
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 18 August 2022
Sec. Neurotrauma
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.861688
 Lennart Riemann†
Lennart Riemann† Obada T. Alhalabi†
Obada T. Alhalabi† Andreas W. Unterberg
Andreas W. Unterberg Alexander Younsi* and The CENTER-TBI investigators and participants
Alexander Younsi* and The CENTER-TBI investigators and participantsObjective: Spine injury is highly prevalent in patients with poly-trauma, but data on the co-occurrence of spine trauma in patients with traumatic brain injury (TBI) are scarce. In this study, we used the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) database to assess the prevalence, characteristics, and outcomes of patients with TBI and a concurrent traumatic spinal injury (TSI).
Methods: Data from the European multi-center CENTER-TBI study were analyzed. Adult patients with TBI (≥18 years) presenting with a concomitant, isolated TSI of at least serious severity (Abbreviated Injury Scale; AIS ≥3) were included. For outcome analysis, comparison groups of TBI patients with TSI and systemic injuries (non-isolated TSI) and without TSI were created using propensity score matching. Rates of mortality, unfavorable outcomes (Glasgow Outcome Scale Extended; GOSe < 5), and full recovery (GOSe 7–8) of all patients and separately for patients with only mild TBI (mTBI) were compared between groups at 6-month follow-up.
Results: A total of 164 (4%) of the 4,254 CENTER-TBI core study patients suffered from a concomitant isolated TSI. The median age was 53 [interquartile range (IQR): 37–66] years and 71% of patients were men. mTBI was documented in 62% of cases, followed by severe TBI (26%), and spine injuries were mostly cervical (63%) or thoracic (31%). Surgical spine stabilization was performed in 19% of cases and 57% of patients were admitted to the ICU. Mortality at 6 months was 11% and only 36% of patients regained full recovery. There were no significant differences in the 6-month rates of mortality, unfavorable outcomes, or full recovery between TBI patients with and without concomitant isolated TSI. However, concomitant non-isolated TSI was associated with an unfavorable outcome and a higher mortality. In patients with mTBI, a negative association with full recovery could be observed for both concomitant isolated and non-isolated TSI.
Conclusion: Rates of mortality, unfavorable outcomes, and full recovery in TBI patients with and without concomitant, isolated TSIs were comparable after 6 months. However, in patients with mTBI, concomitant TSI was a negative predictor for a full recovery. These findings might indicate that patients with moderate to severe TBI do not necessarily exhibit worse outcomes when having a concomitant TSI, whereas patients with mTBI might be more affected.
Traumatic brain injury (TBI) contributes to the global burden of disease in a sizeable manner (1). The incidence of TBI has risen in the past years (2) and is estimated to become even more relevant with increasing events of traffic accidents and falls of the elderly (3, 4).
Traumatic brain injury can be complicated by additional injuries, such as traumatic spinal injuries (TSIs). When studying patients with spinal cord injury, the rate of concomitant TBI was estimated between 40 and 74% (5, 6). TBI in most of these patients was classified as mild (7). It is postulated that in the context of spine trauma, simultaneous TBI events are underdiagnosed (8). Unsurprisingly, TBI pertaining to spinal cord injury was found to be most frequent when the cervical and thoracic spine are affected (9).
Although various reports on TBI from a spinal injury perspective exist, little is known about the converse case of concomitant isolated spine trauma in patients suffering primarily from TBI. A recent meta-analysis found the rate of concomitant TSI in patients with TBI to be at around 13%, with cervical spinal injury amounting to almost half of the injuries diagnosed (10). This consolidates previous reports on cervical spine injury in larger patient cohorts with TBI (11). Indeed, patients with severe TBI were found to be at a particularly higher risk for sustaining injuries to the cervical spine (12).
Previous literature, while epidemiologically describing the prevalence of and risk factors for concomitant TBI and TSI, rarely elucidates the neurological outcomes of affected patients. In a retrospective analysis, patients with simultaneous TBI and TSI were reported to show increased motor deficits and limited functional gains in rehabilitation (13). Nevertheless, the question whether patients with concomitant TBI and TSI bear an inherent risk for a worse neurological outcome or a higher rate of mortality has yet to be tackled by prospectively collected observational data.
This study hence aimed at assessing the prevalence and characteristics of patients with TBI and concurrent, isolated TSI and comparing outcomes of such patients with TBI only in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) cohort.
In the present study, data collected as part of the CENTER-TBI core study were analyzed. CENTER-TBI is a European multi-center, observational, longitudinal cohort study of patients presenting with TBI of all severities. Patients were eligible for enrollment when presenting with a clinical diagnosis of TBI to a participating study center within 24 h and when a computed tomography (CT) scan was performed at admission. Informed consent was required from all patients and had to be obtained prior to enrollment. The study protocol adhered to all national and local ethical committee requirements of participating study centers. Patients were enrolled from December 2014 to December 2017 in 59 centers across Europe and Israel. More details on the CENTER-TBI study and main descriptive findings have been published elsewhere (14, 15).
For this study, we included adult CENTER-TBI core study patients (i.e., 18 years or older) with TBI that presented with a concomitant, isolated TSI. TSI was defined by an Abbreviated Injury Scale (AIS) score of ≥3 (indicating an injury of at least serious severity) in the cervical, thoracic, or lumbar spine. To study the impact of the TSI separately from poly-traumatic injuries, patients were excluded when also suffering from serious injuries (also defined as an AIS score of ≥3) in other body regions, namely, injuries to the thorax and chest, abdomen, pelvis, upper and lower extremities, or skin. As a complementary investigation, the same analyses were repeated for patients with non-isolated TSI, i.e., those with spine injuries (AIS scores ≥3) and concomitant injuries (AIS scores ≥3) in any of the other body regions. Primary outcome parameters were mortality [i.e., Glasgow Outcome Scale Extended (GOSe) = 1], unfavorable outcomes (i.e., GOSe < 5), and full recovery (i.e., GOSe = 7–8). All data were retrieved from the CENTER-TBI core study database in version 3.0 via the accessing tool Neurobot (RRID: SCR_ 017004).
Patient characteristics were analyzed using descriptive statistics. Continuous variables are reported as medians and interquartile ranges (INRs), while ordinal and categorical variables are presented as numbers and frequencies unless stated otherwise. The completeness of data is reported in Supplementary Table S1. Prior to outcome analysis, multiple imputation with 100 imputed datasets was used to address missing data in the control variables (age, sex, baseline Glasgow Coma Scale [GCS], performed cranial surgery, intracranial CT abnormality (mass lesion, extra-axial hematoma, epidural hematoma, acute subdural hematoma, chronic and subacute subdural hematoma, a subdural collection of mixed density, contusion, traumatic axonal injury, traumatic subarachnoid hemorrhage, intraventricular hemorrhage, midline shift, or cisternal compression), and American Society of Anesthesiologists [ASA] class) and the primary outcome variables (GOSe). Missing data were assumed to be missing at random. GCS and GOSe were defined as ordinal variables. The mortality, unfavorable outcomes, and full recovery of the variables were subsequently derived from imputed GOSe scores. After multiple imputation, propensity score matching with the above-named control variables and GOSe at 6-month follow-up as outcome variable was performed to create a matched comparison group of patients with TBI without concomitant TSI. The control variables were chosen a priori based on clinical expertise. Matching was performed within each imputed dataset. Effect estimates of concomitant TSI to outcomes were analyzed using weighted logistic regression models in each dataset. Additionally, logistic multivariable regression with (isolated or non-isolated) TSI as predictor and adjustment for the same control variables used in the propensity score analysis were performed for the three outcomes as a complementary analysis. Finally, effect estimates from each model were pooled according to Rubin's rules (16). The statistical software R was used for all analyses (https://www.r-project.org/ - version 4.1.1) (17).
A total of 164 adult patients with TBI and concomitant TSI were included in this study, representing about 4% of the entire CENTER-TBI study population (Figure 1). The median age in this subgroup of patients with simultaneous head and isolated spine injury was 53 years (IQR: 37–66 years) and 116 (71%) were men. The majority of injuries were caused by either incidental falls (47%, n = 77) or by road-traffic incidents (42%, n = 68). Alcohol intoxication confirmed by increased alcohol blood levels was found in 16% of patients (n = 26) and suspected in another 8% (n = 13). Most patients were brought to the hospital by ambulance (76%, n = 123) or by helicopter (12%, n = 19). Some patients even presented as walk-ins or drop-offs (6%, n = 9). Endotracheal intubation at the scene of an accident was performed in 22% of patients (n = 33). In total, 86% of patients (n = 141) were directly transported to the study center, while the remainder were referred to the study center from another hospital (see also Figure 2).
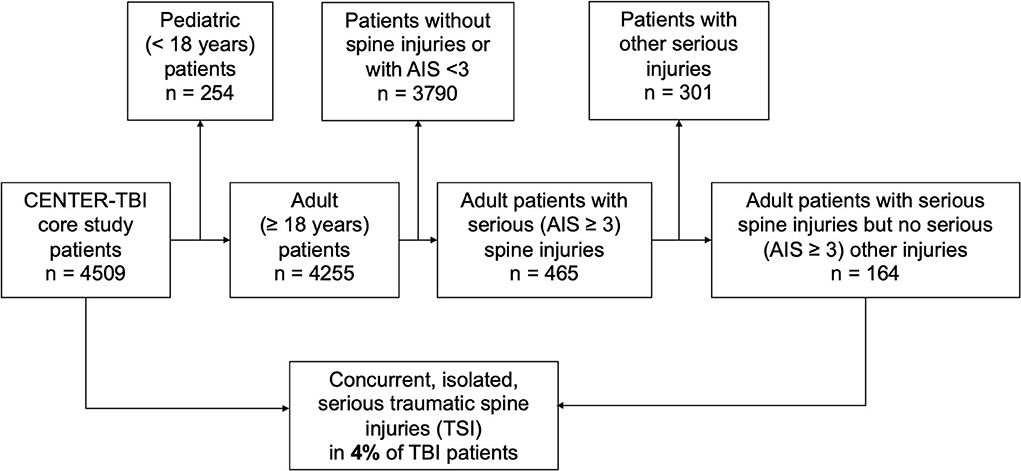
Figure 1. Study design. Adult patients in the Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI) database were screened. Patients with poly-trauma were excluded. Only patients sustaining TBI along with isolated traumatic spine injury (TSI) without the presence of further trauma were included in the analyses.
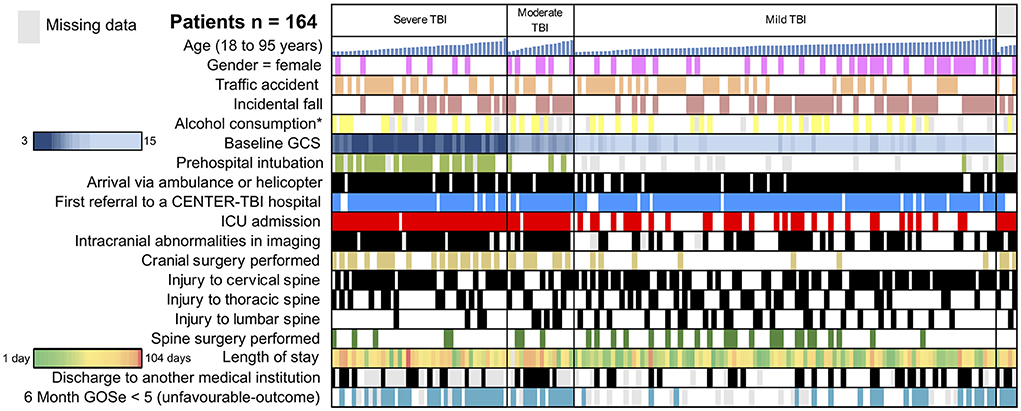
Figure 2. Traumatic brain injury (TBI)-traumatic spine injury (TSI) study cohort. Selected variables of the 164 patients included in the analysis are depicted. Rows represent representative variables, and each column represents one patient. Sub-cohorts are separated based on TBI severity (severe TBI: GCS < 8; moderate TBI: GCS 9–12; and mild TBI: GCS 13–15). In the sub-cohorts, patients are sorted by age from youngest (19 years) to oldest (95 years). A heat-map was utilized to visualize the length of stay (green: short, red: long) and GCS (dark blue: 15, light blue: 3). ICU, intensive care unit; GOSe, Glasgow Outcome Scale Extended. *Confirmed or suspected alcohol consumption. Gray represents missing data.
Upon admission at the study center, severe TBI (GCS 3–8) was present in 26% of patients (n = 42), while moderate or mild TBI (mTBI) was documented in 10% (n = 16) and 62% (n = 101) of cases, respectively. A traumatic spine injury of at least serious severity was located in the lumbar spine in 32 patients (20%), in the thoracic spine in 51 patients (31%), and in the cervical spine in 104 patients (63%). In 21 patients (13%), more than one region of the spine was affected (e.g., both cervical and thoracic spine injuries). The majority of patients (57%, n = 93) were admitted to the ICU, while 63 patients (38%) were admitted to the regular ward. Among patients admitted to the ICU, the requirement for mechanical ventilation was named as the primary reason in 40 patients (43%), followed by the need for frequent neurological observations in 22 patients (24%) and neurosurgical intervention in 13 patients (14%). Spine stabilization surgery was performed in 32 patients (20%). During the hospital stay, respiratory complications were documented in 14 patients (12%), making it the most common type of complication. Further complications included seizures in 5 patients (4%), cardiac complications in 4 patients (3%), and urinary tract infections in 6 patients (5%). Patients with TBI and concomitant TSI stayed in the hospital for a median of 9 (3–20) days. Most patients could be discharged home (56%, n = 70), while 26 patients (21%) were discharged to a rehabilitation facility, and 24 patients (19%) were transferred to another hospital (see also Figure 2).
In this cohort of patients with TBI and concomitant TSI, 18 of 164 patients were dead after 6 months, yielding a mortality rate of 11%. Of those, 13 died in the ICU. In 9 patients, the initial head injury was documented as the cause of death whereas secondary intracranial damage was documented in 2 patients. For the remaining deceased patients, no cause of death was documented. A total of 48 patients (29%) were considered to have an unfavorable outcome (GOSe < 5). Approximately, one-third of the patients achieved a full recovery (GOSe 7 or 8). To compare the outcomes of TBI patients with and without isolated, concomitant TSI, we performed propensity score matching with subsequent weighted logistic regression to estimate the effect of the simultaneous spine injury on patient outcomes. Patients were matched with age, sex, baseline GCS, performed cranial surgery, intracranial CT abnormalities, and ASA class as covariables (see Supplementary Tables S2, S3 for balance statistics and exemplary descriptions of the matched cohorts). In the outcome analysis, the presence of an isolated, concomitant TSI was neither significantly associated with mortality [β = −0.12 (−0.84 to 0.59), p = 0.732] nor with unfavorable outcomes [β = 0.28 (−0.21 to 0.77), p = 0.270], or full recovery [β = −0.29 (−0.76 to 0.18), p = 0.228]. Similar results were obtained in the logistic regression analysis (Supplementary Table S4), which showed that an isolated TSI, when controlling for age, sex, baseline GCS, performed cranial surgery, intracranial CT abnormalities, and ASA class, was neither a significant predictor of full recovery (p = 0.084) nor of unfavorable outcomes (p = 0.184) or death (p = 0.355) in our cohort. To put these results into a broader context, we performed a similar analysis but examined patients with TBI and concomitant TSI in conjunction with systemic injuries (i.e., non-isolated TSI) instead of an isolated TSI. In comparison with a matched cohort, TSI with concomitant systemic injuries in patients with TBI was negatively associated with full recovery (p ≤ 0.001), but not with unfavorable outcomes (p = 0.130) or mortality (p = 0.282). In logistic regression analysis, a TSI with systemic injuries was a significant negative predictor of full recovery (p < 0.001) and unfavorable outcomes (p = 0.003), but not mortality (p = 0.355; Supplementary Table S4).
When only patients with mTBI were included in a subgroup analysis, no significant associations between isolated TSI and full recovery [β = −0.468 (−1.127 to 0.190), p-value = 0.160] and unfavorable outcomes [β = 0.899 (−0.175 to 1.973), p-value = 0.099] were seen when using propensity score matching. In the logistic regression analysis, isolated TSI was a significant negative predictor of full recovery [β = −0.507 (−0.994 to 0.012), p = 0.042] and a predictor of unfavorable outcomes [β = 0.770 (0.145–1.394), p = 0.016]. In mTBI patients with TSI and systemic injuries, TSI was significantly associated with unfavorable outcomes [β = 0.853 (0.006–1.699), p-value = 0.048] and inversely with full recovery [β = −1.311 (−1.925 to −0.698), p-value < 0.001] in the propensity score-matching analysis. Similarly, in the logistic regression analysis, a TSI in mTBI patients with systemic injuries was significantly associated with unfavorable outcomes [β = 1.150 (0.610–1.691), p < 0.001] and, in a negative direction, with full recovery [β = −1.345 (−1.772 to −0.918), p < 0.001]. The outcome analysis was not performed for mortality in the subgroup analysis of patients with mTBI due to the very low mortality rate (i.e., zero, and three patients among mTBI patients with isolated and non-isolated TSI were dead at the follow-up timepoint after 6 months, respectively) in this subgroup.
While there is a wealth of epidemiological data on TBI studied from an SCI perspective, the potential role of a simultaneous TSI in exacerbating neurological deficits in patients with TBI remains largely unexplored. This study reported on concomitant TSI using data from a large prospectively followed up cohort of patients presenting with TBI as their main diagnosis and provided propensity-matching analyses to determine the influence of such injury on their global functional outcomes.
In this cohort, the rate of patients with TBI sustaining further isolated injury of at least serious intensity to the spine was found to be 4%. The rift between our current findings and previous analyses indicating higher rates of TSI in patients with TBI of up to 13% (10) could well be attributed to differences in the applied methodology, especially as to what is defined as an “injury.” One key difference could be the AIS used in this study. The AIS is a standardized tool to reliably classify injuries and assess their severity (18, 19). Patients with TBI were regarded to have suffered a concomitant TSI when the AIS score of the cervical, thoracic, or lumbar spine satisfied at least serious severity. In this functional outcome-oriented analysis, thresholds for defining TSI were set as a trade-off between including patients with very minor and clinically negligible injuries that would otherwise skew the analysis and over-estimate TSI in patients with TBI vs. solely including patients undergoing surgical spinal stabilization and hence overlooking patients sustaining TSI with a “relevant” burden of disease that was managed non-surgically.
In a similar vein, an analysis excluding patients showing further injuries beyond TSI was envisaged to help eliminate possible confounders through further injuries (for example, to the skeletal system), and, therefore, yield a less-biased analysis that could compare characteristics and outcomes of isolated TSI+TBI vs. TBI-only patients. Indeed, further propensity-matching and logistic regression analyses compared patients with TBI, and systemic injuries (that included TSI) did show systemic injuries to be associated with unfavorable outcomes and to prevent full recovery.
Confirming data in previous studies, more than 60% of the spinal injuries diagnosed in our cohort were cervical (10). This was previously linked to the physiological bio-mechanical proximity of the cervical spine to the head (20), rendering concomitant injury to the cervical spine in TBI cases more likely than to other regions of the spine (10). Regarding injury causes, incidental falls and road traffic accidents accounted for the majority of TBIs (47 and 40%, respectively). On the one hand, the rate of road traffic accidents seems to be higher in this cohort than what has been previously reported in (isolated) TBI in high-income countries (21), which lends grounds for speculation that road accidents (which are usually poly-traumas in nature), might contribute to an increased risk of concomitant injury, especially with previous studies showing a high proportion of SCI patients with TBI to be victims of road traffic accidents (9). In addition, motor traffic accidents and herein old age, in particular, have been associated with higher odds of cervical spine injury (22). On the other hand, the larger proportion of incidental falls confirms a worldwide trend of increasing TBI rates secondary to falls of the elderly (4). In this cohort, patients with suspected or confirmed alcohol use amounted to 26%. Indeed, alcohol has been previously shown to be the strongest risk factor for clinical TBI in patients with SCI (7), hinting at the possibility that this could be another factor that fosters concomitant injury.
Missed diagnosis of simultaneous spinal injury in patients with TBI was deemed detrimental in the past and accounted for further neurological deterioration (11), especially because patients suffering severe TBI are difficult to assess clinically and possess a higher risk of sustaining injuries to the cervical spine (12). In terms of prognosis, this observation is, however, not reflected by the data we present, in which outcomes were comparable between TBI+TSI and TBI-only patients. Rather, it is conceivable that the probability of missing relevant spine trauma in the wake of comprehensive CT and MRI imaging [that was less available 20 years earlier (11)] in the participating study centers should be low. This is further supported by the fact that in this cohort, 86% of the patients were primarily transported to a more specialized trauma center (part of the CENTER-TBI study group) where the availability of the necessary infrastructure for diagnosis and treatment of spine trauma (especially spine stabilization surgery) is expected to be higher. It is therefore advisable that given the relevant rate of TSI in patients with TBI and the complex spine surgery these patients might potentially require, patients with TBI are primarily presented to specialized trauma centers of maximum care, especially when concomitant TSI is suspected. This effect could indeed be of even more relevance in the context of patients with mTBI since our analysis demonstrated how in the case of the subgroup of patients with mTBI, isolated TSI (or TSI in conjunction with systemic injuries) does indeed hinder full recovery and negatively influence outcomes.
Although most of the patients in the TBI+TSI cohort were admitted to the ICU, there was no significant difference in mortality when comparing them to patients with isolated TBI in our study. This hints at the possibility that in TBI patients with concomitant TSI, the intracranial injury still represents the main prognosis-limiting factor, especially in severe and moderate TBI. The disparity between the findings on patients with mTBI and all patients of the cohort emphasizes on how the prognosis of patients with moderate and severe TBI is limited by their cranial injury and how TSI becomes more relevant in patients sustaining mTBI, that are otherwise less limited in terms of their neurological outcomes. The question is to whether the necessity of intubation and mechanical ventilation is a result of loss of consciousness owing to TBI or of respiratory failure secondary to injury of the cervical spine remains and cannot be explored using the data provided, although previous reports have indicated the presence of the latter patient group (12).
Similarly, two-thirds of the patients in the TBI+TSI cohort showed a favorable outcome (divided in half between complete recovery and incomplete recovery with a GOSe > 5), leaving a third with unfavorable outcomes in our current analysis. Interestingly, an older study estimated patients with the recovery of neurological function after severe and moderate TBI and concomitant cervical TSI (no mTBIs included) to be at about a third (11), which is very comparable to the data we present. Apart from that, little data have been provided in previous epidemiological studies on the specific functional outcomes of TBI patients with concomitant TSI. This once again emphasizes the importance of the data presented in this study, in which the rates of mortality and unfavorable outcomes in TBI patients with concomitant TSI were comparable to the respective rates observed in a matched group of TBI patients without TSI.
In summary, this analysis of prospective observational data sheds light on the current prognosis of patients suffering from TBI with a concomitant isolated or non-isolated TSI in the CENTER-TBI participating centers, showing an outcome that is comparable with what is known in the literature (15). The data presented underscore the role of specialized trauma care centers in preventing further neurological deterioration owing to concomitant TSI especially in patients with mTBI through early detection and adequate therapy of spine trauma.
Several important limitations must be noted. As an observational study focused on TBI, in general, no additional information on the exact nature of the spine injury or its treatment (that included surgical details) was recorded in the CENTER-TBI database. Thus, injuries to the spinal cord could have been present in some patients but not in others, potentially leading to a considerable heterogeneity for the variable “spine trauma.” This should be considered when interpreting our current results. Additional studies are needed to assess how different types of spine and spinal cord injuries relate to outcomes in patients with TBI. To the same end, detailed parameters assessing specifically the spine function, such as motor and sensory function of the extremities, as well as the function of the autonomic nerve system, were not available but would be desirable both for the description of the baseline clinical status and for the evaluation of recovery at follow-up. In terms of outcome analysis, the matching process is dependent on the chosen covariables and unmeasured covariables that might play an important role are not accounted for. Finally, as only TBI patients with concomitant spine trauma were included, the sample sizes of the different subgroups of patients in our analyses were limited. Larger cohorts are needed for a more robust generalizability and to possibly detect more subtle effects of a concomitant spine injury on outcomes in patients with TBI.
The data analyzed in this study was obtained from the CENTER-TBI database; the following licenses/restrictions apply: Upon a reasonable request, access to the dataset must first be reviewed and approved by the CENTER-TBI Management Committee and should be directed to https://www.center-tbi.eu/data.
Ethical approval was obtained for each recruiting site. A complete list is given on https://www.center-tbi.eu/project/ethical-approval or available as a Supplementary file. All patients had to give their written informed consent before enrollment in CENTER-TBI.
Study concept and design and data collection and analysis: LR and AY. Data interpretation and reviewing and editing: LR, OA, AU, and AY. Writing the manuscript: LR and OA. Supervision: AY. All authors approved the final version of the submitted manuscript.
CENTER-TBI was supported by the European Union 7th Framework program (EC Grant 602150). Additional funding was obtained from the Hannelore Kohl Stiftung (Germany), from OneMind (USA), and Integra LifeSciences Corporation (USA). Those funders were not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Cecilia Åkerlund1, Krisztina Amrein2, Nada Andelic3, Lasse Andreassen4, Audny Anke5, Anna Antoni6, Gérard Audibert7, Philippe Azouvi8, Maria Luisa Azzolini9, Ronald Bartels10, Pál Barzó11, Romuald Beauvais12, Ronny Beer13, Bo-Michael Bellander14, Antonio Belli15, Habib Benali16, Maurizio Berardino17, Luigi Beretta9, Morten Blaabjerg18, Peter Bragge19, Alexandra Brazinova20, Vibeke Brinck21, Joanne Brooker22, Camilla Brorsson23, Andras Buki24, Monika Bullinger25, Manuel Cabeleira26, Alessio Caccioppola27, Emiliana Calappi 27, Maria Rosa Calvi9, Peter Cameron28, Guillermo Carbayo Lozano29, Marco Carbonara27, Simona Cavallo17, Giorgio Chevallard30, Arturo Chieregato30, Giuseppe Citerio31, 32, Hans Clusmann33, Mark Coburn34, Jonathan Coles35, Jamie D. Cooper36, Marta Correia37, Amra Cović 38, Nicola Curry39, Endre Czeiter24, Marek Czosnyka26, Claire Dahyot-Fizelier40, Paul Dark41, Helen Dawes42, Véronique De Keyser43, Vincent Degos16, Francesco Della Corte44, Hugo den Boogert10, Bart Depreitere45, Ðula Ðilvesi46, Abhishek Dixit47, Emma Donoghue22, Jens Dreier48, Guy-Loup Dulière49, Ari Ercole47, Patrick Esser42, Erzsébet Ezer50, Martin Fabricius51, Valery L. Feigin52, Kelly Foks53, Shirin Frisvold54, Alex Furmanov55, Pablo Gagliardo56, Damien Galanaud16, Dashiell Gantner28, Guoyi Gao57, Pradeep George58, Alexandre Ghuysen59, Lelde Giga60, Ben Glocker61, Jagoš Golubovic46, Pedro A. Gomez 62, Johannes Gratz63, Benjamin Gravesteijn64, Francesca Grossi44, Russell L. Gruen65, Deepak Gupta66, Juanita A. Haagsma64, Iain Haitsma67, Raimund Helbok13, Eirik Helseth68, Lindsay Horton 69, Jilske Huijben64, Peter J. Hutchinson70, Bram Jacobs71, Stefan Jankowski72, Mike Jarrett21, Ji-yao Jiang58, Faye Johnson73, Kelly Jones52, Mladen Karan46, Angelos G. Kolias70, Erwin Kompanje74, Daniel Kondziella51, Evgenios Kornaropoulos47, Lars-Owe Koskinen75, Noémi Kovács76, Ana Kowark77, Alfonso Lagares62, Linda Lanyon58, Steven Laureys78, Fiona Lecky79, 80, Didier Ledoux78, Rolf Lefering81, Valerie Legrand82, Aurelie Lejeune83, Leon Levi84, Roger Lightfoot85, Hester Lingsma64, Andrew I.R. Maas43, Ana M. Castaño-León62, Marc Maegele86, Marek Majdan20, Alex Manara87, Geoffrey Manley88, Costanza Martino89, Hugues Maréchal49, Julia Mattern90, Catherine McMahon91, Béla Melegh92, David Menon47, Tomas Menovsky43, Ana Mikolic64, Benoit Misset78, Visakh Muraleedharan58, Lynnette Murray28, Ancuta Negru93, David Nelson1, Virginia Newcombe47, Daan Nieboer64, József Nyirádi2, Otesile Olubukola79, Matej Oresic94, Fabrizio Ortolano27, Aarno Palotie95, 96, 97, Paul M. Parizel98, Jean-François Payen99, Natascha Perera12, Vincent Perlbarg16, Paolo Persona100, Wilco Peul101, Anna Piippo-Karjalainen102, Matti Pirinen95, Dana Pisica64, Horia Ples93, Suzanne Polinder64, Inigo Pomposo29, Jussi P. Posti 103, Louis Puybasset104, Andreea Radoi 105, Arminas Ragauskas106, Rahul Raj102, Malinka Rambadagalla107, Isabel Retel Helmrich64, Jonathan Rhodes108, Sylvia Richardson109, Sophie Richter47, Samuli Ripatti95, Saulius Rocka106, Cecilie Roe110, Olav Roise111, 112, Jonathan Rosand113, Jeffrey V. Rosenfeld114, Christina Rosenlund115, Guy Rosenthal55, Rolf Rossaint77, Sandra Rossi100, Daniel Rueckert61, Martin Rusnák116, Juan Sahuquillo105, Oliver Sakowitz90, 117, Renan Sanchez-Porras117, Janos Sandor118, Nadine Schäfer81, Silke Schmidt119, Herbert Schoechl120, Guus Schoonman121, Rico Frederik Schou122, Elisabeth Schwendenwein6, Charlie Sewalt64, Ranjit D. Singh101, Toril Skandsen123, 124, Peter Smielewski26, Abayomi Sorinola125, Emmanuel Stamatakis47, Simon Stanworth39, Robert Stevens126, William Stewart127, Ewout W. Steyerberg64, 128, Nino Stocchetti129, Nina Sundström130, Riikka Takala131, Viktória Tamás125, Tomas Tamosuitis132, Mark Steven Taylor20, Braden Te Ao52, Olli Tenovuo103, Alice Theadom52, Matt Thomas87, Dick Tibboel133, Marjolein Timmers74, Christos Tolias134, Tony Trapani28, Cristina Maria Tudora93, Andreas Unterberg90, Peter Vajkoczy 135, Shirley Vallance28, Egils Valeinis60, Zoltán Vámos50, Mathieu van der Jagt136, Gregory Van der Steen43, Joukje van der Naalt71, Jeroen T.J.M. van Dijck 101, Inge A. van Erp101, Thomas A. van Essen101, Wim Van Hecke137, Caroline van Heugten42, Dominique Van Praag138, Ernest van Veen64, Thijs Vande Vyvere137, Roel P. J. van Wijk101, Alessia Vargiolu32, Emmanuel Vega83, Kimberley Velt64, Jan Verheyden137, Paul M. Vespa139, Anne Vik123, 140, Rimantas Vilcinis132, Victor Volovici67, Nicole von Steinbüchel38, Daphne Voormolen64, Petar Vulekovic46, Kevin K.W. Wang141, Daniel Whitehouse47, Eveline Wiegers64, Guy Williams47, Lindsay Wilson69, Stefan Winzeck47, Stefan Wolf142, Zhihui Yang113, Peter Ylén143, Alexander Younsi90, Frederick A. Zeiler47, 144, Veronika Zelinkova20, Agate Ziverte60, Tommaso Zoerle27
1Department of Physiology and Pharmacology, Section of Perioperative Medicine and Intensive Care, Karolinska Institutet, Stockholm, Sweden
2János Szentágothai Research Centre, University of Pécs, Pécs, Hungary
3Division of Surgery and Clinical Neuroscience, Department of Physical Medicine and Rehabilitation, Oslo University Hospital and University of Oslo, Oslo, Norway
4Department of Neurosurgery, University Hospital Northern Norway, Tromso, Norway
5Department of Physical Medicine and Rehabilitation, University Hospital Northern Norway, Tromso, Norway
6Trauma Surgery, Medical University Vienna, Vienna, Austria
7Department of Anesthesiology & Intensive Care, University Hospital Nancy, Nancy, France
8Raymond Poincare hospital, Assistance Publique – Hopitaux de Paris, Paris, France
9Department of Anesthesiology & Intensive Care, S Raffaele University Hospital, Milan, Italy
10Department of Neurosurgery, Radboud University Medical Center, Nijmegen, The Netherlands
11Department of Neurosurgery, University of Szeged, Szeged, Hungary
12International Projects Management, ARTTIC, Munchen, Germany
13Department of Neurology, Neurological Intensive Care Unit, Medical University of Innsbruck, Innsbruck, Austria
14Department of Neurosurgery & Anesthesia & intensive care medicine, Karolinska University Hospital, Stockholm, Sweden
15NIHR Surgical Reconstruction and Microbiology Research Centre, Birmingham, UK
16Anesthesie-Réanimation, Assistance Publique – Hopitaux de Paris, Paris, France
17Department of Anesthesia & ICU, AOU Città della Salute e della Scienza di Torino - Orthopedic and Trauma Center, Torino, Italy
18Department of Neurology, Odense University Hospital, Odense, Denmark
19BehaviourWorks Australia, Monash Sustainability Institute, Monash University, Victoria, Australia
20Department of Public Health, Faculty of Health Sciences and Social Work, Trnava University, Trnava, Slovakia
21Quesgen Systems Inc., Burlingame, CA, United States
22Australian & New Zealand Intensive Care Research Centre, Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, Australia
23Department of Surgery and Perioperative Science, Umeå University, Umeå, Sweden
24Department of Neurosurgery, Medical School, University of Pécs, Hungary and Neurotrauma Research Group, János Szentágothai Research Centre, University of Pécs, Hungary
25Department of Medical Psychology, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany
26Brain Physics Lab, Division of Neurosurgery, Dept of Clinical Neurosciences, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom
27Neuro ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan, Italy
28ANZIC Research Centre, Monash University, Department of Epidemiology and Preventive Medicine, Melbourne, Victoria, Australia
29Department of Neurosurgery, Hospital of Cruces, Bilbao, Spain
30NeuroIntensive Care, Niguarda Hospital, Milan, Italy
31School of Medicine and Surgery, Università Milano Bicocca, Milano, Italy
32NeuroIntensive Care, ASST di Monza, Monza, Italy
33Department of Neurosurgery, Medical Faculty RWTH Aachen University, Aachen, Germany
34Department of Anesthesiology and Intensive Care Medicine, University Hospital Bonn, Bonn, Germany
35Department of Anesthesia & Neurointensive Care, Cambridge University Hospital NHS Foundation Trust, Cambridge, United Kingdom
36School of Public Health & PM, Monash University and The Alfred Hospital, Melbourne, VIC, Australia
37Radiology/MRI department, MRC Cognition and Brain Sciences Unit, Cambridge, United Kingdom
38Institute of Medical Psychology and Medical Sociology, Universitätsmedizin Göttingen, Göttingen, Germany
39Oxford University Hospitals NHS Trust, Oxford, United Kingdom
40Intensive Care Unit, CHU Poitiers, Potiers, France
41University of Manchester NIHR Biomedical Research Centre, Critical Care Directorate, Salford Royal Hospital NHS Foundation Trust, Salford, United Kingdom
42Movement Science Group, Faculty of Health and Life Sciences, Oxford Brookes University, Oxford, United Kingdom
43Department of Neurosurgery, Antwerp University Hospital and University of Antwerp, Edegem, Belgium
44Department of Anesthesia & Intensive Care, Maggiore Della Carità Hospital, Novara, Italy
45Department of Neurosurgery, University Hospitals Leuven, Leuven, Belgium
46Department of Neurosurgery, Clinical centre of Vojvodina, Faculty of Medicine, University of Novi Sad, Novi Sad, Serbia
47Division of Anaesthesia, University of Cambridge, Addenbrooke's Hospital, Cambridge, United Kingdom
48Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
49Intensive Care Unit, CHR Citadelle, Liège, Belgium
50Department of Anaesthesiology and Intensive Therapy, University of Pécs, Pécs, Hungary
51Departments of Neurology, Clinical Neurophysiology and Neuroanesthesiology, Region Hovedstaden Rigshospitalet, Copenhagen, Denmark
52National Institute for Stroke and Applied Neurosciences, Faculty of Health and Environmental Studies, Auckland University of Technology, Auckland, New Zealand
53Department of Neurology, Erasmus MC, Rotterdam, the Netherlands
54Department of Anesthesiology and Intensive care, University Hospital Northern Norway, Tromso, Norway
55Department of Neurosurgery, Hadassah-hebrew University Medical center, Jerusalem, Israel
56Fundación Instituto Valenciano de Neurorrehabilitación (FIVAN), Valencia, Spain
57Department of Neurosurgery, Shanghai Renji hospital, Shanghai Jiaotong University/school of medicine, Shanghai, China
58Karolinska Institutet, INCF International Neuroinformatics Coordinating Facility, Stockholm, Sweden
59Emergency Department, CHU, Liège, Belgium
60Neurosurgery clinic, Pauls Stradins Clinical University Hospital, Riga, Latvia
61Department of Computing, Imperial College London, London, United Kingdom
62Department of Neurosurgery, Hospital Universitario 12 de Octubre, Madrid, Spain
63Department of Anesthesia, Critical Care and Pain Medicine, Medical University of Vienna, Austria
64Department of Public Health, Erasmus Medical Center-University Medical Center, Rotterdam, The Netherlands
65College of Health and Medicine, Australian National University, Canberra, ACT, Australia
66Department of Neurosurgery, Neurosciences Centre & JPN Apex trauma centre, All India Institute of Medical Sciences, New Delhi, India
67Department of Neurosurgery, Erasmus MC, Rotterdam, the Netherlands
68Department of Neurosurgery, Oslo University Hospital, Oslo, Norway
69Division of Psychology, University of Stirling, Stirling, United Kingdom
70Division of Neurosurgery, Department of Clinical Neurosciences, Addenbrooke's Hospital & University of Cambridge, Cambridge, United Kingdom
71Department of Neurology, University of Groningen, University Medical Center Groningen, Groningen, Netherlands
72Neurointensive Care, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
73Salford Royal Hospital NHS Foundation Trust Acute Research Delivery Team, Salford, United Kingdom
74Department of Intensive Care and Department of Ethics and Philosophy of Medicine, Erasmus Medical Center, Rotterdam, The Netherlands
75Department of Clinical Neuroscience, Neurosurgery, Umeå University, Umeå, Sweden
76Hungarian Brain Research Program - Grant No. KTIA_13_NAP-A-II/8, University of Pécs, Pécs, Hungary
77Department of Anaesthesiology, University Hospital of Aachen, Aachen, Germany
78Cyclotron Research Center, University of Liège, Liège, Belgium
79Centre for Urgent and Emergency Care Research (CURE), Health Services Research Section, School of Health and Related Research (ScHARR), University of Sheffield, Sheffield, UK
80Emergency Department, Salford Royal Hospital, Salford, United Kingdom
81Institute of Research in Operative Medicine (IFOM), Witten/Herdecke University, Cologne, Germany
82VP Global Project Management CNS, ICON, Paris, France
83Department of Anesthesiology-Intensive Care, Lille University Hospital, Lille, France
84Department of Neurosurgery, Rambam Medical Center, Haifa, Israel
85Department of Anesthesiology & Intensive Care, University Hospitals Southhampton NHS Trust, Southhampton, United Kingdom
86Cologne-Merheim Medical Center (CMMC), Department of Traumatology, Orthopedic Surgery and Sportmedicine, Witten/Herdecke University, Cologne, Germany
87Intensive Care Unit, Southmead Hospital, Bristol, Bristol, Uinted Kingdom
88Department of Neurological Surgery, University of California, San Francisco, CA, United States
89Department of Anesthesia & Intensive Care, M. Bufalini Hospital, Cesena, Italy
90Department of Neurosurgery, University Hospital Heidelberg, Heidelberg, Germany
91Department of Neurosurgery, The Walton centre NHS Foundation Trust, Liverpool, United Kingdom
92Department of Medical Genetics, University of Pécs, Pécs, Hungary
93Department of Neurosurgery, Emergency County Hospital Timisoara, Timisoara, Romania
94School of Medical Sciences, Örebro University, Örebro, Sweden
95Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland
96Analytic and Translational Genetics Unit, Department of Medicine; Psychiatric & Neurodevelopmental Genetics Unit, Department of Psychiatry; Department of Neurology, Massachusetts General Hospital, Boston, MA, United States
97Program in Medical and Population Genetics; The Stanley Center for Psychiatric Research, The Broad Institute of MIT and Harvard, Cambridge, MA, United States
98Department of Radiology, University of Antwerp, Edegem, Belgium
99Department of Anesthesiology & Intensive Care, University Hospital of Grenoble, Grenoble, France
100Department of Anesthesia & Intensive Care, Azienda Ospedaliera Università di Padova, Padova, Italy
101Dept. of Neurosurgery, Leiden University Medical Center, Leiden, The Netherlands and Dept. of Neurosurgery, Medical Center Haaglanden, The Hague, Netherlands
102Department of Neurosurgery, Helsinki University Central Hospital
103Division of Clinical Neurosciences, Department of Neurosurgery and Turku Brain Injury Centre, Turku University Hospital and University of Turku, Turku, Finland
104Department of Anesthesiology and Critical Care, Pitié -Salpêtrière Teaching Hospital, Assistance Publique, Hôpitaux de Paris and University Pierre et Marie Curie, Paris, France
105Neurotraumatology and Neurosurgery Research Unit (UNINN), Vall d'Hebron Research Institute, Barcelona, Spain
106Department of Neurosurgery, Kaunas University of technology and Vilnius University, Vilnius, Lithuania
107Department of Neurosurgery, Rezekne Hospital, Latvia
108Department of Anaesthesia, Critical Care & Pain Medicine NHS Lothian & University of Edinburg, Edinburgh, United Kingdom
109Director, MRC Biostatistics Unit, Cambridge Institute of Public Health, Cambridge, United Kingdom
110Department of Physical Medicine and Rehabilitation, Oslo University Hospital/University of Oslo, Oslo, Norway
111Division of Orthopedics, Oslo University Hospital, Oslo, Norway
112Institue of Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway
113Broad Institute, Cambridge MA Harvard Medical School, Boston MA, Massachusetts General Hospital, Boston MA, United States
114National Trauma Research Institute, The Alfred Hospital, Monash University, Melbourne, VIC, Australia
115Department of Neurosurgery, Odense University Hospital, Odense, Denmark
116International Neurotrauma Research Organisation, Vienna, Austria
117Klinik für Neurochirurgie, Klinikum Ludwigsburg, Ludwigsburg, Germany
118Division of Biostatistics and Epidemiology, Department of Preventive Medicine, University of Debrecen, Debrecen, Hungary
119Department Health and Prevention, University Greifswald, Greifswald, Germany
120Department of Anaesthesiology and Intensive Care, AUVA Trauma Hospital, Salzburg, Austria
121Department of Neurology, Elisabeth-TweeSteden Ziekenhuis, Tilburg, Netherlands
122Department of Neuroanesthesia and Neurointensive Care, Odense University Hospital, Odense, Denmark
123Department of Neuromedicine and Movement Science, Norwegian University of Science and Technology, NTNU, Trondheim, Norway
124Department of Physical Medicine and Rehabilitation, St. Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
125Department of Neurosurgery, University of Pécs, Pécs, Hungary
126Division of Neuroscience Critical Care, John Hopkins University School of Medicine, Baltimore, USA
127Department of Neuropathology, Queen Elizabeth University Hospital and University of Glasgow, Glasgow, United Kingdom
128Dept. of Department of Biomedical Data Sciences, Leiden University Medical Center, Leiden, Netherlands
129Department of Pathophysiology and Transplantation, Milan University, and Neuroscience ICU, Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano, Italy
130Department of Radiation Sciences, Biomedical Engineering, Umeå University, Umeå, Sweden
131Perioperative Services, Intensive Care Medicine and Pain Management, Turku University Hospital and University of Turku, Turku, Finland
132Department of Neurosurgery, Kaunas University of Health Sciences, Kaunas, Lithuania
133Intensive Care and Department of Pediatric Surgery, Erasmus Medical Center, Sophia Children's Hospital, Rotterdam, The Netherlands
134Department of Neurosurgery, Kings college London, London, United Kingdom
135Neurologie, Neurochirurgie und Psychiatrie, Charité – Universitätsmedizin Berlin, Berlin, Germany
136Department of Intensive Care Adults, Erasmus MC– University Medical Center Rotterdam, Rotterdam, the Netherlands
137icoMetrix NV, Leuven, Belgium
138Psychology Department, Antwerp University Hospital, Edegem, Belgium
139Director of Neurocritical Care, University of California, Los Angeles, United States
140Department of Neurosurgery, St.Olavs Hospital, Trondheim University Hospital, Trondheim, Norway
141Department of Emergency Medicine, University of Florida, Gainesville, Florida, United States
142Department of Neurosurgery, Charité – Universitätsmedizin Berlin, corporate member of Freie Universität Berlin, Humboldt-Universität zu Berlin, and Berlin Institute of Health, Berlin, Germany
143VTT Technical Research Centre, Tampere, Finland
144Section of Neurosurgery, Department of Surgery, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, MB, Canada
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.861688/full#supplementary-material
1. James SL, Theadom A, Ellenbogen RG, Bannick MS, Mountjoy-Venning WC, Lucches LR. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:56–87. doi: 10.1016/S1474-4422(18)30415-0
2. Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill Summ. (2017) 66:1–16. doi: 10.15585/mmwr.ss6609a1
3. Maas AIR, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. (2008) 7:728–41. doi: 10.1016/S1474-4422(08)70164-9
4. Iaccarino C, Carretta A, Nicolosi F, Morselli C. Epidemiology of severe traumatic brain injury. J Neurosurg Sci Oct. (2018) 62:535–41. doi: 10.23736/S0390-5616.18.04532-0
5. Macciocchi S, Seel RT, Thompson N, Byams R, Bowman B. Spinal cord injury and co-occurring traumatic brain injury: assessment and incidence. Arch Phys Med Rehabil Jul. (2008) 89:1350–7. doi: 10.1016/j.apmr.2007.11.055
6. Tolonen A, Turkka J, Salonen O, Ahoniemi E, Alaranta H. Traumatic brain injury is under-diagnosed in patients with spinal cord injury. J Rehabil Med Oct. (2007) 39:622–6. doi: 10.2340/16501977-0101
7. Hagen EM Eide GE, Rekand T, Gilhus NE, Gronning M. Traumatic spinal cord injury and concomitant brain injury: a cohort study. Acta Neurol Scand Suppl. (2010) (190):51–7. doi: 10.1111/j.1600-0404.2010.01376.x
8. Sharma B, Bradbury C, Mikulis D, Green R. Missed diagnosis of traumatic brain injury in patients with traumatic spinal cord injury. J Rehabil Med Apr. (2014) 46:370–3. doi: 10.2340/16501977-1261
9. Budisin B, Bradbury CC, Sharma B, Hitzig SL, Mikulis D, Craven C, et al. Traumatic brain injury in spinal cord injury: frequency and risk factors. J Head Trauma Rehabil Jul-Aug. (2016) 31:E33–42. doi: 10.1097/HTR.0000000000000153
10. Pandrich MJ, Demetriades AK. Prevalence of concomitant traumatic cranio-spinal injury: a systematic review and meta-analysis. Neurosurg Rev Feb. (2020) 43:69–77. doi: 10.1007/s10143-018-0988-3
11. Holly LT, Kelly DF, Counelis GJ, Blinman T, McArthur DL, Cryer HG. Cervical spine trauma associated with moderate and severe head injury: incidence, risk factors, and injury characteristics. J Neurosurg Apr. (2002) 96:285–91. doi: 10.3171/spi.2002.96.3.0285
12. Tian HL, Guo Y, Hu J, Rong BY, Wang G, Gao WW, et al. Clinical characterization of comatose patients with cervical spine injury and traumatic brain injury. J Trauma Acute Care Surg. (2009) 67:1305–10. doi: 10.1097/TA.0b013e31819db57c
13. Macciocchi SN, Bowman B, Coker J, Apple D, Leslie D. Effect of co-morbid traumatic brain injury on functional outcome of persons with spinal cord injuries. Am J Phys Med Rehabil Jan. (2004) 83:22–6. doi: 10.1097/01.PHM.0000104661.86307.91
14. Maas AI, Menon DK, Steyerberg EW, Citerio G, Lecky F, Manley GT, et al. Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury (CENTER-TBI): a prospective longitudinal observational study. Neurosurgery Jan. (2015) 76:67–80. doi: 10.1227/NEU.0000000000000575
15. Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol Oct. (2019) 18:923–34. doi: 10.1016/S1474-4422(19)30232-7
16. Gladitz J. Rubin, Donald B.: multiple imputation for nonresponse in surveys. John Wiley & Sons, Chichester – New York – Brisbane – Toronto – Singapore 1987, xxx, 258 S., 6 Abb., £ 30.25, ISSN 0271-6232. Biom J. (1989) 31:131–2. doi: 10.1002/bimj.4710310118
17. Team RC. R: A Language Environment for Statistical Computing. (2021). Available online at: https://www.r-project.org/ (accessed January 12, 2022).
18. Gennarelli TA, Wodzin E. AIS 2005: a contemporary injury scale. Injury Dec. (2006) 37:1083–91. doi: 10.1016/j.injury.2006.07.009
19. Salottolo K, Settell A, Uribe P, Akin S, Slone DS, O'Neal E, et al. The impact of the AIS 2005 revision on injury severity scores and clinical outcome measures. Injury Sep. (2009) 40:999–1003. doi: 10.1016/j.injury.2009.05.013
20. Swartz EE, Floyd RT, Cendoma M. Cervical spine functional anatomy and the biomechanics of injury due to compressive loading. J Athl Train Jul-Sep. (2005) 40:155–61.
21. Dewan MC, Rattani A, Gupta S, Baticulon RE, Hung YC, Punchak M, et al. Estimating the global incidence of traumatic brain injury. J Neurosurg. (2018) 1:1–18. doi: 10.3171/2017.10.JNS17352
Keywords: traumatic brain injury, traumatic spine injury, outcome, CENTER-TBI, spine trauma
Citation: Riemann L, Alhalabi OT, Unterberg AW, Younsi A and The CENTER-TBI investigators and participants (2022) Concomitant spine trauma in patients with traumatic brain injury: Patient characteristics and outcomes. Front. Neurol. 13:861688. doi: 10.3389/fneur.2022.861688
Received: 25 January 2022; Accepted: 28 June 2022;
Published: 18 August 2022.
Edited by:
Wiliam Panenka, University of British Columbia, CanadaReviewed by:
Mathieu van der Jagt, Erasmus Medical Center, NetherlandsCopyright © 2022 Riemann, Alhalabi, Unterberg, Younsi and The CENTER-TBI investigators and participants. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alexander Younsi, YWxleGFuZGVyLnlvdW5zaUBtZWQudW5pLWhlaWRlbGJlcmcuZGU=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.