
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 22 July 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.869880
 Ji Jin1†
Ji Jin1† Geng Guo2*†
Geng Guo2*† Yeqing Ren2†
Yeqing Ren2† Biao Yang2
Biao Yang2 Yongqiang Wu2
Yongqiang Wu2 Shule Wang2
Shule Wang2 Yanqi Sun2
Yanqi Sun2 Xiaogang Wang2
Xiaogang Wang2 Yuxiao Wang1
Yuxiao Wang1 Jianzhong Zheng1
Jianzhong Zheng1Intracranial aneurysm is a severe cerebral disorder involving complicated risk factors and endovascular coiling is a common therapeutic selection for intracranial aneurysm. The recurrence is a clinical challenge in intracranial aneurysms after coil embolization. With this study, we provided a meta-analysis of the risk factors for the recurrence of intracranial aneurysm after coil embolization. Nine studies were included with a total of 1,270 studies that were retrieved from the database. The sample size of patients with intracranial aneurysms ranged from 241 to 3,530, and a total of 9,532 patients were included in the present meta-analysis. The intracranial aneurysms that occurred in middle cerebral artery (MCA) (OR = 1.09, 95% CI: 1.03–1.16, P = 0.0045) and posterior circulation (OR = 2.01, 95% CI: 1.55–2.60, P = 0.000) presented the significantly higher risk of recurrence after coil embolization. Meanwhile, intracranial aneurysms of size > 7 mm (OR = 5.38, 95%CI: 3.76–7.70, P = 0.000) had a significantly higher risk of recurrence after coil embolization. Moreover, ruptured aneurysm (OR = 2.86, 95% CI: 2.02–4.04, P = 0.000) and subarachnoid hemorrhage (SAH) (OR = 1.57, 95% CI: 1.20–2.06, P = 0.001) was positively correlated with the risk of recurrence after coil embolization. In conclusion, this meta-analysis identified the characteristics of intracranial aneurysms with MCA, posterior circulation, size > 7 mm, ruptured aneurysm, and SAH as the risk factors of recurrence after coil embolization for intracranial aneurysms.
The cerebral arterial aneurysm is a severe and prevalent disorder, which is a leading cause of sudden neurological disability secondary to rupture (1, 2). Intracranial aneurysms are among the most popular non-traumatic risk factors of subarachnoid hemorrhage (SAH) with an increasing incidence, resulting in a heavy economic and social burden globally (3, 4). Even though the patients accept therapy, many cases will ultimately die or suffer from a cognitive disability or severe neurological (5, 6). The treatment of intracranial aneurysms is associated with multiple unexpected risk factors (7, 8). Endovascular coiling has become a prevalent therapeutic selection for intracranial aneurysm patients in many hospitals (9, 10). Nevertheless, the recurrence of the intracranial aneurysm treated by endovascular coiling is still a crucial problem in the clinic (11). Numerous risk factors are involved in the events of recurrence and rebleeding (12, 13), which are poorly understood.
In this study, we were interested in the exploration of the correlation of risk factors, such as gender, smoking, posterior circulation, anterior cerebral artery (ACA), interior carotid artery (ICA), middle cerebral artery (MCA), aneurysms of size, aneurysms of the neck, ruptured aneurysm, and SAH, with the recurrence of intracranial aneurysms after coil embolization.
The inclusion criteria were as follows: the study type is a retrospective study; the language is limited to English.
Exclusion criteria: duplicate publication; research without full text, research without the needed information of this study, incomplete information, or inability to conduct data extraction; animal experiments; reviews and systematic reviews.
In this meta-analysis, we searched Pubmed, Embase, and Cochrane Library from the establishment of the database to December 2020. The search terms are mainly: “Intracranial Aneurysm” “Brain Aneurysm” “Anterior Communicating Artery Aneurysm” “Basilar Artery Aneurysm” “Cerebral Aneurysm” and “Coil embolization” “recurrence”. Keywords were combined with Boolean operators to increase search sensitivity and specificity.
The literature search, screening, and information extraction were all independently completed by two researchers. When there were doubts or disagreements, the decision was made after discussion or consultation with a third party. The data extraction included the author, year, study area, research type, number of cases, and the OR and 95%CI of age, smoking, posterior circulation, ACA (anterior cerebral artery), ICA (interior carotid artery), MCA (middle cerebral artery), Ruptured aneurysm, SHA (subarachnoid hemorrhage), aneurysm size > 7mm and aneurysm neck > 4mm for the prediction of recurrence after coil embolization for intracranial aneurysms. Aneurysm recurrence was defined as inflow into a previously completely occluded aneurysm or growth of an incompletely occluded aneurysm (aneurysm recanalization) (14).
Two authors (GENG GUO and JIANZHONG ZHENG) independently conducted literature quality evaluations using the NOS (Newcastle-Ottawa Scale) for the retrospective study. When the opinions are inconsistent, it is decided through discussion or consultation with a third person. The meta-analysis was performed based on the related items of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement (PRISMA statement).
Following eligibility verification, data about the risk factors, such as gender, smoking, posterior circulation, ACA, ICA, MCA, aneurysms of size, aneurysms of the neck, ruptured aneurysm, and SAH, were extracted from the manuscript text, patient demographic tables, and on-line tables and figures. STATA 15.1 was used to analyze the data. Odds ratio (OR; 95% Cl) was used to analyze the risk factors of no-reflow/slow-flow. Higgins index (I2) is used to evaluate heterogeneity. If the heterogeneity test is P ≥ 0.1 and I2 ≤ 50%, it indicates that there is homogeneity between studies, and the fixed effects model is used for combined analysis; if P < 0.1, I2 > 50%, it indicates that the study is heterogeneous, and we use sensitivity analysis to find the source of heterogeneity. If the heterogeneity is still large, we use the random-effects model or give up the combination of results and use descriptive analysis. A funnel plot was used to analyze publication bias.
In this study, a total of 1,270 studies were retrieved from the database. After eliminating duplicate studies, 638 were obtained. After browsing titles and abstracts, 385 studies were obtained. Finally, nine studies were meta-analyzed through full-text reading (Figure 1).
A total of nine retrospective studies were included in this meta-analysis. The sample size of patients with intracranial aneurysms ranged from 241 to 3,530, and a total of 9,532 patients were included in the present meta-analysis. Patients in four studies were from China, patients in two studies were from Korea, and the others were from Europe and America. The NOS score used for quality assessment is all above seven and meets the requirements. The baseline characteristics quality assessment of the included studies is shown in Table 1.
We first explored the correlation between gender (female) and recurrence after coil embolization for intracranial aneurysms. There are 4 studies, including 4,558 patients, that reported the association between gender (female) and recurrence after coil embolization for intracranial aneurysms. Since there is no significant heterogeneity (I2 = 0.0%, P = 0.793 > 0.1), a meta-analysis was conducted through a fixed-effects model. The pooled results show that there is no significant association between gender (female) and recurrence after coil embolization for intracranial aneurysms (OR = 0.96, 95% CI: 0.77–1.19, P = 0.707 > 0.05; Figure 2A). We also pooled the results through a random-effects model (I2 = 62.5%, P = 0.103 > 0.1), thus we found that there is no significant association between smoking and recurrence after coil embolization for intracranial aneurysms (OR = 1.61, 95% CI: 0.59–4.34, P = 0.351 > 0.05; enrolling 579 patients; Figure 2B).
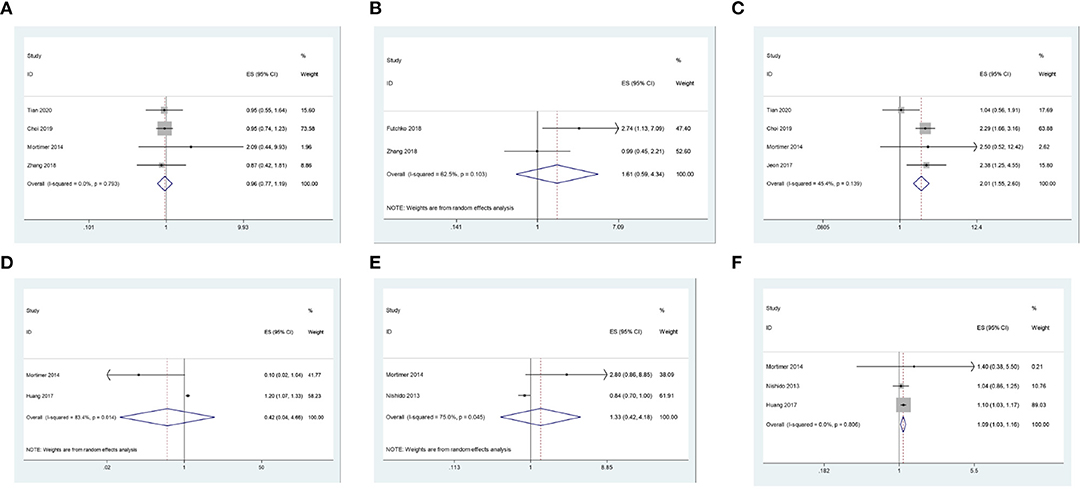
Figure 2. (A) The correlation between gender and recurrence after coil embolization for intracranial aneurysms. (B) The correlation between smoking and recurrence after coil embolization for intracranial aneurysms. (C) The correlation between posterior circulation and recurrence after coil embolization for intracranial aneurysms. (D) The correlation between ACA and recurrence after coil embolization for intracranial aneurysms. (E) The correlation between ICA and recurrence after coil embolization for intracranial aneurysms. (F) The correlation between MCA and recurrence after coil embolization for intracranial aneurysms.
We continue to explore the correlation between the location of the aneurysm and the recurrence of intracranial aneurysms after coil embolization. There are four studies, including 5,145 patients, that reported the association between posterior circulation and recurrence after coil embolization for intracranial aneurysms. Since there is no significant heterogeneity (I2 = 45.4%, P = 0.139 > 0.1), a meta-analysis was conducted through a fixed-effects model. The pooled results indicate that intracranial aneurysms that occur in posterior circulation have a significantly higher risk of recurrence after coil embolization (OR = 2.01, 95% CI: 1.55–2.60, P = 0.000 < 0.05; Figure 2C). There are two studies, including 899 patients, that reported the association between ACA and its recurrence after coil embolization for intracranial aneurysms. Since there is significant heterogeneity (I2 = 83.4%, P = 0.014 < 0.1), a meta-analysis was conducted through a random-effects model. The polled results indicate that there is no significant association between ACA and recurrence after coil embolization for intracranial aneurysms (OR = 0.42, 95% CI: 44–4.66, P = 0.483.05; Figure 2D). Additionally, pooled results also show that there is no significant association between ICA and recurrence after coil embolization for intracranial aneurysms (OR = 1.33, 95% CI: 0.42–4.18, P = 0.627 > 0.05; enrolling 2,056 patients) from a random-effects model (I2 = 75.0%, P = 0.045 < 0.1; Figure 2E). However, pooled results show that intracranial aneurysms that occur in MCA have a significantly higher risk of recurrence after coil embolization (OR = 1.09, 95% CI: 1.03–1.16, P = 0.004 < 0.05; enrolling 2,714 patients) from a fixed-effects model (I2 = 0.0%, P = 0.806 > 0.1; Figure 2F).
In addition, we explored the correlation between disease characteristics and recurrence after coil embolization. There are four studies, including 2,065 patients, that reported the association between aneurysms of size > 7 mm and their recurrence after coil embolization for intracranial aneurysms. Since there is no significant heterogeneity (I2 = 0.0%, P = 0.866 > 0.1), a meta-analysis was conducted through a fixed-effects model. The pooled results indicate that intracranial aneurysms of size > 7 mm have a significantly higher risk of recurrence after coil embolization. (OR = 5.38, 95% CI: 3.76–7.70, P = 0.000 < 0.05; Figure 3A). There are two studies, including 3,771 patients, that reported the association between aneurysms of neck > 4 mm and recurrence after coil embolization for intracranial aneurysms. Since there is significant heterogeneity (I2 = 72.2%, P = 0.058 < 0.1), a meta-analysis was conducted through a random-effects model. The pooled results indicate that there is no significant association between aneurysms of neck > 4 mm and recurrence after coil embolization for intracranial aneurysms (OR = 2.08, 95% CI: 0.50–8.70, P = 0.315 > 0.05; Figure 3B).
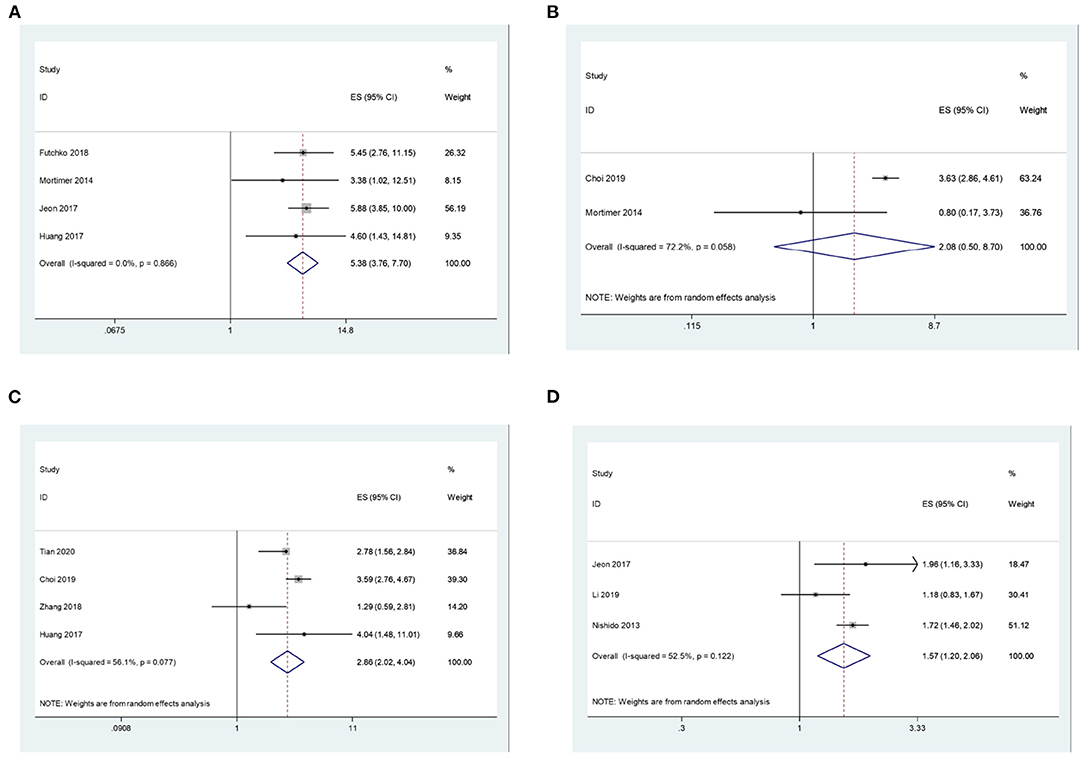
Figure 3. (A) The correlation between aneurysms of size > 7 mm and recurrence after coil embolization for intracranial aneurysms. (B) The correlation between aneurysms of neck > 4 mm and recurrence after coil embolization for intracranial aneurysms. (C) The correlation between ruptured aneurysm and recurrence after coil embolization for intracranial aneurysms. (D) The correlation between SAH and recurrence after coil embolization for intracranial aneurysms.
Lastly, pooled results show that ruptured aneurysm (OR = 2.86, 95% CI: 2.02–4.04, P = 0.000 < 0.05; enrolling 4,975 patients) and SAH (OR = 1.57, 95% CI: 1.20–2.06, P = 0.001 < 0.05; enrolling 4,020 patients) can all significantly increase the risk of recurrence after coil embolization with random effects models (I2 = 56.1%, P = 0.077 < 0.1; I2 = 52.5%, P = 0.122 > 0.1; Figures 3C,D).
The funnel plot of this study is shown in Supplementary Figure S1. The funnel plot is basically symmetrical, indicating that there is no obvious publication bias in this study.
Sensitivity analysis eliminates each included study one by one and performs a summary analysis on the remaining studies to assess whether a single included study has an excessive impact on the results of the entire meta-analysis. The results showed that none of the studies had an excessive impact on the results of the meta-analysis, indicating that the results of the remaining studies are stable and reliable.
Intracranial aneurysm is a prevalent cerebral disorder with severe injury and coil embolization has been identified as a prevalent therapeutic method in the intracranial aneurysm. In the present study, we identified the risk factors of recurrence of intracranial aneurysm after coil embolization using a meta-analysis based on nine studies.
In our meta-analysis, we found that there is no significant association between gender and smoking with recurrence after coil embolization for intracranial aneurysms. However, it has been reported that smoking is harmful to the clinical outcomes of intracranial aneurysm patients using coil embolization, and aneurysms are more prevalent in women than men (21). The influence of gender and smoking on the recurrence after coil embolization for intracranial aneurysms may be complicated and are affected by other factors. It has been identified that ACA, ICA, and MCA are close factors of intracranial aneurysms (22–27). Previous investigation has shown that posterior circulation is a risk factor for coil embolization of unruptured aneurysms (28). The behavior of posterior circulation has been proved to associate with PcoA aneurysms recanalization (29). Our analysis showed that MCA bifurcation and posterior circulation aneurysms presented a higher recurrence risk. It has been reported that endovascular treatment of wide-neck MCA and basilar apex aneurysms resulted in a core lab adjudicated Raymond Roy (14) occlusion rate of 30.6% and self-reported results at follow-up favor better angiographic outcomes (30). It suggests the necessity and significance of novel endovascular devices specifically designed to treat complex intracranial aneurysms.
Our data also revealed that ruptured aneurysm and SAH were positively correlated with the risk of recurrence after coil embolization. Recently, a study of the comparison between outcomes of endovascular and surgical treatments of ruptured anterior communicating artery aneurysms show that aneurysms with the first presentation of SAH secondary to a ruptured anterior communicating artery aneurysm treated by endovascular coiling have an increased risk of recurrence vs. those treated with clipping (31). These findings may provide some reference for treatment decisions of a multi-disciplinary team. It has been identified that the size of intracranial aneurysms significantly affects the selection and treatment effectiveness of intracranial aneurysms (32–35). Consistently, our analysis showed that intracranial aneurysms of size > 7 mm have a significantly higher risk of recurrence after coil embolization.
There are still some limitations in the current study. In this work, we provided a meta-analysis of the risk factors for recurrence of intracranial aneurysms after coil embolization. Despite the crucial risk of rebleeding or symptomatic recurrences needing retreatment in the model, we did not describe them in our analysis because there was no such detailed data in the literature. Meanwhile, the aspect of time and the aneurysms recurrence were not considered in the current study because there was no such detailed data in the literature, which is crucial for treatment decisions. In this study, we only included retrospective studies. Other types of studies should be considered in future investigations.
This meta-analysis identified the characteristics of intracranial aneurysms with MCA, posterior circulation, size > 7 mm, ruptured aneurysm, and SAH as the risk factors of recurrence after coil embolization for intracranial aneurysms. Our finding enriches the understanding of the recurrence of intracranial aneurysms after coil embolization in patients, providing the theoretical reference for the clinical application of coil embolization for intracranial aneurysms. Meanwhile, it is crucial to design novel and specific endovascular devices for the treatment of these complex intracranial aneurysms and attenuate their recurrence.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GG, JJ, YR, and JZ designed the study and wrote the manuscript. GG, YR, BY, YWu, SW, YS, XW, and YWa performed the analysis. All authors contributed to the article and approved the submitted version.
This study was supported by the Scientific Research Foundation of Shanxi Intelligence Institute of Big Data Technology and Innovation (SIBD-2020-YL0052).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.869880/full#supplementary-material
Supplementary Figure S1. Funnel plot for evaluating the publication bias of this meta-analysis.
1. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13:393–404. doi: 10.1016/S1474-4422(14)70015-8
2. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. (2016) 12:699–713. doi: 10.1038/nrneurol.2016.150
3. Matsushige T, Sakamoto S, Ishii D, Shinagawa K, Shimonaga K, Hosogai M, et al. Safety and efficacy of a new outreach distal access catheter, TACTICS, for coil embolization of unruptured intracranial aneurysms. Interv Neuroradiol. (2018) 24:482–8. doi: 10.1177/1591019918774888
4. Ihn YK, Shin SH, Baik SK, Choi IS. Complications of endovascular treatment for intracranial aneurysms: management and prevention. Interv Neuroradiol. (2018) 24:237–45. doi: 10.1177/1591019918758493
5. Nevzati E, Rey J, Coluccia D, Gruter BE, Wanderer S., vonGunten M, et al. Aneurysm wall cellularity affects healing after coil embolization: assessment in a rat saccular aneurysm model. J Neurointerv Surg. (2020) 12:621–5. doi: 10.1136/neurintsurg-2019-015335
6. Cai ZQ, Chai SH, Wei XL, You KZ, Li J, Zhang DM. Comparison of postsurgical clinical sequences between completely embolized and incompletely embolized patients with wide nicked intracranial aneurysms treated with stent assisted coil embolization technique: a STROBE-compliant study. Medicine. (2018) 97:e10987. doi: 10.1097/MD.0000000000010987
7. Choi HH, Lee SH, Yeon EK, Yoo DH, Cho YD, Cho WS, et al. Determination of aneurysm volume critical for stability after coil embolization: a retrospective study of 3530 aneurysms. World Neurosurg. (2019) 132:e766–74. doi: 10.1016/j.wneu.2019.08.020
8. Kim SS, Park H, Lee KH, Jung S, Yoon CH, Kim SK, et al. Utility of low-profile visualized intraluminal support junior stent as a rescue therapy for treating ruptured intracranial aneurysms during complicated coil embolization. World Neurosurg. (2020) 135:e710–e5. doi: 10.1016/j.wneu.2019.12.110
9. Haffaf I, Clarencon F, Shotar E, Rolla-Bigliani C, Vande Perre S, Mathon B, et al. Medina embolization device for the treatment of intracranial aneurysms: 18 months' angiographic results. J Neurointerv Surg. (2019) 11:516–22. doi: 10.1136/neurintsurg-2018-014110
10. Yamagami K, Hatano T, Nakahara I, Ishii A, Ando M, Chihara H, et al. Long-term outcomes after intraprocedural aneurysm rupture during coil embolization of unruptured intracranial aneurysms. World Neurosurg. (2020) 134:e289–97. doi: 10.1016/j.wneu.2019.10.038
11. Suzuki T, Genkai N, Nomura T, Abe H. Assessing the hemodynamics in residual cavities of intracranial aneurysm after coil embolization with combined computational flow dynamics and silent magnetic resonance angiography. J Stroke Cerebrovasc Dis. (2020) 29:105290. doi: 10.1016/j.jstrokecerebrovasdis.2020.105290
12. Tian Z, Liu J, Zhang Y, Zhang Y, Zhang X, Zhang H, et al. Risk factors of angiographic recurrence after endovascular coil embolization of intracranial saccular aneurysms: a retrospective study using a multicenter database. Front Neurol. (2020) 11:1026. doi: 10.3389/fneur.2020.01026
13. Son W, Kang DH. Risk factor analysis of delayed intracerebral hemorrhage after coil embolization of unruptured cerebral aneurysms. Front Neurol. (2020) 11:584596. doi: 10.3389/fneur.2020.584596
14. Futchko J, Starr J, Lau D, Leach MR, Roark C, Pandey AS, et al. Influence of smoking on aneurysm recurrence after endovascular treatment of cerebrovascular aneurysms. J Neurosurg. (2018) 128:992–8. doi: 10.3171/2016.12.JNS161625
15. Mortimer AM, Marsh H, Klimczak K, Joshi D, Barton H, Nelson RJ, et al. Is long-term follow-up of adequately coil-occluded ruptured cerebral aneurysms always necessary? A single-center study of recurrences after endovascular treatment. J Neurointerv Surg. (2015) 7:373–9. doi: 10.1136/neurintsurg-2014-011152
16. Jeon JP, Cho YD, Yoo DH, Moon J, Lee J, Cho WS, et al. Risk factor analysis of recanalization timing in coiled aneurysms: Early versus late recanalization. AJNR Am J Neuroradiol. (2017) 38:1765–70. doi: 10.3174/ajnr.A5267
17. Li H, Gao BL, Li CH, Wang JW, Liu JF, Yang ST. Endovascular retreatment of cerebral aneurysms previously treated with endovascular embolization. J Neurol Surg A Cent Eur Neurosurg. (2020) 81:207–12. doi: 10.1055/s-0039-1685513
18. Zhang Q, Jing L, Liu J, Wang K, Zhang Y, Paliwal N, et al. Predisposing factors for recanalization of cerebral aneurysms after endovascular embolization: a multivariate study. J Neurointerv Surg. (2018) 10:252–7. doi: 10.1136/neurintsurg-2017-013041
19. Nishido H, Piotin M, Bartolini B, Pistocchi S, Redjem H, Blanc R. Analysis of complications and recurrences of aneurysm coiling with special emphasis on the stent-assisted technique. AJNR Am J Neuroradiol. (2014) 35:339–44. doi: 10.3174/ajnr.A3658
20. Huang DZ, Jiang B, He W, Wang YH, Wang ZG. Risk factors for the recurrence of an intracranial saccular aneurysm following endovascular treatment. Oncotarget. (2017) 8:33676–82. doi: 10.18632/oncotarget.16897
21. Ahmad S. Clinical outcome of endovascular coil embolization for cerebral aneurysms in Asian population in relation to risk factors: a 3-year retrospective analysis. BMC Surg. (2020) 20:104. doi: 10.1186/s12893-020-00756-1
22. King RM, Chueh JY, van der Bom IM, Silva CF, Carniato SL, Spilberg G, et al. The effect of intracranial stent implantation on the curvature of the cerebrovasculature. Am J Neuroradiol. (2012) 33:1657–62. doi: 10.3174/ajnr.A3062
23. Yamada S, Ishikawa M, Yamamoto K, Ino T, Kimura T, Kobayashi S, et al. Aneurysm location and clipping vs. coiling for development of secondary normal-pressure hydrocephalus after aneurysmal subarachnoid hemorrhage: Japanese stroke databank. J Neurosurg. (2015) 123:1555–61. doi: 10.3171/2015.1.JNS142761
24. Poncyljusz W, Kubiak K. Initial experience with LVIS EVO stents for the treatment of intracranial aneurysms. J Clin Med. (2020) 9:3966. doi: 10.3390/jcm9123966
25. Twitchell S, Abou-Al-Shaar H, Reese J, Karsy M, Eli IM, Guan J, et al. Analysis of cerebrovascular aneurysm treatment cost: retrospective cohort comparison of clipping, coiling, and flow diversion. Neurosurg Focus. (2018) 44:E3. doi: 10.3171/2018.1.FOCUS17775
26. Koyanagi M, Ishii A, Imamura H, Satow T, Yoshida K, Hasegawa H, et al. Long-term outcomes of coil embolization of unruptured intracranial aneurysms. J Neurosurg. (2018) 129:1492–8. doi: 10.3171/2017.6.JNS17174
27. Takeshima Y, Kaku Y, Nishi T, Mukasa A, Yamashiro S. Multiple cerebral aneurysms associated with neurofibromatosis type 1. J Stroke Cerebrovasc Dis. (2019) 28:e83–91. doi: 10.1016/j.jstrokecerebrovasdis.2019.04.019
28. Son W, Kang DH. Is vertebral artery and posterior inferior cerebellar artery dominance a risk factor for thromboembolism during coil embolization of unruptured posterior circulation aneurysms? World Neurosurg. (2020) 138:e743–8. doi: 10.1016/j.wneu.2020.03.065
29. Choi HH, Cho YD, Yoo DH, Lee SH, Yeon EK, Kang HS, et al. Comparative analysis of coil embolization in posterior and anterior communicating artery aneurysms. J Neurointerv Surg. (2019) 11:790–5. doi: 10.1136/neurintsurg-2018-014490
30. De Leacy RA, Fargen KM, Mascitelli JR, Fifi J, Turkheimer L, Zhang X, et al. Wide-neck bifurcation aneurysms of the middle cerebral artery and basilar apex treated by endovascular techniques: a multicentre, core lab adjudicated study evaluating safety and durability of occlusion (BRANCH). J Neurointerv Surg. (2019) 11:31–6. doi: 10.1136/neurintsurg-2018-013771
31. Harris L, Hill CS, Elliot M, Fitzpatrick T, Ghosh A, Vindlacheruvu R. Comparison between outcomes of endovascular and surgical treatments of ruptured anterior communicating artery aneurysms. Br J Neurosurg. (2021) 35:313–8. doi: 10.1080/02688697.2020.1812517
32. Lee J, Cho YD, Yoo DH, Kang HS, Cho WS, Kim JE, et al. Does stent type impact coil embolization outcomes in extended follow-up of small-sized aneurysms (<10 mm)? Neuroradiology. (2018) 60:747–56. doi: 10.1007/s00234-018-2022-4
33. Damiano RJ, Tutino VM, Paliwal N, Patel TR, Waqas M, Levy EI, et al. Aneurysm characteristics, coil packing, and post-coiling hemodynamics affect long-term treatment outcome. J Neurointerv Surg. (2020) 12:706–13. doi: 10.1136/neurintsurg-2019-015422
34. Burkhardt JK, Srinivasan V, Srivatsan A, Albuquerque F, Ducruet AF, Hendricks B, et al. Multicenter postmarket analysis of the neuroform atlas stent for stent-assisted coil embolization of intracranial aneurysms. Am J Neuroradiol. (2020) 41:1037–42. doi: 10.3174/ajnr.A6581
Keywords: intracranial aneurysm, coil embolization, recurrence, meta-analysis, risk factors
Citation: Jin J, Guo G, Ren Y, Yang B, Wu Y, Wang S, Sun Y, Wang X, Wang Y and Zheng J (2022) Risk Factors for Recurrence of Intracranial Aneurysm After Coil Embolization: A Meta-Analysis. Front. Neurol. 13:869880. doi: 10.3389/fneur.2022.869880
Received: 11 February 2022; Accepted: 23 May 2022;
Published: 22 July 2022.
Edited by:
Gustavo J. Rodriguez, Texas Tech University Health Sciences Center El Paso, United StatesReviewed by:
Faheem G. Sheriff, Texas Tech University Health Science Center, United StatesCopyright © 2022 Jin, Guo, Ren, Yang, Wu, Wang, Sun, Wang, Wang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Geng Guo, Z3VvZ2VuZzk3M0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.