- 1Child Neurology and Psychiatry Unit, Department of Human Neuroscience, Sapienza University of Rome, Rome, Italy
- 2Department of Dynamic and Clinical Psychology, and Health Studies, Sapienza University of Rome, Rome, Italy
Background: This paper aimed to evaluate the frequency of observation of genetically determined developmental encephalopathies with epilepsy and movement disorders in a specialistic center, the distribution of etiologies and presenting clinical hallmarks, and the mean times for the achievement of molecular genetic diagnosis.
Patients and Methods: Retrospective data about clinical phenotypes, etiology, and diagnostic pathways were collected in all the genetically confirmed patients with developmental encephalopathies with epilepsy and movement disorders referred to our institution between 2010 and 2020. The cohort was divided into two groups according to the predominant movement disorder type: 1) Group A: patients with hyperkinetic movement disorders; 2) Group B: patients with hypokinetic movement disorders. Both groups were analyzed in terms of developmental, epileptic, and movement disorder phenotypes.
Results: The cohort included 69 patients (Group A = 53; Group B = 16). The etiological spectrum was heterogeneous with a predominance of Rett and Angelman syndrome in Group A and neurodegenerative disorders in Group B. A moderate/severe intellectual disability was assessed in 58/69 patients (mean age at the first signs of developmental impairment = 1,87 ± 1,72 years). Group A included patients with an earlier onset of epileptic seizures (2,63 ± 3,15 vs. 4,45 ± 5,55 years of group B) and a predominant generalized motor semiology of seizures at the onset. Focal seizures were the main initial epileptic manifestations in Group B. Seizures were noticed earlier than movement disorders in Group A while the opposite occurred in Group B. A higher increase in molecular genetic diagnosis was obtained in the last five years. Mean diagnostic delay was longer in Group B than in Group A (12,26 ± 13,32 vs. 5.66 ± 6.41 years). Chorea as an initial movement disorder was associated with a significantly longer diagnostic delay and a higher age at etiological diagnosis.
Conclusions: This study suggested: (a) a higher frequency of genetic defects involving neurotransmission, neuronal excitability, or neural development in patients with hyperkinetic movement disorders; (b) a higher frequency of neurodegenerative courses and a longer diagnostic delay in patients with hypokinetic movement disorders.
Introduction
The spectrum of genetic developmental encephalopathies presenting with epilepsy and movement disorders has significantly expanded in the last decade with the characterization of more than 100 monogenic disorders, and several genotype-phenotype correlation studies prominently focusing on single genes, specific pathogenic copy number variants, or non-mendelian conditions (1, 2).
The increasing availability of next generation sequencing techniques resulted in a higher number of etiological diagnoses, but also highlighted the importance of a careful evaluation of phenotypes for the interpretation of molecular data (3, 4).
The present single tertiary center study retrospectively analyzed the distribution of etiologies and presented phenotypes in a cohort of patients with these complex pediatric-onset disorders. The study aimed to evaluate the frequency of observation of these conditions in a specialistic center, their most frequent clinical hallmarks, and the mean times for the achievement of molecular genetic diagnosis in the last decade (during the age of introduction and diffusion of next generation sequencing methods).
Patients and Methods
We retrospectively collected 69 patients (35 females and 34 males) with genetically confirmed developmental encephalopathies with epilepsy and movement disorders, who were referred to our Institution and received a genetic diagnosis between January 2010 and January 2020.
Medical records and video recordings for movement disorder characterization were reviewed and data were collected in a standardized digital form to include demographic information, perinatal history, developmental milestones, predominant seizure, and movement disorder subtypes, onset and evolution of seizures and movement disorders during the follow-up, the severity of developmental impairment, age at the diagnosis, diagnostic delay (time between the onset of symptoms and the molecular genetic diagnosis), EEG patterns at onset and during the follow-up, neuroimaging features, relevant neurophysiologic and laboratory investigations, pathogenic or likely pathogenic gene or copy number variants.
Seizure types and epilepsy syndromes were classified according to the 2017 classification by ILAE Commission for Classification and Terminology (5, 6).
Patients were subdivided into two groups, patients with hyperkinetic (Group A) and patients with hypokinetic (Group B) movement disorders according to the same criteria that are currently adopted for adults (7). Hyperkinetic movements (i.e., unwanted and excess movements) included dystonia, chorea, athetosis, myoclonus, tremor, tics, ataxia, and stereotypies. Hypokinetic movements (i.e., decrease in the number of movements) included hypokinetic-rigid syndrome or parkinsonism (8, 9).
In both groups, we evaluated the timing for the achievement of the etiological diagnosis through a comparison between temporal parameters (age at the onset of seizures, age at the onset of movement disorders, age at the molecular genetic diagnosis and diagnostic delay) and clinical manifestations (seizure types and type of movement disorder).
We also analyzed the distribution of the diagnostic delay during the years in which the molecular diagnoses were made. The temporal periods were divided in 4 intervals: PERIOD 1: 2016-2020; PERIOD 2: 2011- 2015; PERIOD 3 = 2006-2010; PERIOD 4 = before 2005. This last temporal clustering was introduced to minimize the burden of the different diagnostic yields associated with the various molecular genetic technologies that were available over the years. These different methods included Sanger sequencing of specific single genes, array CGH, targeted gene panels for epilepsy and/or movement disorders, clinical exome, or whole-exome sequencing. Single gene variants were classified according to the American College of Medical Genetics criteria (3, 10).
Statistical analysis was performed using SPSS 26.0 statistical software package for Windows. Data were reported as frequencies and percentages for discrete variables, as well as means and standard deviations for continuous variables. Chi-squared test (χ2) was used to reveal differences between and within groups in the discrete variables investigated, while a one-way analysis of variance (ANOVA) was carried out to test between and within groups differences in continuous variables. Bonferroni correction was applied to the post-hoc tests. The significance level for all statistical tests was set a priori to α = 0.05.
Results Cohort Composition
Sixty-nine patients (35 females and 34 males), with a mean age of 16.26 ± 9.18 years, with a confirmed molecular genetic diagnosis, were selected. Group A included 53 patients (29 females and 24 males) while 16 patients (6 females and 10 males) belonged to Group B.
The mean age at the molecular genetics diagnosis was 8.75 ± 8.743 years with a mean diagnostic delay of 6.21 ± 8.05 years.
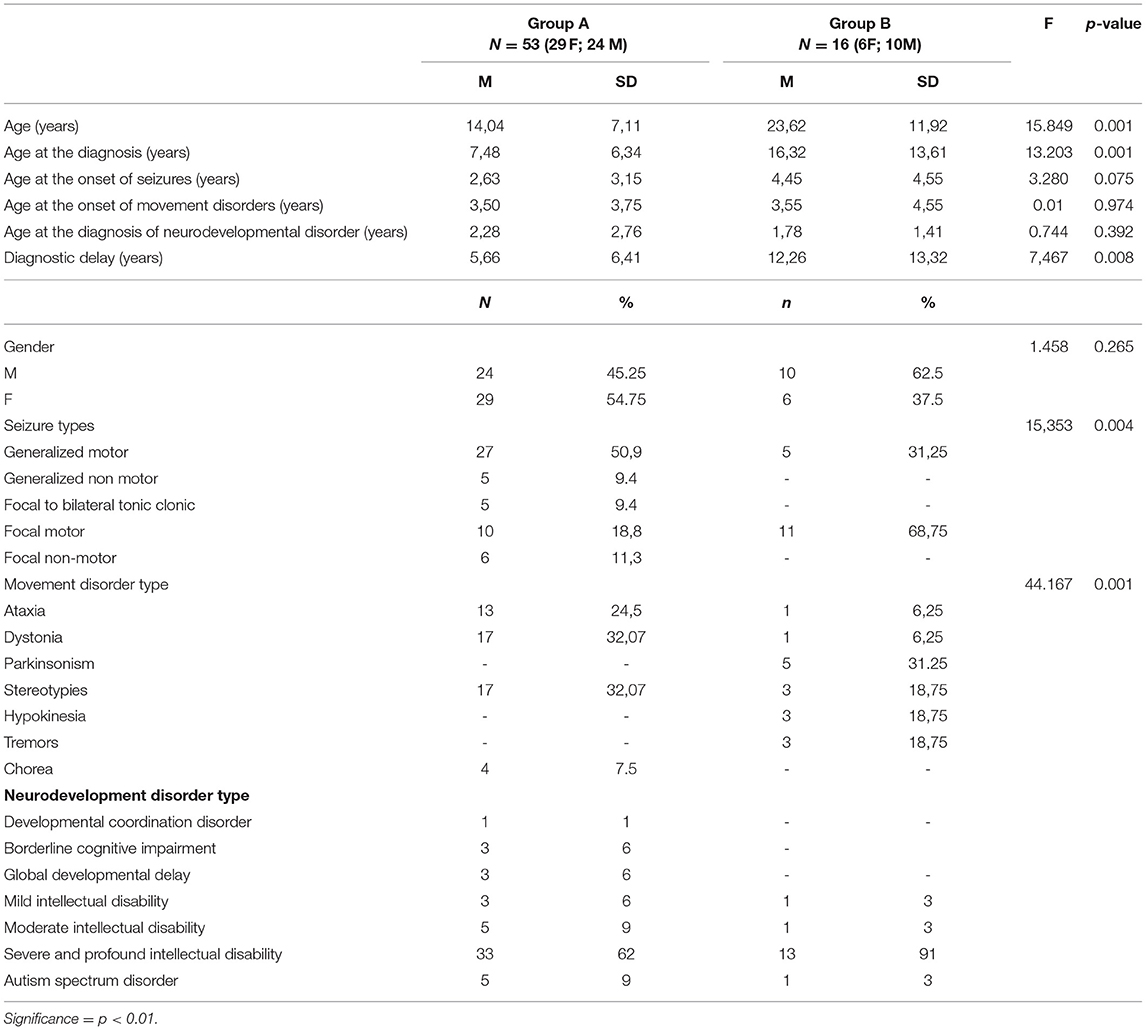
Tables 1, 2 summarize the demographic, genetic, and clinical data of patients with hyperkinetic (Group A) and hypokinetic movement disorders (Group B).
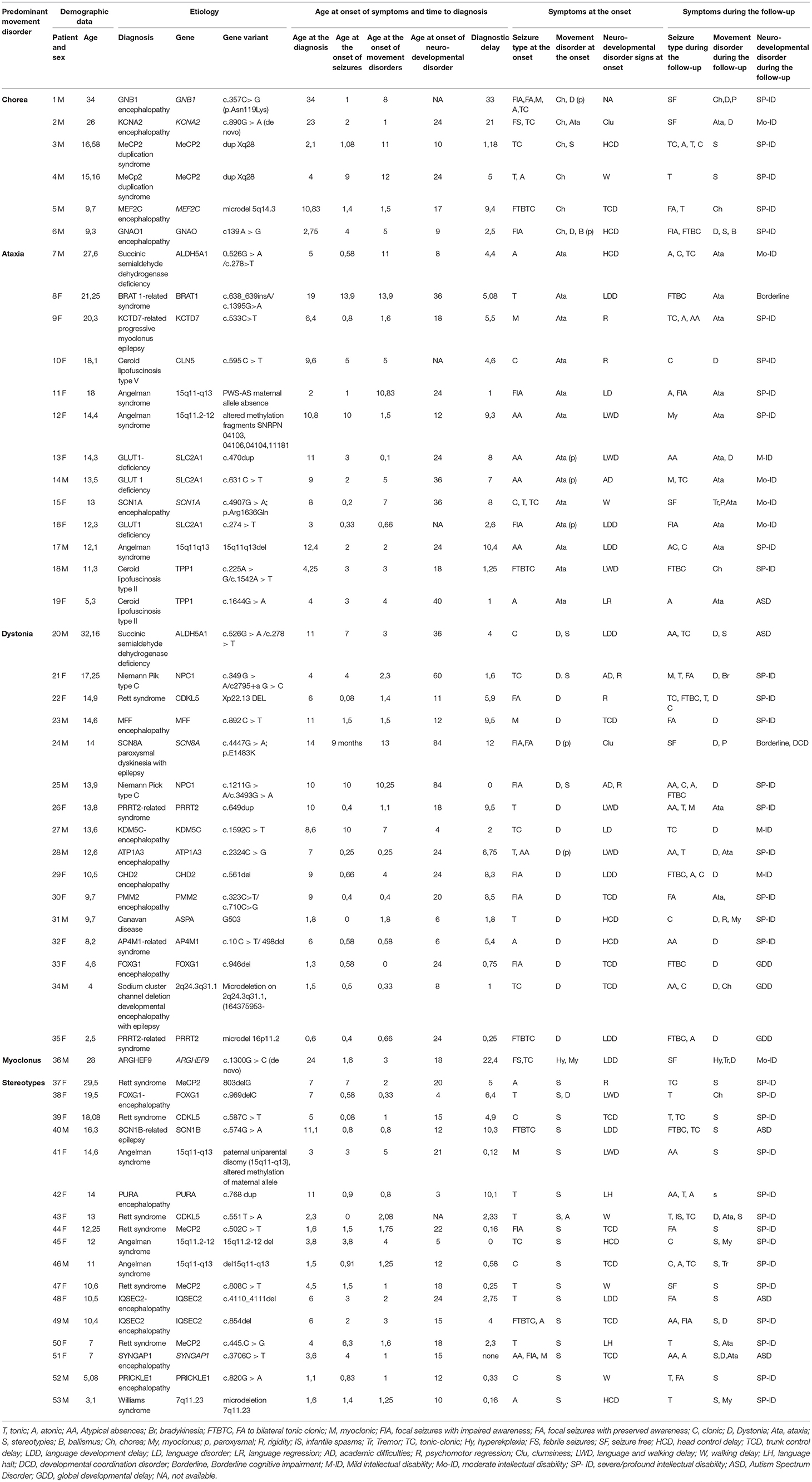
Table 1. Demographic, clinical, and molecular genetics features of patients with developmental encephalopathies presenting with epilepsy and hyperkinetic movement disorders (Group A).
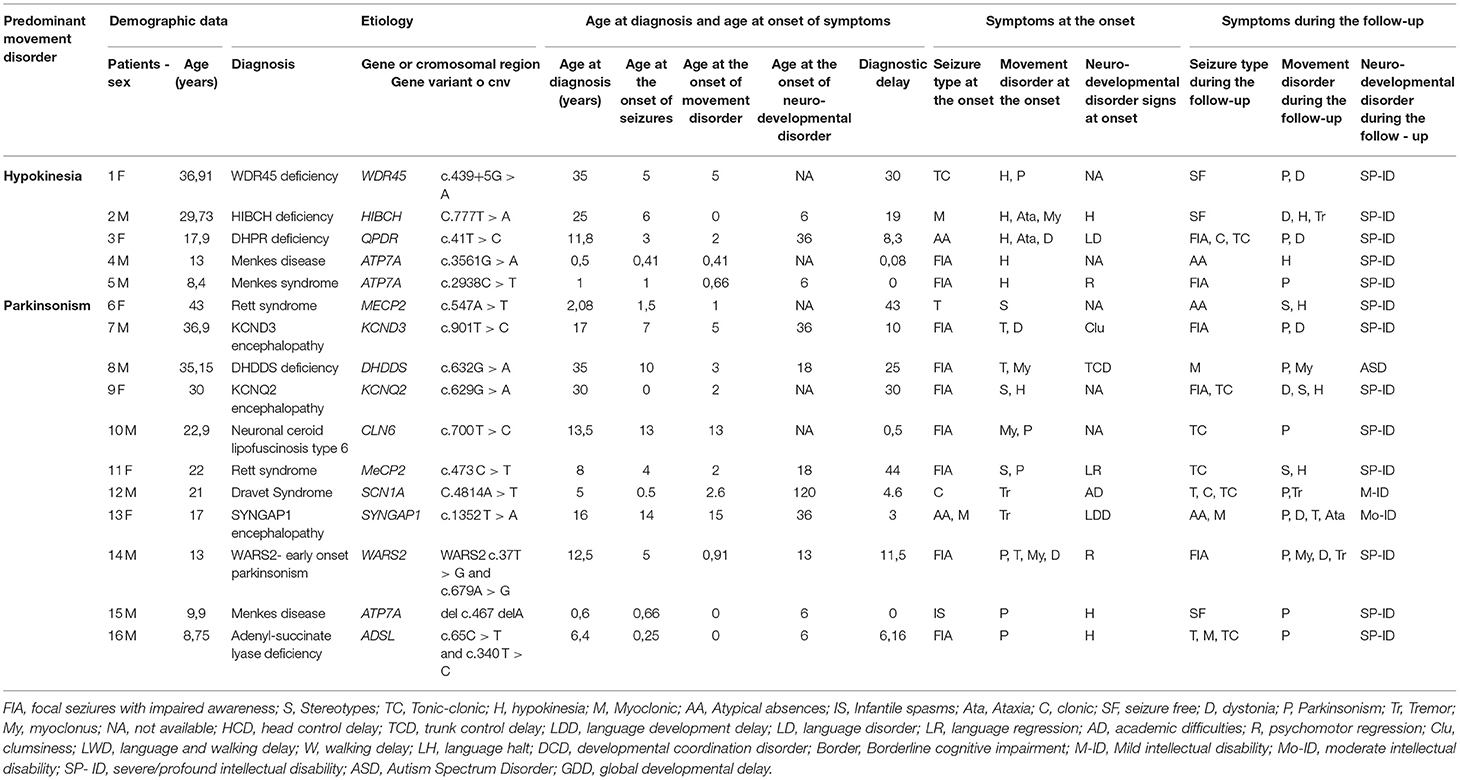
Table 2. Demographic, clinical, and molecular genetics features of patients with developmental encephalopathies presenting with epilepsy and hypokinetic movement disorders (Group B).
Table 3 summarizes both groups: (a) demographic data; (b) temporal distribution of clinical presentations (e.g., age at the onset of each cluster of symptoms) and diagnostic steps (e.g., age at the diagnosis and diagnostic delay); (c) distribution of seizure and movement disorder types.
In Group B the mean patient age was almost double compared to that of Group A (Table 3).
A neurodegenerative course was observed in 30/53 patients of Group A and 14/16 patients of Group B.
Etiological Spectrum
The distribution of genetic etiologies according to the different presenting seizure and movement disorder types in the whole cohort is summarized in Figure 1, Table 4.
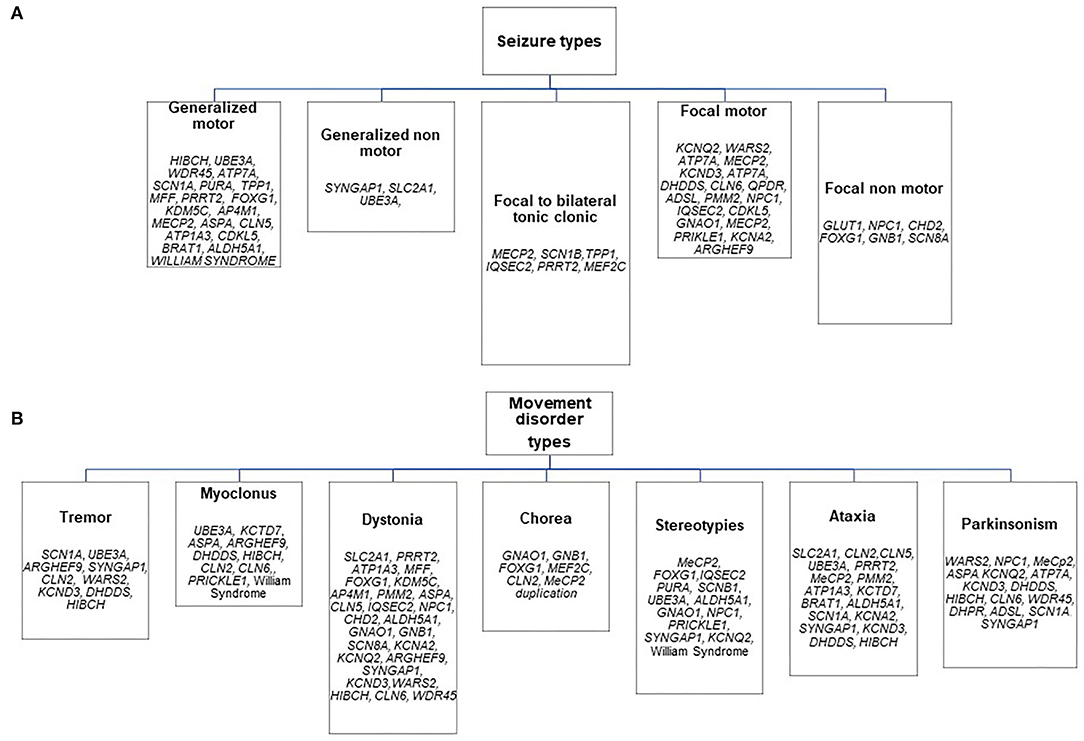
Figure 1. Distribution of genetic etiologies according to presenting seizure (A) and movement disorder (B) types in the whole sample.
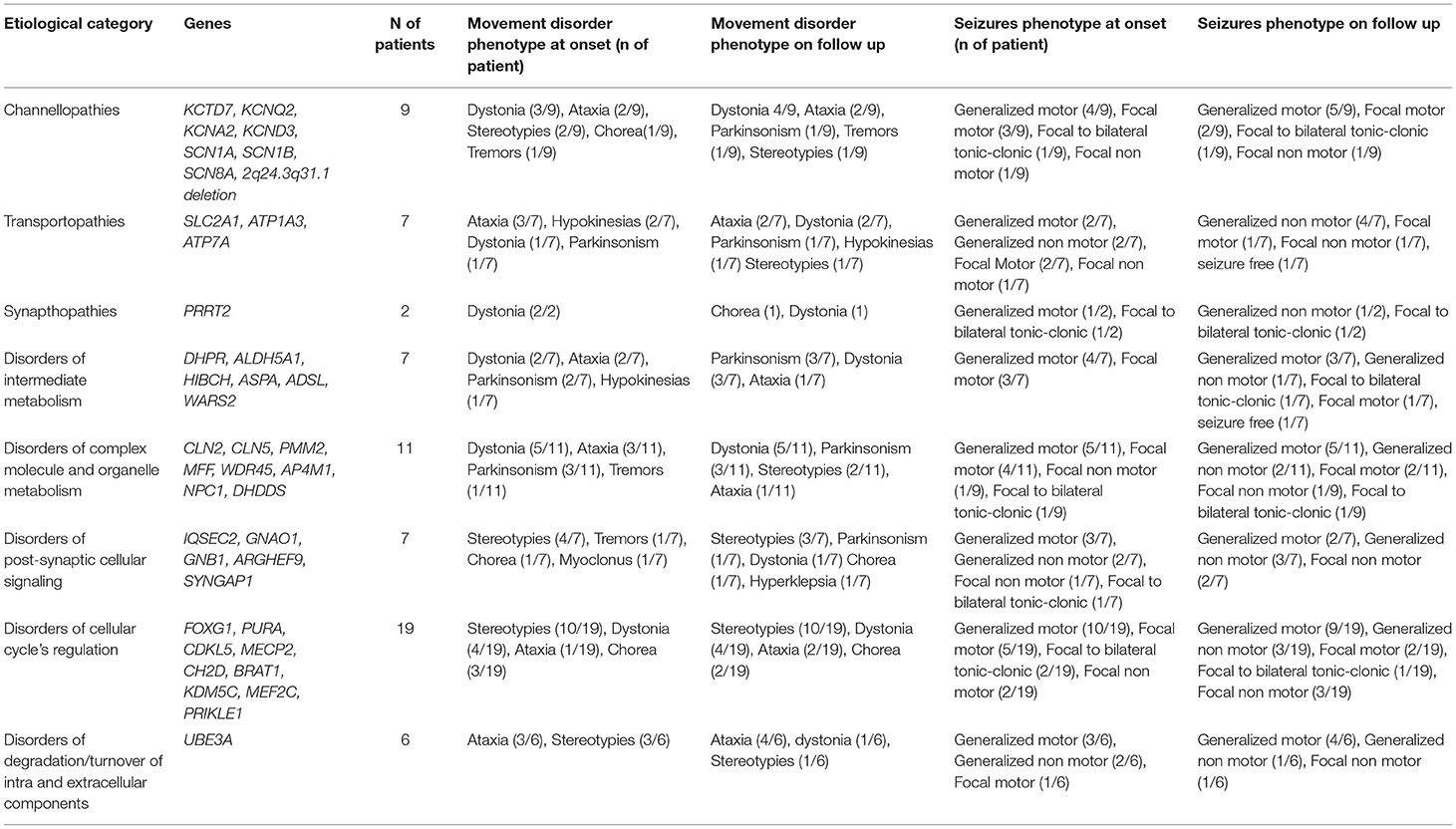
Table 4. Distribution of epilepsy and movement disorder phenotypes at onset and during the follow-up in the different functional groups of genetic etiologies.
Almost half of the cases carried pathogenic variants of genes involved in the regulation of the cellular cycle and intracellular metabolism (Table 4).
In Group A the most frequent diseases were Angelman Syndrome (6/53) and typical (5/53 patients harboring pathogenic MeCP2 variants) or Atypical Rett Syndrome (8/53 patients with pathogenic variants in CDKL5, FOXG1, IQSEC2, PURA or MEF2C genes), accounting for 35% of diagnosis (19/53). A metabolic disorder was diagnosed in 22% of patients (13/53). Sodium and potassium channelopathies (5/23 patients), synaptopathies (3/53), and postsynaptic signaling disorders (3/53) accounted for most of the remaining cases.
In Group B 10/16 patients had a metabolic disorder (3/9 with Menkes disease), 3/16 a sodium or potassium channelopathy (SCN1A, KCNQ2, KCND3), 2/16 Rett syndrome, and 1/16 a synaptopathy (Table 2). All metabolic disorders described in this group, except for DHPR deficiency, had a neurodegenerative course and 5/16 were associated to brand new genes or genes described in the last decade (e.g., WARS2, KCND3, DHDDS, HIBCH, WDR45).
A subset of genes including MeCP2, SCN1A, and SYNGAP1 was identified both in Group A and B in patients with different ages and disease stages (Tables 1, 2). Coherently hypokinetic features have been reported as late features in these conditions, preceded by hyperkinetic movement disorders.
Developmental History
A complete developmental history was available for 62/69 patients. The mean age at the first signs of neurodevelopmental impairment was lower than the onset of seizures and movement disorders without significant differences between Group A and Group B (Table 3).
No developmental milestones were achieved in 16/69 patients while 48/69 patients experienced different degrees of delay in one or more developmental milestones (Table 3). Motor impairment was variable with an autonomous walking that was achieved in 34/69 patients while 18/69 were wheelchair bounded and 17/69 were bedridden.
A profound-severe to moderate cognitive impairment was diagnosed in 58/69 patients (71% of patients in Group A vs. 94% of patients in Group B). Developmental regression was observed in 8 patients (Patients 9, 10, 19, 21, 22, 25, 37 in Table 1 and patient 14 in Table 2). An autism spectrum disorder was diagnosed in 12% of patients.
The developmental impairment was borderline in 2 patients belonging to group A (patients 8 and 24 in Table 1). Patient 8 in Table 1 was a female carrying a pathogenic variant of BRAT1 gene who had also a less severe epilepsy phenotype compared to other cases previously reported in the literature harboring the same variant (11). Patient 24 in Table 1 presented with a previously reported SCN8A variant associated with a benign childhood focal epilepsy, paroxysmal dyskinesia, and borderline cognitive functioning with minor coordination issues (12).
Epilepsy Phenotype
Seizure onset occurred during infancy or early childhood in 82,7% of patients. Motor seizures accounted for 82,61% of initial epileptic manifestations with almost half of the patients presenting with a generalized semiology while the frequency of non-motor seizures tended to double on follow-up (Table 4).
Patients belonging to Group A experienced an earlier onset of seizures than the ones of group B even if the difference in terms of age at the onset was not statistically significant (Table 3).
The initial epileptic manifestations mainly included generalized motor seizures in Group A (with a predominance of tonic seizures accounting for 21% of cases) and focal motor seizures with impaired awareness in Group B (56% of the cases) (Tables 1–3). In Group A, seven patients became seizure-free (13,2%), and none in Group B (Tables 1, 2).
These clinical patterns were associated with a concurrent predominance of multifocal (58,5% of patients in Group A) and focal (43% of patients in Group B) interictal EEG abnormalities. EEG was generally non-specific in terms of consistency with the epilepsy phenotype reported in the literature for the considered gene, with two significant exceptions in Group A (Table 1: Patients 9 and 19 who had early onset photosensitivity at low frequencies in association, respectively, with a neuronal ceroid lipofuscinosis type 2 and KCTD7-related progressive myoclonus epilepsy) and in two patients of Group B (Table 2: Patient 9 who had focal abnormalities during neonatal age as frequently reported in KCNQ2 encephalopathy and Patient 15 with Menkes disease who experienced a West syndrome at the age of 8 months).
The response to antiepileptic treatments was poor in most patients with the best response to valproate (27.65% of patients in Group A and 28,57% in Group B). A ketogenic diet resulted in a dramatic seizure reduction in 3 patients with Glut1 deficiency (Table 1: patients 13, 14, and 16) and one patient with adenyl-succinate lyase deficiency (Table 2: patient 16). The treatment with carbamazepine resulted in a mild improvement of paroxysmal dystonia in one patient with ATP1A3 encephalopathy (Table 1: patient 28) while a ketogenic diet was associated with an improvement of ataxic gait in 3 patients with Glut1 deficiency (Table 1: patients 13, 14, and 16). Valproate induced a worsening of tremors in 5 patients (Table 1: patients 13, 36, and 46; Table 2: patients 2 and 13).
Movement Disorder Phenotype
The onset of movement disorders was noticed in the age-range 1–6 years in 57,97% of cases and before the age of 12 months in 24,63% of patients. A relevant number of patients presented with more than one subtype of hyperkinetic movement disorder (11 out of 53 in Group A) or hyper and hypokinetic features observed simultaneously at disease onset or sequentially during the follow-up (7 out of 16 in Group B). In 7 out of 16 patients belonging to Group B, parkinsonism was preceded by hyperkinetic movement disorders (Patients 2, 4, 6, 7, 9, 11, 13 in Table 2).
Stereotypes and dystonia were the most frequent hyperkinetic movement disorders at the onset in Group A (17 patients for both symptoms: Tables 1, 3). The first ones were predominant in the disorders of the cellular cycle's regulation while dystonia was the presenting signs of different groups of disorders involving all the steps of cellular signaling and metabolism (Figure 1; Table 4). Paroxysmal movement disorders were observed at the onset in 3 patients with GLUT1 deficiency who presented with episodic ataxias and in 1 patient with ATP1A3-related dystonic attacks (Patients 13, 14, 16, and 28 in Table 1).
In Group A, only 2 patients (Patients 6 and 18 in Table 1) received pharmacological treatments for movement disorders with a limited response, while a few therapeutic strategies were attempted in Group B in which the most relevant positive effects were observed in 2 patients (Patient 3 and 14 in Table 2) who received dopaminergic agents to treat, respectively, WARS2-related parkinsonism and a DHPR deficiency.
Time to Achieve a Molecular Genetic Diagnosis
The present study failed to demonstrate that specific combinations of seizure or movement disorder types might have been associated with an earlier etiological diagnosis (Tables 3, 5, 6). Diagnostic delay was longer in Group B than in Group A (Tables 3, 5). The analysis of the distribution of genetic diagnosis per year and diagnostic delay showed a higher gain in diagnostic yield of molecular genetic investigations in the last 5 years (Figures 2A,B).

Table 5. Differences between Group A and Group B in terms of diagnostic delay and temporal periods in which the molecular genetic diagnosis was made.
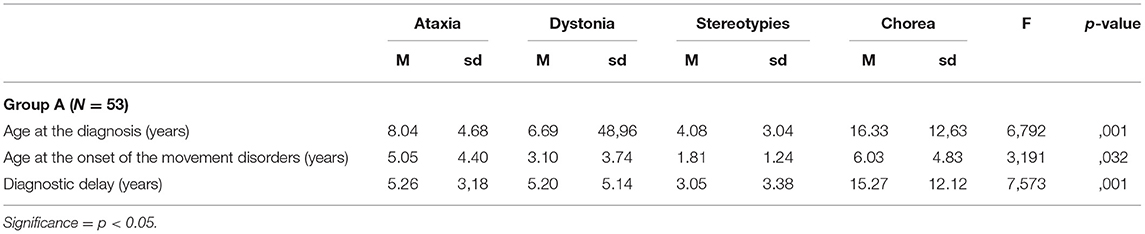
Table 6. Differences in terms of age at diagnosis, age at the onset of movement disorders, and diagnostic delay according to the different hyperkinetic movement disorders that were observed in patients of Group A.
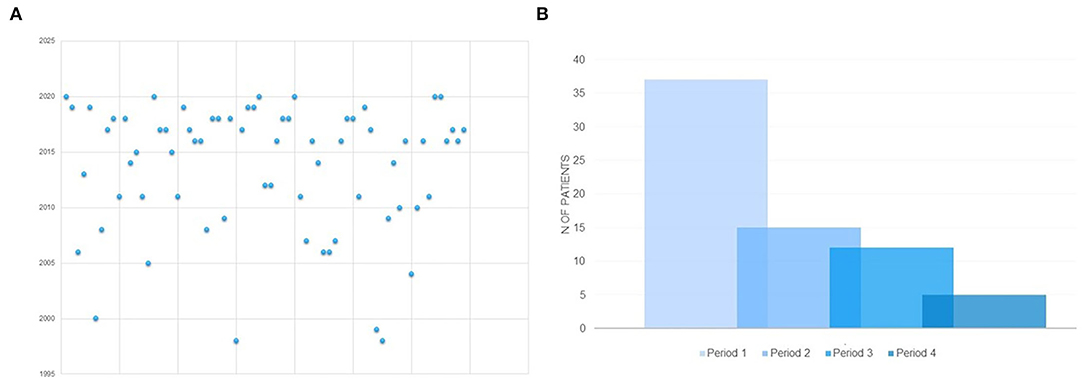
Figure 2. (A) Distribution of molecular genetic diagnosis per year in the whole sample. (B) Distribution of molecular genetic diagnosis according to temporal periods in which the molecular genetic diagnosis was made. PERIOD 1: 2016-2020; PERIOD 2: 2011- 2015; PERIOD 3 = 2006-2010; PERIOD 4 = before 2005.
Analysis of variance and post-hoc tests with Bonferroni correction for multiple comparisons evidenced, in Group A, a significantly longer diagnostic delay and higher age at molecular genetic diagnosis in patients who presented with chorea at the onset if compared with the ones presenting with ataxia, dystonia, and stereotypies (Table 6).
Discussion
The literature provides few epidemiological data about the distribution of molecular genetic diagnosis of developmental encephalopathies with epilepsy and movement disorders in the pediatric populations while several recent studies reported detailed and updated genotype-phenotype correlations for about 60 single-gene related disorders other than historically well-known patterns such as the ones of Rett or Angelman syndrome (1, 13–18). The studies including epidemiological data were often hardly comparable because of several methodological differences in terms of analyzed cohorts, inclusion and exclusion criteria, and modalities of data collection (1, 2). Most of these data were not statistically significant because of the rare or ultrarare prevalence of the analyzed diseases.
A recent systematic review of 49 papers identified 27 neonatal-onset single gene-related diseases presenting with severe epileptic and developmental encephalopathies and a predominance of hyperkinetic movement disorders in more than 85% of the cases, with neurometabolic conditions not included in the analysis (2). The proportion of neurometabolic diseases in a Canadian cohort of 197 patients referring to a single pediatric epilepsy center, who underwent targeted gene panels or whole-exome sequencing, accounted for 13% of cases (19). In the same cohort, a co-occurrent movement disorder was reported in almost one-fourth of the cases with a large predominance of dystonia (8,6% of the patients) (19). In a smaller Japanese cohort of 11 patients, 9 different monogenic diseases presenting with early-onset hyperkinetic movement disorders were diagnosed in 7 infants who had a West syndrome and in 2 children with a non-syndromic epileptic encephalopathy (20). Trump et al. (21) identified movement disorders in 4 out of 71 patients with a confirmed genetically determined epilepsy while Cordeiro et al. (22) reported an epileptic syndrome in 13 out of 21 patients with a genetically determined movement disorder.
The herein-reported single center retrospective cohort analysis depicted the heterogeneous clinical panorama and the diagnostic yield that may be observed in a specialistic setting.
The analysis of etiologies suggested a predominant association of chorea with a subset of genes or CNV involved in post-synaptic signaling (e.g., GNAO1, GNB1, FOXG1, MEF2C, CLN2, MeCP2 duplication) and a high quote of the pathogenic gene or chromosomal variants associated with neurodegenerative and metabolic diseases among patients presenting with myoclonus and parkinsonism.
Patients with hyperkinetic movement disorders, that were diagnosed in our center, presented with an earlier onset of epileptic seizures, a predominant generalized motor semiology of seizures at the onset, and prominence of multifocal EEG abnormalities. The prominent phenotypic features of patients with hypokinetic movement disorders included a later onset of seizures, a higher frequency of focal seizures at the onset, and focal EEG abnormalities.
The onset of seizures was noticed earlier than the onset of movement disorders in patients presenting with hyperkinetic movement disorders while the opposite occurred in patients with hypokinetic manifestations.
A possible explanation might be represented by an underdiagnosis of movement disorders because of their benign nature (e.g., stereotypes) or lack of awareness, vs. the higher social and clinical alarm induced by seizures. A second explanation could rely upon the different etiological spectrum observed in hyperkinetic and hypokinetic groups. In most patients from Group B (60 vs. 20% in Group A), epilepsy is the result or the by-product of a neurodegenerative process, and therefore it can appear later in the disease course, while in most patients from Group A (42/53) epilepsy is one of the first signs of a developmental and epileptic encephalopathy related to disfunction of genes involved in neurotransmission, neuronal excitability, or neural development.
A significant diagnostic delay was experienced in patients in which chorea represented the main movement disorder at the onset. This delay may result from different factors: (a) the highest tendency of physicians, especially if specific expertise in rare diseases lacks, to consider chorea mainly as a symptom of acquired diseases (e.g., Sydenham Chorea or other autoimmune disorders) with a possible underestimation of genetic etiologies; (b) the late diagnosis in patients carrying variants in novel disease-causing genes that were discovered up to several years after the onset of symptoms and the first clinical evaluation of patients in which pathogenic single gene variants or chromosomal aberrations were reported for the first time in the literature) (23).
The significantly longer diagnostic delay in Group B is probably due to the extremely rare occurrence of hypokinetic movement disorders in pediatric ages, especially in patients studied before the era of next generation sequencing. An extensive educational campaign focused on the peculiar phenotypic features and the availability of reliable biochemical (e.g., CSF neurotransmitter measurement in the disorders of monoamine metabolism or urinary copper in Menkes disease) and neuroimaging (e.g., cerebellar atrophy in Neuronal Ceroid Lipofuscinosis or lactate peak at HMRS in Menkes disease) biomarkers might increase the knowledge of genetically determined hypokinetic movement disorders among pediatric neurologists (13).
The limits of the study are strictly correlated with its retrospective design, the rarity of the explored diseases, and the heterogeneous spectrum of genetic etiologies while a bias might be represented by the inclusion of patients with an established molecular genetic diagnosis only (and the subsequent exclusion of patients with comparable spectra but without molecular genetic confirm). Moreover, this study did not systematically explore the longitudinal changes of epilepsy and movement disorder phenotypes from infancy into adulthood with a subsequent possible loss of useful information about prognostic implications even if these details are already available in the literature for some single-gene diseases (24–26). A further limit may be correlated with the current unavailability of an acceptable classification for movement disorders taking into account specific pediatric peculiarities (i.e., the high quote of mixed movement disorders, disorders of tone and postures, weakness, ataxia, and apraxia).
Conclusions
This paper explored the presenting patterns of genetically determined encephalopathies with epilepsy and movement disorders and highlighted some relevant clinical and diagnostic issues including (a) a more frequent etiological role of abnormalities of genes/chromosomal regions involved in neurotransmission, neuronal excitability, or neural development in patients with hyperkinetic movement disorders; (b) a higher frequency of neurodegenerative courses and a longer diagnostic delay in patients with hypokinetic movement disorders.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
MM and SG contributed to the conception and design of the study and wrote the first draft of the manuscript. SC, AR, and LC contributed to data collection and data analysis and wrote some sections of the manuscript. VL revised the manuscript. All authors contributed to the manuscript and read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Papandreou A, Danti FR, Spaull R, Leuzzi V, Mctague A, Kurian MA. The expanding spectrum of movement disorders in genetic epilepsies. Dev Med Child Neurol. (2020) 62:178–91. doi: 10.1111/dmcn.14407
2. Spagnoli C, Fusco C, Percesepe A, Leuzzi V, Pisani F. Genetic neonatal-onset epilepsies and developmental/epileptic encephalopathies with movement disorders: a systematic review. Int J Mol Sci. (2021) 22:4202. doi: 10.3390/ijms22084202
3. Foo JN, Liu J, Tan EK. Next-generation sequencing diagnostics for neurological diseases/disorders: from a clinical perspective. Hum Genet. (2013) 132:721–34. doi: 10.1007/s00439-013-1287-2
4. Fogel BL, Lee H, Strom SP, Deignan JL, Nelson SF. Clinical exome sequencing in neurogenetic and neuropsychiatric disorders. Ann N Y Acad Sci. (2016) 1366:49–60. doi: 10.1111/nyas.12850
5. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:522–30. doi: 10.1111/epi.13670
6. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
7. Fahn S. Classification of movement disorders. Mov Disord. (2011) 26:947–57. doi: 10.1002/mds.23759
8. Sanger TD, Chen D, Fehlings DL, Hallett M, Lang AE, Mink JW, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. (2010) 25:1538–49. doi: 10.1002/mds.23088
9. Singer HS, Mink JW, Gilbert DL, Jankovic J. Movement Disorders in Childhood, 2nd ed. Philadelphia, PA: Butterworth-Heinemann (Elsevier) (2015).
10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med. (2015) 17:405–24. doi: 10.1038/gim.2015.30
11. Nuovo S, Baglioni V, De Mori R, Tardivo S, Caputi C, Ginevrino M, et al. Clinical variability at the mild end of BRAT1-related spectrum: Evidence from two families with genotype-phenotype discordance. Hum Mutat. (2021) 43:67–73. doi: 10.1002/humu.24293
12. Gardella E, Becker F, Møller RS, Schubert J, Lemke JR, Larsen LH, et al. Benign infantile seizures and paroxysmal dyskinesia caused by an SCN8A mutation. Ann Neurol. (2016) 79:428–36. doi: 10.1002/ana.24580
13. Mastrangelo M. Epilepsy in inherited neurotransmitter disorders: Spotlights on pathophysiology and clinical management. Metab Brain Dis. (2021) 36:29–43. doi: 10.1007/s11011-020-00635-x
14. Danti FR, Galosi S, Romani M, Montomoli M, Carss KJ, Raymond FL, et al. GNAO1 encephalopathy: Broadening the phenotype and evaluating treatment and outcome. Neurol Genet. (2017) 3:e143. doi: 10.1212/NXG.0000000000000143
15. Wolff M, Johannesen KM, Hedrich UBS, Masnada S, Rubboli G, Gardella E, et al. Genetic and phenotypic heterogeneity suggest therapeutic implications in SCN2A-related disorders. Brain. (2017) 140:1316–36. doi: 10.1093/brain/awx054
16. Döring JH, Saffari A, Bast T, Brockmann K, Ehrhardt L, Fazeli W, et al. The phenotypic spectrum of PRRT2-associated paroxysmal neurologic disorders in childhood. Biomedicines. (2020) 8:456. doi: 10.3390/biomedicines8110456
17. Vetro A, Nielsen HN, Holm R, Hevner RF, Parrini E, Powis Z, et al. ATP1A2- and ATP1A3-associated early profound epileptic encephalopathy and polymicrogyria. Brain. (2021) 21:awab052. doi: 10.1093/brain/awab052
18. Bourque DK, Cordeiro D, Nimmo GAM, Kobayashi J, Mercimek-Andrews S. Phenotypic and genotypic spectrum of glucose transporter-1 deficiency syndrome. Can J Neurol Sci. (2021) 12:1–5. doi: 10.1017/cjn.2021.3
19. Costain G, Cordeiro D, Matviychuk D, Mercimek-Andrews S. Clinical application of targeted next-generation sequencing panels and whole exome sequencing in childhood epilepsy. Neuroscience. (2019) 418:291–310. doi: 10.1016/j.neuroscience.2019.08.016
20. Kobayashi Y, Tohyama J, Kato M, Akasaka N, Magara S, Kawashima H, et al. High prevalence of genetic alterations in early-onset epileptic encephalopathies associated with infantile movement disorders. Brain Dev. (2016) 38:285–92. doi: 10.1016/j.braindev.2015.09.011
21. Trump N, McTague A, Brittain H, Papandreou A, Meyer E, Ngoh A, et al. Improving diagnosis and broadening the phenotypes in early-onset seizure and severe developmental delay disorders through gene panel analysis. J Med Genet. (2016) 53:310–7. doi: 10.1136/jmedgenet-2015-103263
22. Cordeiro D, Bullivant G, Siriwardena K, Evans A, Kobayashi J, Cohn RD, et al. Genetic landscape of pediatric movement disorders and management implications. Neurol Genet. (2018) 4:e265. doi: 10.1212/NXG.0000000000000265
23. Mastrangelo M, Mei D, Cesario S, Fioriello F, Bernardini L, Brinciotti M, et al. A novel developmental encephalopathy with epilepsy and hyperkinetic movement disorders associated with a deletion of the sodium channel gene cluster on chromosome 2q24.3. Parkinsonism Relat Disord. (2019) 68:1–3. doi: 10.1016/j.parkreldis.2019.09.016
24. Larson AM, Shinnick JE, Shaaya EA, Thiele EA, Thibert RL. Angelman syndrome in adulthood. Am J Med Genet A. (2015) 167A:331–44. doi: 10.1002/ajmg.a.36864
25. Bartolini E, Campostrini R, Kiferle L, Pradella S, Rosati E, Chinthapalli K, Palumbo P. Epilepsy and brain channelopathies from infancy to adulthood. Neurol Sci. (2020) 41:749–61. doi: 10.1007/s10072-019-04190-x
Keywords: developmental and epileptic encephalopathies, movement disorders, neurogenetic disorders, next generation sequencing-NGS, phenotypes
Citation: Mastrangelo M, Galosi S, Cesario S, Renzi A, Campea L and Leuzzi V (2022) Presenting Patterns of Genetically Determined Developmental Encephalopathies With Epilepsy and Movement Disorders: A Single Tertiary Center Retrospective Cohort Study. Front. Neurol. 13:855134. doi: 10.3389/fneur.2022.855134
Received: 14 January 2022; Accepted: 23 May 2022;
Published: 20 June 2022.
Edited by:
Wang-Tso Lee, National Taiwan University Hospital, TaiwanReviewed by:
Christian M. Korff, HUG, SwitzerlandBruria Ben-Zeev, Sheba Medical Center, Israel
Piero Pavone, University of Catania, Italy
Copyright © 2022 Mastrangelo, Galosi, Cesario, Renzi, Campea and Leuzzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Leuzzi, dmluY2Vuem8ubGV1enppQHVuaXJvbWExLml0
†These authors have contributed equally to this work
 Mario Mastrangelo
Mario Mastrangelo Serena Galosi
Serena Galosi Serena Cesario
Serena Cesario Alessia Renzi
Alessia Renzi Lucilla Campea
Lucilla Campea Vincenzo Leuzzi
Vincenzo Leuzzi