- 1Department of Neurology, Hartford Hospital, Hartford, CT, United States
- 2Department of Neurology, University of Connecticut, Farmington, CT, United States
- 3Department of Research, Hartford Hospital, Hartford, CT, United States
Introduction: Understanding the potential embolic source in young patients with ESUS may improve the diagnosis and treatment of such patients.
Hypothesis: Potential embolic sources (PES) differ in young vs. older patients with ESUS, and, therefore, not all patients with ESUS have the same risk profile for stroke recurrence.
Methods: Young patients (age 18-49) with ESUS, who were admitted to our stroke center from 2006 to 2019, were identified retrospectively and matched with next consecutive older patients (age 50–99) with ESUS by admission date. PES were categorized as atrial cardiopathy, AFib diagnosed during follow-up, left ventricular disease (LVD), cardiac valvular disease (CVD), PFO or atrial septal aneurysm (ASA), and arterial disease. Patients, who had cancer or thrombophilia, were excluded. The type and number of PES and stroke recurrence rates were determined and compared between young and older patients.
Results: In young patients (55.3% women, median age 39 years), the most common PES was PFO/ASA, and the rate of other PES was low (2–7%). Half of the young patients (54.1%) had a single PES, only 10% had multiple PES, and 35.3% of young patients did not have any PES identified. In older patients (41.7% women, median age 74 years), the 3 most common PES were atrial cardiopathy (38.1%), LVD (35.7%), and arterial disease (23.8%). Nearly half of older patients (42.9%) had multiple PES. The rate of stroke recurrence tended to be lower in young patients as compared to older patients (4.9 vs. 11.4%, p = 0.29). During a median follow-up of 3 years, only 3 young patients (4.9%) had a recurrent stroke, and two of them had unclosed PFO. There were no recurrent strokes among young patients with no PES identified.
Conclusions: It was noted that PES differ in patients with ESUS according to age and differences in recurrence. PFO is the only common PES in young patients with ESUS. Future studies prospectively evaluating PES in both age groups are needed.
Introduction
The concept of embolic stroke of undetermined source (ESUS) was introduced by the cryptogenic stroke/ESUS international working group in 2014 (1). In patients with ESUS, the causal etiology might not be identified, but there are many potential embolic sources, also referred to as potential embolic sources (PES) (2, 3). These underlying PES are heterogeneous, but it is hypothesized that emboli are either cardiogenic, arteriogenic, or paradoxical from the veins. At present, the main challenge in ESUS remains how to decide on optimal secondary stroke prevention in the absence of a known stroke mechanism. The efficacy of antithrombotic agents for secondary prevention in ESUS was tested in the NAVIGATE ESUS and RE-SPECT ESUS trials. Results showed that anticoagulation was not superior to aspirin for the prevention of stroke in patients with ESUS (4, 5). Ischemic stroke in young adults is usually defined clinically by an age threshold of <50 at stroke occurrence, based on different risk factors and clinical characteristics between young and older patients with stroke (6). Approximately 15–20% of patients with ESUS are young patients (7, 8). However, previous RCTs and studies included mainly older patients at a mean age of around 65 years (2–5). There is limited information about PES and the associated risk of recurrent stroke in young patients with ESUS. In this retrospective study, we evaluated the rate and overlap degree of previously reported PES and assessed the rate of stroke recurrence per PES in young patients with ESUS and compared them with older patients with ESUS.
Materials and Methods
We retrospectively reviewed young patients (age 18–49) with ESUS who were admitted and followed at Hartford Hospital from 2006 to 2019. Each young patient was matched with the next consecutive older patient (age 50–99) with ESUS by admission date within the same period.
The ESUS was defined as non-lacunar brain infarct in the absence of extracranial or intracranial atherosclerosis causing ≥50% luminal stenosis in arteries supplying the area of infarcts, established major cardioembolic source, other specific cause (e.g., vasculitis/vasculopathy, dissection, vasospasm, or thrombophilia) according to the criteria proposed by the Cryptogenic Stroke/ESUS International Working Group and previous studies (1). The minimal standard evaluation was required for the diagnosis of ESUS, which included transthoracic or transesophageal echocardiography, CTA/MRA of head and neck, 12-lead EKG, at least 24 hours of continuous heart rhythm monitoring, and screening for inherited thrombophilia in patients younger than 50-year-old (1, 9). We did not include cancer as PES as hypercoagulability and embolism are both possible mechanisms of cancer-related stroke.
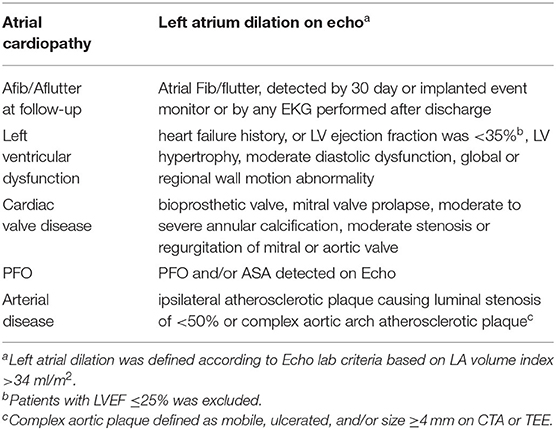
According to previous studies, we used the definitions of PES described in Table 1: categorized as atrial cardiopathy, AFib diagnosed during follow-up, left ventricular disease (LVD), cardiac valvular disease (CVD), PFO and/or Atrial septal aneurysm (ASA), and arterial disease (1–3). In a single patient, there may be none, single, or multiple PES. Atrial cardiopathy was defined as left atrial (LA) volume index >34 ml/m2 according to American Society of Echocardiography guidelines (10). AFib was diagnosed by EKG, 30-day or implanted heart rhythm monitor during follow-up.
Baseline and clinical stroke characteristics were compared between young and older patients using proportions for categorical variables and medians with interquartile range for continuous variables. The rate of different types of PES and numbers of PES were determined and compared between young and older patients with ESUS. After discharge, patients were followed up at Hartford Healthcare for at least one year with the available medical record. Patients who did not follow with Hartford Healthcare for at least one year were considered as lost to follow-up and were not included in the study. The recurrent ischemic stroke was diagnosed by repeated brain MRI or head CT during follow-up. The rate of stroke recurrence during follow-up was assessed by different types of PES, numbers of PES, and comparison with young and older patients with ESUS. All comparisons were performed using the Pearson Chi-square test with SPSS and p < 0.05 as the significance level.
Results
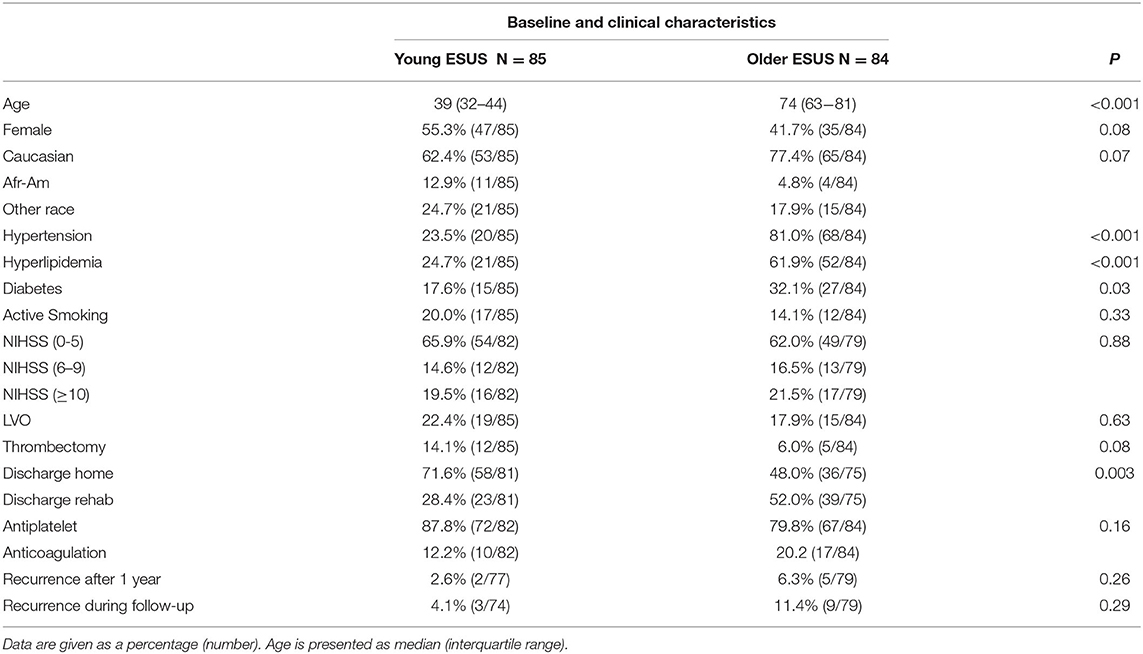
A total of 85 young patients with ESUS (55.3% women, median age 39 years) were identified and matched with 84 older patients (41.7% women, median age 74 years). The baseline and clinical characteristics of patients are summarized in Table 2. Young patients with ESUS tended to be women and ethnic minorities as compared to older patients.
As expected, a lower proportion of young patients with ESUS had hypertension, hyperlipidemia, and diabetes, although both young and older patients with ESUS had a similar rate of active smoking. The initial NIHSS at admission as well as the rate of large vessel occlusion were similar in young and older patients with ESUS, with young patients tending to receive mechanical thrombectomy. Young patients were more likely to be discharged home compared to older patients. Most young (87.8%) and older (79.8%) patients with ESUS were treated with antiplatelet agents with lower rates of anticoagulation among younger patients. Young patients with ESUS tended to have a lower rate of recurrent embolic stroke during follow-up as compared to older patients.
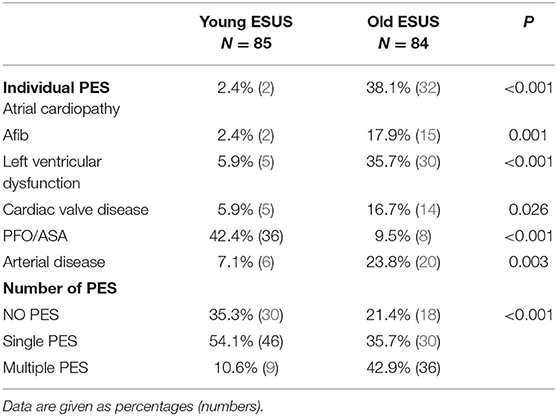
The most common PES in young patients with ESUS was PFO, with or without an atrial septal defect (ASA) (42.4%). Other types of PES were only identified in 2–7% of young patients with ESUS (Table 3). About half of young patients (54.1%) with ESUS were found to have a single PES, only 10% had multiple PES and about one-third (35.3%) did not have any PES identified (Table 3). In older patients with ESUS, the 3 most common PES were atrial cardiopathy (38.1%), LVD (35.7%), and arterial disease (23.8%). wDuring follow up, AFib was detected in 17.9% of older patients (about 32% of older patients with ESUS had a 30-day monitor and 11% had implanted monitors) as compared to in 2.4% of young patients with ESUS (~20% of young patients with ESUS had a 30-day monitor and 5% received implanted monitor). As compared to young patients, at least one PES was identified in 78.6% of older patients with ESUS. Nearly half (42.9%) of older patients had multiple PES (Table 3).
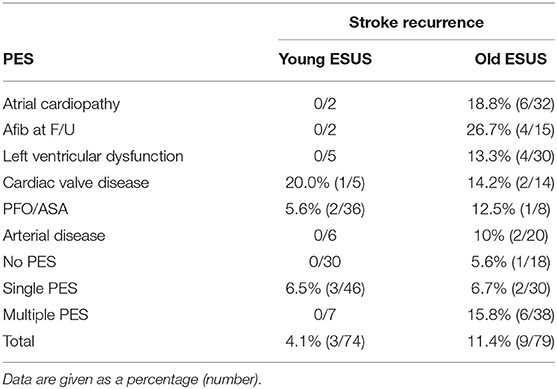
The rate of recurrent embolic stroke by PES is summarized in Table 4. During a median follow-up of 3 years, 13% of young patients with ESUS and 6% of older patients with ESUS were lost follow-up. About one out of 10 (11.4%) older patients with ESUS had a recurrent stroke. Older patients who were found to have A-fib during follow-up tended to have the highest rate of stroke recurrence (26.7%). Older patients who had multiple PES had a rate of 15.8% stroke recurrence compared to those who had single or no PES at 6.7% and 5.6%, respectively. Only one out of 25 young patients with ESUS (4.1%) had a recurrent stroke. Among the three young patients who had a recurrent stroke, two patients had PFO without closure and one patient had cardiac valve disease. There were no recurrent strokes among young patients with no PES identified.
Discussion
In this observational study, PFO and/or ASA were the only common PES in young patients with ESUS and found in nearly half (42%) of young patients. In contrast, PFO and/or ASA were detected in less than 10% of older patients, albeit many older patients who usually underwent transthoracic echocardiography without bubble study. Other PES, such as atrial cardiopathy, LVD, arterial disease, AFib, and cardiac valve disease, were frequently identified in about 15–40% of older patients, but they were uncommonly detected in less than 10% of young patients. Overall, young patients with ESUS had a lower burden and less overlap of PES than older patients. Half (54%) of young patients had single PES, and one-third of young patients did not have any PES identified, whereas nearly half of older patients had two or three PES. Our findings are consistent with a recent study that identified three main clusters of patients with ESUS according to clinical characteristics and cerebrovascular risk factors. Those clusters were correlated to the potential arteriogenic, cardiogenic, and paradoxical source of embolic stroke. One cluster was associated with PFO in patients at young ages (11).
According to a systemic review including most studies of older patients, ESUS is associated with a recurrent stroke rate of 4.5% per year (8). Among the young patients registered to the Helsinki Young Stroke Registry, young patients with ESUS had a relatively low cumulative risk of recurrent stroke that is about 1.95% annually over a median follow-up time of 10 years (7). Again, our study showed young patients with ESUS tended to have a lower rate of stroke recurrence as compared with older patients with ESUS. Only three young patients had a recurrent stroke, and two of them had PFO without closure. In recent years, emerging evidence indicates that paradoxical embolism through PFO is a potential causal mechanism of ESUS (12). PFO with large shunting and/or ASA was associated with a high risk for recurrent stroke (12, 13). Two recent RCTs found that percutaneous closure of PFO in appropriately selected patients decreases the risk of recurrent stroke in young patients with ESUS (14, 15). Only one patient with cardiac valve disease had a recurrent stroke for other PES. The rate of other PES-associated stroke recurrence is difficult to be interpreted in our study because of their low detection rate in young patients with ESUS. As compared to young patients, older patients who were found to have A-fib during follow-up tended to have the highest rate of stroke recurrence (26.7%). And all other PES-associated stroke recurrence rate was 10–20% in older patients with ESUS. However, given half of older patients had multiple PES, it is difficult to determine the PES that are potentially implicated in each patient. Previous studies found that older patients with multiple PES were not at increased risk of recurrent stroke as compared to patients who had single PES (2, 3). The finding may indicate high-risk PES, but not multiple low-risk PES, play the key role in stroke recurrence. Young patients with ESUS had less overlap of PES; our study showed that among 10% of young patients who had two PES identified, none of them had a recurrent stroke.
The low rate of PES in young patients with ESUS is partly explained by the low rate of traditional cerebrovascular risk factors. In addition, according to recommendations from the international ESUS working group, only minimal standard evaluation is required to rule out established etiology, other diagnostic tests such as malignancy screening, hypercoagulable testing, 30-day or implanted rhythm monitors, TEE, and special MRIs may be considered on a case-by-case basis (1). PES detection rate may be higher if all the young patients underwent TEE, prolonged cardiac monitoring, and more advanced diagnostic workup. It is important to note, however, that occult AFib was a rare finding in younger patients with ESUS in our study, and of low yield. High-resolution MRI had higher sensitivity and specificity in detecting vessel wall abnormality such as atheroma and dissection (16, 17). If high-resolution MRI can be used in routine practice, it may help to identify more PES in young patients. Furthermore, young stroke patients have unique risk factors such as migraines, the use of oral contraceptive pills, or illicit drugs (18–20). Mechanisms other than embolisms such as hypercoagulability, vasospasm, and in-situ thrombosis may be the cause of cryptogenic stroke in young patients. Cancer, especially active cancer is considered a risk factor for ischemic stroke in young adults (21, 22). The hypercoagulable state is the most important mechanism of malignancy-associated ischemic stroke and other potential mechanisms include marantic endocarditis, the effect of chemotherapy or radiotherapy, accelerated atherosclerosis, and intravascular disease (Lymphoma) (22). Inherited thrombophilia is a group of disorders including factor V Leiden or Prothrombin mutation, deficiency of Antithrombin, protein C and protein S, and hyperhomocysteinemia caused by mutations in methylenetetrahydrofolate reductase (23, 24). Studies have shown an inconsistent or weak association between inherited thrombophilia and ischemic stroke (23–25), although a moderately stronger risk was reported in a subgroup of younger patients (25, 26). Our study focused on ESUS with embolism as a likely cause of stroke and we did not include patients with malignancy or inherited thrombophilia, in which a hypercoagulable state is a potential mechanism. However, malignancy and thrombophilia are important risk factors for cryptogenic stroke in young adults and they can cause imaging patterns similar as embolic stroke. Of note, our study shows PES was not identified in one-third of young patients with ESUS, and none of those patients had a recurrent stroke. Although the causal mechanism remains unknown, this finding is reassuring that stroke recurrence was rare in these young patients with ESUS without PES identified.
The findings of our study suggest young patients differ from older patients with ESUS. Although the two groups have similar stroke severity and rate of large vessel occlusion, they are different regarding risk factors, possible embolic source, and stroke recurrence. Current RCTs testing antithrombotics for stroke prevention in ESUS mainly focused on older patients and the finding would not be well-applicable to young patients (5, 6). Severe atrial cardiopathy was identified as an independent risk factor in the absence of AFib in patients with ESUS (27). However, due to the low prevalence of atrial cardiopathy in younger patients, the ongoing ARCADIA (Atrial Cardiopathy and Antithrombotic Drugs in Prevention after Cryptogenic Stroke) trial only enrolls patients with ESUS older than 45 years (28). PFO is the only common PES in young patients with ESUS. Given the low rate of other PES, their roles in stroke recurrence are less significant in young patients with ESUS. Future studies in young patients with ESUS should focus on PFO-related stroke to better define features of high-risk PFO and identify appropriate patients to benefit from PFO closure as it will further lower stroke recurrence.
Strengths and Limitations
Our study is the first study to assess PES and its associated rate of stroke recurrence in young patients with ESUS over 15 years. However, due to a single-center design, we were unable to estimate the risk of recurrent stroke by PES given the relatively small number of patients. One common limitation of all PES studies is that the rate and overlap of PES are usually underestimated. PES in our study was defined according to criteria used in NAVIGATE ESUS study and reviewed by an international ESUS working group (1, 2). Many recently identified PES such as new biomarkers of atrial cardiopathy, including NT-proBNP and ECG indexes (28–30), pulmonary arteriovenous fistula, and carotid web, were not included in the study (31, 32). Another common limitation in PES studies is that the criteria also likely include many low-risk PES. Those PES are usually associated with a very low rate of stroke and are often likely incidental without clinical significance. In the future, a prospective multi-center study better defined PES, with a sufficient sample size and completion of extensive diagnostic tests, is needed to assess the PES burden and associated risk of stroke recurrence in young patients with ESUS.
In conclusion, PES differs in patients with ESUS according to age. PFO is the only common PES in young patients with ESUS. Young patients have less burden and overlap with other PES that are commonly identified in older patients. Future research should continue to identify high-risk PES specific to young patients and verify features of high-risk PFO.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by IRB of Research Center at Hartford Hospital. Written informed consent for participation was not required for this study in accordance with the National Legislation and the Institutional Requirements.
Author Contributions
YH: study design, data collection, and manuscript writing and revision. AN: study design and manuscript revision. MA: manuscript revision. IS: data analysis and manuscript revision. AE: data collection. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank Dr. Walter N. Kernan at Yale-New Haven Health for his suggestions on study design.
References
1. Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O'Donnell MJ, et al. Cryptogenic Stroke/ESUS international working group. embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. (2014) 13:429–38. doi: 10.1016/S1474-4422(13)70310-7
2. Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J Am Heart Assoc. (2019) 8:e012858. doi: 10.1161/JAHA.119.012858
3. Ntaios G, Pearce LA, Veltkamp R, Sharma M, Kasner SE., Korompoki E, et al. NAVIGATE ESUS investigators potential embolic sources and outcomes in embolic stroke of undetermined source in the NAVIGATE-ESUS trial. Stroke. (2020) 51:1797–804. doi: 10.1161/STROKEAHA.119.028669
4. Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke. (2009) 40:1195–203. doi: 10.1161/STROKEAHA.108.529883
5. Hart RG, Sharma M, Mundl H, Kasner SE, Bangdiwala SI, Berkowitz SD. el al. NAVIGATE ESUS Investigators. rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. (2018) 378:2191–201.
6. Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, et al. RE-SPECT ESUS steering committee and investigators. dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. (2019) 380:1906–17. doi: 10.1056/NEJMoa1813959
7. Martinez-Majander N, Aarnio K, Pirinen J, Lumikari T, Nieminen T, Lehto M, et al. Embolic strokes of undetermined source in young adults: baseline characteristics and long-term outcome. Eur J Neurol. (2018) 25:535–41. doi: 10.1111/ene.13540
8. Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: a systematic review and clinical update. Stroke. (2017) 48:867–72. doi: 10.1161/STROKEAHA.116.016414
9. Morris JG, Singh S, Fisher M. Testing for inherited thrombophilias in arterial stroke: can it cause more harm than good? Stroke. (2010) 41:2985–90. doi: 10.1161/STROKEAHA.110.595199
10. Tan BYQ, Ho JSY, Sia CH, Boi Y, Foo ASM, Dalakoti M, et al. Left atrial volume index predicts new-onset atrial fibrillation and stroke recurrence in patients with embolic stroke of undetermined source. Cerebrovasc Dis. (2020) 49:285–91. doi: 10.1159/000508211
11. Lattanzi S, Rinaldi C, Pulcini A, Corradetti T, Angelocola S, Zedde ML, et al. Clinical phenotypes of embolic strokes of undetermined source. Neurol Sci. (2021) 42:297–300. doi: 10.1007/s10072-020-04700-2
12. Goel SS, Tuzcu EM, Shishehbor MH, de Oliveira EI, Borek PP, Krasuski RA, et al. Morphology of the patent foramen ovale in asymptomatic versus symptomatic (stroke or transient ischemic attack) patients. Am J Cardiol. (2009) 103:124–9. doi: 10.1016/j.amjcard.2008.08.036
13. Mas JL, Arquizan C, Lamy C, Zuber M, Cabanes L, Derumeaux G, et al. Patent Foramen Ovale and Atrial Septal Aneurysm Study Group. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. (2001) 345:1740–6. doi: 10.1056/NEJMoa011503
14. Søndergaard L, Kasner SE, Rhodes JF, Andersen G, Iversen HK, Nielsen-Kudsk JE, et al. Gore REDUCE clinical study investigators. patent foramen ovale closure or antiplatelet therapy for cryptogenic stroke. N Engl J Med. (2017) 377:1033–42. doi: 10.1056/NEJMoa1707404
15. Mas JL, Derumeaux G, Guillon B, Massardier E, Hosseini H, Mechtouff L, et al. CLOSE investigators. patent foramen ovale closure or anticoagulation vs. antiplatelets after stroke. N Engl J Med. (2017) 377:1011–21. doi: 10.1056/NEJMoa1705915
16. Yuan X, Cui X, Gu H, Wang M, Dong Y, Cai S, et al. Evaluating cervical artery dissections in young adults: a comparison study between high-resolution MRI and CT angiography. Int J Cardiovasc Imaging. (2020) 36:1113–9. doi: 10.1007/s10554-020-01799-4
17. Freilinger TM, Schindler A, Schmidt C, Grimm J, Cyran C, Schwarz F, et al. Prevalence of nonstenosing, complicated atherosclerotic plaques in cryptogenic stroke. JACC Cardiovasc Imaging. (2012) 5:397–405. doi: 10.1016/j.jcmg.2012.01.012
18. Bousser MG. Estrogens, migraine, and stroke. Stroke. (2004) 35:2652–6. doi: 10.1161/01.STR.0000143223.25843.36
19. Roach RE, Helmerhorst FM, Lijfering WM, Stijnen T, Algra A, Dekkers OM. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. (2015) 2015:CD011054. doi: 10.1002/14651858.CD011054.pub2
20. Fonseca AC, Ferro JM. Drug abuse and stroke. Curr Neurol Neurosci Rep. (2013) 13:325. doi: 10.1007/s11910-012-0325-0
21. Aarnio K, Joensuu H, Haapaniemi E, Melkas S, Kaste M, Tatlisumak T, et al. Cancer in young adults with ischemic stroke. Stroke. (2015) 46:1601–6. doi: 10.1161/STROKEAHA.115.008694
22. Navi BB, Kasner SE, Elkind MSV, Cushman M, Bang OY, DeAngelis LM. Cancer and embolic stroke of undetermined source. Stroke. (2021) 52:1121–30. doi: 10.1161/STROKEAHA.120.032002
23. Chiasakul T, De Jesus E, Tong J, Chen Y, Crowther M, Garcia D, et al. Inherited thrombophilia and the risk of arterial ischemic stroke: a systematic review and meta-analysis. J Am Heart Assoc. (2019) 8:e012877. doi: 10.1161/JAHA.119.012877
24. Ho GY, Eikelboom JW, Hankey GJ, Wong CR, Tan SL, Chan JB, et al. Methylenetetrahydrofolate reductase polymorphisms and homocysteine-lowering effect of vitamin therapy in Singaporean stroke patients. Stroke. (2006) 37:456–60. doi: 10.1161/01.STR.0000199845.27512.84
25. Alhazzani AA, Kumar A, Selim M. Association between factor v gene polymorphism and risk of ischemic stroke: an updated meta-analysis. J Stroke Cerebrovasc Dis. (2018) 27:1252–61. doi: 10.1016/j.jstrokecerebrovasdis.2017.12.006
26. Jiang B, Ryan KA, Hamedani A, Cheng Y, Sparks MJ, Koontz D, et al. Prothrombin G20210A mutation is associated with young-onset stroke: the genetics of early-onset stroke study and meta-analysis. Stroke. (2014) 45:961–7. doi: 10.1161/STROKEAHA.113.004063
27. Kamel H, Okin PM, Elkind MS, Iadecola C. Atrial fibrillation and mechanisms of stroke: time for a new model. Stroke. (2016) 47:895–900. doi: 10.1161/STROKEAHA.115.012004
28. Kamel H, Longstreth WT Jr, Tirschwell DL, Kronmal RA, Broderick JP, Palesch YY, et al. The AtRial cardiopathy and antithrombotic drugs in prevention after cryptogenic stroke randomized trial: rationale and methods. Int J Stroke. (2019) 14:207–14. doi: 10.1177/1747493018799981
29. Bahit MC, Sacco RL, Easton JD, Meyerhoff J, Cronin L, Kleine E, et al. RE-SPECT ESUS Steering Committee and Investigators. predictors of atrial fibrillation development in patients with embolic stroke of undetermined source: an analysis of the RE-SPECT ESUS Trial. Circulation. (2021) 144:1738–46. doi: 10.1161/CIRCULATIONAHA.121.055176
30. Li TYW, Yeo LLL, Ho JSY, Leow AS, Chan MY, Dalakoti M, et al. Association of electrocardiographic p-wave markers and atrial fibrillation in embolic stroke of undetermined source. Cerebrovasc Dis. (2021) 50:46–53. doi: 10.1159/000512179
31. Sousa S, Vasco Costa N, Carmona C, Coimbra É, Pita F. Recurrent stroke in a young woman with a single pulmonary arteriovenous fistula: an unusual association. Case Rep Neurol. (2017) 9:293–8. doi: 10.1159/000484682
Keywords: ESUS, potential embolic source, PFO, ischemic stroke in young adults, AFib
Citation: Hou Y, Elmashad A, Staff I, Alberts M and Nouh A (2022) Potential Embolic Sources Differ in Patients With Embolic Stroke of Undetermined Source According to Age: A 15-Year Study. Front. Neurol. 13:860827. doi: 10.3389/fneur.2022.860827
Received: 23 January 2022; Accepted: 14 April 2022;
Published: 17 May 2022.
Edited by:
Bing Tian, Naval Medical University, ChinaReviewed by:
Ching-Hui Sia, National University of Singapore, SingaporeSimona Lattanzi, Marche Polytechnic University, Italy
Copyright © 2022 Hou, Elmashad, Staff, Alberts and Nouh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amre Nouh, YW1yZS5ub3VoQGhoY2hlYWx0aC5vcmc=
 Yan Hou
Yan Hou Ahmed Elmashad
Ahmed Elmashad Ilene Staff3
Ilene Staff3 Amre Nouh
Amre Nouh