochlea and immune system. For instance, the number and morphology of hair cells of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice were well-preserved not differing from the hair cells of the Nlrp3 D301NneoR /+; Gfi1+/+ mice. It is possible that the mice in this study did not survive long enough for hair cell damage from prolonged cochlear inflammation to manifest. Future studies using a different Cre mouse that generates milder phenotype and longer survival could show different outcomes. Based on the finding that only around 30% of patients with autoinflammatory inner ear disease respond to anakinra therapy (20) and the hearing in others deteriorate to the level that requires cochlear implantation, the damage caused by the inflammation on the cochlea can be progressive, irreversible and could lead to eventual hair cell damage.
The hearing thresholds of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice were clearly worse than those of normal controls (Figure 1). A previous study regarding Gfi1Cre/+ mice demonstrated early-onset mild progressive hearing loss, which might serve as a source of bias when interpreting the ABR results (14). However, the difference in hearing thresholds between Gfi1Cre/+ mice and wild-type (Gfi1+/+) mice in that study was limited to 32 kHz until 2 months of life, meaning that intrinsic hearing deficit due to haploinsufficiency of Gfi1 did not have a significant impact within <1 month of life. Accordingly, there was no difference in hearing thresholds between Nlrp3+/+; Gfi1Cre/+ mice and Nlrp3 D301NneoR /+; Gfi1+/+ mice (P > 0.05 at all frequencies by the Mann-Whitney test), although the mean hearing threshold of Nlrp3+/+; Gfi1Cre/+ mice at 32 kHz appeared slightly higher than those of Nlrp3 D301NneoR /+; Gfi1+/+ mice. On the contrary, Nlrp3 D301NneoR /+; Gfi1Cre/+ mice showed significantly worse hearing than mice expressing normal Nlrp3, irrespective of the loss of one copy of the Gfi1gene (Figure 1).
Middle ear inflammation observed in the MR images of the Nlrp3 D301NneoR /+; Gfi1Cre/+ mice could also have contributed to the elevated hearing thresholds. Indeed, prior studies of ABR threshold measurement in mouse models of otitis media have quantified at most 15 to 30 dB threshold shift depending on the tested frequency (21, 22). However, six out of the 10 Nlrp3 D301NneoR /+; Gfi1Cre/+ mice in this study did not show any ABR responses in all four tested frequencies even to the strongest stimuli (100 dB SPL). Thus, it is conceivable that a substantial portion of the elevation of hearing thresholds was due to the inner ear dysfunction, not the middle ear inflammation.
Cochlear inflammation was evident on the contrast-enhanced MR images of the Nlrp3 D301NneoR /+; Gfi1Cre/+ mouse. MRI studies of patients with CAPS or non-syndromic autoinflammatory inner ear disease have also identified cochlear enhancement (7–9). Intravenously injected contrast material can reach the inner ear via small vessels that supply the labyrinth and cochlea (23). Capillary networks present in the stria vascularis and spiral ligament form the blood-labyrinth barrier, composed of endothelial cells with tight-junctions surrounded by pericytes and resident macrophages (24). During inflammatory process of the cochlea, elevated levels of cytokines can increase capillary permeability thereby compromising the integrity of the blood-labyrinth barrier (25). The contrast material can enter the perilymphatic and endolymphatic spaces more freely than in the non-inflammatory state.
Immunohistochemical analyses using the anti-human NLRP3 polyclonal antibody revealed the widespread presence of Nlrp3, not limited to expression in the spiral ganglion, which has been reported in the literature (26). Rather, in the cochlea of Nlrp3 D301NneoR /+; Gfi1Cre/+ mice, Nlrp3 was expressed strongly in the spiral prominence, the organ of Corti, and the spiral ganglion neurons. Nlrp3D301NneoR/+; Gfi1Cre/+ mice carry one copy of a wild-type Nlrp3 allele that would result in similar expression of Nlrp3 as seen in Nlrp3D301NneoR/+; Gfi1+/+ mice. The other floxed Nlrp3 allele would lead to the overexpression of Nlrp3 in Cre-expressing cells. Although Gfi1 is known to be expressed in hair cells in the late embryonic and postnatal inner ear (27), a previous validation of a Gfi1Cre/+ mouse model found that Cre-mediated recombination was not entirely hair cell-specific, but was also present in CD45+ monocytes/macrophages (14). A cell type-specific RNA-sequencing study of gene expression during mouse inner ear development also identified Gfi1 expression in the surrounding cells of the cochlea at P4 (28). Thus, in the current study, overexpression of Nlrp3 was observed in cells in which Gfi1 was once expressed during the developmental period—that is, in hair cells and the regions of the cochlea where CD45+ cells can be found (in the organ of Corti, the basilar membrane, the spiral ligament, spiral limbus, and the neural region) (29).
The immunoreactivity to Nlrp3 in the cochlear sections differed the most in the inner sulcus and the outer sulcus region between the wildtype and mutant mice, displaying the significant staining exclusively from the mutant mice. The greatest difference in immunoreactivity to Nlrp3 in the cochlear sections between the wildtype and mutant mice was at the region of the inner sulcus and the outer sulcus, with significant positive staining occurring exclusively in the mutant mice. In the spiral prominence and the spiral ganglion neurons, both types of mice showed some degree of immunoreactivity, but it was stronger in the Nlrp3D301NneoR/+; Gfi1Cre/+ mice. The aforementioned areas in which we observed differences in immunoreactivity, we could attribute the findings to the ramifications of the gain-of-function variant of Nlrp3. Contrastingly, in the hair cells, the degree of Nlrp3 immunostaining was similar in both types of mice, precluding differentiation of the true immunostaining from staining artifacts. Absence of immunostaining of Nlrp3 from hair cells in the whole mount preparations—unlike in the frozen section—further complicated interpretation of immunostaining in the hair cells. This issue awaits further clarification through validation of the findings on a knockout mouse, which will be done in forthcoming studies.
Il-1β, a downstream product of Nlrp3 inflammasome activation (8), is known to form a positive feedback loop that potentially primes nearby cells in a paracrine manner (30, 31). In the inner sulcus and the outer sulcus region, Gfi1 expression was not anticipated, but overexpression of Nlrp3 was nonetheless evident. In these sites, the induced Il-1β from neighboring cells expressing mutant Nlrp3 could have enhanced the priming step to increase endogenous Nlrp3 expression. Future studies including co-localization of Nlrp3 with Il-1β and treatment with Il-1β antagonists would provide further insights.
Intriguingly, as was seen in Figure 6, the morphology of the organ of Corti in Nlrp3D301NneoR/+; Gfi1Cre/+ mice gradually normalized from P12 (significant difference between Nlrp3D301NneoR/+; Gfi1Cre/+ and Nlrp3D301NneoR/+; Gfi1+/+ mice) to P20, which might suggest that the effect of Nlrp3D301NneoR/+ on the development of inner ear is transient in the early postnatal period and further indicate that the elevation of the hearing threshold might not be solely due to the anatomical alterations. In further research, we need to generate a new mouse model by breeding Cre mice of specific immune cell population with Nlrp3 knock-in mice, which could express Nlrp3, thereby coming one step closer to endogenous Nlrp3 expression. By doing so, we could elucidate the specific immune cell population associated with the development of autoinflammatory hearing loss in an animal model with a longer lifespan, which could enable a more detailed characterization of hearing loss and immune system dysregulation.
Nlrp3 D301NneoR /+; Gfi1Cre/+ mice, an animal model of Nlrp3 expression driven by the Gfi1 promoter, is the first mouse model to show quantifiable hearing loss and overexpression of Nlrp3 in the cochlea. This overexpression is potentially associated with hearing loss, and this model could also provide a valuable tool to investigate the link between mutant Nlrp3 overexpression and hearing loss, further revealing the underlying mechanism of inflammasome activation-mediated hearing loss.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
The animal study was reviewed and approved by Institutional Animal Care and Use Committee of Seoul National University Bundang Hospital.
BK and BC contributed to the conception and design of the study. YK, MK, and JH organized the data and conducted the experiments. YK, S-YL, JK, BK, and BC performed the data analysis. YK, S-YL, BK, and BC wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This study was supported by the Basic Science Research Program through the NRF funded by the Ministry of Education (Grants 2021R1A2C2092038 and 2018R1A2B2001054 to BC), the Korea Government (MSIT) (No. 2021R1C1C1007980 to BK), also by SNUBH Intramural Research Funds (16-2020-0005 and 14-2021-0003 to BC), and the Institute of Information Communication Technology Planning and Evaluation (IITP) grant funded by the Korea Government (MSIT) [No. 2020-0-01441, Artificial Intelligence Convergence Research Center (Chungnam National University) to BK].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Gfi1Cre knock-in mice and Nlrp3D301NneoR knock-in mice were kindly provided for this study by Dr. Jinwoong Bok at the Department of Anatomy, Yonsei University and by Dr. Je-Wook Yu at the Department of Microbiology and Immunology, Yonsei University, respectively.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.890256/full#supplementary-material
Supplementary Figure 1. Validation of the anti-NLRP3 antibody on human embryonic kidney (HEK) 293 cells transfected with NLRP3 (Myc-DDK-tagged) cDNA. The anti-NLRP3 antibody showed specific binding to the HEK 293 cells transfected with NLRP3 (red color in the bottom panel) and not to the mock transfection control (top panel).
Supplementary Figure 2. Hair cell count between Nlrp3 D301NneoR /+/+; Gfi1Cre/+ mice and Nlrp3 D301NneoR /+; Gfi1+/+ mice. Number of inner (A) and outer (B) hair cells in segments spanning 1% of the whole cochlear length in P15 Nlrp3 D301NneoR /+/+; Gfi1Cre/+ mice (n = 6 cochleae) and Nlrp3 D301NneoR /+; Gfi1+/+ mice (n = 6 cochleae) were not different.
Supplementary Figure 3. Cochlear morphology of Nlrp3+/+; Gfi1Cre/+ mice at P15. Sections of the cochlea of Nlrp3+/+; Gfi1Cre/+ mice did not show collapsed morphology of the organ of Corti, unlike the Nlrp3D301N/+; Gfi1Cre/+ mice. P15, postnatal day 15.
Supplementary Figure 4. Differences in the morphology of Nlrp3D301N/+; Gfi1+/+ mice compared to Nlrp3D301N/+; Gfi1Cre/+ mice. At P11, two Nlrp3 D301NneoR /+; Gfi1Cre/+ mice were smaller (2.8 g and 3.1 g vs. 6.8 g) and showed more extensive areas of skin inflammation with hair loss than the Nlrp3D301N/+; Gfi1+/+mouse (left). P11, postnatal day 11.
1. Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp JJI. Nalp3 forms an Il-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity. (2004) 20:319–25. doi: 10.1016/S1074-7613(04)00046-9
2. Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, et al. Critical role for Nalp3/Cias1/cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. (2006) 24:317–27. doi: 10.1016/j.immuni.2006.02.004
3. Guarda G, Zenger M, Yazdi AS, Schroder K, Ferrero I, Menu P, et al. Differential expression of Nlrp3 among hematopoietic cells. J Immunol. (2011) 186:2529–34. doi: 10.4049/jimmunol.1002720
4. Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of ProIL-?. Mol Cell. (2002) 10:417–26. doi: 10.1016/S1097-2765(02)00599-3
5. Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in Cias1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet. (2002) 71:198–203. doi: 10.1086/341357
6. Kuemmerle-Deschner JB, Koitschev A, Ummenhofer K, Hansmann S, Plontke SK, Koitschev C, et al. Hearing loss in Muckle-Wells syndrome. Arthritis Rheum. (2013) 65:824–31. doi: 10.1002/art.37810
7. Ahmadi N, Brewer CC, Zalewski C, King KA, Butman JA, Plass N, et al. Cryopyrin-associated periodic syndromes: otolaryngologic and audiologic manifestations. Otolaryngol Head Neck Surg. (2011) 145:295–302. doi: 10.1177/0194599811402296
8. Nakanishi H, Kawashima Y, Kurima K, Chae JJ, Ross AM, Pinto-Patarroyo G, et al. Nlrp3 mutation and cochlear autoinflammation cause syndromic and nonsyndromic hearing loss Dfna34 responsive to Anakinra therapy. Proc Natl Acad Sci USA. (2017) 114:E7766–75. doi: 10.1073/pnas.1702946114
9. Kim BJ, Kim YH, Lee S, Han JH, Lee SY, Seong J, et al. Otological aspects of Nlrp3-related autoinflammatory disorder focusing on the responsiveness to Anakinra. Rheumatology. (2021) 60:1523–32. doi: 10.1093/rheumatology/keaa511
10. Kelley N, Jeltema D, Duan Y, He YJ. The Nlrp3 inflammasome: an overview of mechanisms of activation and regulation. Int J Mol Sci. (2019) 20:3328. doi: 10.3390/ijms20133328
11. Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo Cias1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (Nomid): a new member of the expanding family of Pyrin-associated autoinflammatory diseases. Arthritis Rheum. (2002) 46:3340–8. doi: 10.1002/art.10688
12. Kim YH, Kim BJ, Han J, Choi BY, Lee S. Long-term efficacy of Anakinra in cryopyrin-associated periodic syndrome: focus on destructive arthropathy. J Clin Immunol. (2021) 41:1936–9. doi: 10.1007/s10875-021-01099-z
13. Kim BJ, Kim YH, Han JH, Lee SY, Carandang M, Lee DH, et al. Outcome of cochlear implantation in Nlrp3-related autoinflammatory inner ear disorders. Otol Neurotol. (2021) 42:e168–71. doi: 10.1097/MAO.0000000000002933
14. Matern M, Vijayakumar S, Margulies Z, Milon B, Song Y, Elkon R, et al. Gfi1cre mice have early onset progressive hearing loss and induce recombination in numerous inner ear non-hair cells. Sci Rep. (2017) 7:1–13. doi: 10.1038/srep42079
15. Yang H, Gan J, Xie X, Deng M, Feng L, Chen X, et al. Gfi1-Cre knock-in mouse line: a tool for inner ear hair cell-specific gene deletion. Genesis. (2010) 48:400–6. doi: 10.1002/dvg.20632
16. Cox BC, Liu Z, Lagarde MMM, Zuo J. Conditional gene expression in the mouse inner ear using Cre-Loxp. J Assoc Res Otolaryngol. (2012) 13:295–322. doi: 10.1007/s10162-012-0324-5
17. Akil O, Oursler A, Fan K, Lustig LR. Mouse auditory brainstem response testing. Bio Protoc. (2016) 6:e1768. doi: 10.21769/BioProtoc.1768
18. Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. (2004) 197:1–10. doi: 10.1016/j.heares.2004.04.016
19. Bonar SL, Brydges SD, Mueller JL, McGeough MD, Pena C, Chen D, et al. Constitutively activated Nlrp3 inflammasome causes inflammation and abnormal skeletal development in mice. PLoS ONE. (2012) 7:e35979. doi: 10.1371/journal.pone.0035979
20. Sibley CH, Plass N, Snow J, Wiggs EA, Brewer CC, King KA, et al. Sustained response and prevention of damage progression in patients with neonatal-onset multisystem inflammatory disease treated with anakinra: a cohort study to determine three-and five-year outcomes. Arthritis Rheum. (2012) 64:2375–86. doi: 10.1002/art.34409
21. Han F, Yu H, Zhang J, Tian C, Schmidt C, Nava C, et al. Otitis media in a mouse model for down syndrome. Int J Exp Pathol. (2009) 90:480–8. doi: 10.1111/j.1365-2613.2009.00677.x
22. MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. (2006) 219:12–23. doi: 10.1016/j.heares.2006.05.012
23. Song CI, Pogson JM, Andresen NS, Ward BK. Mri with gadolinium as a measure of blood-labyrinth barrier integrity in patients with inner ear symptoms: a scoping review. Front Neurol. (2021) 12:746. doi: 10.3389/fneur.2021.662264
24. Zhang W, Dai M, Fridberger A, Hassan A, DeGagne J, Neng L, et al. Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid–blood barrier. Proc Natl Acad Sci USA. (2012) 109:10388–93. doi: 10.1073/pnas.1205210109
25. Ichimiya I, Yoshida K, Hirano T, Suzuki M, Mogi G. Significance of spiral ligament fibrocytes with cochlear inflammation. Int J Pediatr Otorhinolaryngol. (2000) 56:45–51. doi: 10.1016/S0165-5876(00)00408-0
26. Chen P, He L, Pang X, Wang X, Yang T, Wu H. Nlrp3 is expressed in the spiral ganglion neurons and associated with both syndromic and nonsyndromic sensorineural deafness. Neural Plast. (2016) 2016:3018132. doi: 10.1155/2016/3018132
27. Wallis D, Hamblen M, Zhou Y, Venken KJ, Schumacher A, Grimes HL, et al. The zinc finger transcription factor Gfi1, implicated in lymphomagenesis, is required for inner ear hair cell differentiation and survival. Development. (2003) 130:221–32. doi: 10.1242/dev.00190
28. Scheffer DI, Shen J, Corey DP, Chen Z-Y. Gene expression by mouse inner ear hair cells during development. J Neurosci. (2015) 35:6366–80. doi: 10.1523/JNEUROSCI.5126-14.2015
29. Dong Y, Zhang C, Frye M, Yang W, Ding D, Sharma A, et al. Differential fates of tissue macrophages in the cochlea during postnatal development. Hear Res. (2018) 365:110–26. doi: 10.1016/j.heares.2018.05.010
30. Gritsenko A, Green JP, Brough D, Lopez-Castejon G. Mechanisms of Nlrp3 priming in inflammaging and age related diseases. Cytokine Growth Factor Rev. (2020) 55:15–25. doi: 10.1016/j.cytogfr.2020.08.003
Keywords: autoinflammatory inner ear disease, Nlrp3, inflammasome, hearing loss, Gfi1Cre
Citation: Kim Y, Lee S-Y, Kim MY, Park K, Han JH, Kim JH, Kim BJ and Choi BY (2022) Auditory Phenotype and Histopathologic Findings of a Mutant Nlrp3 Expression Mouse Model. Front. Neurol. 13:890256. doi: 10.3389/fneur.2022.890256
Received: 05 March 2022; Accepted: 19 May 2022;
Published: 24 June 2022.
Edited by:
Bohua Hu, University at Buffalo, United StatesReviewed by:
Celia Zhang, University of the Pacific, United StatesCopyright © 2022 Kim, Lee, Kim, Park, Han, Kim, Kim and Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bong Jik Kim, Ym9uZ2ppay5raW1AY251LmFjLmty; Byung Yoon Choi, Y2hvaWJ5MjAxMEBnbWFpbC5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.