
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 28 April 2022
Sec. Neuro-Otology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.883749
A correction has been applied to this article in:
Corrigendum: COVID-19 and sudden sensorineural hearing loss: a systematic review
A growing body of evidence suggests that patients with the 2019 Coronavirus disease (COVID-19) have a risk of developing sudden sensorineural hearing loss (SSNHL). The pathogenesis of COVID-19-related SSNHL remains unclear. This systematic review examined whether COVID-19 causes an increased incidence of SSNHL and the clinical characteristics of patients with COVID-19-related SSNHL according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines. SSNHL usually developed between a few days and 2 months after the diagnosis of COVID-19, and a proportion of patients developed it before the diagnosis of COVID-19. The literature is inconsistent regarding whether COVID-19 causes an increased incidence of SSNHL, and this matter remains unclear. This review included 23 patients with COVID-19-related SSNHL, all adult patients with an average age of 43.1 years. Of these patients, 60.9% had accompanying tinnitus symptoms. Glucocorticoids are the preferred medication to treat COVID-19-related SSNHL. Intratympanic administration may be considered to reduce the side effects of the drug. Hearing tests are suggested when hearing loss is suspected in COVID-19 individuals, and if SSNHL is detected, prompt and aggressive treatment is vital. Large-scale, multicenter research on the pathophysiology, treatment, and prognosis of COVID-19- related SSNHL should be conducted in the future.
A novel infectious disease, 2019 Coronavirus disease (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a highly transmissible and pathogenic human coronavirus (1). Scientific and clinical evidence suggested that COVID-19 can affect multiple organ systems (2). SARS-CoV-2 can also invade the central and peripheral nervous system to cause various neurological diseases (3).
Sudden sensorineural hearing loss (SSNHL) is defined as the sudden onset of sensorineural hearing loss (SNHL) without an identifiable etiology, with at least three consecutive frequency losses ≥ 30 dB within 72 h (4). The annual incidence of SSNHL varies between 5 and 27 cases per 100,000 persons (5). The cause of SSNHL is unclear. However, the possible causes include viral infection, circulatory abnormalities, autoimmune disease, labyrinthine membrane rupture, and central nervous system (CNS) anomalies (5, 6).
Strong evidence suggests that viral infection is a cause of SSNHL (7). It may be related to direct viral access to the labyrinth or the cochlear nerve, reactivation of latent virus inside spiral ganglia, and immunoregulation of systemic viral infections (7). Lassa fever, mumps, adenovirus, and other viruses have caused SSNHL (8–10). Growing data indicate that patients with COVID-19 are prone to SSNHL (7, 11–13). However, many aspects of COVID-19-related SSNHL have remained unclear.
The purpose of this review was to evaluate the impact of COVID-19 on the incidence of SSNHL and to provide an understanding of the clinical characteristics of COVID-19-related SSNHL.
A systematic review was conducted based on the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines (14). Ethics committee approval was not required for this literature review.
We systematically searched literature databases, including PubMed, Scopus, Web of Science, and Embase, using variations of the descriptors for “COVID-19,” “SARS-CoV-2,” “SSNHL” with AND/OR. Searches were not restricted to studies by date, publication status, or language. The retrieval scheme was mainly based on a combination of medical subject headings (MeSH) terms and free words. The final search was performed on February 22, 2022. Two reviewers (JW and KZ) independently assessed the eligibility of the studies and extracted the data.
The inclusion criteria were original studies or case reports, human studies, and studies written in English. Included patients required confirmation of COVID-19 by polymerase chain reaction testing or antibody testing. Irrelevant articles, non-English language articles, review papers, letters without data, commentary, studies of SSNHL after COVID-19 vaccine, and abstracts without full text were excluded.
Two reviewers (JW and KZ) extracted data using a well-designed Excel (Microsoft Inc., USA) spreadsheet. The following data were included: authors, year of publication, the country in which the study was conducted, study design, study purpose, assessment period, main results, conclusions, sample size, age and gender, time of onset, side affected, symptoms, audiological assessment, treatments, and treatment outcome.
The two reviewers (JW and KZ) independently scored each study. The quality of studies for inclusion in the review was assessed using the National Institutes of Health quality assessment tool for case series studies, as well as observational cohort and cross-sectional studies (10). Based on the rating of each study, its quality rating can be divided into three categories: good, fair, and poor. Any disagreement was resolved by consensus through the authors' discussions.
There were eventually 26 studies included in this systematic review. Ten papers worldwide have investigated various aspects of whether COVID-19 contributes to the increased incidence of SSNHL. These studies are summarized in Table 1. Sixteen articles studied the clinical characteristics and therapeutic outcomes of COVID-19-related SSNHL. Of these 16 studies, 13 were case reports, and 3 were case series. The clinical characteristics of the patients with COVID-19-related SSNHL are synthesized in Table 2. A flowchart of the literature search is presented in Figure 1.
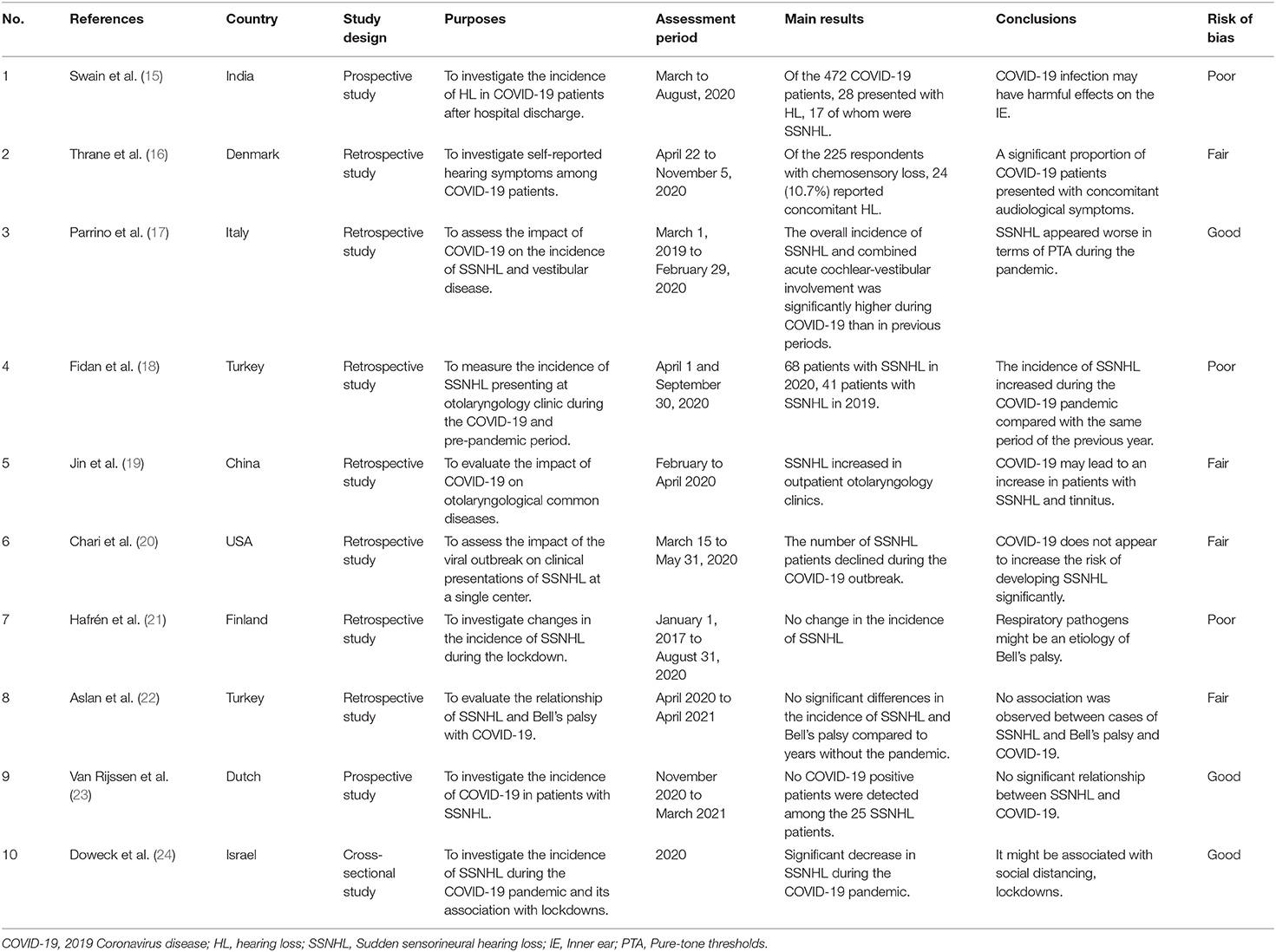
Table 1. A summary of studies on the relationship between 2019 Coronavirus disease (COVID-19) and the incidence of sudden sensorineural hearing loss (SSNHL).
Although case reports of COVID-19-related SSNHL have increased, it remains unknown whether SARS-CoV-2 has contributed to the increased incidence of SSNHL worldwide.
Swain et al. investigated the audiological performance of 472 COVID patients and found that 24 (5.08%) patients had SNHL, most of which were SSNHL (15). Based on self-reported email questionnaires, a retrospective observational study from Denmark showed that over 10% of COVID-19 patients with self-reported chemosensory loss complained of hearing loss (HL) (16). However, the patients included in the study did not receive objective diagnostic and audiological tests, thus affecting the credibility of the study.
Studies from around the world have independently evaluated the impact of the COVID-19 pandemic on the incidence of SSNHL in a single medical center, with inconsistent results. Data from an Italian audiology tertiary referral center showed that the incidence of SSNHL and combined acute cochlear-vestibular involvement was significantly higher during the COVID-19 pandemic than in previous periods, with a more severe clinical presentation of pure-tone audiometry (PTA) (17). A study from Turkey found an increased incidence of SSNHL during the COVID-19 epidemic, with 60.3% of subjects having signs compatible with COVID-19 (18). A large tertiary hospital in China also found an increase in SSNHL visits to the outpatient and emergency departments during the COVID-19 pandemic compared to the past (19). However, several studies found no increase in the incidence of SSNHL after the COVID-19 outbreak, concluding that SARS-CoV-2 does not appear to have a significant risk of SSNHL development (20–23). However, these study data are based on the number of patients who visited the hospital. It is possible that some patients were reluctant to visit the hospital for worry of being infected with COVID-19, thus affecting the accuracy of the incidence of SSNHL (20). Van Rijssen et al. tested 25 patients with SSNHL in a large Dutch teaching hospital for COVID-19 with no positive cases, and concluded no significant relationship between SSNHL and COVID-19 (23). Doweck et al. used data from Clalit Health Services to compare the incidence of SSNHL during the COVID-19 pandemic in Israel with the incidence in 2018 and 2019, and found that the incidence of SSNHL decreased during the COVID-19 pandemic compared with the time before the COVID-19 outbreak (24). It might be related to the decrease in community physicians and emergency department visits (24).
There were 23 patients, 11 males and 12 females. The male to female ratio was 0.92. The average age of the patients was 43.1 years (range 18–67 years). The age distribution is shown in Figure 2. Of these patients, six were affected on the left side, eight on the right side, and nine on the bilateral side.
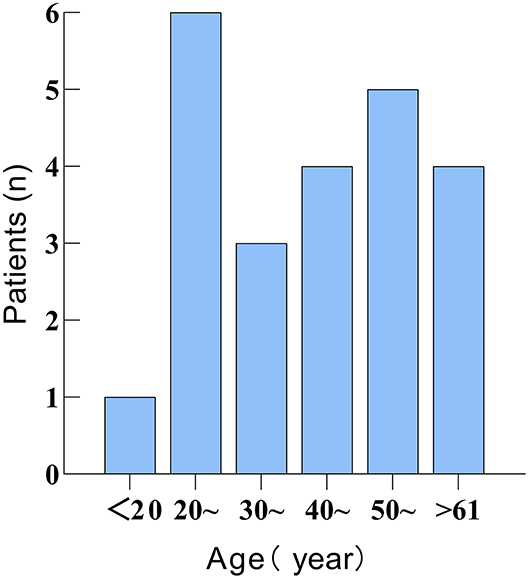
Figure 2. Figure showing the age distribution of 23 patients with 2019 Coronavirus disease (COVID-19)-related sudden sensorineural hearing loss (SSNHL).
COVID-19- related SSNHL is more common in adults, and audio-vestibular symptoms typically manifest following the diagnosis of COVID-19 or during the rehabilitation period. The time from confirmation of COVID-19 to the onset of SSNHL also varied, which could be anywhere from a few days to 2 months. However, SSNHL in some patients occurred days or 10's of days before confirmation of COVID-19. We speculated that COVID-19 in these patients might have also occurred before SSNHL, confirming COVID-19 somewhat late.
Of these 23 patients, only 4 (17.4%) presented with HL symptoms solely, while the majority (82.6%) were accompanied by one or more other symptoms. The concomitant symptoms in these patients included tinnitus (n = 14, 60.9%), vertigo (n = 3, 13.0%), dizziness (n = 2, 8.7%), ear pain (n = 1, 4.0%), and peripheral facial nerve palsy (n = 1, 4.0%). The distribution of accompanying symptoms in patients with COVID-19-related SSNHL is shown in Figure 3. As can be seen, tinnitus is the most common symptom associated with SSNHL.
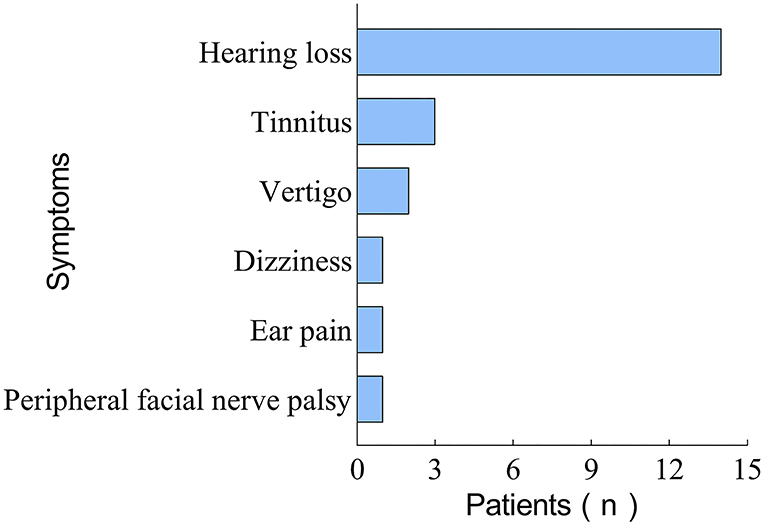
Figure 3. Figure showing the distribution characteristics of concomitant symptoms in 23 patients with COVID-19-related SSNHL.
In most cases, SSNHL is not the first sign of COVID-19 (11). However, there are certain exceptions. Chern et al. described a case of an 18-year-old SSNHL patient who presented to an otology clinic with bilateral auditory fullness and vertigo. The patient was identified with COVID-19 a few weeks later using SARS-CoV-2 IgG antibodies (7). Some individuals have also experienced chemoreceptor dysfunction symptoms, such as anosmia and loss of taste, before developing SSNHL (13, 15).
Most patients with COVID-19-related SSNHL showed normal MRI findings, while some developed an intra-labyrinthine hemorrhage (7, 13). Ozer et al. reported a case of SSNHL with peripheral facial paralysis in a COVID-19 patient, suggesting a link between SSNHL and isolated facial paralysis (25).
In these studies, PTA was the most commonly used method of audiological assessment (6, 13, 35). Several studies employed tympanometry and tuning fork tests to identify COVID-19-related SSNHL (6, 35). Due to isolation of patients, telephone consultations were also used to provide an initial assessment of the patient's condition (13). Auditory brainstem response (ABR), otoacoustic emission, and electroneuronography were applied in a few studies (25, 28). Two studies used a head-shaking test or video head impulse testing to identify vertigo (11, 27). The patients' word recognition scores (WRS) were assessed using the speech audiometry method in two studies (6, 7). Also, tinnitus handicap inventory and dizziness handicap inventory were used in one study (11).
Glucocorticoids were the most commonly used therapeutic agents for COVID-19-related SSNHL, and the vast majority of patients were treated with glucocorticoids. Corticosteroids can be delivered orally, intravenously, or intramuscularly via systemic and/or intratympanic (IT) routes (12, 25, 27). In one study, five patients received hyperbaric oxygen therapy (HBOT) and oral mesoglycan as an adjunctive treatment (11). Patients whose hearing did not improve after treatment or who had a history of SSNHL for over 8 weeks were given cochlear implants (CI) and/or hearing aids to enhance their hearing (12, 13, 28). The treatment was not mentioned in the article for one patient (13).
The cure rate of COVID-19-related SSNHL is unknown since most available studies are case reports or case series with limited sample numbers. Among these 23 cases of COVID-19-related SSNHL, two patients (8.7%) recovered completely, 12 patients (52.2%) recovered partially, 1 (4.3%) case improved slightly, 6 (26.1%) patients did not improve, one patient (4.3%) recovered completely on one side and partially on the other, and one patient (4.3%) with an unknown outcome. The treatment outcomes are presented in Figure 4. Ricciardiello et al. treated and followed up on five individuals with COVID-19-related SSNHL, most of whom recovered partial auditory function (11). Shah et al. described four patients with COVID-19-related SSNHL, two of whom did not improve following treatment with glucocorticoids (13).
This systematic review examined whether COVID-19 leads to an increased incidence of SSNHL by using the available literature. However, the COVID-19 pandemic varied across countries, and the findings were inconsistent and even contradictory. Therefore, it remains unknown whether COVID-19 contributes to the high incidence of SSNHL.
All 23 patients with COVID-19-related SSNHL, included in this systematic review, were adults, with no significant differences in the number of incidences by age group. Pediatric SSNHL is uncommon, and there have been no reports of COVID-19-related SSNHL in children (36). Tinnitus, one of the most common sensorineural disorders, is associated with SSNHL in 66–93% of cases (37). In this review, we found that 60.9% of patients with COVID-19-related SSNHL had tinnitus symptoms similar to the presentation of regular SSNHL.
COVID-19-related SSNHL can occur on one or both sides; however, it is more common on one side (11, 15). Bilateral SSNHL is relatively uncommon (7). However, the likelihood of binaural morbidity in SSNHL was significantly higher in COVID-19 patients than in the general population. Shah et al. studied 4 COVID-19-related SSNHL patients referred to their otolaryngology clinic, 3 of whom presented with bilateral symptoms (13). It may relate to the fact that ototoxicity symmetrically affects both ears (12).
Early audiological assessments and monitoring are critical when suspected or detected with sensorineural hearing loss (SNHL) (38). PTA, otoacoustic emissions, and ABR can all be used to assess the circumstances of patients with COVID-19-related SSNHL (15). PTA helps determine the type and severity of HL. In the era of COVID-19, telemedicine can reduce outpatient follow-up visits, reduce the risk of cross-infection, and improve patient satisfaction (39). According to physical distance requirements and Centers for Disease Control and Prevention guidelines, conventional hearing services are at moderate to high risk for COVID-19 infection (40). As a result, traditional auditory service deliveries are highly restricted. In this context, tele-audiometry has become a workable option for audiometric examination, with a high level of accuracy that is not significantly different from traditional audiometric testing (41). A study by Shilo et al. based on smartphone vibration and uHear App hearing tests also confirmed that the telemedicine model for diagnosing SSNHL is valid and reliable (42). In addition, some improvements can be made to the hearing test technologies to minimize contact with the patient. A recent study has shown that PTA combined with the digits-in-noise test, without bone-conduction thresholds, can distinguish between SNHL and conductive HL (40). Since this hearing test method does not require traditional bone-conduction audiometry in a closed sound booth, it is valuable in preventing cross-contamination.
According to the clinical practice guidelines of the American Academy of Otolaryngology-Head and Neck Surgery, glucocorticoids can be used as initial treatment for SSNHL patients within 2 weeks after the onset of the disease (4). Since there are contradictory results on adverse reactions to corticosteroids in patients with COVID-19, IT administration is a beneficial option (6). A clinical study compared the therapeutic efficacy of IT corticosteroid administration vs. intravenous corticosteroids in COVID-19-related SSNHL, and found comparable efficacy of both treatments (43). However, IT administration did not cause systemic side effects compared to intravenous corticosteroids (43). Therefore, patients with COVID-19-related SSNHL can be treated with IT corticosteroids as soon as they are diagnosed (6).
A recent systematic review and meta-analysis published in JAMA Otolaryngology-Head and Neck Surgery showed, including over 20 years of literature data, that HBOT as part of a comprehensive treatment for patients with SSNHL significantly improves hearing outcomes (44). However, HBOT sessions should be adjusted to meet COVID-19 prevention and control requirements for infection identification, distancing, and isolation, to avoid breathing with an internal chamber atmosphere (45). Moreover, pediatric patients can be safely treated with HBOT (46). Thus, HBOT can be applied while meeting the protection of medical staff and patients to improve the outcomes.
Patients who do not regain their hearing after active treatment may consider hearing aids or CI to improve their hearing. In patients with poor physical condition, CI procedures can be conducted under local anesthesia with analgesia to limit the risk of anesthesia (12).
Individuals with HL may experience challenges, such as difficulty perceiving speech, leading to communication and social difficulties (47). Surgical face masks and face shields were essential for personal protection during the COVID-19 era. However, this impaired the speech perception in individuals with moderate-to-severe HL (48). The pandemic has exacerbated the situation, worsening patients' mental health, particularly in rural areas (49). As a result, the entire society should pay attention to people with HL by giving practical support in various areas such as socialization, detection, treatment, and rehabilitation.
Otolaryngology is considered a high-risk unit for COVID-19 (50). During the ongoing pandemic, there are many infected individuals with atypical symptoms. Each symptom should be considered for association with COVID-19 (51). Kilic et al. detected one positive patient for SARS-CoV-2 among five male patients with unilateral SSNHL as the only complaint in an outpatient otolaryngology clinic (35). SSNHL may be the first symptom of COVID-19. Therefore, all patients with SSNHL and/or acute vestibular disease should be screened for SARS-CoV-2 infection (17, 52). In addition, patients in the otolaryngology outpatient clinic should receive enough attention to their anxiety and stress levels (53).
The majority of the extant research is based on retrospective studies with limited sample sizes, and large-scale prospective investigations are lacking. As a result, large-scale, multicenter research on the pathophysiology, treatment, and prognosis of COVID-19-related SSNHL should be conducted. In the available literature, pure tone audiometry is mainly used to detect COVID-19-related SSNHL, while speech audiometry is rarely used to examine patients' WRS. Speech audiometry may be considered to evaluate the effect of SARS-CoV-2 on cognitive processes.
SARS-CoV-2 has the potential to cause damage to the audio-vestibular system, resulting in SSNHL. However, the exact mechanisms by which SARS-CoV-2 affects the audio-vestibular system remain unclear. Although numerous investigations have been conducted on COVID-19-related SSNHL, they are fragmented and unsystematic. The true prevalence of SSNHL in COVID-19 patients around the world is unknown. Glucocorticoids are the preferred medication to treat COVID-19-related SSNHL. Hearing testing is recommended when HL is suspected in COVID-19 individuals, and if SSNHL is identified, prompt and vigorous treatment is critical.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
XM and JS conceptualized and drafted the manuscript. JW and KZ critically reviewed the literature and extracted data. XM revised the draft manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the Science and Technology Development Project of the Bureau of Science and Technology of Wuxi, China (Grant number CSZ0N1622).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
COVID-19, 2019 Coronavirus disease; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; SSNHL, Sudden sensorineural hearing loss; CNS, Central nervous system; ACE2, Angiotensin-converting enzyme 2; TMPRSS2, Transmembrane protease serine subtype 2; ME, Middle ear; IE, Inner ear; HC, Hair cells; MRI, Magnetic resonance imaging; SNHL, Sensorineural hearing loss; WRS, Word recognition scores; PTA, Pure-tone audiometry; HL, hearing loss; HBOT, Hyperbaric oxygen therapy.
1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. (2021) 19:141–54. doi: 10.1038/s41579-020-00459-7
2. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
3. Wan D, Du T, Hong W, Chen L, Que H, Lu S, et al. Neurological complications and infection mechanism of SARS-COV-2. Signal Transduct Target Ther. (2021) 6:406. doi: 10.1038/s41392-021-00818-7
4. Chandrasekhar SS, Tsai Do BS, Schwartz SR, Bontempo LJ, Faucett EA, Finestone SA, et al. Clinical practice guideline: sudden hearing loss (update). Otolaryngol Head Neck Surg. (2019) 161(1_suppl):S1–45. doi: 10.1177/0194599819859885
5. Li C, Zhou H, Feng Y, Zhao Y, Wang J, Chen Z, et al. Coagulation states in patients with sudden sensorineural hearing loss evaluated by thromboelastography. Otolaryngol Head Neck Surg. (2021) 164:1280–6. doi: 10.1177/0194599820965240
6. Rahimi V, Asiyabar MK, Rouhbakhsh N. Sudden hearing loss and coronavirus disease 2019: the role of corticosteroid intra-tympanic injection in hearing improvement. J Laryngol Otol. (2021) 135:464–6. doi: 10.1017/S0022215121001080
7. Chern A, Famuyide AO, Moonis G, Lalwani AK. Bilateral sudden sensorineural hearing loss and intralabyrinthine hemorrhage in a patient with COVID-19. Otol Neurotol. (2021) 42:e10–e4. doi: 10.1097/MAO.0000000000002860
8. Cummins D, McCormick JB, Bennett D, Samba JA, Farrar B, Machin SJ, et al. Acute sensorineural deafness in Lassa fever. J Am Med Assoc. (1990) 264:2093–6. doi: 10.1001/jama.1990.03450160063030
9. Comacchio F, D'Eredità R, Marchiori C. MRI evidence of labyrinthine and eighth-nerve bundle involvement in mumps virus sudden deafness and vertigo. ORL J Otorhinolaryngol Relat Spec. (1996) 58:295–7. doi: 10.1159/000276856
10. National Heart, Lung, and Blood Institute. Study Quality Assessment Tools. (2014). Available online at: https://www.nhlbi.nih.gov/health-topics/studyquality-assessment-tools (accessed March 10, 2022).
11. Ricciardiello F, Pisani D, Viola P, Cristiano E, Scarpa A, Giannone A, et al. Sudden sensorineural hearing loss in mild COVID-19: case series and analysis of the literature. Audiol Res. (2021) 11:313–26. doi: 10.3390/audiolres11030029
12. Degen C, Lenarz T, Willenborg K. Acute profound sensorineural hearing loss after COVID-19 pneumonia. Mayo Clin Proc. (2020) 95:1801–3. doi: 10.1016/j.mayocp.2020.05.034
13. Shah SM, Rocke J, France K, Izzat M. Sudden sensorineural hearing loss in covid-19: a case series from the wrightington, wigan and leigh teaching hospitals, United Kingdom. Med J Malaysia. (2021) 76:55–9.
14. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
15. Swain SK, Pani SR. Incidence of hearing loss in COVID-19 patients: a covid hospital-based study in the eastern part of India. Intern J Cur Res Rev. (2021) 13:103–7. doi: 10.31782/IJCRR.2021.13329
16. Thrane JF, Britze A, Fjaeldstad AW. Incidence and duration of self-reported hearing loss and tinnitus in a cohort of COVID-19 patients with sudden chemosensory loss: a STROBE observational study. Eur Ann Otorhinolaryngol Head Neck Dis. (2021) 2021:S1879-7296(21)00224-6. doi: 10.1016/j.anorl.2021.07.012
17. Parrino D, Frosolini A, Toninato D, Matarazzo A, Marioni G, de Filippis C. Sudden hearing loss and vestibular disorders during and before COVID-19 pandemic: an audiology tertiary referral centre experience. Am J Otolaryngol. (2022) 43:103241. doi: 10.1016/j.amjoto.2021.103241
18. Fidan V, Akin O, Koyuncu H. Rised sudden sensorineural hearing loss during COVID-19 widespread. Am J Otolaryngol. (2021) 42:102996. doi: 10.1016/j.amjoto.2021.102996
19. Jin L, Fan K, Tan S, Liu S, Wang Y, Yu S. Analysis of the characteristics of outpatient and emergency diseases in the department of otolaryngology during the “COVID-19” pandemic. Sci Prog. (2021) 104:368504211036319. doi: 10.1177/00368504211036319
20. Chari DA, Parikh A, Kozin ED, Reed M, Jung DH. Impact of COVID-19 on presentation of sudden sensorineural hearing loss at a single institution. Otolaryngol Head Neck Surg. (2021) 165:163–5. doi: 10.1177/0194599820974685
21. Hafrén L, Saarinen R, Lundberg M. Effects of social distancing on the incidence of Bell's palsy and sudden sensorineural hearing loss. Acta Otolaryngol. (2022) 142:220–3. doi: 10.1080/00016489.2021.2025425
22. Aslan M, Çiçek MT. Can isolated sudden sensorineural hearing loss (SSNHL) and idiopathic acute facial paralysis (Bell's palsy) be symptoms of COVID-19? Am J Otolaryngol. (2021) 42:103129. doi: 10.1016/j.amjoto.2021.103129
23. van Rijssen LB, Derks W, Hoffmans R, van Looij MA, van Maanen JP, van Monsjou HS, et al. No COVID-19 in patients with sudden sensorineural hearing loss (SSNHL). Otol Neurotol. (2022) 43:170–3. doi: 10.1097/MAO.0000000000003438
24. Doweck I, Yanir Y, Najjar-Debbiny R, Shibli R, Saliba W. Sudden sensorineural hearing loss during the COVID-19 pandemic. J Am Med Assoc Otolaryngol Head Neck Surg. (2022) 2022:e214105. doi: 10.1001/jamaoto.2021.4105
25. Ozer F, Alkan O. Simultaneous sudden hearing loss and peripheral facial paralysis in a patient with covid-19. Ear Nose Throat J. (2021) 2021:1455613211028094. doi: 10.1177/01455613211028094
26. Gunay E, Kozan G, Yuksel E, Mizrakli A, Aslan O, Kavak S, et al. A case of peritoneal dialysis in which SARS-CoV-2 was diagnosed by sudden hearing loss. Ren Fail. (2021) 43:325–6. doi: 10.1080/0886022X.2021.1882493
27. Beckers E, Chouvel P, Cassetto V, Mustin V. Sudden sensorineural hearing loss in COVID-19: a case report and literature review. Clin Case Rep. (2021) 9:2300–4. doi: 10.1002/ccr3.4019
28. Gerstacker K, Speck I, Riemann S, Aschendorff A, Knopf A, Arndt S. Deafness after COVID-19? HNO. (2021) 69:92–5. doi: 10.1007/s00106-021-01041-0
29. Guigou C, Schein AD, Blanchard C, Folia M. Sudden sensorineural hearing loss and SARS-CoV-2: Don’t forget the standard work-up! Eur Ann Otorhinolaryngol Head Neck Dis. (2021) 138:219–20. doi: 10.1016/j.anorl.2021.02.010
30. Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. (2020) 13:238419. doi: 10.1136/bcr-2020-238419
31. Lamounier P, Franco Gonçalves V, Ramos HVL, Gobbo DA, Teixeira RP, Dos Reis PC, et al. A 67-year-old woman with sudden hearing loss associated with SARS-CoV-2 infection. Am J Case Rep. (2020) 21:e927519. doi: 10.12659/AJCR.927519
32. Pokharel S, Tamang S, Pokharel S, Mahaseth RK. Sudden sensorineural hearing loss in a post-COVID-19 patient. Clin Case Rep. (2021) 9:4956. doi: 10.1002/ccr3.4956
33. Edwards M, Muzaffar J, Naik P, Coulson C. Catastrophic bilateral sudden sensorineural hearing loss following COVID-19. BMJ Case Rep. (2021) 14:e243157. doi: 10.1136/bcr-2021-243157
34. Lang B, Hintze J, Conlon B. Coronavirus disease 2019 and sudden sensorineural hearing loss. J Laryngol Otol. (2020) 134:1026–8. doi: 10.1017/S0022215120002145
35. Kilic O, Kalcioglu MT, Cag Y, Tuysuz O, Pektas E, Caskurlu H, et al. Could sudden sensorineural hearing loss be the sole manifestation of COVID-19? An investigation into SARS-COV-2 in the etiology of sudden sensorineural hearing loss. Int J Infect Dis. (2020) 97:208–11. doi: 10.1016/j.ijid.2020.06.023
36. Pitaro J, Bechor-Fellner A, Gavriel H, Marom T, Eviatar E. Sudden sensorineural hearing loss in children: etiology, management, and outcome. Int J Pediatr Otorhinolaryngol. (2016) 82:34–7. doi: 10.1016/j.ijporl.2015.12.022
37. Ding X, Zhang X, Huang Z, Feng X. The characteristic and short-term prognosis of tinnitus associated with sudden sensorineural hearing loss. Neural Plast. (2018) 2018:6059697. doi: 10.1155/2018/6059697
38. Marcoux A. Emerging Reports of Hearing Loss During the COVID-19 Pandemic and the Importance of Accessibility to Audiological Services. (2020). Lynge, WS Audiology.
39. Dai Z, Wang Y, Hang C, Zhu K, Meng X. Telemedicine for ear diseases with the smartphone otoscopes via WeChat in the COVID-19 era. Am J Otolaryngol. (2021) 42:102997. doi: 10.1016/j.amjoto.2021.102997
40. De Sousa KC, Smits C, Moore DR, Myburgh HC, Swanepoel DW. Pure-tone audiometry without bone-conduction thresholds: using the digits-in-noise test to detect conductive hearing loss. Int J Audiol. (2020) 59:801–8. doi: 10.1080/14992027.2020.1783585
41. Linkenheimer ES. Telehealth audiology and virtual hearing screenings: a case report. Semin Hear. (2021) 42:152–7. doi: 10.1055/s-0041-1731695
42. Shilo S, Ungar OJ, Handzel O, Abu Eta R, Shapira U, Muhanna N, et al. Telemedicine for patients with unilateral sudden hearing loss in the COVID-19 era. J Am Med Assoc Otolaryngol Head Neck Surg. (2022) 148:166–72. doi: 10.1001/jamaoto.2021.3672
43. Tsuda T, Hanada Y, Wada K, Fujiwara E, Takeda K, Nishimura H. Efficacy of intratympanic glucocorticoid steroid administration therapy as an initial treatment for idiopathic sudden sensorineural hearing loss during the COVID-19 pandemic. Ear Nose Throat J. (2021) 2021:1455613211032534. doi: 10.1177/01455613211032534
44. Joshua TG, Ayub A, Wijesinghe P, Nunez DA. Hyperbaric oxygen therapy for patients with sudden sensorineural hearing loss: a systematic review and meta-analysis. J Am Med Assoc Otolaryngol Head Neck Surg. (2021) 148:5–11. doi: 10.1001/jamaoto.2021.2685
45. Narozny W, Skorek A, Tretiakow D. Does treatment of sudden sensorineural hearing loss in patients with COVID-19 require anticoagulants? Otolaryngol Head Neck Surg. (2021) 165:236–7. doi: 10.1177/0194599820988511
46. El Hawa AAA, Bekeny JC, Phillips NW, Johnson-Arbor K. Hyperbaric oxygen therapy for paediatric patients: an unintended consequence of the COVID-19 pandemic. J Wound Care. (2021) 30:S24–s8. doi: 10.12968/jowc.2021.30.Sup9.S24
47. Seifi Ala T, Graversen C, Wendt D, Alickovic E, Whitmer WM, Lunner T. An exploratory study of EEG alpha oscillation and pupil dilation in hearing-aid users during effortful listening to continuous speech. PLoS ONE. (2020) 15:e0235782. doi: 10.1371/journal.pone.0235782
48. Homans NC, Vroegop JL. The impact of face masks on the communication of adults with hearing loss during COVID-19 in a clinical setting. Int J Audiol. (2021) 2021:1–6. doi: 10.1080/14992027.2021.1952490
49. Wilson HL, Crouch J, Schuh M, Shinn J, Bush ML. Impacts of the COVID-19 pandemic on communication and healthcare access for adults with hearing loss. Otol Neurotol. (2021) 42:1156–64. doi: 10.1097/MAO.0000000000003203
50. Meng X, Deng Y, Dai Z, Meng Z. COVID-19 and anosmia: a review based on up-to-date knowledge. Am J Otolaryngol. (2020) 41:102581. doi: 10.1016/j.amjoto.2020.102581
51. Kalcioglu MT, Cag Y, Kilic O, Tuysuz O. Can COVID-19 cause sudden sensorineural hearing loss? Int J Infect Dis. (2020) 101:205. doi: 10.1016/j.ijid.2020.09.1468
52. Narozny W, Skorek A, Tretiakow D. Should patients with sudden deafness be tested for COVID19? Auris Nasus Larynx. (2021) 48:797–8. doi: 10.1016/j.anl.2021.01.025
Keywords: COVID-19, SARS-CoV-2, sudden sensorineural hearing loss, inner ear, incidence, glucocorticoids
Citation: Meng X, Wang J, Sun J and Zhu K (2022) COVID-19 and Sudden Sensorineural Hearing Loss: A Systematic Review. Front. Neurol. 13:883749. doi: 10.3389/fneur.2022.883749
Received: 25 February 2022; Accepted: 30 March 2022;
Published: 28 April 2022.
Edited by:
Agnieszka J. Szczepek, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Guillermo Plaza, Fuenlabrada University Hospital, SpainCopyright © 2022 Meng, Wang, Sun and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangming Meng, eGlhbmdtaW5nX21lbmdAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.