- 1Sport and Physical Activity Research Centre, Faculty of Education, Health and Wellbeing, University of Wolverhampton, Wolverhampton, United Kingdom
- 2Functional Architecture of Mammals in their Environment Laboratory, Department of Physical Education and Sport Science, University of Thessaly, Volos, Greece
- 3First Department of Neurology, Medical School, National and Kapodistrian University of Athens, Eginition University Hospital, Athens, Greece
- 4School of Physical Education Physiotherapy and Dance, Federal University of Rio Grande do Sul (UFRGS), Porto Alegre, Brazil
Objectives: The aim of the present systematic review and meta-analysis was to synthesize evidence associated with the functional and clinical effectiveness of rhythmic cueing, dance, or resistance training (RT) on motor and non-motor parameters in Parkinson's Disease patients, and to provide a comparative perspective not offered by existing systematic reviews.
Methodology: Eligibility criteria for selecting studies retained no restrictions in methodological design and included interventions of rhythmic cueing, dance, RT, and measurements of motor and non-motor parameters. Animal studies, reviews, editorials, conferences, magazines, and gray literature articles were excluded. Two independent investigators searched Cochrane Library, Medline, PubMed, and SPORTDiscus from the date of their inception until 1 June 2021. The ROBINS-I tool was employed for the non-randomized controlled trials, and the updated for Risk of Bias 2 tool of Cochrane Library used for randomized controlled trials. For meta-analyses, the RevMan 5.4.13 software was used. For incompatible meta-analysis studies, a narrative data synthesis was conducted.
Results: A total of 49 studies included in the systematic review involving 3767 PD participants. Meta-analyses revealed that rhythmic cueing training assists gait velocity (p = 0.01), stride length (p = 0.01), and motor symptoms (p = 0.03). Similarly, dance training benefits stride length (p = 0.05), lower extremity function-TUG (p = 0.01), and motor symptoms (p = 0.01), whilst RT improves lower extremity function-TUG (p = 0.01), quality of life (p = 0.01), knee flexion (p = 0.02), and leg press (p = 0.01). Subgroup analyses have shown non-significant differences in gait velocity (p = 0.26), stride length (p = 0.80), functional mobility-TUG (p = 0.74), motor symptoms-UPDRS-III (p = 0.46), and quality of life-PDQ39 (p = 0.44).
Conclusion: Rhythmic cueing, dance, or RT positively affect the examined outcomes, with rhythmic cueing to be associated with three outcomes (Gait, Stride, and UPDRS-III), dance with three outcomes (TUG, Stride, and UPDRS-III), and RT with two outcomes (TUG and PDQ-39). Subgroup analyses confirmed the beneficial effects of these forms of exercise. Clinicians should entertain the idea of more holistic exercise protocols aiming at improving PD manifestations.
International Prospective Register of systematic reviews (PROSPERO) (registration number: CRD42020212380).
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative disorder, which is mainly characterized by the loss of dopaminergic neurons in the substantia Nigra pars compacta (SNpc) of the midbrain and the accumulation of Lewy bodies and Lewy neuritis (1). Being the second most common neurodegenerative disorder (2), PD affects approximately 10 million people worldwide (3). It is estimated that by 2040 this number will increase over 12 million (4), with aging, as well as genetic and environmental factors contributing to its development (5). Physical exercise accompanied by healthy lifestyle has been shown to exert beneficial effects on the progression of the disease [(6–8)].
Some of the most common non-motor manifestations of PD include sleeping disorders, cognitive impairment (e.g., difficulties in concentrating, learning, remembering, and thinking), anxiety, depression, and lack of motivation (9). Motor manifestations include resting tremor, bradykinesia, freezing of gait, rigidity, and postural impairment. In PD, nigrostriatal degeneration resulting in basal ganglia dysfunction is critically associated with impaired synchronization of regular and periodical movement patterns (10, 11).
Auditory cues are beats that indicate a rhythmic schema, which usually consists of a monotonous tapping. Auditory cues can be any kind of rhythmic stimulation (12), while all beats are by default strong (13). For instance, the use of voice for counting, or syllabi (ya, ta, ta), or use of a tambourine or a metronome, or to move according to the meter of a music piece i.e., 2/4 or 4/4 time. When rhythmic schema is established, it can continue to exist in the listener's mind even when the source of rhythm is paused (13, 14). People usually synchronize their actions through an innate rhythmic entrainment (13), and in a healthy brain, this procedure is related to subcortico-thalamo-cortical network including the pre-supplementary and supplementary motor areas, basal ganglia, and cerebellum (12). Basal ganglia, and especially the putamen, is critically implicated in the sequencing of rhythmic stimuli, and potentially the ‘feeling of the beat’ (13). Acoustic cues may enhance the connectivity between auditory perception and movement, since rhythm enables the activation of neural circuits associated with motor processing (13). Given that PD patients display difficulties in performing automatized movements, the use of external cues appears to be beneficial (15). Rhythm, as a form of external cue, therefore, seems to reduce the dependence on deficient automatized processes (16) that characterize PD pathophysiology, since movement could be synchronized to the regular expectation of a beat (13).
Indeed, a systematic review, containing 50 studies with 1,892 PD participants, revealed the beneficial effects of external rhythmical cues on gait (17). However, another systematic review underlined the lack of consistency in studies with rhythmic auditory stimulation in most components such as participants, exercise intervention, duration, or design (12).
According to Malloch and Trevarthen (18), rhythm usually stands between music and dance, interacts between music and movement/dance, and forms the first step toward musicality. Dance itself is an activity as old as human civilization (19, 20), and in ancient Greece, it was used to improve or maintain health, especially in older people (21). Studies in dance displayed different methodological characteristics, such as type of dance, duration of intervention, and group comparisons (22). However, recent literature indicates that dance can improve selected motor and non-motor elements, such as gait, cognition, quality of life (QoL), and mood (22, 23), as it increases - brain-derived neurotrophic factor (BDNF) levels that, inter alia, trigger dopamine's production, an important aspect of PD pathophysiology (22, 24, 25). In addition, neurophysiological evidence via functional magnetic resonance imaging (fMRI) has shown that dance is associated with enhanced functional connectivity between premotor cortex and basal ganglia, while electroencephalogram (EEG) studies have demonstrated that Tango might alter muscle synergy during balance and walking testing (26). It has been found that dance provides environmental enrichment that positively affects social and emotional states by stimulating diverse sensory functions during dancing, such as audition, vision, proprioception and tactile perception, balance, and vestibular control that might affect several aspects of motor function, mood, and cognitive impairment of PD patients (25). Although the neuroprotective effects of dance in PD have not been adequately examined, it has been proposed that BDNF upregulation and other molecular pathways may underlie the dance-mediated enhancement of neuronal activation in disrupted sensory-motor areas in PD, thereby resulting in the improvement of motor symptoms (25).
Resistance training (RT) is a renowned part of disease-prevention and disease-therapy protocols (27). It averts muscle loss, as muscle can increase its size through hypertrophy at any age, and improves muscular strength and gait components (2, 28, 29). Muscular weakness is a resultant of PD, as inhibition activation of motor neurons leads to muscle mass losses (7). Gait disturbances, poor balance, falls, and bradykinesia also seem to be associated with lack of strength, muscular imbalances, and differences between left and right sides (2, 30).
Indeed, a review with 401 participants examining the effects of progressive RT on physical function and balance in people with PD demonstrated that after 10 weeks of such training (2–3 times per week at moderate intensity) significantly improved strength, balance, and motor symptoms (28). Other studies found that RT should be combined with different forms of training in order to improve parameters such as balance or gait (2, 29), while there was also evidence that RT improves lower limb strength but not gait and balance (31). It should be stressed that research on RT in relation to PD is rather limited with different characteristics and methodological heterogeneity such as study design, randomization, and/or measurements (2).
Previous systematic reviews have individually examined rhythmic cueing, dance, or RT in relation to PD symptomatology. However, it is not yet entirely clear with which of these three methods would provide the most benefits for different clinical aspects of PD. Therefore, the aim of the present systematic review and meta-analysis was to synthesize evidence associated with the functional and clinical effectiveness of rhythmic cueing, dance, or RT on motor and non-motor parameters in patients with PD. It is anticipated that the findings would form the basis for a new protocol synthesis aiming at improving PD symptoms, through the development of more holistic exercise interventions.
Methodology
The present work was conducted according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines. It was registered with the International Prospective Register of systematic reviews (PROSPERO) (registration number: CRD42020212380).
Eligibility criteria
We considered the studies of any methodological design, which included experimental groups attended any form of rhythmic cueing intervention, any type of dance, or any form of RT, in PD patients. There were no restrictions regarding the duration of interventions. Key outcome domains were gait velocity/speed, stride length, stride time, strength of lower limbs, motor symptoms, functional parameters, QoL, cognition, state of mood, and sleep disorders. Eligible control situation considered either an appropriate control group (non-active or usual care for PD) or baseline measurements that were comparable with post-intervention measurements. Animal studies, case reports, reviews, editorials, conferences, and magazine papers were excluded.
Eligibility criteria for participants were Hoehn & Yahr (H&Y) PD rating scale I–IV (32). We applied no restrictions on disease duration, age, gender, and type of drug therapy, except for stable antiparkinsonian medication. Patients with other neurological problems or deep brain stimulation, cancer, cardiovascular disease, poor visual or auditory capability, and musculoskeletal problems were excluded.
Search and selection strategy
PubMed, Medline, Cochrane Library (trials), and SPORTDiscus were searched from the date of their inception until 1 June 2021. The key words (algorithm) used can be found in the supplement (33). The article selection was undertaken by two researchers (CK and MB). Any discrepancies have been resolved through discussion by a third researcher acting as referee (PCD). In the first step of the selection process, retrieved articles that were obviously irrelevant to our research question were excluded based on screening of titles and abstracts. Considering the aim of the current systematic review, we then checked the full texts of the remaining publications in order to select the eligible ones. Both steps were based on our inclusion and exclusion criteria.
Data extraction
CK and MB extracted the data from the eligible studies. One referee (PCD) ensured that all the necessary data are listed in tables. These included: (1) First author name and date of publication for identification, (2) Methodological design of each study, (3) Population characteristics sample size, groups, age, gender (if available), and H&Y PD rating scale (32), (4) Intervention (type, duration, and frequency), and (5) Eligible outcomes. Outcomes were continuously presented in mean and standard deviation (SD) of unified PD rating scale part III (UPDRS-III), Timed up and go test (TUG), ten meters walk test (TMWT), gait (velocity/speed), stride length, stride duration, PD questionnaire (PDQ39) score, strength of lower limbs, Montreal cognitive assessment (MoCa), sleep disorders (PSQI), and Brunel mood state (BRUMS). Fill the above outcomes were considered as “critical and meaningful,” according to 2022 Cochrane handbook for systematic reviews (34). Included outcomes encompassed the most frequent motor and non-motor parameters that affect every-day life of people with PD (35, 36). It is noteworthy that PDQ-39 is a valid questionnaire to assess quality of life in PD (37), UPDRS-III is an effective scale to assess motor symptoms in PD (38), whereas TUG is a common test to measure functional mobility in PD (39, 40). Similarly, MoCa is a widely used test to detect even mild cognitive impairments in patients with PD (41, 42). The BRUMS (43) evaluates 6 mood states (tension, depression, anger, vigor, fatigue, and mental confusion) in different populations, including PD patients and elderly people (44–47). The extracted data used for the meta-analyses can be found in the supplement in an open depository (33).
Risk of bias
The evaluation of the methodological quality of the eligible studies was independently completed by two researchers (CK and MB). Any conflicts arose between the two researchers, assessment and evaluation, were resolved by the referee researcher (PCD) via discussion. The ROBINS-I tool was used for non-randomized controlled trials (48), and the updated Risk of Bias 2 (ROB2) tool of Cochrane Library used for randomized controlled trials (RCT) (49).
Data synthesis and prospective meta-analysis
For seven eligible studies (50–56), a narrative data synthesis was conducted due to unsuitable data for a meta-analysis, as means and standard deviations (SD) were not included, and we were not able to retrieve the data from the corresponding authors. In addition, two studies (52, 53) were included in the narrative data synthesis due to non-parametric data reported. It has been advised that non-parametric and parametric data should not be mixed in a meta-analysis (34). Finally, a further study (57) provided data for sleep disorders, but this entry appeared only once in the outcomes, and, as such, no meta-analysis could be conducted (34).
For the eligible publications with data suitable for a meta-analysis, a continuous random effect model was employed, with means and SD, to assess motor and non-motor symptoms between experimental and control groups or baseline and post measurements. For the motor and non-motor events, a dichotomous inverse variance random effect model meta-analysis (i.e., odds ratio) was used to assess the effects (acute or chronic) of rhythmic cueing, dance, and/or RT interventions, in patients with PD, against the incidence of an adverse effect or positive effect in a group of patients not exposed to the aforementioned interventions. For all meta-analyses, the RevMan 5.4.13 software was used (58). Outcomes in four eligible studies (53, 59–61) were reported in figures, and therefore, the WebPlotDigitizer (62) software was used to extract data for the meta-analysis. For the eligible studies (59, 63–65) with reported outcomes as means and standard errors, conversions into standard deviations were achieved using the following equation: Standard deviation = standard error (34). The 95% confidence interval and heterogeneity between the eligible studies were evaluated using the I2 statistic. A statistically significant result for heterogeneity was considered when p < 0.10, while interpretation of I2 index was based on the Cochrane Library Handbook (34). Finally, the standardized mean difference (SMD) was used in cases where meta-analysis included studies that assessed the same outcome but used different measurement scales. Publication bias was assessed using funnel plots, but only for those meta-analyses that include >10 studies/entries (34).
In the comparisons of group of different dance styles (64) or rhythmic cueing (66, 67), pre measurements data were considered as a control situation, and post measurements data were considered as an experimental situation. For the eligible studies (59, 60, 63, 68–72) that compared interventions of dance or RT with other activities, only dance or resistance group was considered. Control groups receiving usual care treatment were considered as appropriate, unless physical activity was part of their usual care treatment. In the absence of appropriate control group (active or healthy) (73–83) or control group (84–86), comparisons focused on pre and post measurements of experimental groups. In one study (78) that comparisons focused on less affected and most affected leg, the latter was considered. In the context of gait measurements, self-selected speed or preferred rhythm (79, 87) was chosen since these two parameters are closer to normality.
Finally, we conducted subgroup analyses to compare each one of the outcomes among rhythmic cueing, dance, and RT. In particular, gait velocity, stride length, functional mobility-TUG, Qol-PDQ-39, and motor symptoms UPDRS-III have been analyzed.
Confidence in cumulative evidence
Meta-analyses quality of evidence was judged via the Grading of Recommendations Assessment, Development and Evaluation (GRADE) analysis (34, 88). Following previous guidelines (34, 88), we considered as an optimal information size more than 110 participants for each meta-analysis. This was based on a power analysis of a conventional sample size using three single trials (59, 66, 89).
Results
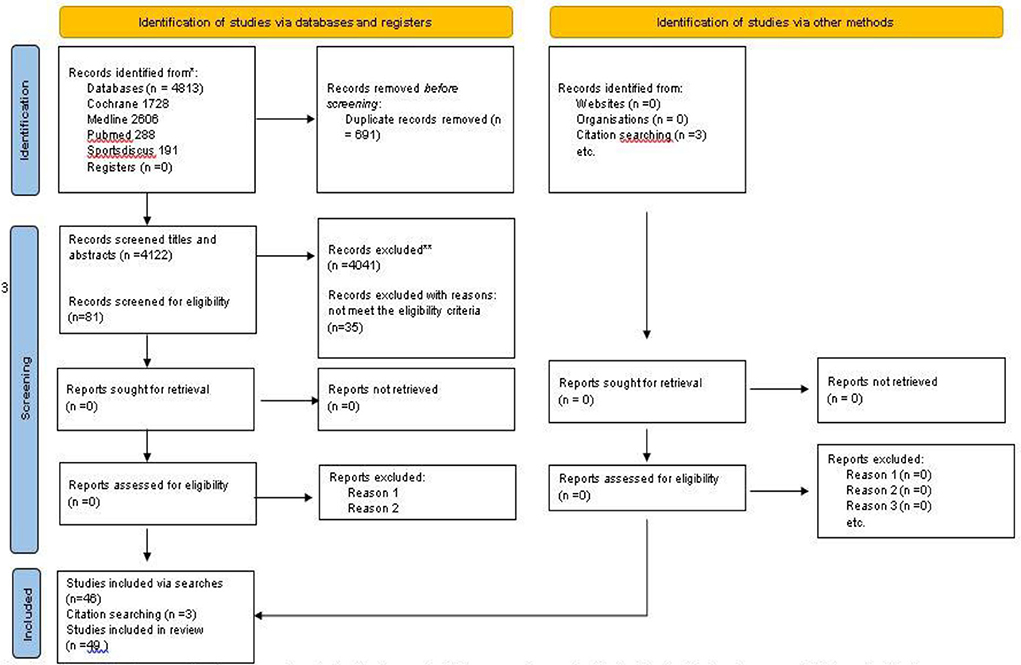
Prisma flow diagram shows information regarding article selection and characteristics of included studies (Figure 1).
We included publications from 1996 to 2021 which involved 3,767 participants (933 for rhythmic cueing, 1,470 for dance, and 1,364 for RT). Eight RCTs, two CTs, and one cohort study examined the effect of rhythmic cueing (rhythmical sounds, metronome, rhythmic styles) on PD. 10 RCTs and 12 CTs examined the effect of western theatrical (ballet, contemporary, jazz) social (Waltz, Foxtrot, Tango, Salsa, Samba, Forro), and Folklore (Irish, Sardinian, and Turo) dance protocols on PD. Twelve RCTs and four CTs studies examined the effect of RT protocols on PD. Interventions ranged from one session for a period of 24 months. The characteristics of the eligible studies are available in the supplement (Supplementary Table S1, pages 5–34) in an open depository (33).
Search and selection outcomes
Of the 4,813 retrieved publications, 691 were duplicates and 4,039 were excluded. Of the remaining 134 publications, 53 were reviews and conference papers and 35 did not fulfill the inclusion criteria. Finally, 46 studies were classified eligible, while three additional eligible studies were found in their reference lists. The total number of eligible studies included in the systematic review was 49.
Risk of bias assessments
Regarding the eligible RCTs, one study displayed high risk of bias (90), 12 were found with some concerns (57, 63, 65, 67–69, 73, 74, 91–94), and 18 studies displayed low risk of bias (53, 55, 60, 61, 64, 66, 70, 72, 75, 79, 81, 82, 89, 95–98), in randomization process. With respect to intervention assignment, two studies showed high risk of bias (64, 90), five studies exhibited some concerns (72, 75, 92, 95, 97), while the remaining studies disclosed low risk of bias (53, 55, 57, 60, 61, 63, 66–70, 73, 74, 79, 81, 82, 89, 91, 93, 94, 96, 98). In relation to intervention adherence, six studies displayed high risk of bias [55, 64, 72, 74, 90, 91[, eight exhibited some concerns (63, 65, 70, 81, 92, 95, 97), and 17 low risk of bias (53, 57, 60, 61, 66–69, 73, 75, 79, 82, 89, 93, 94, 96, 98). Considering missing data, three studies displayed some concerns (53, 57, 72), while the remaining studies showed low risk of bias (55, 60, 61, 63–70, 73–75, 79, 81, 82, 89–98). In relation to bias outcome, five studies exhibited some concerns (72–74, 81, 96), and the remaining studies presented low risk of bias (55, 60, 61, 63–70, 72, 75, 79, 82, 89–95, 97, 98). In bias reported outcomes, two studies presented high risk of bias (60, 90) one study displayed some concerns (81) and 27 studies revealed low risk of bias (53, 55, 57, 61, 63–70, 72–75, 79, 82, 89, 91–98).
Regarding the eligible CTs, two studies displayed moderate risk of bias (51, 80) and the remaining studies low risk (31, 50, 52, 54, 56, 59, 76–78, 83–87, 99–101). For bias selection, one study displayed serious risk of bias (99), nine studies showed moderate risk of bias (50, 51, 54, 59, 76, 77, 83, 87, 101), and nine studies showed low risk of bias (31, 52, 56, 78, 80, 83, 84, 86, 100). Regarding bias classification, seven studies showed moderate risk (23, 50, 76, 78, 83, 85, 99) and 12 studies low risk in bias (31, 51, 52, 54, 56, 59, 77, 80, 84, 86, 87, 100). For association to bias deviation of intervention, all studies (23, 31, 50–52, 54, 56, 59, 76–78, 80, 83–87, 99, 100) displayed low risk. Three studies displayed moderate risk (80, 85, 100), and 16 studies low risk in bias missing data (23, 31, 50–52, 54, 56, 59, 76–78, 83, 84, 86, 87, 99). For bias outcome, one study displayed some concerns (76), 14 studies moderate (23, 31, 50, 51, 54, 56, 76–78, 80, 83, 85, 87, 99), and five studies (51, 52, 84, 86, 100) displayed low risk. In bias reported results, three studies showed moderate risk (50, 56, 85) and 16 (23, 31, 51, 52, 54, 59, 76–78, 80, 83, 84, 86, 87, 99, 100) displayed low risk of bias. Risk of bias outcomes can be found in Supplementary Tables S2, S3 and Supplementary Figures S1, S2 (33).
Narrative data synthesis
In relation to the effects of rhythmic cueing on PD, one study examined the acute effects of rhythmic auditory stimulation (RAS) on gait velocity, indicating that RAS can facilitate locomotion (50). Similarly, another study (54) reported that rhythmic auditory cues significantly increased gait parameters, such as walking velocity and stride length, after 8 weeks of training. However, the use of metronomes did not improve mobility or physical functioning or other aspects of QoL (55).
In relation to the effects of dance, one study revealed that Irish dance may improve QoL (52), but another set of data (53) revealed that Irish dance does not improve QoL. A 12-month classical ballet did not affect gait variability (51), but an 8-month dance for PD did improve functional mobility and QoL in patients with PD (56). With respect to RT, a 12-week progressive RT improved sleep quality in this population (57).
However, the narrative review included a small number of studies, and therefore, it is difficult to evaluate the relevance of the findings.
Meta-analysis outcomes
In the supplement (S) of the following can be found: (a) forest plots of rhythmic cueing (Supplementary Figures S3A–C, S6A,B), (b) forest plots of dance (Supplementary Figures S4A–C, S7A–C), (c) funnel plots of dance 4Ba and 4Ca, and d) RT (Supplementary Figures S5A–D, S8A–D). The data used for the meta-analyses can be found in an open depository (33).
Rhythmic cueing
Meta-analysis results revealed significant effects of rhythmic cueing on gait velocity [(SMD = 0.54, CI = 0.21–0.88, Z = 3.20, I2 = 46%, p = 0.01, (Supplementary Figure S3A)] and stride length [MD = 0.09, CI = 0.03–0.15, Z = 3.08, I2 = 37%, p = 0.01, (Supplementary Figure S3B)], whereas no significant effects have been observed on stride time [SMD = 0.21, CI = −0.57 to 0.14, Z = 1.17, I2 = 0%, p = 0.20, (Supplementary Figure S6A)] in PD patients. Furthermore, rhythmic cues significantly improved motor symptoms-UPDRS-III [MD = −3.94, CI = (−7.47) – (−0.41), Z = 2.19, I2 = 7%, p = 0.03, (Supplementary Figure S3C)]. No effects of rhythmic cueing have been observed on functional mobility-TUG [MD = 2.31, CI = −7.83, 3.21, Z = 0.82, I2 = 75%, p = 0.41, (Supplementary Figure S6B)].
Dance
Dance interventions for PD significantly improved stride length [MD = 0.07, CI = 0–0.15, Z = 1.97, I2 = 0%, p = 0.05, (Supplementary Figure S4A], functional mobility-TUG [MD = −1.26, CI = (−1.77) -(−0.75), Z = 4.82, I2 = 0% p = 0.01, (Supplementary Figure S4B)], and motor symptoms-UPDRS-III [MD = −5.38, CI = (−8.44) – (−2.32), Z = 3.44, I2 = 79%, p = .01, (Supplementary Figure S4C)]. On the contrary, no significant effects have been observed on gait velocity [SMD = 0.19, CI = −0.06, 0.44, Z = 1.52, I2 = 0%, p = .13 (Supplementary Figure S7A)], quality of life-PDQ39 [MD = −2.19, CI = −6.21, 1.84, Z = 1.07, I2 = 34%, p = 0.29 (Supplementary Figure S7B)], and cognition-MoCa [MD = 0.60, CI = −0.78, 1.97, Z = 0.85, I2 = 0%, p = 0.13 (Supplementary Figure S7C)].
Resistance training
Significant positive effects of RT in PD have been observed on functional mobility-TUG [MD = −1.75, CI = (−3.07)-(−0.44), Z = 2.61, I2 = 81%, p = 0.01, (Supplementary Figure S5A)], quality of life-PDQ-39 [SMD = 0.38, CI = (−0.67)–(−0.09), Z = 2.58, I2 = 31%, p = 0.01, (Supplementary Figure S5B)], leg press [SMD = 3.51, CI = 1.50–5.52, Z = 3.42, I2 = 91%, p = 0.01, (Supplementary Figure S5C)], and knee flexion [SMD = 1.00, CI = 0.18–1.82, Z = 2.40, I2 = 65%, p = 0.02, (Supplementary Figure S5D)]. No significant effects have been found on gait velocity/speed [SMD = 0.32, CI = −0.13, 0.77, Z = 1.37, I2 = 50%, p = 0.17, (Supplementary Figure S8A)], stride length [MD = 0.05, CI = −0.05, 0.16, Z = 1.96, I2 = 0%, p = 0.34, (Supplementary Figure S8B)], in motor symptoms-UPDRS-III [MD = −2.74 CI = −5.55, 0.07, Z = 1.91, I2 = 1%, p = 0.06, (Supplementary Figure S3B)], and knee extension [SMD = 0.88, CI = −0.54, 2.30, Z = 1.22, I2 = 91%, p = 0.22, (Supplementary Figure S8D)].
Subgroup analyses of the outcomes between rhythmic cueing, dance, and resistance training
Subgroup analyses have shown non-significant differences between groups (Rhythmic cueing, Dance, RT) in gait velocity [SMD = 0.37, CI = 0.19, 0.56, I2 = 26.2%, p = 0.26, Figure 2A], while we found a significant overall effect [Z = 3.94, p < 0.0001]. Non-significant differences between groups have been observed (Rhythmic cueing, Dance, RT) in stride length [SMD = 0.09, CI = 0.05, 0.13, I2 = 0 %, p = 0.80, Figure 2B], while we observed a significant overall effect [Z = 4.45, p < 0.00001]. Similarly, non-significant differences have been found between groups (Rhythmic cueing, Dance, RT) in functional mobility-TUG [MD = −1.36, CI = (−2.02, −0.69), I2 = 0%, p = 0.74, Figure 2C], but a significant overall effect [Z = 3.97, p < 0.0001]. Non-significant differences between groups have been revealed in motor symptoms-UPDRS-III [MD = −4.62, CI = (−6.96, −2.28), I2 = 0%, p = 0.46], while we detected a significant overall effect [Z = 3.87, p < 0.0001, Figure 2D]. Non-significant subgroup (Dance, RT) differences have further been observed for quality of life-PDQ-39 [MD = −36, CI = (−6.02, −0,89), I2 = 0%, Figure 2E], coupled with a significant overall effect [Z = 2.64, p < 0.008].

Figure 2. (A) Subgroup analysis of rhythmic cueing, dance and RT of Gait Velocity. (B) Subgroup analysis of rhythmic cueing, dance, and RT of stride length. (C) Subgroup analysis of rhythmic cueing, dance, and RT of functional mobility - TUG. (D) Subgroup analysis of rhythmic cueing, dance, and RT of motor symptoms - UPDRS-III. (E) Subgroup analysis of dance and RT of QoL-PDQ-39.
Confidence in cumulative evidence outcomes
GRADE analysis outcomes can be found in the supplement (Supplementary Table S4) in an open depository (33). The meta-analyses of the effects of rhythmic cueing on gait velocity (#1) and stride length (#2) displayed moderate quality, while on stride time (#3), the quality was very low. The meta analysis of the effects of rhythmic cueing on functional mobility TUG (#4) and motor symptoms-UPDRS-III (#5) displayed low quality. The meta-analyses of the effect of dance on gait velocity (#6), stride length (#7), and functional mobility-TUG (#8) exhibited moderate quality. The meta-analyses for motor symptoms-UPDRS-III (#10) exhibited very low quality, whereas QoL-PDQ39 (#10) and cognition-MoCa (#11) exhibited moderate quality. The meta-analyses focused on the effects of RT displayed moderate quality for gait velocity (#12) and very low for stride length (#13); yet, low quality for functional mobility-TUG (#14) and moderate for motor symptoms-UPDRS-III (#15). The meta-analyses of the effects of RT displayed moderate quality for QoL-PDQ39 (#16), low for leg press (#17), very low for knee flexion (#18), and low quality for knee extension (#19).
Discussion
The aim of the present systematic review and meta-analysis was to synthesize evidence associated with the functional and clinical effectiveness of rhythmic cueing, dance, or RT on motor and non-motor parameters in patients with PD. We found that the aforementioned forms of exercise positively affect the examined outcomes, with rhythmic cueing to be associated with three outcomes (Gait, Stride, and UPDRS-III), dance with three (TUG, Stride, and UPDRS-III), and RT with two outcomes (TUG and PDQ-39). However, there is no sufficient evidence to recommend which of these interventions has the greatest effects.
Completeness of evidence
Rhythmic cueing
There was sufficient evidence to assess the effects of rhythmic cueing on gait velocity (nine included in meta-analysis/nine eligible) and stride length (nine included in meta-analysis/nine eligible). The sample was of optimal information size (>110), and GRADE analysis displayed moderate quality of evidence, indicating that rhythmic cueing could be treated as an effective intervention for improving gait characteristics (12, 17). Similarly, there was sufficient evidence to assess the effects of rhythmic cueing on motor symptoms-UPDRS-III (four included in meta-analysis/nine eligible), but the sample size was relatively small (<110), and GRADE analysis displayed low quality of evidence.
Dance
There was sufficient evidence (>110 participants) assessing the effects of dance protocols on functional mobility-TUG (11 included in meta-analysis/19 eligible), motor symptoms-UPDRS-III (13 included in meta-analysis/19 eligible), and stride length (five included in meta-analysis/19 eligible) in patients with PD. Although GRADE analysis revealed moderate quality for functional mobility-TUG, very low for motor symptoms-UPDRS-III, and moderate quality for stride length, findings indicate the efficacy of dance for improving mobility in this population (22, 102).
Resistance training (RT)
There was sufficient evidence assessing the effects of RT on QoL-PDQ39 (eight included in meta-analysis/16 included studies) with a sample size of >110 and functional mobility-TUG (eight included in meta-analysis/16 eligible) with a sample size of >110. Although GRADE analysis displayed moderate for QoL-PDQ39 and low quality for functional mobility-TUG, it could be argued that RT seems to regulate the majority of parameters associated with daily life. Also, there was sufficient evidence for leg press (four included in meta-analysis/16 eligible) with a sample size of >110, and to a lesser extent for knee flexion (three included in meta-analysis/16 eligible) with a sample size of <110. The aforementioned findings suggest that RT may activate cellular adaptive mechanisms thus, improving muscle strength (2, 103).
Subgroup analysis of the outcomes for rhythmic cueing, dance, and resistance training
Gait velocity, stride length, functional mobility-TUG, motor symptoms UPDRS-III, and Qol-PDQ-3 outcomes were analyzed. Stride time outcome has been detected in rhythm group only, and therefore, was excluded from the subgroup analysis. Also, cognition-MoCa was excluded from the subgroup analysis as it was only detected in the dance group. Similarly, knee flexion, knee extension, and leg press outcomes were detected in RT group only, and they were not included in the subgroup analyses.
Comparative perspective and applicability of evidence
Subgroup analyses have shown that all three forms of exercise are effective in patients with PD, supporting our hypothesis referring to a holistic approach. This stems from the fact that only outcome common to all three forms of exercise were incorporated in these analyses (Figures 2A–E).
Furthermore, meta-analyses have shown that rhythm cueing improves gait parameters, such as gait velocity, stride length, and motor symptoms, whereas dance seems to improve stride length, motor symptoms, and functional mobility. RT helps to improve QoL, functional mobility and, at the same time, enhances muscular strength in lower limbs. These findings support the notion that a protocol combining rhythmic cues, dance, and RT would probably provide a more holistic approach for improving PD manifestation.
We may theorize that the non-significant effects of dance on QoL could be attributed to the fact that dance is a complicated activity (104), especially for people who experience cognitive impairment in attention, visuospatial skills, and memory. For instance, Western theatrical dance or social dances are complicated activities containing movement combinations, whereas each class may include sections such as rhythm part, improvization, mime, and choreographies. Given that PD symptoms vary from person to person with some patients experiencing cognitive decline, the perception and understanding of movements in a dance class may be stressful for some patients. Relatively, on the one hand, recent systematic reviews examining the impact of dance on QoL revealed contradictory results suggesting that further research is needed (22, 104). On the other hand, a 2021 systematic review provided positive evidence on the effect of dance on quality of life, but the sample size was rather small and prevented generalization (105). An explanation for the aforementioned results may be the complexity of dance activity itself, which renders existing questionnaires not sensitive enough to fully capture elements of QoL (104).
Strengths and limitations
To the best of our knowledge, this is the first systematic review and meta-analysis on the effects of rhythmic cues, dance, or RT on PD patients. We searched appropriate databases to develop the key word algorithms, using standardized indexing terms, MeSH terms, and truncations, in order to retrieve publications relevant to our research question (34), while two independent investigators performed the searching, selection, data extraction, and risk of bias assessments.
The current narrative data synthesis included a relatively small number of studies (nine out of 50), which may impose a difficulty to merge their findings with those from the meta-analyses. We did not detect eligible articles for evaluating the state of mood - BRUMS. If more commonly used measures of mood were included in the search, then some effects of the interventions may have been found.
Other limitations include variations in methodological designs, while there was no material indicating whether protocols were designed according to participants' symptomatology. Also, eligible studies did not differentiate disease stages. None of the eligible studies examined fatigue factors, and we detected no information regarding the intensities of dance interventions in most studies. Duration, frequency, and intensity of physical activities are crucial, as fatigue may be an inhibitory factor in parkinsonian populations, similar to that in athletic populations (106, 107). Finally, the eligible dance studies included different dance genres with little information on the structure and/or content.
Conclusions
The present systematic review and meta-analysis indicates that rhythmic cues, dance, or RT positively affect the examined outcomes, with rhythmic cueing to be associated with three outcomes (Gait, Stride, and UPDRS-III), dance with three (TUG, Stride, and UPDRS-III), and RT with two outcomes (TUG and PDQ-39). Subgroup analyses confirmed the beneficial effects of these forms of exercise. Clinicians should entertain the idea of more holistic exercise protocols aiming at improving PD manifestations. Future studies should consider (a) implementation of exercise protocols based on PD patients' symptomatology and disease duration, and (b) standardization of test protocols.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
CK and YK did the conceptualization. CK and PD designed the algorithm. CK and MB did article selection and Risk of Bias and the evaluation of the methodological quality of the eligible studies was independently completed. PD acted as referee. CK did the statistical analysis, edited tables and pictures, and wrote the primary manuscript. AH contributed to the methodology and the article. MW, SP, and EA contributed to the article. CK, PD, and YK reviewed the final manuscript. All authors approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.875178/full#supplementary-material
References
1. Mhyre TR, Boyd JT, Hamill RW, Maguire-Zeiss KA. Parkinson's disease. Subcell Biochem. (2012) 65:389–455. doi: 10.1007/978-94-007-5416-4_16
2. Roeder L, Costello JT, Smith SS, Stewart IB, Kerr GK. Effects of resistance training on measures of muscular strength in people with parkinson's disease: a systematic review and meta-analysis. PLoS ONE. (2015) 10:e0132135. doi: 10.1371/journal.pone.0132135
3. Ou Z, Pan J, Tang S, Duan D, Yu D, Nong H, et al. Global trends in the incidence, prevalence, and years lived with disability of parkinson's disease in 204 countries/territories from 1990 to 2019. (2021) 9:6847. doi: 10.3389/fpubh.2021.776847
4. Dorsey ER, Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol. (2018) 75:9–10. doi: 10.1001/jamaneurol.2017.3299
5. Bloem BR, Okun MS, Klein C. Parkinson's disease. Lancet. (2021). doi: 10.1016/S0140-6736(21)00218-X
6. Dorsey ER, Sherer T, Okun MS, Bloem BR. The emerging evidence of the Parkinson pandemic. J Parkinson's Dis. (2018) 8:S3–S8. doi: 10.3233/JPD-181474
7. Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. (2008) 23:631–40. doi: 10.1002/mds.21922
8. Allen L. Are we facing a noncommunicable disease pandemic? J Epidemiol Glob Health. (2017) 7:5–9. doi: 10.1016/j.jegh.2016.11.001
9. Watson GS, Leverenz JB. Profile of cognitive impairment in Parkinson's disease. Brain Pathol. (2010) 20:640–5. doi: 10.1111/j.1750-3639.2010.00373.x
10. Alexander GE. Biology of Parkinson's disease: pathogenesis and pathophysiology of a multisystem neurodegenerative disorder. Dialogues Clin Neurosci. (2004) 6:259–80. doi: 10.31887/DCNS.2004.6.3/galexander
11. Wichmann T, Dostrovsky JO. Pathological basal ganglia activity in movement disorders. Neuroscience. (2011) 198:232–44. doi: 10.1016/j.neuroscience.2011.06.048
12. Forte R, Tocci N, De Vito G. The impact of exercise intervention with rhythmic auditory stimulation to improve gait and mobility in parkinson disease: an umbrella review. Brain Sci. (2021) 11:685. doi: 10.3390/brainsci11060685
13. Nombela C, Hughes LE, Owen AM, Grahn JA. Into the groove: can rhythm influence Parkinson's disease? Neurosci Biobehav Rev. (2013) 37:2564–70. doi: 10.1016/j.neubiorev.2013.08.003
15. Wu T, Hallett M, Chan P. Motor automaticity in Parkinson's disease. Neurobiol Dis. (2015) 82:226–34. doi: 10.1016/j.nbd.2015.06.014
16. HOssner EJO, Wenderoth NI. Attentional focus and motor learning: a review of 10 years of research. E-J Bewegung und Training. (2007) 1:1–64.
17. Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci Rep. (2018) 8:506. doi: 10.1038/s41598-017-16232-5
18. Malloch S, Trevarthen C. (Eds.). Communicative Musicality: Exploring the Basis of Human Companionship. Oxford: Oxford University Press (2009).
20. Hagel SAP. David: the dance of the muses. Choral Theory Anc Greek Poetics Gnomon. (2009) 81:294–7. doi: 10.17104/0017-1417_2009_4_294
21. Byl Simon. Andrée CATRYSSE. Les Grecs et la vieillesse d'Homère à Epicure. In: L'antiquité classique 2004. Available online at: https://www.persee.fr/doc/antiq_0770-2817_2004_num_73_1_2554_t1_0463_0000_2
22. Dos Santos Delabary M, Komeroski IG, Monteiro EP, Costa RR, Haas AN. Effects of dance practice on functional mobility, motor symptoms and quality of life in people with Parkinson's disease: a systematic review with meta-analysis. Aging Clin Exp Res. (2018) 30:727–35. doi: 10.1007/s40520-017-0836-2
23. Kalyani HHN, Sullivan K, Moyle G, Brauer S, Jeffrey ER, Roeder L, et al. Effects of dance on gait, cognition, and dual-tasking in parkinson's disease: a systematic review and meta-analysis. J Parkinson's Dis. (2019) 9:335–49. doi: 10.3233/JPD-181516
24. Barnish MS, Barran SM. A systematic review of active group-based dance, singing, music therapy and theatrical interventions for quality of life, functional communication, speech, motor function and cognitive status in people with Parkinson's disease. BMC Neurol. (2020) 20:371. doi: 10.1186/s12883-020-01938-3
25. Bearss KA, DeSouza JFX. Parkinson's disease motor symptom progression slowed with multisensory dance learning over 3-years: a preliminary longitudinal investigation. Brain Sci. (2021) 11:895. doi: 10.3390/brainsci11070895
26. Claus I, Muhle P, Czechowski J, Ahring S, Labeit B, Suntrup-Krueger S, et al. Expiratory muscle strength training for therapy of pharyngeal dysphagia in Parkinson's disease. Mov Disord. (2021). doi: 10.1002/mds.28552
27. Metsios GS, Brodin N, Vlieland T, Van den Ende CHM, Stavropoulos-Kalinoglou A, Fatouros I, et al. Position statement on exercise dosage in rheumatic and musculoskeletal diseases: the fole of the IMPACT-RMD toolkit. Mediterranean J Rheumatol. (2021) 32:378–85. doi: 10.31138/mjr.32.4.378
28. Chung CL, Thilarajah S, Tan D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson's disease: a systematic review and meta-analysis. Clin Rehabil. (2016) 30:11–23. doi: 10.1177/0269215515570381
29. Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson's disease: a systematic review. J Physio. (2013) 59:7–13. doi: 10.1016/S1836-9553(13)70141-3
30. Robichaud JA, Pfann KD, Comella CL, Brandabur M, Corcos DM. Greater impairment of extension movements as compared to flexion movements in Parkinson's disease. Exp Brain Res. (2004) 156:240–54. doi: 10.1007/s00221-003-1782-0
31. Tillman A, Muthalib M, Hendy AM, Johnson LG, Rantalainen T, Kidgell DJ, et al. Lower limb progressive resistance training improves leg strength but not gait speed or balance in Parkinson's disease: a systematic review and meta-analysis. Front Aging Neurosci. (2015) 7:40. doi: 10.3389/fnagi.2015.00040
32. Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology. (1998) 50:318–16. doi: 10.1212/WNL.50.2.318
33. Karpodini C, Dinas PC, Wyon M, Maria B, Haas AN, Angelopoulou E, et al. Supplementary Material Frontiers Karpodini et al. (2022). Available online at: https://doi.org/10.6084/m9.figshare.19601425
34. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Review of Interventions version 6.2 (updated February 2021). Available online at: www.training.cochrane.org/handbook
35. Opara J, Małecki A, Małecka E, Socha T. Motor assessment in Parkinson‘s disease. Ann Agric Environ Med. (2017) 24:411–5. doi: 10.5604/12321966.1232774
36. Tibar H, El Bayad K, Bouhouche A, Ait Ben Haddou EH, Benomar A, Yahyaoui M, et al. Non-motor symptoms of parkinson's disease and their impact on quality of life in a cohort of moroccan patients. Front Neurol. (2018) 9:170. doi: 10.3389/fneur.2018.00170
37. Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. (1998) 245 Suppl 1:S10–4. doi: 10.1007/PL00007730
38. Society IPaMD. MDS-Unified Parkinson's Disease Rating Scale (MDS-UPDRS) Available online at: https://www.movementdisorders.org/MDS/MDS-Rating-Scales/MDS-Unified-Parkinsons-Disease-Rating-Scale-MDS-UPDRS.htm
39. Morris S, Morris ME, Iansek R. Reliability of measurements obtained with the Timed “Up & Go” test in people with Parkinson disease. Phys Ther. (2001) 81:810–8. doi: 10.1093/ptj/81.2.810
40. Buisseret F, Catinus L, Grenard R, Jojczyk L, Fievez D, Barvaux V, et al. Timed up and go and six-minute walking tests with wearable inertial sensor: one step further for the prediction of the risk of fall in elderly nursing home people. Sensors. (2020) 20:3207. doi: 10.3390/s20113207
41. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kedziora-Kornatowska K. Is the montreal cognitive assessment (MoCA) test better suited than the mini-mental state examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-Ana Psych polska. (2016) 50:1039–52. doi: 10.12740/PP/45368
42. Hoops S, Nazem S, Siderowf AD, Duda JE, Xie SX, Stern MB, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. (2009) 73:1738–45. doi: 10.1212/WNL.0b013e3181c34b47
43. Terry PC, Potgieter JR, Fogarty GJ. The stellenbosch mood scale: a dual-language measure of mood. Int J Sport Exe Psychol. (2003) 1:231–45. doi: 10.1080/1612197X.2003.9671716
44. Terry PC, Parsons-Smith RL, Terry VR. Mood responses associated with COVID-19 restrictions. Front Psychol. (2020) 11:9598. doi: 10.3389/fpsyg.2020.589598
45. D'Oliveira A, De Souza LC, Langiano E, Falese L, Diotaiuti P, Vilarino GT, et al. Home physical exercise protocol for older adults, applied remotely during the COVID-19 pandemic: protocol for randomized and controlled trial. Front Psychol. (2022) 13 :8495. doi: 10.3389/fpsyg.2022.828495
46. Sroykham W, Wongsawat Y. Effects of brain activity, morning salivary cortisol, and emotion regulation on cognitive impairment in elderly people. Medicine. (2019) 98:e16114. doi: 10.1097/MD.0000000000016114
47. Jéssica Moratelli KHA Leonessa Boing Melissa de Carvalho Souza Vieira Adriana Adriana Coutinho de Azevedo Guimarães Adapted functional training versus Mat Pilates in motor and non-motor symptoms of individuals with Parkinson's disease: study protocol for a randomized controlled clinical trial Functional training versus Mat Pilates in Parkinson's disease. Res Square. (2021) 27:6291. doi: 10.21203/rs.3.rs-62891/v1
48. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res Ed). (2016) 355:i4919. doi: 10.1136/bmj.i4919
49. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed). (2019) 366:l4898. doi: 10.1136/bmj.l4898
50. McIntosh GC, Brown SH, Rice RR, Thaut MH. Rhythmic auditory-motor facilitation of gait patterns in patients with Parkinson's disease. J Neurol Neurosurg Psych. (1997) 62:22–6. doi: 10.1136/jnnp.62.1.22
51. McGill A, Houston S, Lee RYW. Effects of a ballet-based dance intervention on gait variability and balance confidence of people with Parkinson's. Arts Health. (2018) 5:1–14. doi: 10.1080/17533015.2018.1443947
52. Shanahan J, Morris ME, Bhriain ON, Volpe D, Richardson M, Clifford AM. Is Irish set dancing feasible for people with Parkinson's disease in Ireland? Complement Ther Clin Pract. (2015) 21:47–51. doi: 10.1016/j.ctcp.2014.12.002
53. Shanahan J, Morris ME, Bhriain ON, Volpe D, Lynch T, Clifford AM. Dancing for Parkinson disease: a randomized trial of irish set dancing compared with usual care. Arc Phy Med Rehabil. (2017) 98:1744–51. doi: 10.1016/j.apmr.2017.02.017
54. Ford MP, Malone LA, Nyikos I, Yelisetty R, Bickel CS. Gait training with progressive external auditory cueing in persons with Parkinson's disease. Arch Phys Med Rehabil. (2010) 91:1255–61. doi: 10.1016/j.apmr.2010.04.012
55. Elston J, Honan W, Powell R, Gormley J, Stein K. Do metronomes improve the quality of life in people with Parkinson's disease? A pragmatic, single-blind, randomized cross-over trial. Clin Rehabil. (2010) 24:523–32. doi: 10.1177/0269215509360646
56. Heiberger L, Maurer C, Amtage F, Mendez-Balbuena I, Schulte-Monting J, Hepp-Reymond MC, et al. Impact of a weekly dance class on the functional mobility and on the quality of life of individuals with Parkinson's disease. Front Aging Neurosci. (2011) 3:14. doi: 10.3389/fnagi.2011.00014
57. Silva-Batista C, de Brito LC, Corcos DM, Roschel H, de Mello MT, Piemonte MEP, et al. Resistance training improves sleep quality in subjects with moderate Parkinson's disease. J Strength Condition Res. (2017) 31:2270–7. doi: 10.1519/JSC.0000000000001685
58. Cochrane. RevMan5. (2021) Available online at: https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman/revman-5-download
59. Rawson KS, McNeely ME, Duncan RP, Pickett KA, Perlmutter JS, Earhart GM. Exercise and Parkinson disease: comparing tango, treadmill, and stretching. J Neurol Phys Therapy. (2019) 43:26–32. doi: 10.1097/NPT.0000000000000245
60. Cherup NP, Buskard ANL, Strand KL, Roberson KB, Michiels ER, Kuhn JE, et al. Power vs strength training to improve muscular strength, power, balance and functional movement in individuals diagnosed with Parkinson's disease. Exp Gerontol. (2019) 128:110740. doi: 10.1016/j.exger.2019.110740
61. Leal LCP, Abrahin O, Rodrigues RP, Silva MCR, Araújo APM, Sousa EC, et al. Low-volume resistance training improves the functional capacity of older individuals with Parkinson's disease. Geriat Gerontol Int. (2019) 19:635–40. doi: 10.1111/ggi.13682
62. Rohatgi A,. WebPlotDigitizer. (2021). Available online at: https://apps.automeris.io/wpd/ (accessed June 2022).
63. Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson's disease: a preliminary study. J Neurol Phys Therapy. (2007) 31:173–9. doi: 10.1097/NPT.0b013e31815ce78b
64. Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson's disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. (2009) 41:475–81. doi: 10.2340/16501977-0362
65. Vieira de. Moraes Filho A, Chaves SN, Martins WR, Tolentino GP, de Cássia Pereira Pinto Homem R, Landim de Farias G, et al. Progressive resistance training improves bradykinesia, motor symptoms and functional performance in patients with Parkinson's Disease. Clin Intervent Aging. (2020) 15:87–95. doi: 10.2147/CIA.S231359
66. Murgia M, Pili R, Corona F, Sors F, Agostini TA, Bernardis P, et al. The use of footstep sounds as rhythmic auditory stimulation for gait rehabilitation in Parkinson's disease: a randomized controlled trial. Front Neurol. (2018) 9:348. doi: 10.3389/fneur.2018.00348
67. Braun Janzen T, Haase M, Thaut MH. Rhythmic priming across effector systems: a randomized controlled trial with Parkinson's disease patients. Human Mov Sci. (2019) 64:355–65. doi: 10.1016/j.humov.2019.03.001
68. Carvalho A, Barbirato D, Araujo N, Martins JV, Cavalcanti JL, Santos TM, et al. Comparison of strength training, aerobic training, and additional physical therapy as supplementary treatments for Parkinson's disease: pilot study. Clin Intervent Aging. (2015) 10:183–91. doi: 10.2147/CIA.S68779
69. Silva-Batista C, Corcos DM, Roschel H, Kanegusuku H, Gobbi LT, Piemonte ME, et al. Resistance training with instability for patients with Parkinson's disease. Med Sci Sports Exerc. (2016) 48:1678–87. doi: 10.1249/MSS.0000000000000945
70. Strand KL, Cherup NP, Totillo MC, Castillo DC, Gabor NJ, Signorile JF. Periodized resistance training with and without functional training improves functional capacity, balance, and strength in Parkinson's disease. J Stren Condition Res. (2021). doi: 10.1519/JSC.0000000000004025
71. Volpe D, Signorini M, Marchetto A, Scutari A, Zambito Marsala S, Piggott C, et al. Irish set dance improves mobility, balance and quality of life in Parkinson's disease. Mov Disord. (2012) 27:S178. doi: 10.1002/mds.25051
72. Poier D, Rodrigues Recchia D, Ostermann T, Büssing A. A randomized controlled trial to investigate the impact of tango argentino versus tai chi on quality of life in patients with parkinson disease: a short report. Comp Med Res. (2019) 26:398–403. doi: 10.1159/000500070
73. Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair. (2011) 25:626–35. doi: 10.1177/1545968311401627
74. De Icco R, Tassorelli C, Berra E, Bolla M, Pacchetti C, Sandrini G. Acute and chronic effect of acoustic and visual cues on gait training in parkinson's disease: a randomized, controlled study. Parkinson's Dis. (2015) 2015:978590. doi: 10.1155/2015/978590
75. Thaut MH, Rice RR, Braun Janzen T, Hurt-Thaut CP, McIntosh GC. Rhythmic auditory stimulation for reduction of falls in Parkinson's disease: a randomized controlled study. Clin Rehabili. (2018) 33:34–43. doi: 10.1177/0269215518788615
76. Scandalis TA, Bosak A, Berliner JC, Helman LL, Wells MR. Resistance training and gait function in patients with Parkinson's disease. Am J Phys Med Rehabil. (2001) 80:38–43. doi: 10.1097/00002060-200101000-00011
77. Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. (2006) 21:1444–52. doi: 10.1002/mds.20997
78. Dibble LE, Hale TF, Marcus RL, Gerber JP, LaStayo PC. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: a preliminary study. Parkinson Rel Disord. (2009) 15:752–7. doi: 10.1016/j.parkreldis.2009.04.009
79. Santos L, Fernandez-Rio J, Winge K, Barragán-Pérez B, González-Gómez L, Rodríguez-Pérez V, et al. Effects of progressive resistance exercise in akinetic-rigid Parkinson's disease patients: a randomized controlled trial. Eu J Phy Rehabil Med. (2017) 53:651–63. doi: 10.23736/S1973-9087.17.04572-5
80. Demonceau M, Maquet D, Jidovtseff B, Donneau AF, Bury T, Croisier JL, et al. Effects of twelve weeks of aerobic or strength training in addition to standard care in Parkinson's disease: a controlled study. Eur J Phys Rehabil Med. (2017) 53:184–200. doi: 10.23736/S1973-9087.16.04272-6
81. Rios Romenets S, Anang J, Fereshtehnejad SM, Pelletier A, Postuma R. Tango for treatment of motor and non-motor manifestations in Parkinson's disease: a randomized control study. Complement Therap Med. (2015) 23:175–84. doi: 10.1016/j.ctim.2015.01.015
82. Solla P, Cugusi L, Bertoli M, Cereatti A, Della Croce U, Pani D, et al. Sardinian folk dance for individuals with parkinson's disease: a randomized controlled pilot trial. J Alternat Complemen Med. (2019) 25:305–6. doi: 10.1089/acm.2018.0413
83. Hausdorff JM, Lowenthal J, Herman T, Gruendlinger L, Peretz C, Giladi N. Rhythmic auditory stimulation modulates gait variability in Parkinson's disease. Eur J Neurosci. (2007) 26:2369–75. doi: 10.1111/j.1460-9568.2007.05810.x
84. Westheimer O, McRae C, Henchcliffe C, Fesharaki A, Glazman S, Ene H, et al. Dance for PD: a preliminary investigation of effects on motor function and quality of life among persons with Parkinson's disease (PD). J Neural Trans. (2015) 122:1263–70. doi: 10.1007/s00702-015-1380-x
85. Hackney ME, Earhart GM. Short duration, intensive tango dancing for Parkinson disease: an uncontrolled pilot study. Complemen Therap Med. (2009) 17:203–7. doi: 10.1016/j.ctim.2008.10.005
86. Harrison EC, Earhart GM, Leventhal D, Quinn L, Pietro M. A walking dance to improve gait speed for people with Parkinson disease: a pilot study. Neurodegener Dis Manag. (2020) 10:301–8. doi: 10.2217/nmt-2020-0028
87. Dos Santos Delabary M, Monteiro EP, Donida RG, Wolffenbuttel M, Peyré-Tartaruga LA, Haas AN. Can Samba and Forró Brazilian rhythmic dance be more effective than walking in improving functional mobility and spatiotemporal gait parameters in patients with Parkinson's disease? BMC Neurol. (2020) 20:305. doi: 10.1186/s12883-020-01878-y
88. Schünemann H, Brozek J, Guyatt G, Oxman Ae. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (2013). Available online at: https://gdt.gradepro.org/app/handbook/handbook.html#h.ged5uqebmir9
89. de Lima TA, Ferreira-Moraes R, Alves W, Alves TGG, Pimentel CP, Sousa EC, et al. Resistance training reduces depressive symptoms in elderly people with Parkinson disease: a controlled randomized study. Scand J Med Sci Sports. (2019) 29:1957–67. doi: 10.1111/sms.13528
90. Thaut MH, McIntosh GC, Rice RR, Miller RA, Rathbun J, Brault JM. Rhythmic auditory stimulation in gait training for Parkinson's disease patients. Mov Disord. (1996) 11:193–200. doi: 10.1002/mds.870110213
91. de Bruin N, Doan JB, Turnbull G, Suchowersky O, Bonfield S, Hu B, et al. Walking with music is a safe and viable tool for gait training in Parkinson's disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinson's Dis. (2010) 2010:483530. doi: 10.4061/2010/483530
92. Kunkel D, Fitton C, Roberts L, Pickering RM, Roberts HC, Wiles R, et al. A randomized controlled feasibility trial exploring partnered ballroom dancing for people with Parkinson's disease. Clin Rehabil. (2017) 31:1340–50. doi: 10.1177/0269215517694930
93. Lee HJ, Kim SY, Chae Y, Kim MY, Yin C, Jung WS, et al. Turo (Qi Dance) Program for Parkinson's disease patients: randomized, assessor blind, waiting-list control, partial crossover study. Explore. (2018) 14:216–23. doi: 10.1016/j.explore.2017.11.002
94. Hass CJ, Buckley TA, Pitsikoulis C, Barthelemy EJ. Progressive resistance training improves gait initiation in individuals with Parkinson's disease. Gait Posture. (2012) 35:669–73. doi: 10.1016/j.gaitpost.2011.12.022
95. Volpe D, Signorini M, Marchetto A, Lynch T, Morris ME. A comparison of Irish set dancing and exercises for people with Parkinson's disease: a phase II feasibility study. BMC Geriatr. (2013) 13:54. doi: 10.1186/1471-2318-13-54
96. Hashimoto H, Takabatake S, Miyaguchi H, Nakanishi H, Naitou Y. Effects of dance on motor functions, cognitive functions, and mental symptoms of Parkinson's disease: a quasi-randomized pilot trial. Complem Therap Med. (2015) 23:210–9. doi: 10.1016/j.ctim.2015.01.010
97. Morris ME, Taylor NF, Watts JJ, Evans A, Horne M, Kempster P, et al. A home program of strength training, movement strategy training and education did not prevent falls in people with Parkinson's disease: a randomised trial. J Physioth. (2017) 63:94–100. doi: 10.1016/j.jphys.2017.02.015
98. Ferreira RM, Alves WMGdC, de Lima TA, Alves TGG, Alves Filho PAM, Pimentel CP, et al. The effect of resistance training on the anxiety symptoms and quality of life in elderly people with Parkinson's disease: a randomized controlled trial. Arquivos de neuro-psiquiatria. (2018) 76:499–506. doi: 10.1590/0004-282x20180071
99. Kalyani HH, Sullivan KA, Moyle GM, Brauer S, Jeffrey ER, Kerr GK. Dance improves symptoms, functional mobility and fine manual dexterity in people with Parkinson disease: a quasi-experimental controlled efficacy study. Eu J Phy Rehabil Med. (2020) 08:6069. doi: 10.23736/S1973-9087.20.06069-4
100. Krishnamurthi N, Murphey C, Driver-Dunckley E. comprehensive Movement and Motion training program improves mobility in Parkinson's disease. Aging Clin Exp Res. (2020) 32:633–43. doi: 10.1007/s40520-019-01236-0
101. Kalyani HHN, Sullivan KA, Moyle G, Brauer S, Jeffrey ER, Kerr GK. Impacts of dance on cognition, psychological symptoms and quality of life in Parkinson's disease. NeuroRehabilitation. (2019) 45(2):273-83. doi: 10.3233/NRE-192788
102. Sharp K, Hewitt J. Dance as an intervention for people with Parkinson's disease: a systematic review and meta-analysis. Neurosci Biobehav Rev. (2014) 47:445–56. doi: 10.1016/j.neubiorev.2014.09.009
103. David FJ, Rafferty MR, Robichaud JA, Prodoehl J, Kohrt WM, Vaillancourt DE, et al. Progressive resistance exercise and Parkinson's disease: a review of potential mechanisms. Parkinson's Dis. (2012) 2012:124527. doi: 10.1155/2012/124527
104. Carapellotti AM, Stevenson R, Doumas M. The efficacy of dance for improving motor impairments, non-motor symptoms, and quality of life in Parkinson's disease: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0236820. doi: 10.1371/journal.pone.0236820
105. Wu VX, Chi Y, Lee JK, Goh HS, Chen DYM, Haugan G, et al. The effect of dance interventions on cognition, neuroplasticity, physical function, depression, and quality of life for older adults with mild cognitive impairment: a systematic review and meta-analysis. Int J Nurs Stud. (2021) 122:104025. doi: 10.1016/j.ijnurstu.2021.104025
106. Wyon MA, Koutedakis Y. Muscular fatigue: considerations for dance. J Dance Med Sci. (2013) 17:63–9. doi: 10.12678/1089-313X.17.2.63
Keywords: Parkinson's disease, rhythm, dance, strength, systematic review, meta-analysis
Citation: Karpodini CC, Dinas PC, Angelopoulou E, Wyon MA, Haas AN, Bougiesi M, Papageorgiou SG and Koutedakis Y (2022) Rhythmic cueing, dance, resistance training, and Parkinson's disease: A systematic review and meta-analysis. Front. Neurol. 13:875178. doi: 10.3389/fneur.2022.875178
Received: 23 February 2022; Accepted: 14 July 2022;
Published: 09 August 2022.
Edited by:
Hannes Devos, University of Kansas, United StatesReviewed by:
Gregory Youdan, Brown University, United StatesJudith Bek, University of Toronto, Canada
Copyright © 2022 Karpodini, Dinas, Angelopoulou, Wyon, Haas, Bougiesi, Papageorgiou and Koutedakis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Claire Chrysanthi Karpodini, Qy5DLkthcnBvZGluaTJAd2x2LmFjLnVr; Y2xhaXJlX2thcnBvZGluaUBvdXRsb29rLmNvbQ==
 Claire Chrysanthi Karpodini
Claire Chrysanthi Karpodini Petros C. Dinas
Petros C. Dinas Efthalia Angelopoulou
Efthalia Angelopoulou Matthew A. Wyon
Matthew A. Wyon Aline Nogueira Haas
Aline Nogueira Haas Maria Bougiesi
Maria Bougiesi Sokratis G. Papageorgiou
Sokratis G. Papageorgiou Yiannis Koutedakis
Yiannis Koutedakis