
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 28 February 2022
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.835812
This article is part of the Research Topic Updating Amyloid Neuropathy Knowledge: From Diagnosis to Treatment View all 5 articles
 Vincenzo Di Stefano1*
Vincenzo Di Stefano1* Ewan Thomas2
Ewan Thomas2 Valerio Giustino2
Valerio Giustino2 Salvatore Iacono1
Salvatore Iacono1 Angelo Torrente1
Angelo Torrente1 Guglielmo Pillitteri2
Guglielmo Pillitteri2 Andrea Gagliardo1
Andrea Gagliardo1 Antonino Lupica1
Antonino Lupica1 Antonio Palma2
Antonio Palma2 Giuseppe Battaglia2
Giuseppe Battaglia2 Filippo Brighina1
Filippo Brighina1Purpose: Hereditary transthyretin amyloidosis with polyneuropathy (ATTRv) is caused by mutations in the TTR gene, leading to misfolded monomers that aggregate generating amyloid fibrils. The clinical phenotype is heterogeneous, and characterized by a multisystemic disease affecting the sensorimotor and autonomic functions along with other organs.
Materials and Methods: All the patients were assessed by complete neurological assessment, neurophysiological evaluation, of the median nerve, and handgrip analysis. The data are presented as means and standard deviations. Parametric and non-parametric assessments have been performed to identify differences between groups. Pearson's correlation has been carried out when appropriate.
Results: Twenty patients with ATTRv (66.1 ± 8.4 years; eight females) and 30 controls (61.1 ± 11.6 years; 16 females) were enrolled. Handgrip strength was reduced in patients with ATTR in both right and left hands compared to the controls. Significant differences were found between patients and controls in the right (handgrip right, HGSR TTR 21.1 ± 13 kg vs. HGSR Control 29.4 ± 11.3 kg, p = 0.017) and left (handgrip left, HGSL TTR 22.2 ± 10.7 kg. vs. HGSL Control 31 ± 11.3 kg, p = 0.007). NIS and CMAP amplitude of the median nerve were related to HGS measures for both hands in patients with ATTRv.
Conclusions: The progression of bilateral carpal tunnel syndrome is related to neurophysiological data in the median nerve in ATTRv. Also, handgrip measures might represent an important tool for the assessment of disease progression in ATTRv. We propose using a combination of CMAP amplitude and HGS for the assessment of hand motor strength in ATTRv.
Hereditary transthyretin amyloidosis with polyneuropathy (ATTRv) is caused by mutations in the TTR gene, leading to misfolded monomers that aggregate generating amyloid fibrils (1). The clinical phenotype is heterogeneous, and is characterized by a multisystemic disease affecting the sensorimotor and autonomic functions along with other organs.
Carpal tunnel syndrome (CTS) is a well-known symptom of ATTRv and appears very early in the disease course, causing hand clumsiness and sensory deficits associated with weakness and hypotrophy in tenar muscles (2). Also, CTS can be the first sign of the disease and is often present in middle-aged carriers of TTR variants. Moreover, hand weakness progresses along with aggregation of amyloid fibrils in peripheral nerves and the carpal ligament (2). As a consequence, patients with ATTRv often complain of severe neuropathy in the median nerve with significant limitations and implications in the quality of life due to difficulties in the execution of simple movements, such as turning a key in a lock or signing a document.
It is a well-known fact that ATTRv, if untreated, can lead to death within 7–10 years of disease onset (1); however, recent advancements in diagnosis and treatment of this unfavorable condition have surprisingly improved so much that patients with ATTRv might not only survive longer but also enjoy better prognosis and quality of life (3, 4). Indeed, while the first therapies discovered aimed to stabilize the tetramer of TTR (even if new amyloid fibrils might accumulate in tissues of patients), the most recent drugs for the treatment of ATTRv are based on RNA-interference mechanisms, and they almost completely stop the production of TTR as a result of complete stabilization of the disease and mild recovery from symptoms of neuropathy (4–6). These unprecedent successes on the treatment of ATTR demand more accurate and punctual evaluation of ATTRv, even with the aid of specific tools. Conventional neurophysiology is routinely performed on patients with ATTRv patients showing length-dependent axonal sensorimotor polyneuropathy that usually starts in the lower limbs and subsequently extends to the upper limbs (7). However, the involvement of the upper limb is early and begins in the median nerve with CTS even decades before disease onset (8).
Handgrip strength (HGS) measurement is an instrument that allows for the estimation of overall muscle strength, a physical characteristic strictly connected to carrying out of activities of daily living (ADL) (9). Indeed, decrease in handgrip strength, common in some conditions such as carpal tunnel patients, can negatively affect the quality of life (QoL) (10). Moreover, it is widely recognized that HGS represents a valid marker for physical health, and it is considered a predictor for risk of falls in the elderly (11).
In this study we explored the role of nerve conduction studies (NCSs) and HGS for the assessment of strength in patients with ATTRv, and compared our results with those of healthy controls.
In this study, we described the electrophysiological features of the median nerve and strength assessed by handgrip evaluation in a cohort of patients with ATTRv to evaluate the combined use of neurophysiology and handgrip measures to assess the strength of the upper limbs.
The severity of ATTRv polyneuropathy was assessed with the Neuropathy Impairment Score (NIS), a scale assessing three major components (muscle weakness, muscle stretch reflexes, and sensation) in the head and upper and lower limbs (7). We calculated the total score (NIS, muscle weakness, muscle stretch reflexes, and sensation), the motor component alone for the whole body (NIS-W, muscle weakness), and the composite subscore by anatomic region for the upper limbs (right and left NIS-WUL).
Nerve conduction studies (NCSs) were performed on both median nerves for all the subjects enrolled according to standard procedures (i.e., bipolar surface stimulating electrodes delivering rectangular pulses 0.1–0.5 ms in duration and recording electrodes placed over the recording site with a ground electrode placed between recording and stimulation electrodes) (12). The stimulation of the right and left median nerve was performed at wrist and elbow and recording from APB. Distal motor latency (DML), compound motor action potential (CMAP) amplitude and motor conduction velocity (MCV) were analyzed in the NCS.
Maximal isometric handgrip strength for both right and left hands was measured using a mechanical dynamometer in units of kgf (KernMap model 80K1 - Kern®, Kern & Sohn GmbH, Balingen, Germany) in the standardized position recommended by the American Society of Hand Therapists (13), that is, sitting in a chair with a 90° backrest and 90° elbow flexion of the hand to be evaluated (Figure 1). Each participant exerted three trials of 3 s of maximal isometric handgrip strength alternatively for the right and left hands, with a rest period of 180 s between trials and considering the best performance of the three trials for statistical analysis.
Data are presented as means and standard deviations. Shapiro-Wilks was performed to test for normality. Parametric (unpaired Student's t-test) and non-parametric assessments (Mann Whitney U rank tests) were performed to identify differences between groups. Furthermore, Pearson's correlation was carried out on the TTR amyloidosis group to identify associations with clinical variables. Significance was set to p < 0.05 for all analyses. All the analysis were carried out with Jamovi (The jamovi project 2021; jamovi version 1.8.0.1, computer software, retrieved from https://www.jamovi.org). Graphs were created with GraphPad Prism8 (GraphPad Software, San Diego, CA, United States).
Twenty patients affected by TTR amyloidosis (66.1 ± 8.37 years, eight females) and 30 controls (61.1 ± 11.56 years, 16 females) participated in the study. Genetic testing confirmed a mutation in heterozygosis in the TTR gene in all the patients enrolled. F64L (p.F84L) mutation was encountered in 15 patients, followed by E89Q (p.E109Q) in two patients, V122I (p.V142I) in two patients, and H90A (p.H110A) in one patient. The most frequent symptoms were carpal tunnel syndrome (50%), cardiomyopathy (44%), weight loss (38%), and autonomic dysfunction (28%). Controls have been selected among patients' relatives (i.e., brothers, sisters, sons and daughthers) who screened negative to genetic testing for TTR mutations. All the patients underwent complete neurological assessment, neurophysiological evaluation, and handgrip analysis. No significant differences were retrieved for anthropometric characteristics between the two groups (Table 1).
Fifteen patients presented mild symptoms (FAP I), and five had moderate to severe symptoms (FAP II). Patients with ATTRv presented a mean NIS of 37.15 ± 30.71 and NIS-W of 18.54 ± 22.4. In the right upper limb, NIS-WUL scored 3.83 ± 5.49, while in the left it was 3.61 ± 4.9.
When the grip strength of both hands was evaluated, significant differences in the right (handgrip right, HGSR, TTR 21.1 ± 13 kg vs. Control 29.4 ± 11.3 kg, p = 0.017) and left (handgrip left, HGSL, TTR 22.2 ± 10.7 kg vs. Control 31 ± 11.3 kg, p = 0.007, Figure 2) were retrieved between the patients and the controls.
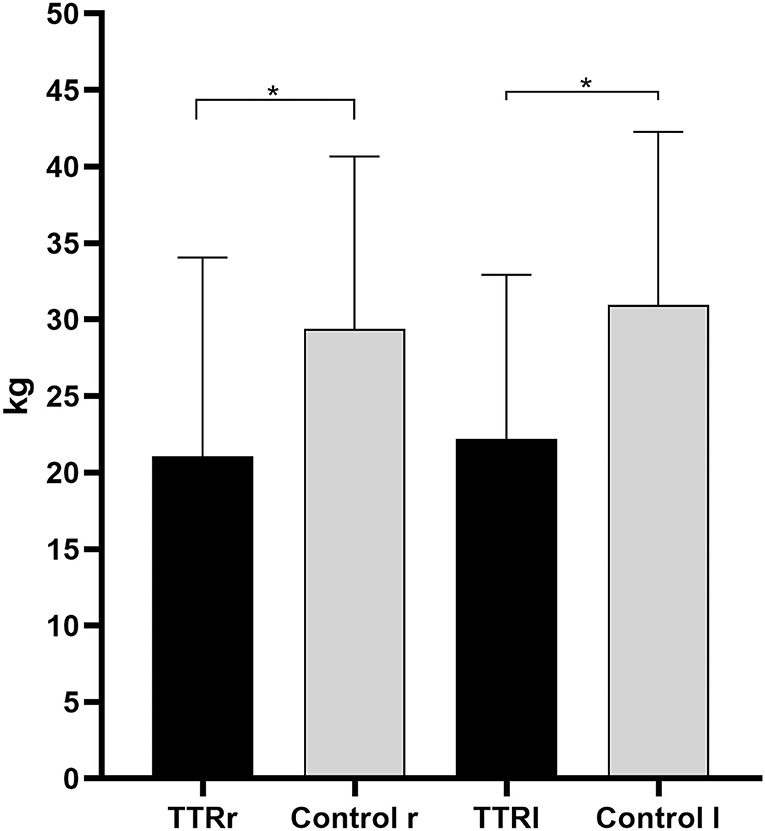
Figure 2. Handgrip measurements of patients with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv) and controls for the right and left hands. TTRr, handgrip strenght in the right; TTRl, handgrip strenght in the left; r, right; l, left. *Mann-Whitney U-test, p < 0.05.
In the right median nerve, a significant difference was reported between the patients with ATTRv and controls for DML and MCV (Table 2). In the left median nerve, the difference was more pronounced and demonstrated in all the parameters (DML, CMAP amplitude, and MCV). A significant correlation was found between the right median nerve DML and CMAP amplitude (−0.34, p = 0.049), as expected, because of the presence of CTS.
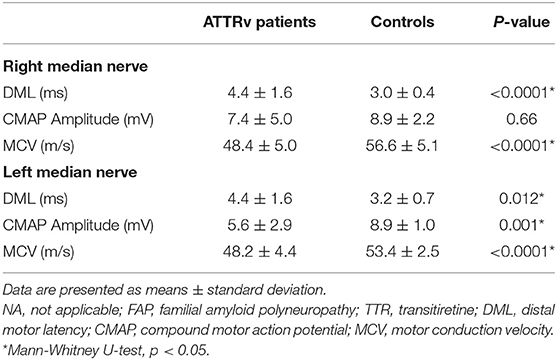
Table 2. Neurophysiological variables relative to the EMG of the median nerve in patients with hereditary transthyretin amyloidosis with polyneuropathy (ATTRv) and controls.
Clinical and neurophysiological variables for the ATTRv group (described in Table 2) have been further analyzed and related to HGS.
Total NIS score was negatively correlated with both HGSR (r = −0.45, p = 0.033) and HGSL (r = −0.5, p = 0.015). Also, the motor subscore NIS-W was negatively correlated with HGSL (r = −0.44, p = 0.035); a similar correlation was present for the right side, but it was not statistically significant (r = −0.39, p = 0.06). NIS-WUL scores were negatively correlated with homolateral HGS measured for both the right (r = 0.43, p = 0.039) and left upper limbs (r = −0.46, p = 0.027).
Total NIS score was negatively correlated with CMAP amplitude of both right (r = −0.58, p = 0.014) and left (r = −0.61, p = 0.01) median nerves. The motor subscore NIS-W was negatively correlated with the CMAP amplitude of the left median nerve (r = −0.44, p = 0.035). NIS-WUL scores were not correlated with any neurophysiological data.
The CMAP amplitude of the right median nerve showed a positive correlation with HGSR (r = 0.54, p = 0.026). Similarly, we found a positive correlation between the CMAP amplitude of the left median nerve and HGSL (r = 0.56, p = 0.02). No significant correlations were established between DML and VCM of both median nerves and HGSR or HGSL.
Nowadays, hereditary transtiretin amyloidosis is a tractable disorder, for which a prompt diagnosis is needed, and biomarkers are on demand (14). Many molecules have been studied for cardiac phenotypes, such as serum retinol-binding protein 4 or B-type natriuretic peptide and transtiretin (15). However, there are only a few studies on biomarkers for nerve damage in ATTRv. Of interest, serum neurofilament light chain has been recently studied in ATTRv (16); however, there are only preliminary results available (14), that show a correlation with the severity of polyneuropathy, thus proposing NfL as a biomarker for nerve damage.
Handgrip test is a rapid, simple, and non-invasive tool that can be used for strength evaluation in patients affected by neuromuscular diseases. Furthermore, it is an attractive tool, because it can be easily performed and does not require expensive equipment (Figure 1). Several studies have shown how handgrip test is altered in polyneuropathies, such as diabetic one, in which patients show a significant reduction of strength (17). Moreover, some authors tried to use it as an outcome measure, showing promising results in CMT (18). However, to date, few authors have performed handgrip test in the evaluation of familial ATTRv showing significant strength reduction (19).
Here, we aim to confirm alteration in handgrip strength in patients affected by ATTRv and correlate HGS data with clinical and neurophysiological findings. In particular, given the high prevalence of carpal tunnel syndrome in ATTRv (2), we argued to correlate the reduced strength due to CTS with neurophysiological alterations NCS of the median nerve.
The present study directly compares neurophysiological data recorded from the median nerve and HGS in patients affected by ATTRv and healthy controls. HGS demonstrated a significant reduction of strength in both the right and left hands in patients compared to controls (Figure 1); also, the entity of strength reduction was similar in the right and left sides. Moreover, The NCSs showed prolonged DML in both right and left median nerves in the patients but not in the controls (Table 2), while CMAP amplitude was different only in left median nerves of the patients ATTRv (p = 0.012). This result might be explained by the high prevalence of CTS in the dominant hand (more often right) of the general population, because the controls also presented a similar mean right CMAP amplitude of the median nerve. Moreover, when present, CTS in ATTRv is usually bilateral, underlining the importance of detecting left (non-dominant) or bilateral CTS in ATTRv as a specific finding of amyloidosis, less often encountered in routine NCS (2, 12). Indeed, while bilateral CTS is an early sign of ATTRv, CTS in the dominant hand is the rule in general population, especially in the elderly (20).
Our data showed that the combined use of neurophysiology and HGS might represent a simple and unexpensive way to assess for motor compromise in ATTRv. As expected, the DML of the right median nerve was negatively correlated to CMAP amplitude but not with HGS in patients with ATTRv. Conversely, the CMAP amplitude of both right and left median nerves was positively correlated with left HGS. Indeed, CMAP amplitude is an expression of the number of motor fibers and, consequently, motor units that can generate maximum strength (21). On the contrary, DML depends on direct compression and local demyelination in the median nerve at wrist in CTS, which is not related with ATTRv itself, and it is not related with motor strength. Hence, we can assume that the reduced strength measured by HGS reflects CMAP amplitude in the median nerve.
Of interest is that relevant correlations have been reported between the CMAP amplitude of the median nerve and both NIS and HGS measures. This finding suggests that reduction in CMAP amplitude of the median nerve might cause a consequent increase in NIS scores and reduction of strength measured by HGS. However, when assessing the upper limbs alone (without considering the score from the lower limbs), correlations between clinical and neurophysiological data disappear; on the contrary, the relationship between NIS-WUL and HGS for both the right and left sides persist. These data support the use of HGS measures, suggesting that they might be more sensitive than neurophysiology alone in the assessment of strength in the upper limbs. Indeed, despite being a very sensitive and reliable tool in the clinical onset, conventional neurophysiology might be less reliable in the follow-up, when motor nerves become not elicitable or too altered to allow an indirect evaluation of residual strength (22, 23). From this perspective, HGS may offer the possibility of qualifying residual strength in the hands and appreciate even little changes that might escape with conventional neurophysiology.
Our study presents some limitations. The selection bias and low number of patients might have led to interpretation errors. Also, the evaluation of isolated median nerves might have carried an underestimation of the contribution of the ulnar nerve in hand weakness in ATTRv. Future studies are needed to validate this promising tool in clinical practice.
Progression of bilateral carpal tunnel syndrome can be shown with neurophysiological data of the median nerve in ATTRv. Also, handgrip measures might represent a simple and cost-effective tool for the assessment of disease progression in ATTR. We propose using a combination of CMAP amplitude and HGS for rapid and simple assessment of motor strength in ATTRv.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
The studies involving human participants were reviewed and approved by Palermo1. The patients/participants provided their written informed consent to participate in this study.
VD, ET, and VG contributed to the conception and design of the study. VD organized the database and performed data collection. SI, AT, AG, and AL collected the clinical and neurophysiological data. ET, GP, and VG evaluated the handgrip data. VD and ET supervised data collection and analysis. ET performed the statistical analysis. VD, SI, AT, ET, and VG wrote the first draft of the manuscript. AP, GB, and FB revised the manuscript. All authors contributed to manuscript revisions, read, and approved the submitted version of the manuscript.
Financial support for article processing fees was provided by Alnylam Pharmaceuticals (Cambridge, MA, USA). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Francesca Gentile, Vincenzo Gulino, and Sonia Tarantino for the aid in performing clinical and instrumental evaluations on patients with ATTR. Financial support for article processing fees was provided by Alnylam Pharmaceuticals (Cambridge, MA, USA).
1. Planté-Bordeneuve V, Said G. Familial amyloid polyneuropathy. Lancet Neurol. (2011) 10:1086–97. doi: 10.1016/S1474-4422(11)70246-0
2. Donnelly JP, Hanna M, Sperry BW, Seitz WH. Carpal tunnel syndrome: a potential early, red-flag sign of amyloidosis. J Hand Surg Am. (2019) 44:868–76. doi: 10.1016/j.jhsa.2019.06.016
3. Russo M, Gentile L, Di Stefano V, Di Bella G, Minutoli F, Toscano A, et al. Use of drugs for attrv amyloidosis in the real world: how therapy is changing survival in a non-endemic area. Brain Sci. (2021) 11:545. doi: 10.3390/brainsci11050545
4. Adams D, Polydefkis M, González-Duarte A, Wixner J, Kristen AV, Schmidt HH, et al. Long-term safety and efficacy of patisiran for hereditary transthyretin-mediated amyloidosis with polyneuropathy: 12-month results of an open-label extension study. Lancet Neurol. (2021) 20:49–59. doi: 10.1016/S1474-4422(20)30368-9
5. Coelho T, Ando Y, Benson MD, Berk JL, Waddington-Cruz M, Dyck PJ, et al. Design and rationale of the global phase 3 NEURO-TTRansform Study of Antisense Oligonucleotide AKCEA-TTR-L Rx (ION-682884-CS3) in hereditary transthyretin-mediated amyloid polyneuropathy. Neurol Ther. (2021) 10:375–89. doi: 10.1007/s40120-021-00235-6
6. Yarlas A, Lovley A, McCausland K, Brown D, Vera-Llonch M, Conceição I, et al. Early data on long-term impact of inotersen on quality-of-life in patients with hereditary transthyretin amyloidosis polyneuropathy: open-label extension of NEURO-TTR. Neurol Ther. (2021) 10:865–86. doi: 10.1007/s40120-021-00268-x
7. Escolano-Lozano F, Barreiros AP, Birklein F, Geber C. Transthyretin familial amyloid polyneuropathy (TTR-FAP): parameters for early diagnosis. Brain Behav. (2018) 8:889. doi: 10.1002/brb3.889
8. Conceição I, González-Duarte A, Obici L, Schmidt HH-J, Simoneau D, Ong M-L, et al. Red-flag symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. (2016) 21:5–9. doi: 10.1111/jns.12153
9. Rijk JM, Roos PRKM, Deckx L, Van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. (2016) 16:5–20. doi: 10.1111/ggi.12508
10. Núñez-Cortés R, Cruz-Montecinos C, Antúnez-Riveros MA, Pérez-Alenda S. Does the educational level of women influence hand grip and pinch strength in carpal tunnel syndrome? Med Hypotheses. (2020) 135:109474. doi: 10.1016/j.mehy.2019.109474
11. Ribom EL, Mellström D, Ljunggren Ö, Karlsson MK. Population-based reference values of handgrip strength and functional tests of muscle strength and balance in men aged 70-80 years. Arch Gerontol Geriatr. (2011) 53:5. doi: 10.1016/j.archger.2010.07.005
12. Di Stefano V, Gagliardo A, Barbone F, Vitale M, Ferri L, Lupica A, et al. Median-to-ulnar nerve communication in carpal tunnel syndrome: an electrophysiological study. Neurol Int. (2021) 13:304–14. doi: 10.3390/neurolint13030031
13. Spijkerman DCM, Snijders CJ, Stijnen T, Lankhorst GJ. Standardization of grip strength measurements. Effects on repeatability and peak force. Scand J Rehabil Med. (1991) 23:203–6.
14. Lupica A, Di Stefano V, Gagliardo A, Iacono S, Pignolo A, Ferlisi S, et al. Inherited neuromuscular disorders: which role for serum biomarkers? Brain Sci. (2021) 11:398. doi: 10.3390/brainsci11030398
15. Arvanitis M, Koch CM, Chan GG, Torres-Arancivia C, LaValley MP, Jacobson DR, et al. Identification of transthyretin cardiac amyloidosis using serum retinol-binding protein 4 and a clinical prediction model. J Am Med Assoc Cardiol. (2017) 2:305–13. doi: 10.1001/jamacardio.2016.5864
16. Louwsma J, Brunger AF, Bijzet J, Kroesen BJ, Roeloffzen WWH, Bischof A, et al. Neurofilament light chain, a biomarker for polyneuropathy in systemic amyloidosis. Amyloid. (2020) 2020:1–6. doi: 10.1080/13506129.2020.1815696
17. Oh TJ, Kang S, Lee JE, Moon JH, Choi SH, Lim S, et al. Association between deterioration in muscle strength and peripheral neuropathy in people with diabetes. J Diabetes Complications. (2019) 33:598–601. doi: 10.1016/j.jdiacomp.2019.04.007
18. Piscosquito G, Reilly MM, Schenone A, Fabrizi GM, Cavallaro T, Santoro L, et al. Responsiveness of clinical outcome measures in Charcot-Marie-Tooth disease. Eur J Neurol. (2015) 22:1556–63. doi: 10.1111/ene.12783
19. Tomás MT, Santa-Clara MH, Monteiro E, Baynard T, Carnero E, Bruno PM, et al. Body composition, muscle strength, functional capacity, and physical disability risk in liver transplanted familial amyloidotic polyneuropathy patients. Clin Transplant. (2011) 25:1436. doi: 10.1111/j.1399-0012.2011.01436.x
20. Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. (2016) 15:1273–84. doi: 10.1016/S1474-4422(16)30231-9
21. Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle. Oxford: Oxford University Press (2014). doi: 10.1093/med/9780199738687.001.0001
22. Uncini A, Aretusi G, Manganelli F, Seliguchi Y, Magy L, Tozza S, et al. Electrodiagnostic accuracy in polyneuropathies: supervised learning algorithms as a tool for practitioners. Neurol Sci. (2020) 41:3719–27. doi: 10.1007/s10072-020-04499-y
Keywords: TTR, hereditary amyloid neuropathy, median nerve, handgrip, hand strength, carpal tunnel syndrome, neurophysiology, nerve conduction study - NCS
Citation: Di Stefano V, Thomas E, Giustino V, Iacono S, Torrente A, Pillitteri G, Gagliardo A, Lupica A, Palma A, Battaglia G and Brighina F (2022) Motor Conduction Studies and Handgrip in Hereditary TTR Amyloidosis: Simple Tools to Evaluate the Upper Limbs. Front. Neurol. 13:835812. doi: 10.3389/fneur.2022.835812
Received: 14 December 2021; Accepted: 10 January 2022;
Published: 28 February 2022.
Edited by:
Giuseppe Vita, University of Messina, ItalyReviewed by:
Marina Grandis, University of Genoa, ItalyCopyright © 2022 Di Stefano, Thomas, Giustino, Iacono, Torrente, Pillitteri, Gagliardo, Lupica, Palma, Battaglia and Brighina. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vincenzo Di Stefano, dmluY2Vuem8xOTY4OUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.