
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 21 October 2022
Sec. Pediatric Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.885897
This article is part of the Research Topic Insights in Pediatric Neurology: 2021 View all 20 articles
 Francesco Pizzo1
Francesco Pizzo1 Alessandra Di Nora1
Alessandra Di Nora1 Alessia Di Mari2
Alessia Di Mari2 Giuseppe Costanza1
Giuseppe Costanza1 Elisabetta Testa1
Elisabetta Testa1 Marianna Strazzieri1
Marianna Strazzieri1 Filippo Greco3
Filippo Greco3 Tiziana Timpanaro3
Tiziana Timpanaro3 Antonio Basile2
Antonio Basile2 Giuseppe Belfiore2
Giuseppe Belfiore2 Andrea Giugno1
Andrea Giugno1 Roberta Rocca1
Roberta Rocca1 Martino Ruggieri3*
Martino Ruggieri3* Agata Fiumara3
Agata Fiumara3 Piero Pavone3*
Piero Pavone3*Background: Guillain-Barrè syndrome (GBS) is an acute immune-mediated disorder affecting peripheral nerves and nerve roots with a variable clinical course and outcome. Epidemiologic analyses have revealed that the incidence of the syndrome increases linearly among the age. The clinical diagnosis of GBS is based on the family history, physical and neurological examination, electrodiagnostic exams, and cerebrospinal fluid analysis with the classical presence of albumin-cytologic dissociation. Prognosis is associated with the severity of clinical signs and the type of peripheral nerves involved.
Methods: This study aims to clarify which clinical features can be used for prognostic purposes. We evaluated the correlation between (1) brain MRI lesions and grade of disability; (2) brain MRI lesions and elevated cerebrospinal fluid (CSF) protein; and (3) increased levels of CSF protein and grade of disability. Statistical analysis extracted from these data indicated a good correlation to be a prognostic indicator in children affected by GBS. We found little evidence regarding laboratory tests, imaging, and prognosis. We enrolled 12 continuous patients who met the Brighton criteria for GBS in this retrospective study. Each patient was clinically evaluated at the time of disease onset to assess the GBS disability score and after 2 weeks.
Results: We estimated Pearson's correlation index to evaluate the possible correlation between MRI and disability and CSF protein levels and disability. The correlation coefficient was 0.92 and 0.85, respectively. In addition, we developed a graph to see the trend of the disability values, proteins in the CSF, and damage assessed with MRI in the 12 patients. It seems that these parameters have a parallel trend and a good correlation in each patient. Finally, we calculated the correlation between MRI and CSF protein values, with an r-value of 0.87. The values suggest a correlation among the MRI score, CSF protein, and prognosis.
Conclusion: The MRI and CSF laboratory parameters can be important tools for the clinician not only for diagnosis but also to evaluate the possible worsening of general conditions or the need to prepare measures to support life parameters. Patients who need ventilatory support could be established early from patients who have less severe GBS and can begin rehabilitation earlier. We suggest MRI should be performed routinely in children with GBS to be able to estimate the evolution of the clinical condition.
Guillain-Barrè syndrome (GBS) is an immune-mediated rapidly developed polyneuropathy whose etiology and pathogenesis are not yet entirely comprehended. Guillain–Barré syndrome (GBS) manifests clinically as acute flaccid paralysis, marked by the symmetrical weakness of the limbs, and hyporeflexia or areflexia, which arrives full harshness within 4 weeks (1).
Sensory symptoms, such as paraesthesia or insensibility, begin distally and have a symmetrical extension.
Among pediatric patients in the zenith phase of the syndrome, 75% are unable to walk unsupported, 30% are quadriplegic, 35–50% show cranial nerves involvement, and 15–20% have respiratory failure and/or autonomic dysfunctions (2, 3). Mortality, or severe disability due to GBS, occurs in ~20% of patients (4).
Epidemiological analyses have documented that the incidence of GBS increases with age. In children from 0 to 9 years, it occurs with an incidence of 0.62 cases per 100,000 person-year (py) (5, 6).
Diagnosis of GBS is based on the patient's family history, physical and neurological assessment with sensorial and motor disturbances, electrodiagnostic exams, and cerebrospinal fluid (CSF) which reveals the classical pattern of albumin-cytologic dissociation.
The prognosis of the GBS is linked to the severity of clinical signs and unclear to the presence of autonomic nerve dysfunction (7). Predictors of poor outcomes are defined as a GBS Disability Scale score ≥3 after either 2 weeks or 6 months (8).
We evaluated the correlation among CSF analysis, imaging, and the severity of the GBS in the acute phase to predict a short-term outcome.
We aimed to evaluate CSF analysis and imaging to determine the severity of the GBS in the acute phase and to predict a short-term outcome.
• We evaluated the possible correlation among MRI radiological classification, the values of proteins in the CSF, and the prognosis of children with GBS.
• The correlation between the increasing values of the radiological classification of the clinic with the GBS disability score and the prognosis was evaluated.
• In addition, we evaluated the correlation between increasing CSF protein levels and symptomatology and prognosis.
A systematic revision of the current literature was conducted using Cochrane, EMBASE, and MEDLINE. In addition to official websites of highly qualified journals which were expected to publish studies related to this topic, for example, the New England Journal of Medicine, The Lancet, PLOS Medicine, Neurology, and Pediatrics were also searched for relevant studies. The words used to search were: “Child; Guillain-Barrè; Neurology; Prognosis; Disability; Laboratory; MRI; cerebrospinal fluid.” We found little evidence regarding laboratory tests, imaging, and prognosis. Following, we cite the most recent evidence regarding prognosis and imaging and prognosis and CSF.
Althubaiti et al. in a recent publication on the prognosis value of MRI in children with GBS advise that brain and spinal MRI is a recommended supportive test but a predictive value for clinical and therapeutic outcomes in the short or long term has not yet been proved (9).
Regarding the proteins in the CSF, we want to mention the most recent evidence proposed by Kasser et al. In their letter to the editor, the authors affirmed CSF protein levels seemed to be potentially associated with the need for mechanical ventilation (10).
The Guillain-Barré syndrome (GBS) disability score is a widely accepted scoring system to evaluate the functional level of patients with GBS. It was originally described in Hughes et al. (11) and since then, diverse variations have emerged in the literature.
The patient's level of disability is reported operating on a scale from 0 to 6: Grade 0 is assigned to the asymptomatic patient; Grade 1 is associated with movement reduction but capable of performing manual work; Grade 2 is assigned to those who walk but are unable to perform work with their hands; Grade 3 concerns patients who need support for walking; Grade 4 is assigned to patients who are bedridden. The last 2 degrees of the disease in which there is respiratory failure or death (Table 1). The GBS disability score is not only a clinical parameter but also provides a prognostic value. Revealed disability at the onset and the acute phase of the disease were signs of poor long-term prognosis.
The Erasmus GBS and van Koningsveld et al. (8–12) evaluated the association between clinical severity at onset and short-term prognosis. They introduced three variables that were predictive of poor outcome at 6 months, and inability to walk independently: age, preceding diarrhea, and GBS disability score at 2 weeks after entry.
Magnetic resonance (MR) imaging also contributes to the diagnosis of GBS by demonstrating anterior and posterior intrathecal spinal nerve roots and cauda equine. Gorson et al. (13) showed a correlation between the severity of enhancement of the nerve roots and the severity of the clinical grade. Yikilmaz et al. (14) described the MR features in children with GBS and introduced a radiological classification based on the patterns of contrast enhancement, as reported in Table 2.
Figure 1 shows one of our patients' axials with a marked enhancement of the anterior and posterior nerve roots and a GBS disability score of 4.
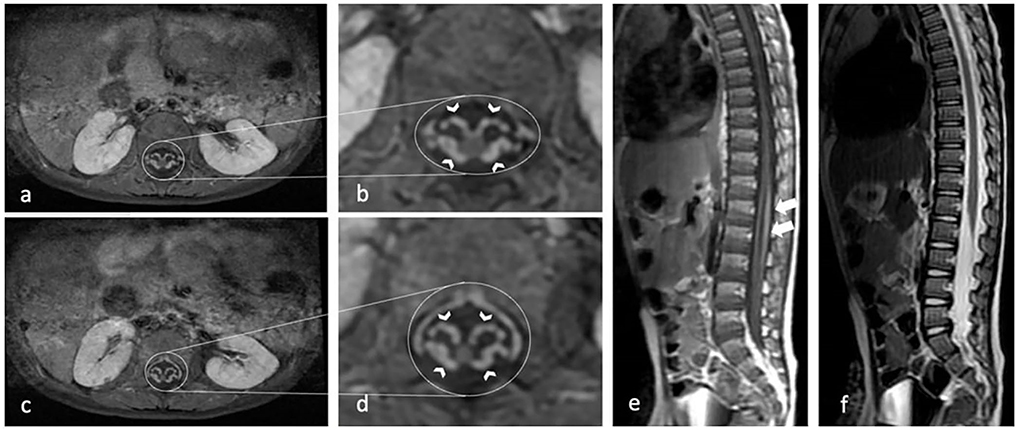
Figure 1. Contrast-enhanced axial T1-weighted MR image shows marked enhancement of the anterior and posterior nerve roots (arrow heads) in the conus medullaris and cauda equina (a–d). Sagittal T1-weighted MR image (e) show mild thickening and moderate contrast enhancement of nerve root in the conus medullaris and cauda equina (white arrows). Sagittal T2-weighted MR image (f) show mild thickening of cauda equina.
We evaluated the chemical-physical characteristics of the CSF in each child, examining appearance, color, number of cells, glucose, proteins, presence of immunoglobulins, and albumin. We examined all these parameters one by one for their correlation with the GBS disability score and therefore with the prognosis.
In this study, the levels of CSF proteins were evaluated according to the increases of 1, 2, and 3 times the basal cut-off value of 45 mg/dl.
The manuscript attempts to evaluate whether a clinical score (the grade of disability of patients), an imaging score (spinal MRI enhancement), and a grade of albumin-cytologic dissociation in CSF are linearly correlated to give a prognostic value of the disease.
We retrospectively enrolled 12 continuous children affected by GBS and diagnosed at the Clinical Pediatrics Department for 10 years from January 2012 to January 2022 (Figure 2). The children were from 1 year to 14 years old, with a mean age of 6.8 years. The gender ratio was 7 boys to 5 girls. Furthermore, all cases examined met the Brighton criteria validated for children (15). Table 1 reports the grade of disability and other clinical features associated with GBS signs observed in the children. We evaluated the following characteristics of the population included in this study:
• Clinical: each patient was clinically evaluated at the time of disease onset assessing the GBS disability score and after 2 weeks. In addition, all the children treated in our center were placed in instrumental clinical follow-up by carrying out checks at 2 weeks to 1 month, 3 months to 6 months, and 1 year from the onset of the disease. In this study, we collected clinical data at onset and 2 weeks after onset (Table 3).
• Imaging: all patients enrolled in this study underwent MR of the brain and spinal cord. Images were evaluated and classified by the pediatric neuroradiology team and assessed by Yakilmaz radiological classification (14).
• Laboratory: lumbar puncture (LP) was performed on average 3 days after symptom onset (Table 4), and all enrolled patients had CSF analyzed.
Patients selected during this period were treated according to guidelines with standard IVIg administration of 1 g/kg daily for 2 days. In addition, patients with clinical follow-up at 2 weeks were included in the study. Data were collected and a statistical analysis was done to determine the presence of a possible correlation among these three variables.
We performed a statistical analysis to measure the potential relationship between the clinical progress of children with GBS, MRI imaging, and CSF protein levels. We measured the correlation index between the clinical and assessed and the GBS and MRI scores according to Yakilmaz Classification. We observed that the CSF parameters that correlated with GBS disability score at 2 weeks were protein levels in the CSF, when these were increased 1, 2, and 3 times the cut-off value of 45 mg/dl as Kerasnoudis et al. reported (16).
We performed an extensive literature search and found no publications evaluating a possible correlation among the clinical severity of GBS, imaging, and laboratory values of CSF proteins combined in the same population.
Two children showed notable signs of respiratory impairment and were intubated. Three children showed involvement of the cranial nerves. Children with respiratory disturbances involving the cranial nerves had a worse clinical course (Table 1). Trigger events were represented by gastrointestinal infections, high airway infections, and vaccination of 3, 8, and 1 cases, respectively (Table 5).
We executed the statistical analysis to measure the potential relationship among the clinical assessment of children with GBS, MRI imaging, and CSF protein levels.
It seems that these parameters have a parallel trend and a good correlation in each patient. The values of r calculated for all correlations are close to unity and therefore indicate a strong correlation value between them. We developed a graph to see the trend of the disability values, proteins in the CSF, and damage assessed with MRI (Figure 4).
The graph (Figure 3) shows the correlation between brain MRI lesions according to Yikilmaz et al. classification (11) and grade of disability. Pearson's correlation index coefficient was 0.92 (R2 = 0.8573).
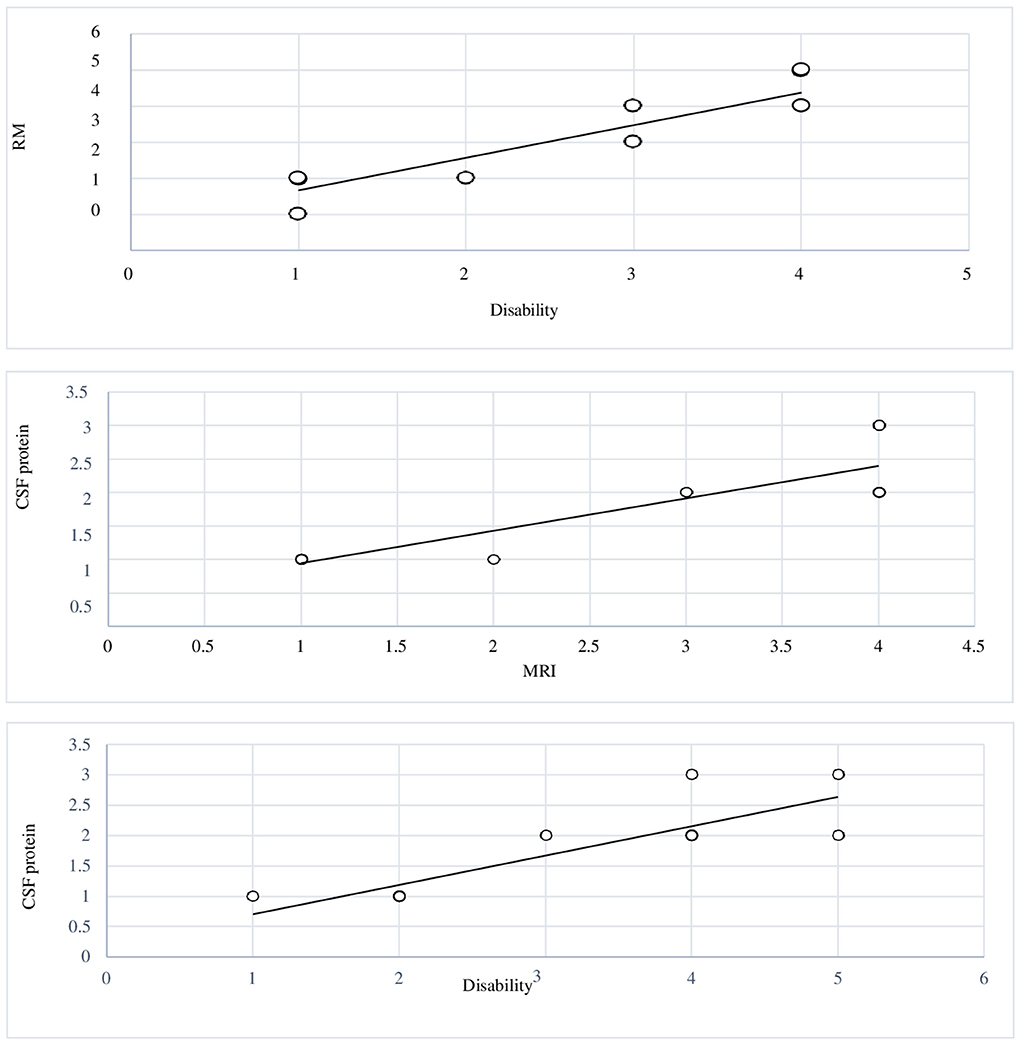
Figure 3. Graphic 1 showing the correlation between MRI brain lesions and levels of disability: Pearson's correlation index coefficient was 0.92. Graphic 2 The graphic showing the correlation between levels of disability and elevated CSF protein; r-value 0.85. Graphic 3 reports the correlation between MRI brain lesions and elevated CSF protein; r-value 0.87.
We assessed the chemical-physical characteristics of the CSF in each patient: appearance, color, the number of cells, glucose, proteins, presence of immunoglobulins, and albumin.
We examined these parameters one by one and the correlation with the GBS disability score and the prognosis.
The single parameter showing the more robust correlation with prognosis was the increase of the proteins in the CSF.
The graph (Figure 3) shows the correlation between levels of disability and increased levels of CSF protein. Pearson's correlation index coefficient was 0.85 (R2 = 0.7342).
Our pediatric neuroradiologists team evaluated and classified magnetic resonance images following the Yakilmaz radiological classification (14). This classification presented in the study was made for descriptive purposes and the potential prognostic use was not investigated.
The graph (Figure 3) reports the correlation between MRI brain lesions and increased levels of CSF protein. The correlation index coefficient had a value of 0.87.
The graph (Figure 4) was performed to evaluate the trend of the three parameters studied (level of disability, MRI brain lesions, and increased levels of CSF protein) in each of the 12 patients. In the graph, the variables considered have a parallel trend in the population studied.
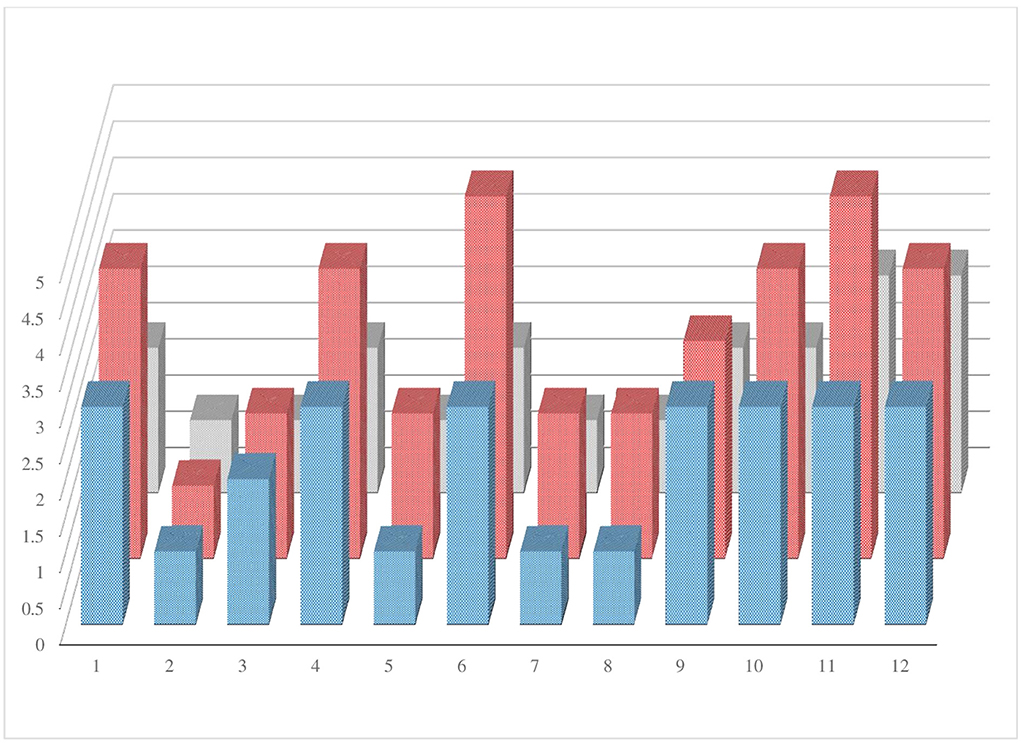
Figure 4. The graphic shows the trend of the three parameters studied (level of disability, MRI brain lesions, and elevated CSF protein) in each patient enrolled.
Staging the pediatric patient affected by GBS assumes an important value both in the general evaluation of the patient and in the prognosis. Currently, prognostic scores are based exclusively on clinical parameters, such as the GBS disability scale and the Erasmus GBS scale. There is no clinical-prognostic score that takes into consideration radiological or laboratory parameters.
To fill this gap, we tried to evaluate the correlation between the imaging staging of GBS on MRI and the chemical-physical examination of the CSF of 12 pediatric patients treated.
In our evaluations, we were able to evaluate a good correlation between them.
We assessed that MRI should be considered an essential and effective exam in the diagnostic and prognostic evaluation process in GBS. The involvement of the anterior and posterior nerve root is associated with severe clinical conditions and, consequently, with a worse prognosis (17).
Brain MRI then should be performed routinely in children with GBS to estimate the possible evolution of the clinical condition.
Regarding the CFS analysis, we observed that among the various elements of cerebral liquor, the best indication for clinical condition and prognostic evaluation were related mainly to the high level of CFS protein, it has also been reported in the literature (18, 19).
The MRI and CSF laboratory parameters can be important tools for the clinician not only for diagnosis but also for having an estimate of the possible worsening of general conditions or the need to prepare measures to support life parameters.
We believe it is important to establish how early the need for ventilatory support in the most severe patient is or to be able to decide to start physiotherapy in less severe cases. The need for this study also arises to give indications to the clinician in the sub-acute phase of the disease.
Our study aimed to predict outcomes in GBS by the use of acute phase clinical features, laboratory analysis, and imaging. We have not found in the literature other studies that use laboratory, imaging, and clinical parameters to stage GBS together.
On the other hand, our systematic search of the literature did not reveal studies that simultaneously evaluate the laboratory aspects of CSF and imaging and their relationship to the clinical conditions of children with GBS.
Especially considering the ongoing International GBS Outcome Study, the potential association among CSF protein, imaging, and disability score may be worthy of further exploration (20).
The study has several limitations in our opinion: the population has a heterogeneous age and this could vary the response to the disease of different patients. In the literature, the early age of onset is a negative prognostic factor (11). The sample is numerically small, and a larger sample of patients coming from different centers would be needed for the next studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted ethically in accordance with the World Medical Association Declaration of Helsinki and approved by the Ethics Committee of the University of Catania, Italy (Ethical Committee Catania 1 Clinical Registration No. 95/2018/PO). Written informed consent was obtained from the parents.
PP, FP, AF, and MR worked with and helped gather patient data. FG, TT, RR, AG, GC, and ADN drafted the present manuscript. AB, GB, and ADM were the radiologist consultant. PP, ET, MS, and AG were responsible for revising the work critically for important intellectual content. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van Doorn PA, Ruts L, Jacobs BC. Clinical features, pathogenesis, and treatment of Guillain-Barré syndrome. Lancet Neurol. (2008) 7:939–50. doi: 10.1016/S1474-4422(08)70215-1
2. Bragazzi NL, Kolahi AA, Nejadghaderi SA, Lochner P, Brigo F, Naldi A, et al. Global, regional, and national burden of Guillain-Barrè syndrome and its underlying causes from 1990 to 2019. J Neuroinflam. (2021) 18:264. doi: 10.1186/s12974-021-02319-4
3. Leonhard SE, Mandarakas MR, Gondim FAA, Bateman K, Ferreira MLB, Cornblath DR, et al. Diagnosis and management of GuillainBarré syndrome in ten steps. Nat Rev Neurol. (2019) 15:671–83. doi: 10.1038/s41582-019-0250-9
4. van den Berg B, Walgaard C, Drenthen J, Bateman K, Ferreira MLB, Cornblath DR, et al. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. (2014) 10:469–82. doi: 10.1038/nrneurol.2014.121
5. McGrogan A, Madle GC, Seaman HE, de Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature reviews. Neuroepidemiology. (2009) 32:150–63. doi: 10.1159/000184748
6. Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barre syndrome: a systematic review and meta-analysis. Neuro-epidemiology. (2011) 36:123e133. doi: 10.1159/000324710
7. Nguyen TP, Taylor RS. Guillain Barre Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing (2022).
8. van Koningsveld R, Steyerberg EW, Hughes RA, Swan AW, van Doorn P, Jacobs BC, et al. clinical prognostic scoring system for Guillain-Barré syndrome. Lancet Neurol. (2007) 6:589–94. doi: 10.1016/S1474-4422(07)70130-8
9. Althubaiti F, Guiomard C, Rivier F, Meyer P, Leboucq N. Prognostic value of contrast-enhanced MRI in Guillain-Barré syndrome in children. Arch Pediatr. (2022) 29:230–5. doi: 10.1016/j.arcped.2022.01.004
10. Kasser S, Hossieny ZS, Arnold WD, Elsheikh B, Palettas M, Kline D, et al. CSF protein level and short-term prognosis in Guillain-Barré syndrome. J Clin Neuromuscul Dis. (2019) 21:118–9. doi: 10.1097/CND.0000000000000259
11. Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. (1978) 2:750–3. doi: 10.1016/S0140-6736(78)92644-2
12. Doets AY, Lingsma HF, Walgaard C, Islam B, Papri N, Amy Davidson A, et al. Predicting outcome in Guillain-Barré syndrome. Neurology. (2022) 98:e518–32. doi: 10.1212/WNL.0000000000013139
13. Gorson KC, Ropper AH, Muriello MA, Blair R. Prospective evaluation of MRI lumbosacral nerve root enhancement in acute Guillain-Barré syndrome. Neurology. (1996) 47:813–7. doi: 10.1212/WNL.47.3.813
14. Yikilmaz A, Doganay S, Gumus H, Per H, Kumandas S, Coskun A. Magnetic resonance imaging of childhood GuillainBarre syndrome. Childs Nerv Syst. (2010) 26:1103–8. doi: 10.1007/s00381-010-1197-8
15. Roodbol J, de Wit MCY, van den Berg B, Kahlmann V, Drenthen J, Catsman-Berrevoets CE, et al. Diagnosis of Guillain-Barré syndrome in children and validation of the Brighton criteria. J Neurol. (2017) 264:856–61. doi: 10.1007/s00415-017-8429-8
16. Kerasnoudis A, Pitarokoili K, Behrendt V, Gold R, Yoon MS. Increased cerebrospinal fluid protein and motor conduction studies as prognostic markers of outcome and nerve ultrasound changes in GuillainBarré syndrome. J Neurol Sci. (2014) 3401:37–43. doi: 10.1016/j.jns.2014.02.019
17. Morgan GW, Barohn RJ, Bazan C 3rd, King RB, Klucznik RP. Nerve root enhancement with MRI in inflammatory demyelinating polyradiculoneuropathy. Neurology. (1993) 43:618–20. doi: 10.1212/WNL.43.3_Part_1.618
18. Brettschneider J, Petzold A, Süssmuth S. Cerebrospinal fluid biomarkers in Guillain-Barré syndrome—where do we stand? J Neurol. (2009) 256:3–12. doi: 10.1007/s00415-009-0097-x
19. Di Capua DB, Lakraj AA, Nowak RJ, Robeson K, Goldstein J, Patwa H, et al. Relationship between cerebrospinal fluid protein levels and electrophysiologic abnormalities in Guillain-Barré syndrome. J Clin Neuromuscul Dis. (2015) 17:47–51. doi: 10.1097/CND.0000000000000091
20. Jacobs BC, van den Berg B, Verboon C, Chavada G, Cornblath DR, Gorson KC, et al. International Guillain-Barré syndrome outcome study: protocol of a prospective observational cohort study on clinical and biological predictors of disease course and outcome in Guillain-Barré syndrome. J Peripher Nervous Syst. (2017) 22:68–76. doi: 10.1111/jns.12209
Keywords: Guillain–Barré syndrome, CSF, childhood, MRI, clinical outcome, neurology-clinical
Citation: Pizzo F, Di Nora A, Di Mari A, Costanza G, Testa E, Strazzieri M, Greco F, Timpanaro T, Basile A, Belfiore G, Giugno A, Rocca R, Ruggieri M, Fiumara A and Pavone P (2022) Case report: Incidence and prognostic value of brain MRI lesions and elevated cerebrospinal fluid protein in children with Guillain-Barré syndrome. Front. Neurol. 13:885897. doi: 10.3389/fneur.2022.885897
Received: 28 February 2022; Accepted: 22 July 2022;
Published: 21 October 2022.
Edited by:
Pasquale Striano, Giannina Gaslini Institute (IRCCS), ItalyReviewed by:
Alessandro Orsini, Pisana University Hospital, ItalyCopyright © 2022 Pizzo, Di Nora, Di Mari, Costanza, Testa, Strazzieri, Greco, Timpanaro, Basile, Belfiore, Giugno, Rocca, Ruggieri, Fiumara and Pavone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martino Ruggieri, bXJ1Z2dpZUB1bmljdC5pdA==; Piero Pavone, cHBhdm9uZUB1bmljdC5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.