
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 03 March 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.845296
This article is part of the Research TopicEndovascular and Interventional Neurology - Case Report Collection 2021View all 12 articles
 Nozomi Otsuka1
Nozomi Otsuka1 Hirohisa Yajima1
Hirohisa Yajima1 Satoru Miyawaki1*
Satoru Miyawaki1* Satoshi Koizumi1
Satoshi Koizumi1 Satoshi Kiyofuji1
Satoshi Kiyofuji1 Hiroki Hongo1
Hiroki Hongo1 Yu Teranishi1
Yu Teranishi1 Taichi Kin1,2
Taichi Kin1,2 Nobuhito Saito1
Nobuhito Saito1Background: A duplicated middle cerebral artery (DMCA) is an anatomical variant that includes duplication of the middle cerebral artery (MCA) and an anomalous vessel originating between the anterior choroidal artery (AChA) and the distal end of the internal carotid artery (ICA). Here, we present a case report of an ICA aneurysm with a DMCA and the AChA originating from the dome, which was successfully treated with clipping.
Case Description: In a 64-year-old man, preoperative angiography revealed an unruptured right ICA aneurysm with a maximum diameter of 4.3 mm, and fusion three-dimensional computer graphics revealed that a DMCA and the AChA originated from the dome. The aneurysm enlarged; therefore, clipping was performed. The closure of the aneurysm while preserving the patency of the DMCA and AChA was identified using intraoperative microvascular Doppler ultrasonography and indocyanine green video angiography. The postoperative course was uneventful, and no ischemic lesions were confirmed on MR imaging.
Conclusion: To the best of our knowledge, this is the first report of an ICA aneurysm with a DMCA and the AChA arising from the dome. In such cases, the anatomy of the DMCA and AChA should be well-characterized before treatment.
A duplicated middle cerebral artery (DMCA) is a normal variation of the middle cerebral artery (MCA), in which the MCA originates between the anterior choroidal artery (AChA) and the distal end of the internal carotid artery (ICA), and this passes into the sylvian fissure and perfuses part of the territory of the MCA (1, 2). The treatment of aneurysms at the origin of the DMCA has been reported previously (3–5). When treating aneurysms arising from the origin of the DMCA, it is important to preserve the AChA which branches nearby. We present a case in which clipping was performed for an ICA aneurysm with a DMCA and the AChA arising from the dome. There are no reports of aneurysms in which both a DMCA and the AChA branch from the dome. This report discusses the anatomical aspects of these aneurysms.
A 64-year-old man was referred to our hospital because of an unruptured right ICA aneurysm that was detected incidentally on time-of-flight MR angiography (Figures 1A,B). Cerebral digital substruction angiography revealed an unruptured aneurysm with a maximum diameter of 4.3 mm at the supraclinoid portion of the right ICA (Figure 1C), and three-dimensional rotational angiography demonstrated that a DMCA and the AChA originated from the dome (Figure 1D). Additionally, fusion three-dimensional computer graphics integrating MR imaging and digital substruction angiography revealed that the DMCA passed through the sylvian fissure along the M1 segment of the MCA (Figure 1E) and perfused the anterior temporal lobe (Figure 1F). The fusion three-dimensional computer graphics was reconstructed using GRID 1.1 (Kompath Inc., Tokyo, Japan), utilizing the multi-threshold technique, as described previously (6–8). In summary, preoperative images were the output from the DICOM (digital imaging communication in medicine) format; they were imported into the image processing software GRID, which implements automatic registration of multiple imaging modalities. The multi-threshold is a method for extracting both thick and thin blood vessels with different threshold values. Microvessels can be visualized with less noise using this method (7). The patient had a family history of subarachnoid hemorrhage. The aneurysm increased to 4.3 mm after 10 years of follow up; it was 3 mm at the time of detection. Therefore, we determined that an intervention was required. The risk of occlusion of the arteries branching from the dome was estimated to be high if endovascular treatment was performed. In order to preserve the incorporated branch arteries, we decided to perform clipping. A right frontotemporal craniotomy was performed using a transsylvian approach to the aneurysm. Intraoperative findings showed that the DMCA and the AChA branched from the dome of the ICA aneurysm (Figure 2A). Two titanium clips were combined and applied to occlude most part of the aneurysm, while confirming the patency of the DMCA and the AChA (Figure 2B). Aneurysm obliteration and the patency of the parent and branch vessels were confirmed using intraoperative microvascular Doppler ultrasonography and indocyanine green video angiography (Figure 2C). Postoperatively, there were no neurological deficits or ischemic lesions on MR imaging.
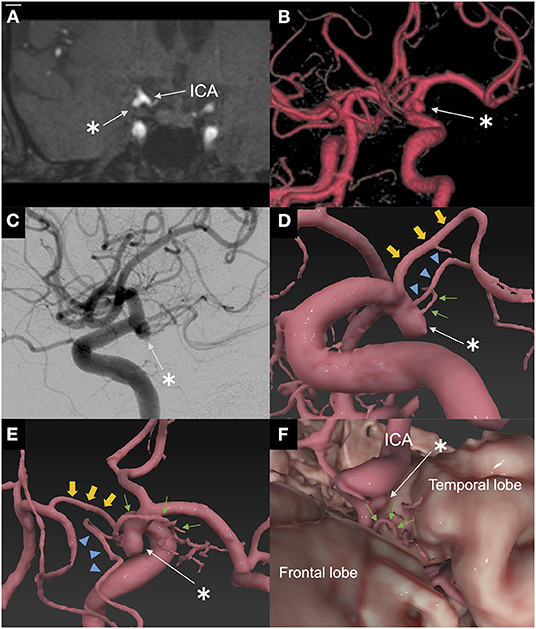
Figure 1. Preoperative imaging. (A) Coronal plane of time-of-flight magnetic resonance angiography on admission. Coronal plane of time-of-flight magnetic resonance angiography showed an unruptured aneurysm of the right internal carotid artery in contact with the temporal lobe. *: aneurysm. (B) Three-dimensional time-of-flight magnetic resonance angiography on admission. Three-dimensional time-of-flight magnetic resonance angiography showed an unruptured aneurysm of the right internal carotid artery supraclinoid portion. *: aneurysm. (C) Lateral view of digital subtraction angiography of the right internal carotid artery. Lateral view of digital subtraction angiography of the right internal carotid artery showing an aneurysm associated with a duplicated middle cerebral artery and anterior choroidal artery. *: aneurysm. (D) Fusion three-dimensional computer graphics integrating MR imaging/MR angiography and three-dimensional rotational angiography. Fusion three-dimensional computer graphics showed that the duplicated middle cerebral artery and the anterior choroidal artery originated from the dome. *: aneurysm; small arrow (green): duplicated middle cerebral artery; arrowhead (blue): anterior choroidal artery; large arrowhead (yellow): posterior communicating artery. (E) Fusion three-dimensional computer graphics. Fusion three-dimensional computer graphics shows that the duplicated middle cerebral artery passed through the sylvian fissure along the M1 segment of the MCA. *: aneurysm; small arrow (green): duplicated middle cerebral artery; arrowhead (blue): anterior choroidal artery; large arrowhead (yellow): posterior communicating artery. (F) Fusion three-dimensional computer graphics. Fusion three-dimensional computer graphics showed that the duplicated middle cerebral artery perfused the anterior temporal lobe. *: aneurysm; small arrow (green): duplicated middle cerebral artery. Fusion three-dimensional computer graphics were reconstructed by GRID 1.1 software (Kompath Inc., Tokyo, Japan).
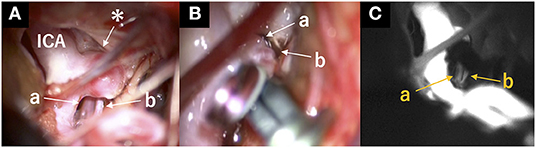
Figure 2. Intraoperative view. (A) Intraoperative view indicated the structures surrounding the aneurysm before clipping. *: aneurysm; a: anterior choroidal artery; b: duplicated middle cerebral artery. (B) Two titanium clips were combined and applied to occlude most part of the aneurysm, while confirming the patency of the DMCA and the AChA. a: Anterior choroidal artery; b: duplicated middle cerebral artery. (C) Patency of the duplicated middle cerebral and anterior choroidal arteries was confirmed using indocyanine green video angiography after clipping. a: anterior choroidal artery; b: duplicated middle cerebral artery.
To our knowledge, this is the first report of an ICA aneurysm with a DMCA and the AChA arising from the dome that was successfully treated without complications. Here, we discuss the anatomical aspects of this aneurysm and the importance of preserving the incorporated branch vessels.
The incidence of DMCA ranges from 0.7 to 2.9% (9, 10). The DMCA arises between the origin of the AChA and the distal end of the ICA. Both the AChA and MCA originate from the cranial division of the ICA, and the AChA is embryologically earlier than the MCA (11). Komiyama et al. (1) described the DMCA as an anomalous early ramification of the early branch of the MCA, which originates from the distal end of the ICA. DMCA is classified into two types, A and B, based on the point of origin. A Type A DMCA arises from the top of the ICA, while a Type B DMCA originates from the ICA between the AChA and proximal portion of the ICA bifurcation (12, 13). Most DMCA aneurysms are type B (5, 14). To the best of our knowledge, 42 cases of DMCA aneurysms have been reported (4, 5, 15–21). Most of these aneurysms are IC-DMCA aneurysms of Type B DMCA. There were five cases of rare variations among the DMCA aneurysms (Table 1) (15, 21–24), including three cases of aneurysms on DMCA. Theoretically, aneurysms that involve AChA, A1, or M1 could be considered as DMCA aneurysms; however, aneurysms that involve AChA, as in the present case, were not detected. Kai et al. (13) estimated that type B DMCA is exposed to higher hemodynamic stress because the angle between the ICA and type B DMCA is sharper than that of type A. Moreover, previous studies have reported that aneurysms associated with type B DMCA are at a high risk of rupture even if they are small in size. Therefore, aggressive treatment could be considered for such aneurysms, as in our case (13, 17, 25).
The differential diagnosis of ICA aneurysm with DMCA and AChA arising from the dome included an aneurysm that involves the double AChA. According to Lasjaunias (26), the origin of the AChA is located posterolateral to the supracavernous portion of the ICA, between the posterior communicating artery and the ICA bifurcation. The AChA passes posterolaterally above the medial part of the uncus, along the optic tract, and laterally curves to reach the lateral geniculate body in the cisternal segment. Usually, the AChA gives off one or two branches that terminate at the medial wall of the temporal lobe. Double AChAs were found in 4–13% of cases (27–29), and their origins were classified into two types. One consists of two separate arteries arising from the ICA, and the other arises from the ICA as a single artery but immediately divides into two trunks. If there are double AChAs, the more distal branch terminates in the medial temporal lobe and the more proximal branch nourishes the remaining anterior choroidal field (29). Aneurysms involving a double AChA, which appear similar to the images of our case, have been reported (30, 31). Generally, the DMCA runs through the sylvian fissure and supplies the anterior and/or middle temporal territories (1). The present case is thought to be a DMCA because the artery originated between the AChA and the distal end of the ICA, passed through the sylvian fissure along the M1 segment of the MCA and perfused the anterior temporal lobe. Embryologically, the DMCA cannot originate proximal to the AChA because the AChA appears earlier in development. Uchino et al. (32) reported a case of DMCA arising from the origin of the AChA. In that case, the common origin of the DMCA and AChA was confirmed, and infundibular dilatation was indicated in the ICA-AChA-DMCA junction. It is assumed that the aneurysm in this case was the result of a DMCA aneurysm involving the AChA, an AChA aneurysm involving a DMCA, or an aneurysm occurring at the common origin of a DMCA and the AChA.
Miyoshi et al. reported a case of aphasia after clipping a DMCA aneurysm (20). DMCAs frequently involve perforating arteries (9, 33). In addition, a DMCA can potentially supply collateral blood flow to the MCA territory in cases of MCA occlusion (34). Thus, blood flow in the DMCA should be preserved. Furthermore, ischemia of the territory of the AChA causes severe neurological deficits (35, 36). Friedman et al. (37) showed that the AChA originates from the dome in 18% of AChA aneurysms, and the ischemic complication rate associated with treatment was even higher in such cases. As mentioned above, when treating aneurysms with a DMCA and the AChA originating from the dome, preserving these important branching vessels should be considered.
To the best of our knowledge, this is the first report of an ICA aneurysm with a DMCA and the AChA arising from the dome. In such cases, the anatomy of the DMCA and AChA must be well-characterized before treatment is initiated.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual for the publication of any potentially identifiable images or data included in this article.
SM certifies that all authors have participated and have been involved in the cases presented and/or in the elaboration of the present manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Komiyama M, Nakajima H, Nishikawa M, Yasui T. Middle cerebral artery variations: duplicated and accessory arteries. AJNR Am J Neuroradiol. (1998) 19:45–9.
2. Teal JS, Rumbaugh CL, Bergeron RT, Segall HD. Anomalies of the middle cerebral artery: accessory artery, duplication, and early bifurcation. Am J Roentgenol Radium Ther Nucl Med. (1973) 118:567–75. doi: 10.2214/ajr.118.3.567
3. Hou K, Xu K, Liu H, Li G, Yu J. The clinical characteristics and treatment considerations for intracranial aneurysms associated with middle cerebral artery anomalies: a systematic review. Front Neurol. (2020) 11:564797. doi: 10.3389/fneur.2020.564797
4. Imahori T, Mizobe T, Fujinaka T, Miura S, Sugihara M, Aihara H, et al. An aneurysm at the origin of a duplicated middle cerebral artery treated by stent-assisted coiling using the “wrapped-candy” low-profile visualized intraluminal support (LVIS) technique: a Technical Case Report and Review of the Literature. World Neurosurg. (2020) 143:353–9. doi: 10.1016/j.wneu.2020.08.046
5. Fujimoto K, Hashimoto H, Uchiyama Y, Maekawa H, Shida Y, Nakagawa I. Duplicated middle cerebral artery aneurysms treated by coil embolization; a report of two cases and literature Review. J Stroke Cerebrovasc Dis. (2021) 30:105773. doi: 10.1016/j.jstrokecerebrovasdis.2021.105773
6. Yoshino M, Nakatomi H, Kin T, Saito T, Shono N, Nomura S, et al. Usefulness of high-resolution 3D multifusion medical imaging for preoperative planning in patients with posterior fossa hemangioblastoma: technical note. J Neurosurg. (2017) 127:139–47. doi: 10.3171/2016.5.Jns152646
7. Saito N, Kin T, Oyama H, Yoshino M, Nakagawa D, Shojima M, et al. Surgical simulation of cerebrovascular disease with multimodal fusion 3-dimensional computer graphics. Neurosurgery. (2013) 60(Suppl. 1):24–9. doi: 10.1227/01.neu.0000430312.71326.6d
8. Kin T, Nakatomi H, Shojima M, Tanaka M, Ino K, Mori H, et al. A new strategic neurosurgical planning tool for brainstem cavernous malformations using interactive computer graphics with multimodal fusion images. J Neurosurg. (2012) 117:78–88. doi: 10.3171/2012.3.Jns111541
9. Crompton MR. The pathology of ruptured middle-cerebral aneurysms with special reference to the differences between the sexes. Lancet. (1962) 2:421–5. doi: 10.1016/s0140-6736(62)90281-7
10. Jain KK. Some observations on the anatomy of the middle cerebral artery. Can J Surg. (1964) 7:134–9.
11. Padget DH. The development of the cranial arteries in the human embryo. Contrib Embryol. (1948) 32:205–61.
12. Cilliers K, Page BJ. Anatomy of the middle cerebral artery: cortical branches, branching pattern and anomalies. Turk Neurosurg. (2017) 27:671–81. doi: 10.5137/1019-5149.Jtn.18127-16.1
13. Kai Y, Hamada J, Morioka M, Yano S, Kudo M, Kuratsu J. Treatment of unruptured duplicated middle cerebral artery aneurysm: case report. Surg Neurol. (2006) 65:190–3; discussion: 193. doi: 10.1016/j.surneu.2005.05.032
14. Stojanović NN, Kostić A, Mitić R, BerilaŽić L. Correlation between multiple cerebral aneurysms and a rare type of segmental duplication of the middle cerebral artery. BMC Neurol. (2020) 20:3. doi: 10.1186/s12883-019-1588-8
15. Mori K, Tamase A, Seki S, Iida Y, Kawabata Y, Nakano T, et al. Duplicated middle cerebral artery associated with aneurysm at M1/M2 bifurcation: a case report. J Med Case Rep. (2018) 12:283. doi: 10.1186/s13256-018-1824-7
16. Iwata M, Kawaguchi S, Manaka H. A case of unruptured cerebral aneurysm arising from duplicate origin of the middle cerebral artery. No Shinkei Geka Neurol Surg. (2020) 48:515–20. doi: 10.11477/mf.1436204221
17. Alliez JR, Manera L. Aneurysm arising at the origin of a duplicated middle cerebral artery. Case Rep Neurol. (2021) 13:446–50. doi: 10.1159/000517366
18. Oh BK, Kim YH, Kim CH, Lee SW, Sung SK, Song GS. A case of an unruptured duplicated middle cerebral artery aneurysm-An unusual presentation of the distal internal carotid artery aneurysm. J Cerebrovasc Endovasc Neurosurg. (2021) 23:240–4. doi: 10.7461/jcen.2021.E2020.10.004
19. Kim JS, Lee CH, Park H, Han JW. An unruptured cerebral aneurysm at the origin of the duplicated middle cerebral artery. J Cerebrovasc Endovasc Neurosurg. (2015) 17:223–6. doi: 10.7461/jcen.2015.17.3.223
20. Miyoshi H, Migita K, Kumano K, Hashimoto N, Toyota A. A case of aphasia after neck clipping of a ruptured aneurysm at the origin of the duplicated middle cerebral artery. No Shinkei Geka Neurol Surg. (2016) 44:959–64. doi: 10.11477/mf.1436203408
21. LaBorde DV, Mason AM, Riley J, Dion JE, Barrow DL. Aneurysm of a duplicate middle cerebral artery. World Neurosurg. (2012) 77:201.e1–4. doi: 10.1016/j.wneu.2011.03.038
22. Uchino M, Kitajima S, Sakata Y, Honda M, Shibata I. Ruptured aneurysm at a duplicated middle cerebral artery with accessory middle cerebral artery. Acta Neurochir. (2004) 146:1373–4; discussion: 1375. doi: 10.1007/s00701-004-0353-x
23. Otani N, Nawashiro H, Tsuzuki N, Osada H, Suzuki T, Shima K, et al. A ruptured internal carotid artery aneurysm located at the origin of the duplicated middle cerebral artery associated with accessory middle cerebral artery and middle cerebral artery aplasia. Surg Neurol Int. (2010) 1:51. doi: 10.4103/2152-7806.69378
24. Takahashi C, Kubo M, Okamoto S, Matsumura N, Horie Y, Hayashi N, et al. “Kissing” aneurysms of the internal carotid artery treated by coil embolization. Neurol Med Chir. (2011) 51:653–6. doi: 10.2176/nmc.51.653
25. Hori E, Kurosaki K, Matsumura N, Yamatani K, Kusunose M, Kuwayama N, et al. Multiple aneurysms arising from the origin of a duplication of the middle cerebral artery. J Clin Neurosci. (2005) 12:812–5. doi: 10.1016/j.jocn.2004.08.033
26. Lasjaunias P, KGtB, Berenstein A. Surgical Neuroangiography. Vol. 2, 2nd ed. Berlin: Springer (2006).
27. Hussein S, Renella RR, Dietz H. Microsurgical anatomy of the anterior choroidal artery. Acta Neurochir. (1988) 92:19–28. doi: 10.1007/bf01401968
28. Morandi X, Brassier G, Darnault P, Mercier P, Scarabin JM, Duval JM. Microsurgical anatomy of the anterior choroidal artery. Surg Radiol Anat. (1996) 18:275–80. doi: 10.1007/bf01627605
29. Saeki N, Rhoton AL Jr. Microsurgical anatomy of the upper basilar artery and the posterior circle of Willis. J Neurosurg. (1977) 46:563–78. doi: 10.3171/jns.1977.46.5.0563
30. Chenin L, Chivot C, Toussaint P, Deramond H, Peltier J. An unusual, duplicate origin of the anterior choroidal artery with aneurysm: a case report. Surg Radiol Anat. (2015) 37:1273–5. doi: 10.1007/s00276-015-1499-3
31. Lee JK, Choi JH, Shin YS. Multiple anterior choroidal arteries and perioperative ischemic complications in unruptured anterior choroidal artery aneurysms treated with microsurgical clipping. Acta Neurochir. (2021) 163:2947–53. doi: 10.1007/s00701-021-04901-4
32. Uchino A, Ito S, Kurita H, Tanaka M. Duplicated middle cerebral artery arising from the origin of the hyperplastic anterior choroidal artery that mimicked aneurysm on routine MR angiography. Neuroradiol J. (2016) 29:106–9. doi: 10.1177/1971400916633711
33. Umansky F, Dujovny M, Ausman JI, Diaz FG, Mirchandani HG. Anomalies and variations of the middle cerebral artery: a microanatomical study. Neurosurgery. (1988) 22:1023–7. doi: 10.1227/00006123-198806010-00008
34. Perez J, Machado C, Scherle C, Hierro D. Duplicated middle cerebral artery. Case Rep. (2009). doi: 10.1136/bcr.06.2009.2035
35. Leys D, Mounier-Vehier F, Lavenu I, Rondepierre P, Pruvo JP. Anterior choroidal artery territory infarcts. Study of presumed mechanisms. Stroke. (1994) 25:837–42. doi: 10.1161/01.str.25.4.837
36. Hupperts RM, Lodder J, Heuts-van Raak EP, Kessels F. Infarcts in the anterior choroidal artery territory. Anatomical distribution, clinical syndromes, presumed pathogenesis and early outcome. Brain. (1994) 117:825–34. doi: 10.1093/brain/117.4.825
Keywords: duplicated middle cerebral artery, cerebral aneurysm, anterior choroidal artery, branch incorporated aneurysm, clipping, fusion three-dimensional computer graphics
Citation: Otsuka N, Yajima H, Miyawaki S, Koizumi S, Kiyofuji S, Hongo H, Teranishi Y, Kin T and Saito N (2022) Case Report: “Clipping” an Internal Carotid Artery Aneurysm With a Duplicated Middle Cerebral Artery and the Anterior Choroidal Artery Arising From the Dome. Front. Neurol. 13:845296. doi: 10.3389/fneur.2022.845296
Received: 31 December 2021; Accepted: 08 February 2022;
Published: 03 March 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Yuanli Zhao, Capital Medical University, ChinaCopyright © 2022 Otsuka, Yajima, Miyawaki, Koizumi, Kiyofuji, Hongo, Teranishi, Kin and Saito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Satoru Miyawaki, c21peWEtbnN1QG0udS10b2t5by5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.