- 1Curtin School of Population Health, Curtin University, Perth, WA, Australia
- 2National Centre for Epidemiology and Population Health, Australian National University, Canberra, ACT, Australia
- 3Centre for Ophthalmology and Visual Science, University of Western Australia, Perth, WA, Australia
- 4Faculty of Science, Medicine and Health, University of Wollongong, Wollongong, NSW, Australia
- 5Illawarra Health and Medical Research Institute, Wollongong, NSW, Australia
- 6Curtin Health Innovation Research Institute (CHIRI), Curtin University, Perth, WA, Australia
Background: The evidence associating consumption of dairy products and risk of MS is contradictory and inconclusive.
Objective: To test associations between dairy consumption and the likelihood of a first clinical diagnosis of central nervous system demyelination (FCD), a common precursor to MS.
Methods: We used data from the 2003–2006 Ausimmune Study, a population-based Australian, multicentre, matched case-control study (272 cases, 519 controls). Total dairy consumption (servings/day) was calculated by summing consumption of milk, cheese and yogurt. Covariate-adjusted treatment effects using augmented inverse probability weighting was used to test for associations with FCD. We conducted sensitivity analyses in the subset of participants who had had a classic first demyelinating event (FDE), defined as a single, first episode of symptoms suggestive of CNS demyelination.
Results: There were no statistically significant associations between total dairy consumption (per one serving/day) and FCD (adjusted OR 1.00; 95% CI 0.93, 1.07; p = 0.979). However, yogurt consumption (vs. no yogurt consumption) was associated with an 11% decreased likelihood of FDE (adjusted OR 0.89; 95% CI 0.89, 0.79; p = 0.046).
Conclusion: While total dairy consumption was not associated with FCD in this Australian case-control study, yogurt consumption was associated with reduced likelihood of FDE.
Introduction
The onset of multiple sclerosis (MS) appears to be influenced by a number of factors, including modifiable lifestyle factors, such as low sun exposure and/or low vitamin D status (1, 2), adiposity in childhood to young adulthood (3), and smoking (4). Although diet is potentially a modifiable lifestyle factor for MS onset (5–13), there is no conclusive evidence on what dietary patterns, whole foods and/or nutrients might play a role.
Some studies have shown that dairy consumption is associated with increased risk of MS (14–17), while others have indicated a protective effect (18, 19) or no association (20). Analysis of observational data from the Nurses' Health Study and the Nurses' Health Study II showed no association between intake of dairy products and risk of MS (20). Retrospective data from the same studies did suggest that women who consumed three or more servings of whole milk per day during adolescence (compared to less than one serving/day) had an increased risk of adult-onset MS (17). However, women reporting greater whole milk intake in adolescence were more likely to live in the northern US as adolescents, where vitamin D synthesis by sun exposure is markedly reduced during the winter months, potentially confounding the association.
Several case-control studies in India (15) and Iran (19, 21, 22) have investigated associations between dairy consumption and risk of MS, but with substantial limitations. In a case-control study in India (63 cases, 63 controls) (15), more people with MS compared with controls had a history of daily dairy consumption, but there was no attempt to control for potential confounding. In contrast, a hospital-based Iranian case-control study (536 cases, 399 controls) found that consumption of dairy products was lower in people with MS than controls (21). However, no covariates were considered and the types of dairy products included were not reported (21).
Building on our previous findings that a healthier dietary pattern, a Mediterranean diet, and higher fish and unprocessed red meat consumption were associated with lower likelihood of FCD (5–9), we aimed to test associations between dairy consumption and the likelihood of FCD using data from the Ausimmune Study (23), an Australian, multicenter, matched case-control study investigating environmental risk factors for a first clinical diagnosis of CNS demyelination (FCD).
Methods
Adults aged 18–59 years were recruited into the 2003–2006 Ausimmune Study from four regions of Australia: Brisbane, Newcastle, Geelong and Western Victoria, and Tasmania. Case participants (n = 282) were recruited from the general population and referred to the study by a range of clinicians (e.g., neurologists, ophthalmologists, and general physicians) operating at different tiers in the referral and diagnostic process (23). This was designed to optimize the proportion of incident cases referred to the study, and the response rate of notified cases was >90%. Eligible cases were diagnosed with CNS demyelination for the first time during the course of the study: classic first demyelinating event (FDE), defined as a single, first, episode of clinical symptoms suggestive of CNS demyelination (n = 216); primary progressive MS at neurological assessment on study entry (n = 18); or, a prior event highly suggestive of CNS demyelination that was neither recognized nor ascribed to demyelination (n = 48). A study neurologist confirmed these diagnoses and the date of onset following a full history and neurologic examination. MRI scans taken prior to diagnosis were available for the majority of case participants and were used to indicate date of onset and to determine whether lesions were present.
Control participants (n = 558) were randomly selected from the Australian Electoral Roll, for which enrolment is mandatory for citizens aged ≥18 years. Between one and four controls were matched to each case participant based on sex, age (± two years) and study region, with a higher number of controls matched to cases in regions where case numbers were expected to be lower (23). Ethics approval was obtained from the Human Research Ethics Committees of the participating institutions. All participants provided written informed consent.
Dietary intake in the 12 months prior to the study interview was assessed using the Dietary Questionnaire for Epidemiological Studies food frequency questionnaire (FFQ) (n = 791) (24). Dairy consumption (grams/day) was reported for milk (full-fat, reduced-fat, and skim), cheese (hard, soft, ricotta, cream cheese, low-fat cheese), and yogurt. We converted intakes in grams/day to servings/day. The amount of milk (full-fat, reduced-fat, and skim), cheese (hard, firm, soft, cream cheese, and low-fat cheese), ricotta/cottage cheese, and yogurt considered as one serving were 1 cup (250), 40, 120 g, and cup (200 g), respectively (25, 26). In line with previous research (27), we did not include butter (commonly classified with fats and oils) (28) or ice-cream (considered an ultra-processed food) (29) in our analyses.
Total dairy consumption (servings/day) was estimated by summing the servings of milk, cheese and yogurt; full-fat dairy consumption by summing the servings of full-fat milk and cheese (including hard, firm, soft, and cream cheese); reduced-fat dairy consumption by summing the servings of low-fat, reduced-fat and skim milk, yogurt and low-fat cheese. Total dairy consumption (servings/day) was also energy-adjusted using the residual method (30). We categorized specific dairy products (full-fat milk, reduced-fat milk, hard/firm cheese, soft cheese, and yogurt) by whether participants consumed the particular product or not (consumption vs. no consumption).
Using self-reported questionnaires, participants indicated highest level of education (up to year 10, year 11 or 12, Technical and Further Education or diploma, or university), history of smoking (yes, no) and history of infectious mononucleosis (yes, no, do not know). Body mass index (BMI) was calculated from height and weight measurements taken at the study interview. Blood samples from participants were used to measure 25-hydroxyvitamin D [25(OH)D] concentration, with seasonal adjustments applied as previously described (1).
Participants with implausible total energy intakes (<3,000 and >20,000 kJ/day) (6) (12 controls; 5 cases) were excluded, leaving a sample of 774 eligible participants. Characteristics of participants whose dairy consumption was below the median were compared with the characteristics of participants whose dairy consumption was at or above the median. Categorical variables were presented as percentage and frequency; normally distributed continuous variables as mean and standard deviation (SD); and non-normally distributed variables as median and interquartile range (IQR). Chi square tests, t-tests and Wilcoxon rank sum tests were used to determine statistically significant associations, as appropriate.
Generalized linear models were used to test unadjusted associations between FCD and dairy consumption. Treatment effect models, using augmented inverse-probability weighting (AIPW), were used to explore associations between FCD and dairy consumption (servings/day) for total, full-fat and reduced-fat dairy, and for specific dairy products (full fat milk, reduced fat milk, hard/firm cheese, soft cheese, and yogurt; consumption vs. no consumption). For all AIPW models, the medians of each type of dairy consumption were the cut points for propensity matching (31). Sex, age and study region were covariates for estimation of FCD in the first part of the equation; the second part of the equation incorporated a logit model to show the difference between below and at/above the median dairy consumption as a function of education, BMI, total energy intake, history of smoking, history of infectious mononucleosis and serum 25(OH)D concentrations in association with FCD. Tests for the overlap assumption of the AIPW matched groups (cases and controls) were performed (32). Coefficients from AIPW outcomes were converted to odds ratios (OR) for reporting purposes. Using the same procedures described above, we conducted a sensitivity analysis including only case participants with an FDE and their matched controls. All final models were bootstrapped (500 replicates) with bias corrected standard errors. Data were analyzed using Stata 14 (33).
Results
Compared with controls, case participants were more likely to have a history of infectious mononucleosis regardless of their dairy consumption (Table 1). Compared to controls, case participants whose total dairy consumption (servings/day) was at or above the median were more likely to have a history of smoking and lower serum 25(OH)D concentrations (Table 1).
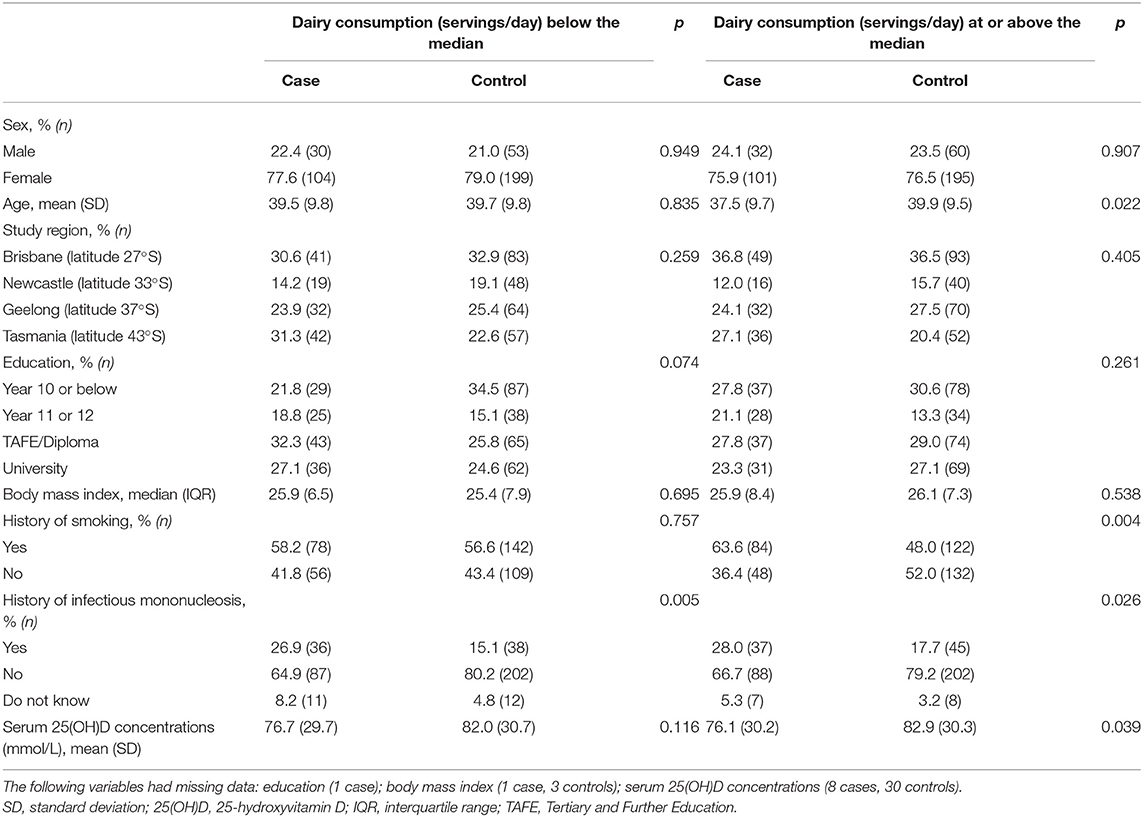
Table 1. Characteristics of participants included in the current study (n = 774; 267 cases, 507 controls) by dairy consumption (servings/day) below the median compared with at or above the median.
All AIPW models met the overlap assumption and tests for balance between cases and controls. Table 2 shows unadjusted, covariate-adjusted and energy-adjusted models for total dairy consumption and the likelihood of FCD and FDE. There were no statistically significant associations. We also found no statistically significant associations between full-fat dairy consumption or reduced-fat dairy consumption and FCD or FDE (Table 3). Similarly, there were no statistically significant associations between consumption of specific dairy products and likelihood of FCD (Table 3). There was no statistical difference between cases and controls in yogurt consumption in unadjusted models; however, there was an 11% decreased likelihood (OR 0.89; 95%CI: 0.79, 1.00) of FDE with yogurt consumption compared to no yogurt consumption in covariate-adjusted and energy-adjusted models (Table 3).

Table 2. AIPWa models showing associations between the likelihood of FCD and FDE with total dairy consumption.
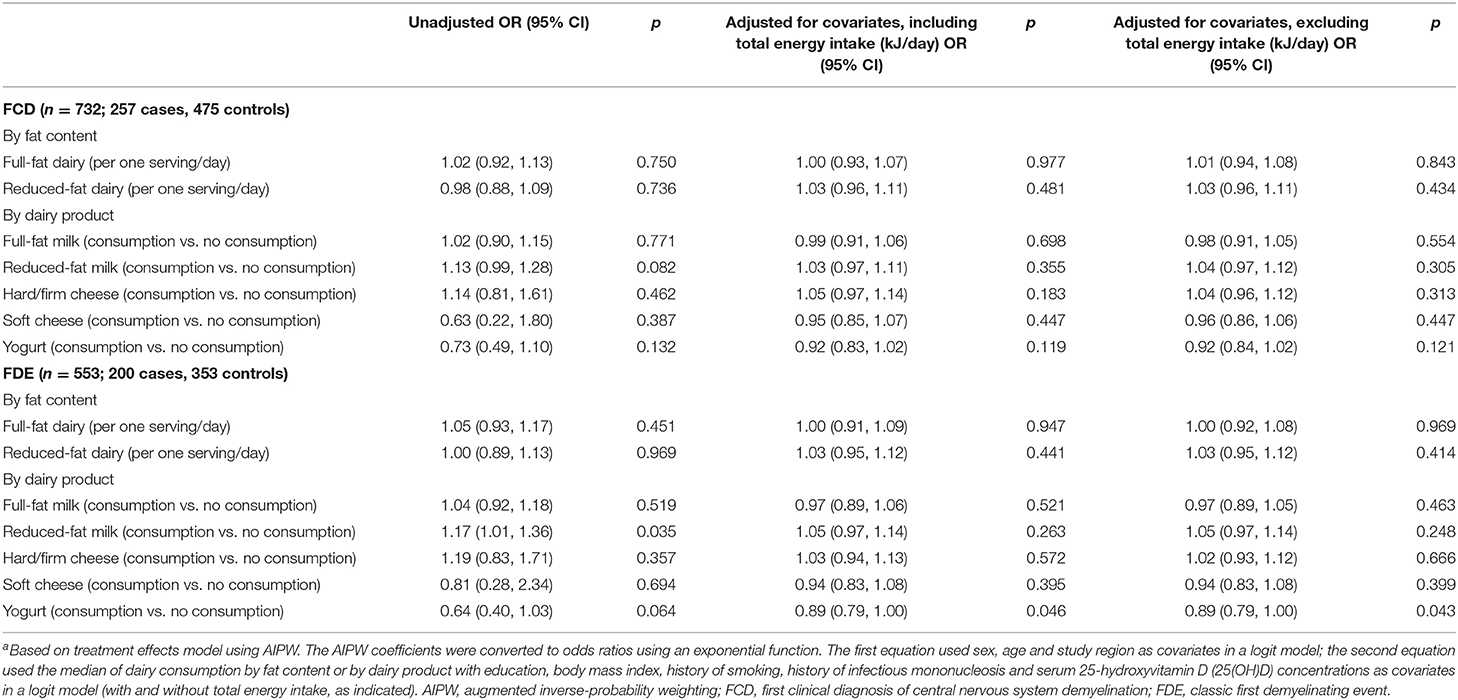
Table 3. AIPWa models showing associations between the likelihood of FCD and FDE with full-fat dairy consumption, reduced-fat dairy consumption, full-fat milk, reduced-fat milk, hard/firm cheese, soft cheese and yogurt.
Discussion
We found no association between any type of dairy consumption and the likelihood of FCD or FDE. However, yogurt consumption (compared with no yogurt consumption) was associated with a small decreased likelihood of FDE. We chose the more statistically powerful treatment effects model based on the AIPW approach (34) rather than conditional logistic regression to examine the associations between FCD and dairy consumption. This approach maximized the chance of finding any significant associations, while the bootstrapping produced robust and bias corrected confidence intervals, decreasing the likelihood of false negative or positive associations.
In our previous study using data from the United States' MS Sunshine Study (602 cases, 653 controls), we found that higher consumption of yogurt between childhood and young adulthood (ages 6–20 years) was protective against adult-onset MS (35). Similarly, an Iranian case-control study (547 cases, 1,057 controls) found that higher consumption of yogurt during adolescence was associated with reduced risk of adult-onset MS (22), while another Iranian case-control study in Iran (660 cases, 421 controls) showed that higher consumption of milk and yogurt was associated with decreased likelihood of MS (19). All three studies reported type of dairy (e.g., milk, yogurt, cheese) and frequency of consumption, but portion size was considered in only one study (22). Nevertheless, those findings are consistent with our current findings in relation to yogurt and FDE.
The association between yogurt and FDE may relate to the probiotic properties of yogurt. Yogurt is often a carrier of probiotics—live microorganisms (e.g., Lactobacillus acidophilus, Bifidobacteria spp.) which, if consumed in adequate amounts, may have health benefits (36). Probiotics potentially modulate the gut microbiota and, given that the gut microbiota may modulate the immune response, gut microbiota may play a role in the pathogenesis of MS (37). As the FFQ used did not collect information on the live culture content or probiotic potential of yogurts consumed, it is uncertain whether the potential links between probiotics, gut microbiota, and inflammation could explain the inverse association between yogurt and FDE seen in our study. Furthermore, it is uncertain why this association was found for FDE and not FCD.
Various mechanisms have been proposed to support either a protective or deleterious effect of dairy consumption on risk of MS, including a protective effect of calcium. There is some evidence from animal studies that low calcium intake may adversely affect myelin lipid synthesis, which has been demonstrated in mouse models (38). However, calcium intake was not associated with MS risk using data from the NHS and NHSII (39). Dairy products, particularly milk, are fortified with vitamin D in some countries (40), and are important contributors to dietary vitamin D intakes in the US and Canada (41, 42). However, the studies on MS risk from the NHS and NHSII showed no statistically significant association between vitamin D intake from food and risk of MS (43). Neither India nor Iran, the settings for the aforementioned case-controls studies (15, 19, 21), appeared to have systematic vitamin D-fortification of dairy products at the time those studies were conducted (44, 45). Although fortification with vitamin D of certain dairy foods is permitted in Australia (46), few products are vitamin D-fortified in practice. Therefore, even if dairy consumption was protective against MS through added vitamin D, this would not likely be observed in our Australian cohort.
A deleterious effect of the milk protein, butyrophilin, has been implicated in MS risk. Two studies have shown molecular mimicry with myelin oligodendrocyte glycoprotein (47, 48), triggering an encephalitogenic T cell response in experimental autoimmune encephalomyelitis (EAE), an animal model for MS (48). However, any link between dairy products, butyrophilin, and myelin oligodendrocyte glycoprotein, remains speculative (47) and the role of encephalitogenic T cells in MS in humans is not well-understood (49).
A strength of our study was the well-characterized sample of people with early MS, with an incident case-control design. However, participants were living in Australia and predominately of European descent; hence, our findings may not be generalizable to other populations. Although we cannot rule out potential residual confounding by other demographic or lifestyle characteristics, such as socioeconomic status, that may be associated with consumption of dairy products, various lifestyle characteristics (e.g., BMI, alcohol intake and physical activity) were not associated with risk of FCD in previous analysis of data from the Ausimmune Study (50). The food consumption data used in this study are subject to the acknowledged limitations of self-reported dietary intake, such as recall bias and measurement error.
In conclusion, we found no association between the likelihood of FCD and total dairy consumption in this Australian population. We did find a small decreased likelihood of FDE associated with yogurt consumption. Dairy foods are an important source of calcium, vitamin D (in some countries), and other vitamins and minerals. Given that many diets promoted for people with MS exclude dairy products, investigating the effect of dairy consumption on MS disease progression, as opposed to onset, is warranted to help develop evidence-based dietary guidance for people with MS.
Data Availability Statement
The data analyzed in this study was obtained from the Ausimmune Study, the following licenses/restrictions apply: The data can be made available for analysis with a collaborative agreement with the Ausimmune Investigator Group. Requests to access these datasets should be directed to Professor Anne-Louise Ponsonby, YW5uZWxvdWlzZS5wb25zb25ieUBmbG9yZXkuZWR1LmF1.
Ethics Statement
The studies involving human participants were reviewed and approved by Barwon Health Research and Ethics Advisory Committee (ref 03/46), Ballarat Health Services and St John of God Health Services Committee, Royal Brisbane and Women's Hospital and Health Service District Office of the Human Research Ethics Committee (ref 2003/093), The University of Queensland Medical Research Ethics Committee (ref 2003000253), The Princess Alexandra Hospital Human Research Ethics Committee (ref 2004/059), The Queensland Institute of Medical Research Human Research Ethics Committee [ref H0511-061 (P950)], Hunter Area Research Ethics Committee (ref 03/06/11/3.07), Southern Tasmania Heath and Medical Research Ethics Committee (ref H7436), The Australian National University Human Research Ethics Committee (ref 2002/111). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LB had primary responsibility for the final content and designed the study. DD, ED, and LB wrote the manuscript. AD analyzed the data and interpreted the results. RL, YP, AD, and Ausimmune Investigator Group provided critical revision of the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Funding
Funding for the Ausimmune Study was provided by the National Multiple Sclerosis Society of the United States of America (NMSS RG 3364A1/2) and the National Health and Medical Research Council of Australia (313901). LB is supported by MS Western Australia (MSWA), an MS Australia Postdoctoral Fellowship, and a Curtin University Research Fellowship. RL is supported by a National Health and Medical Research Council of Australia Senior Research Fellowship. AD is supported by MSWA. Funding bodies had no role in the design or conduct of the study, collection, management, analysis or interpretation of data or preparation, and review or approval of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to acknowledge and thank the people who agreed to participate and the physicians who notified case participants to the Ausimmune Study: Jeffrey Blackie FRACP, Richard Bourke FRACGP, John Cameron MD, Ross Carne MD, Ben Clark FRANZCO, Steven Collins MD, Diana Conrad FRANZCO, Michael Coroneos FRACS, Nicholas Downie FRANZCO, David Floate FRACP, Peter Gates FRACP, Kerryn Green FRACP, Erwin Groeneveld FRANZCO, John Harrison FRANZCO, Michael Haybittel FRANZCO, Robert Henderson FRACP, John Henshaw MMed, James Hurley MD, Dean Jones FRACP, Michael Katekar MBBS, Anthony Kemp FRACP, Mark King FRACP, George Kiroff FRACS, Brett Knight FRACP, Thomas Kraemer FRACP, Cecile Lander FRACP, Jeannette Lechner-Scott FRACP, Andre Loiselle FRACP, Paul McCartney FRANZCO, Pamela McCombe PhD, Mark McGree FRANZCO, David McKnight FRANZCO, Daniel McLaughlin PhD, Satish Nagarajah MBBS, Rob Nightingale FRACP, Terence O'Brien MD, John O'Sullivan MD, Gregory Outteridge FRANZCO, Anthony Pane FRANZCO, Mark Parsons FRACP, Melinda Pascoe FRACP, David Prentice PhD, Richard Ralph FRACGP, Stephen Read FRACP, John Richmond FRACP, Ian Routley FRANZCO, Timothy Ruddle FRANZCO, Noel Saines FRACP, Stan Siejka MBBS (dec), Christopher Staples FRACP, Paul Talman FRACP, Don Todman FRACP, Nitin Verma FRANZCO, Brendan Vote FRANZCO, Michael Waldie FRANZCO, Michael Weetch FRACP, Rodney Westmore FRANZCO, Andrew Wong FRACP.
The local research officers:
Susan Agland BN, Barbara Alexander BN, Marcia Davis MD, Zoe Dunlop BN, Rosalie Scott BN, Marie Steele RN, Catherine Turner MPH&TM, Brenda Wood RN
The Ausimmune Study project officers during the course of the study:
Jane Gresham MA(Int Law), Camilla Jozwick BSc (Hons), Helen Rodgers RN
The Ausimmune Investigator Group includes the following investigators:
Dr Caron Chapman, Barwon Health, Geelong, Victoria, Australia
Prof Alan Coulthard, Royal Brisbane and Women's Hospital and the University of Queensland, Brisbane, Queensland, Australia
Prof Keith Dear, School of Public Health, University of Adelaide, South Australia, Australia
Prof Terry Dwyer, Murdoch Childrens Research Institute, University of Melbourne, Melbourne, Victoria, Australia
Prof Trevor Kilpatrick, Centre for Neuroscience, University of Melbourne, Melbourne, Australia
Prof Robyn Lucas, National Centre for Epidemiology and Population Health, Australian National University, Canberra, Australian Capital Territory, Australia
Prof Tony McMichael (dec), National Centre for Epidemiology and Population Health, The Australian National University, Canberra, Australian Capital Territory, Australia
Prof Anne-Louise Ponsonby, Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Melbourne, VIC, Australia
Prof Bruce Taylor, Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia
A/Prof Patricia Valery, QIMR Berghofer Medical Research Institute, Brisbane, Queensland, Australia
Prof Ingrid van der Mei, Menzies Institute for Medical Research, University of Tasmania, Hobart, Tasmania, Australia
Dr David Williams, Hunter Health, Newcastle, New South Wales, Australia.
References
1. Lucas RM, Ponsonby AL, Dear K, Valery PC, Pender MP, Taylor BV, et al. Sun exposure and vitamin D are independent risk factors for CNS demyelination. Neurology. (2011) 76:540–8. doi: 10.1212/WNL.0b013e31820af93d
2. Ascherio A, Munger KL. Environmental risk factors for multiple sclerosis. Part II: noninfectious factors. Ann Neurol. (2007) 61:504–13. doi: 10.1002/ana.21141
3. Wesnes K, Riise T, Casetta I, Drulovic J, Granieri E, Holmøy T, et al. Body size and the risk of multiple sclerosis in Norway and Italy: the EnvIMS study. Mult Scler. (2015) 21:388–95. doi: 10.1177/1352458514546785
4. O'Gorman C, Broadley SA. Smoking and multiple sclerosis: evidence for latitudinal and temporal variation. J Neurol. (2014) 261:1677–83. doi: 10.1007/s00415-014-7397-5
5. Black LJ, Baker K, Ponsonby AL, van der Mei I, Lucas RM, AusImmune Investigator Group, et al. A higher Mediterranean diet score, including unprocessed red meat, is associated with reduced risk of central nervous system demyelination in a case-control study of Australian adults. J Nutr. (2019) 149:1385–92. doi: 10.1093/jn/nxz089
6. Black LJ, Rowley C, Sherriff J, Pereira G, Ponsonby A-L, Ausimmune Investigator Group, et al. A healthy dietary pattern associates with a lower risk of a first clinical diagnosis of central nervous system demyelination. Mult Scler. (2019) 25:1514–25. doi: 10.1177/1352458518793524
7. Langer-Gould A, Black LJ, Waubant E, Smith J, Wu J, Gonzales E, et al. Seafood, fatty acid biosynthesis genes and multiple sclerosis susceptibility. Mult Scler. (2019) 26:1476–85. doi: 10.1177/1352458519872652
8. Black LJ, Zhao Y, Peng YC, Sherriff JL, Lucas RM, van der Mei I, et al. Higher fish consumption and lower risk of central nervous system demyelination. Eur J Clin Nutr. (2019) 74:818–24. doi: 10.1038/s41430-019-0476-z
9. Black LJ, Bowe GS, Pereira G, Lucas RM, Dear K, van der Mei I, et al. Higher non-processed red meat consumption is associated with a reduced risk of central nervous system demyelination. Front Neurol. (2019) 10:125. doi: 10.3389/fneur.2019.00125
10. Sedaghat F, Jessri M, Behrooz M, Mirghotbi M, Rashidkhani B. Mediterranean diet adherence and risk of multiple sclerosis: a case-control study. Asia Pac J Clin Nutr. (2016) 25:377–84. doi: 10.6133/apjcn.2016.25.2.12
11. Baarnhielm M, Olsson T, Alfredsson L. Fatty fish intake is associated with decreased occurrence of multiple sclerosis. Mult Scler. (2014) 20:726–32. doi: 10.1177/1352458513509508
12. Kampman MT, Wilsgaard T, Mellgren SI. Outdoor activities and diet in childhood and adolescence relate to MS risk above the Arctic Circle. J Neurol. (2007) 254:471–7. doi: 10.1007/s00415-006-0395-5
13. Ghadirian P, Jain M, Ducic S, Shatenstein B, Morisset R. Nutritional factors in the aetiology of multiple sclerosis: a case-control study in Montreal, Canada. Int J Epidemiol. (1998) 27:845–52. doi: 10.1093/ije/27.5.845
14. Swank RL, Lerstad O, Strom A, Backer J. Multiple sclerosis in rural Norway: its geographic and occupational incidence in relation to nutrition. N Engl J Med. (1952) 246:722–8. doi: 10.1056/NEJM195205082461901
15. Khadilkar SSA, Agarwal S. A case control study of environmental risk factors in Indians with multiple sclerosis. Neurol Asia. (2005) 10:47–52.
16. Kotzamani D, Panou T, Mastorodemos V, Tzagournissakis M, Nikolakaki H, Spanaki C, et al. Rising incidence of multiple sclerosis in females associated with urbanization. Neurology. (2012) 78:1728. doi: 10.1212/WNL.0b013e31825830a9
17. Munger KL, Chitnis T, Frazier AL, Giovannucci E, Spiegelman D, Ascherio A. Dietary intake of vitamin D during adolescence and risk of multiple sclerosis. J Neurol. (2011) 258:479–85. doi: 10.1007/s00415-010-5783-1
18. Jahromi SR, Toghae M, Jahromi MJ, Aloosh M. Dietary pattern and risk of multiple sclerosis. Iran J Neurol. (2012) 11:47–53.
19. Abbasi M, Nabavi SM, Fereshtehnejad SM, Jou NZ, Ansari I, Shayegannejad V, et al. Multiple sclerosis and environmental risk factors: a case-control study in Iran. Neurol Sci. (2017) 38:1941–51. doi: 10.1007/s10072-017-3080-9
20. Zhang SM, Willett WC, Hernan MA, Olek MJ, Ascherio A. Dietary fat in relation to risk of multiple sclerosis among two large cohorts of women. Am J Epidemiol. (2000) 152:1056–64. doi: 10.1093/aje/152.11.1056
21. Shaygannejad V, Rezaie N, Paknahad Z, Ashtari F, Maghzi H. The environmental risk factors in multiple sclerosis susceptibility: a case-control study. Adv Biomed Res. (2016) 5:98. doi: 10.4103/2277-9175.183665
22. Abdollahpour I, Sormani MP, Nedjat S, Mansournia MA, van der Mei I. The role of nutritional factors during adolescence in multiple sclerosis onset: a population-based incident case-control study. Nutr Neurosci. (2021) 24:500–7. doi: 10.1080/1028415X.2019.1647689
23. Lucas R, Ponsonby AL, McMichael A, van der Mei I, Chapman C, Coulthard A, et al. Observational analytic studies in multiple sclerosis: controlling bias through study design and conduct. Mult Scler. (2007) 13:827–39. doi: 10.1177/1352458507077174
24. Cancer Council Victoria. Dietary Questionnaire for Epidemiological Studies (DQES v2) User Information Guide. Melbourne, VIC: Cancer Council Victoria (2009).
25. National Health Research Medical Council. The Five Food Groups. (2015). Availabl online at: https://www.eatforhealth.gov.au/food-essentials/how-much-do-we-need-each-day/serve-sizes (accessed November 15, 2021).
26. Food Standards Australia New Zealand. Australian Food Composition Database - Release 1. (2019). Availabl online at: https://www.foodstandards.gov.au/science/monitoringnutrients/afcd/Pages/default.aspx (accessed November 15, 2021).
27. Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi F. Dairy consumption is inversely associated with the prevalence of the metabolic syndrome in Tehranian adults. Am J Clin Nutr. (2005) 82:523–30. doi: 10.1093/ajcn/82.3.523
28. National Health Medical Research Council. Australian Dietary Guidelines. (2015). Availabl online at: https://www.eatforhealth.gov.au (accessed 10 January, 2022).
29. Monteiro CA, Cannon G, Moubarac J-C, Levy RB, Louzada MLC, Jaime PC. The UN Decade of nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr. (2018) 21:5–17. doi: 10.1017/S1368980017000234
30. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65(4 Suppl):1220S−8S. doi: 10.1093/ajcn/65.4.1220S
31. Leuven E, Sianesi B. PSMATCH2: Stata Module to Perform Full Mahalanobis and Propensity Score Matching, Common Support Graphing, and Covariate Imbalance Testing. Chestnut Hill, MA: Boston College Department of Economics (2018).
32. Busso M, DiNardo J, McCrary J. New evidence on the finite sample properties of propensity score reweighting and matching estimates. Rev Econ Stat. (2014) 96:885–97. doi: 10.1162/REST_a_00431
34. Kuo CL, Duan Y, Grady J. Unconditional or conditional logistic regression model for age-matched case-control data? Front Public Health. (2018) 6:57. doi: 10.3389/fpubh.2018.00057
35. Black LJ, Hetherton S, Forkan M, Gonzales EG, Smith JB, Daly A, et al. An exploratory study of diet in childhood and young adulthood and adult-onset multiple sclerosis. Mult Scler. (2021) 27:1611–4. doi: 10.1177/1352458520986964
36. Aryana KJ, Olson DW. A 100-year review: yogurt and other cultured dairy products. J Dairy Sci. (2017) 100:9987–10013. doi: 10.3168/jds.2017-12981
37. Chen J, Chia N, Kalari KR, Yao JZ, Novotna M, Soldan MMP, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. (2016) 6:28484. doi: 10.1038/srep28484
38. Pollet S, Chaix G, Baumann N. Calcium content of mice brain lipids during myelination. Neurosci Lett. (1976) 3:311–4. doi: 10.1016/0304-3940(76)90060-4
39. Cortese M, Chitnis T, Ascherio A, Munger KL. Total intake of different minerals and the risk of multiple sclerosis. Neurology. (2019) 92:e2127–e35. doi: 10.1212/WNL.0000000000006800
40. Cashman KD, Kiely M. Tackling inadequate vitamin D intakes within the population: fortification of dairy products with vitamin D may not be enough. Endocrine. (2016) 51:38–46. doi: 10.1007/s12020-015-0711-x
41. Ahmed M, Ng AP, L'Abbe MR. Nutrient intakes of Canadian adults: results from the Canadian Community Health Survey (CCHS)−2015 public use microdata file. Am J Clin Nutr. (2021) 114:1131–40. doi: 10.1093/ajcn/nqab143
42. Herrick KA, Storandt RJ, Afful J, Pfeiffer CM, Schleicher RL, Gahche JJ, et al. Vitamin D status in the United States, 2011–2014. Am J Clin Nutr. (2019) 110:150–7. doi: 10.1093/ajcn/nqz037
43. Munger KL, Zhang SM, O'Reilly E, Hernan MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. (2004) 62:60–5. doi: 10.1212/01.WNL.0000101723.79681.38
44. Ritu G, Gupta A. Fortification of foods with vitamin D in India. Nutrients. (2014) 6:3601–23. doi: 10.3390/nu6093601
45. Ejtahed H-S, Shab-Bidar S, Hosseinpanah F, Mirmiran P, Azizi F. Estimation of vitamin D intake based on a scenario for fortification of dairy products with vitamin D in a Tehranian population, Iran. J Am Coll Nutr. (2016) 35:383–91. doi: 10.1080/07315724.2015.1022269
46. Food Standards Australia New Zealand. Food Standards Code. (2019). Available online at: http://www.foodstandards.gov.au/code/Pages/default.aspx (accessed November 23, 2021).
47. Guggenmos J, Schubart AS, Ogg S, Andersson M, Olsson T, Mather IH, et al. Antibody cross-reactivity between myelin oligodendrocyte glycoprotein and the milk protein butyrophilin in multiple sclerosis. J Immunol. (2004) 172:661–8. doi: 10.4049/jimmunol.172.1.661
48. Stefferl A, Schubart A, Storch M, Amini A, Mather I, Lassmann H, et al. Butyrophilin, a milk protein, modulates the encephalitogenic T cell response to myelin oligodendrocyte glycoprotein in experimental autoimmune encephalomyelitis. J Immunol. (2000) 165:2859–65. doi: 10.4049/jimmunol.165.5.2859
49. Dong Y, Yong VW. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. (2019) 15:704–17. doi: 10.1038/s41582-019-0253-6
50. Ponsonby AL, Lucas RM, Dear K, van der Mei I, Taylor B, Chapman C, et al. The physical anthropometry, lifestyle habits and blood pressure of people presenting with a first clinical demyelinating event compared to controls: the Ausimmune study. Mult Scler. (2013) 19:1717–25. doi: 10.1177/1352458513483887
Keywords: Ausimmune Study, Australia, nutrition, dairy consumption, multiple sclerosis, diet
Citation: Dieu DYR, Dunlop E, Daly A, Lucas RM, Probst Y and Black LJ (2022) Total Dairy Consumption Is Not Associated With Likelihood of a First Clinical Diagnosis of Central Nervous System Demyelination. Front. Neurol. 13:888559. doi: 10.3389/fneur.2022.888559
Received: 03 March 2022; Accepted: 20 April 2022;
Published: 13 May 2022.
Edited by:
Bianca Weinstock-Guttman, University at Buffalo, United StatesReviewed by:
E. Ann Yeh, University of Toronto, CanadaMerja Hannele Soilu-Hänninen, University of Turku, Finland
Copyright © 2022 Dieu, Dunlop, Daly, Lucas, Probst and Black. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lucinda J. Black, bHVjaW5kYS5ibGFja0BjdXJ0aW4uZWR1LmF1
 Dao Ying Rachel Dieu1
Dao Ying Rachel Dieu1 Eleanor Dunlop
Eleanor Dunlop Robyn M. Lucas
Robyn M. Lucas Yasmine Probst
Yasmine Probst Lucinda J. Black
Lucinda J. Black