Abstract
Introduction:
The relevance of the brush-sign remained poorly documented in large vessel occlusion (LVO). We aimed to assess the relationship between the brush-sign and collateral status and its potential impact on baseline diffusion-weighted imaging–Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) in acute ischemic stroke (AIS) patients eligible to mechanical thrombectomy (MT).
Methods:
Consecutive patients admitted in the Lyon Stroke Center with anterior circulation AIS due to intracranial internal carotid artery (ICA) and/or M1 or M2 segment of the middle cerebral artery (MCA) occlusion eligible for MT were included. The brush-sign was assessed on T2-gradient-echo MRI. Collateral status was assessed on digital subtraction angiography according to the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) score.
Results:
In this study, 504 patients were included, among which 171 (33.9%) patients had a brush-sign. Patients with a brush-sign more frequently had a poor collateral status [72 (42.1%) vs. 103 (30.9%); p = 0.017]. In univariable analysis, a DWI-ASPECTS < 7 was associated with a brush sign. Following multivariable analysis, the brush-sign no longer affected DWI-ASPECTS < 7 while the latter remained associated with younger age [odds ratio (OR) 0.97, 95% CI.96–0.99], male sex (OR 1.79, 95% CI 1.08–2.99), a higher National Institutes of Health Stroke Scale (NIHSS) score (OR 1.16, 95% CI 1.1–1.21), a poor collateral status (OR 9.35, 95% CI 5.59-16.02), MCA segment (OR 2.54, 95% CI 1.25–5.38), and intracranial ICA (OR 3.01, 95% CI 1.16–8) occlusion.
Conclusions and Relevance:
The brush-sign may be a marker of poor collateral status but did not independently predict a lower DWI-ASPECTS.
Clinical Trial Registration:
ClinicalTrials.gov, identifier: NCT04620642.
Introduction
Assessment of vessels using gradient recalled echo T2*-weighted imaging (GRE T2*WI) may provide insights into acute ischemic stroke (AIS) pathophysiology (1–4). The brush-sign refers to the abnormal visibility of enlarged deep medullary veins on GRE T2*WI related to an increase of blood deoxyhemoglobin concentration (3–6). The brush-sign has aroused significant interest in recent years. Previous studies have reported that the brush-sign was a marker of severe ischemia and hemorrhagic transformation risk in the context of intravenous (IV) thrombolytic therapy, while others have highlighted its relationship with a poor prognosis (5, 7–9). The clinical relevance of brush-sign in large vessel occlusion (LVO), and especially its link to the collateral status and baseline lesion volume assessed with diffusion-weighted imaging–Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) remained poorly documented in the context of mechanical thrombectomy (MT). We evaluated this relationship through a large registry of patients with AIS along with LVO eligible for MT.
Materials and Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the local ethics committee and the French Data Protection Authority. All participants have given their agreement to the use of their medical data.
Study Population
The Lyon Registry of Stroke Treated by Thrombolysis or Thrombectomy (RELATE) (NCT NCT04620642) collected data from consecutive patients admitted in the Lyon Stroke Center for AIS eligible for MT and/or IV thrombolysis. For the present study, only patients with anterior circulation AIS with occlusion of the intracranial internal carotid artery (ICA) and/or M1 or M2 segment of the middle cerebral artery (MCA) on admission brain magnetic resonance imaging (MRI) and eligible for MT from January 2015 to October 2020 were considered. Patients treated with IV thrombolysis alone were not included in the present study. Demographic characteristics and medical history as well as the National Institute of Health Stroke Scale (NIHSS) score assessed by board-certified neurologists were collected at admission.
Neuroimaging Protocol
Brain MRIs were obtained with the 1.5 or 3 T Ingenia scanners (Philips Healthcare, Best, The Netherlands). Admission brain MRI protocol included diffusion-weighted-imaging (DWI), GRE T2*WI, fluid-attenuated inversion recovery (FLAIR), and 3D time-of-flight (TOF) MR angiography.
Image Analysis
Two experienced physicians in stroke imaging (LR and NN) blinded to clinical and digital angiography data independently reviewed MRI. The brush-sign was defined as multiple hypointense linear and/or branching structures extending through the affected hemisphere, parallel or perpendicular to the outer wall of the lateral ventricles along the course of deep medullary veins on the baseline T2*-weighted gradient-echo sequence (10). The brush-sign was categorized as absent or present and if present classified as moderate, or obvious (Figure 1) (5). Any discrepancies were resolved by a third expert (ABS). DWI-ASPECTS was assessed on admission DWI sequence without further details on the affected brain regions (11). White matter hyperintensities (WMHs) were assessed on the FLAIR sequence according to the Fazekas scale; both the periventricular and subcortical components of the scale were evaluated (12). Cerebral microbleeds (CMB) were rated on GRE T2*WI according to the MARS criteria (13). The collateral status was determined on digital subtraction angiography (DSA) images according to the score of the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology (ASITN/SIR) and considered as poor for ASITN/SIR score < 3 (14). Reperfusion status was considered as successful if Thrombolysis in Cerebral Infarction (TICI) score was 2b or 3 (14). A CT scan was performed on day 1. Symptomatic intracerebral hemorrhage (sICH) was defined according to the SITS-MOST definition (15).
Figure 1
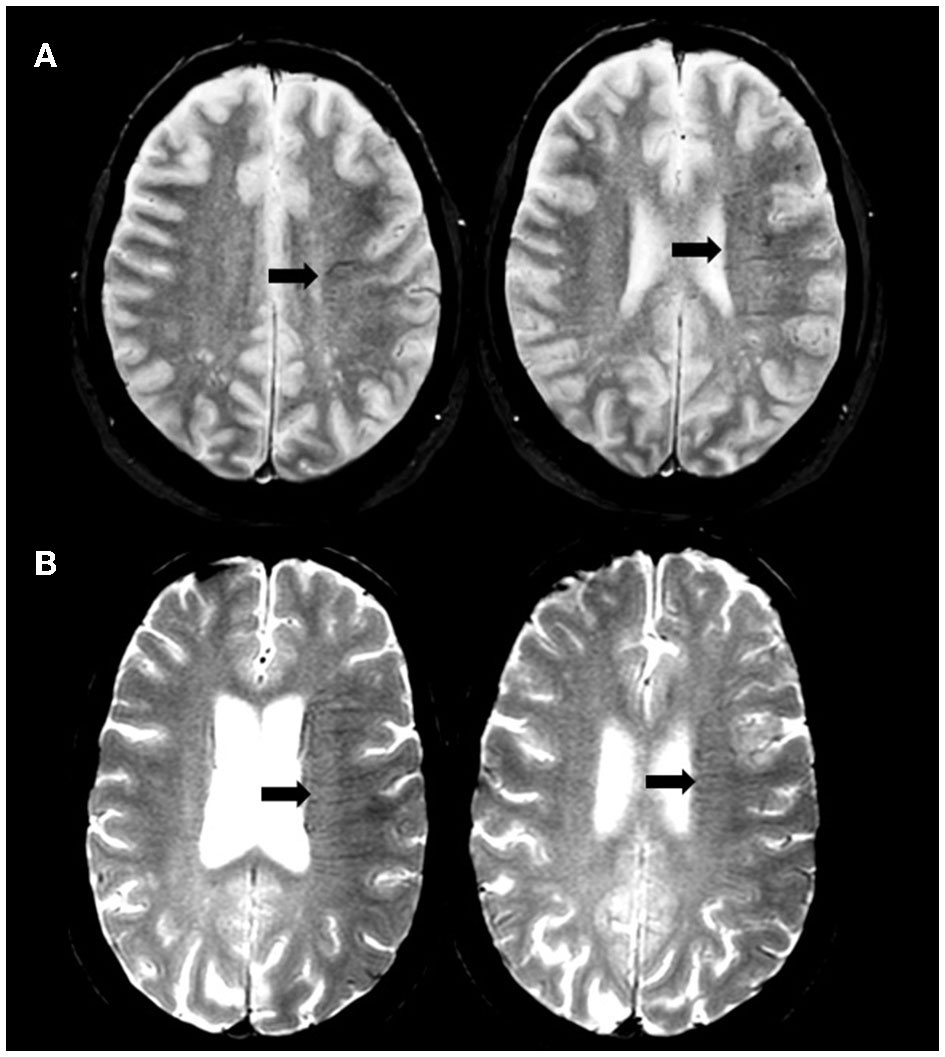
Illustration of brush-sign. Axial gradient-recalled echo T2*-weighted imaging showing moderate (A) and obvious (B) brush-sign.
Statistical Analysis
Continuous variables are expressed as means (SD) or medians (IQR) depending on their distributions, and categorical variables as percentages. Medians were compared using the Mann-Whitney test. Percentages were compared using Fisher's exact test. Interrater agreement for the evaluation of the brush-sign was assessed using the Cohen's kappa coefficient. DWI-ASPECTS was dichotomized at 7, which is a reliable surrogate of DWI volume of 100 ml (16). Univariable and multivariable logistic regression (forward method) models were used to determine the factors associated with DWI-ASPECTS < 7. The same analysis was performed in the subset of patients with M1 MCA segment occlusion. Covariates identified as statistically significant in univariable analyses (p < 0.05) were implemented through a backward procedure with Akaike Information Criterion (AIC) minimization. The interaction between the brush-sign and collateral status to predict DWI-ASPECTS was tested. Statistical testing used a two-tailed α level of 0.05. The data were analyzed with R statistical software version 3.6.1.
Data Availability Statement
Further anonymized data can be made available to qualified investigators on request to the corresponding author.
Results
Study Population
From January 2015 to October 2020, 1,434 patients with anterior circulation AIS with LVO were eligible for MT in the Lyon Stroke Center. Among them, 305 with a CT scan as first-line imaging were excluded. Of the remaining 1,129 patients, 160 patients had uninterpretable MRI data and 465 had non-assessable ASITN/SIR score (anterograde flow, internal carotid artery (ICA) terminus occlusion, and inadequate acquisition) and were therefore excluded (Supplementary Figure 1). These patients did not differ from included patients in terms of age, sex and time from stroke onset to imaging but had a higher admission NIHSS score (17 [12–21] vs. 15 [9–19]; p < 0.001), a lower DWI-ASPECTS (7 [6–8] vs. 7 [6–8]; p < 0.001), a lower rate of M2 [57 (9.1%) vs. 100 (19.8%)] and M1 MCA segments [341 (54.6%) vs. 349 (69.2%)] occlusion and a higher rate of intracranial ICA [215 (34.4%) vs. 55 (10.9%); p for all intracranial occlusion site < 0.001] as well as tandem [155 (24.8%) vs. 45 (7.2%); p <0.001] occlusion compared with included patients. The remaining 504 patients represent the study population. Mean age was 69.4 +/−16 years, 235 (46.6%) were men. Median NIHSS on admission was 15 [9–19] and median DWI-ASPECTS was 7 [6–8]. The median delay from stroke onset to imaging was 106 min [81–153].
Prevalence and Distribution of the Brush-Sign
A total of 171 (33.9%) patients had a brush-sign [moderate, n = 127 (25.2%); obvious, n = 44 (8.7%)]. In the subset of patients with M1 MCA segment occlusion (n = 349), 116 (33,2%) had a brush-sign. Interrater agreement for the evaluation of brush-sign presence was substantial (kappa =0.74). Adjudication was needed for 50 (10%) patients. The main characteristics of the study population are shown in Table 1 according to the brush-sign presence. Patients with a brush-sign were younger, more often male, had a higher NIHSS score, a lower DWI-ASPECTS, a poor collateral status, and received IV thrombolysis more often afterward. The distribution of collateral status and DWI-ASPECTS according to the brush-sign severity are illustrated in Figure 2. The brush-sign was not associated with the severity of WMH, the presence of CMB, intracranial occlusion site, reperfusion status, or the occurrence of sICH. A brush sign was detected in 28% (117/418) and 63% (54/86) of patients who underwent a 1.5T and a 3T MRI, respectively.
Table 1
| Brush-sign presence (n = 171) | Brush-sign absence (n = 333) | p-value | |
|---|---|---|---|
| Age, years | 66.3 +/– 16.0 | 70.9 +/– 15.8 | 0.002 |
| Male | 102 (59.6) | 133 (39.9) | <0.001 |
| NIHSS score | 16 [11–20] | 13 [8–18] | <0.001 |
| Wake-up strokea | 48 (28.2) | 94 (28.3) | 1.000 |
| Magnetic field strength | <0.001 | ||
| 1.5T MRI | 117 (68.4) | 301 (90.4) | |
| 3T MRI | 54 (31.6) | 32 (9.6) | |
| DWI-ASPECTS | 7 [6–8] | 8 [7–8] | <0.001 |
| Fazekas' score ≥3b | 46 (34.6) | 106 (37.5) | 0.647 |
| Cerebral microbleeds ≥1b | 15 (11.3) | 53 (18.8) | 0.074 |
| Poor collateral status | 72 (42.1) | 103 (30.9) | 0.017 |
| Intracranial occlusion site | 0.413 | ||
| M2 MCA segment | 32 (18.7) | 68 (20.4) | |
| M1 MCA segment | 116 (67.8) | 233 (70.0) | |
| Intracranial ICAc | 23 (13.5) | 32 (9.6) | |
| Tandem occlusion | 17 (9.9) | 28 (8.4) | 0.684 |
| IV thrombolysis | 112 (65.5) | 169 (50.8) | 0.002 |
| Successful reperfusion | 123 (71.9) | 243 (73.0) | 0.886 |
| Stroke onset to imaging, mina | 105 [81–137] | 108 [81–167] | 0.133 |
| sICHd | 4 (2.8) | 3 (1.1) | 0.380 |
Main clinical and imaging characteristics according to the presence of the brush-sign.
NIHSS, national institute of health stroke score; MCA, middle cerebral artery; ICA, internal carotid artery; DWI-ASPECTS, diffusion-weighted imaging-Alberta stroke program early CT score; IV, intravenous; sICH, symptomatic intracerebral hemorrhage.
Variables are displayed as absolute number (percentage of column total), mean ± SD, or median (25th−75th percentiles) as appropriate.
Bold values indicate p < 0.05.
Ten patients missing data.
Eighty-eight patients missing data.
Intracranial ICA occlusion was associated with M1 and M2 MCA segment occlusion in 53 and two patients, respectively.
Ninety-two patients missing data.
Figure 2
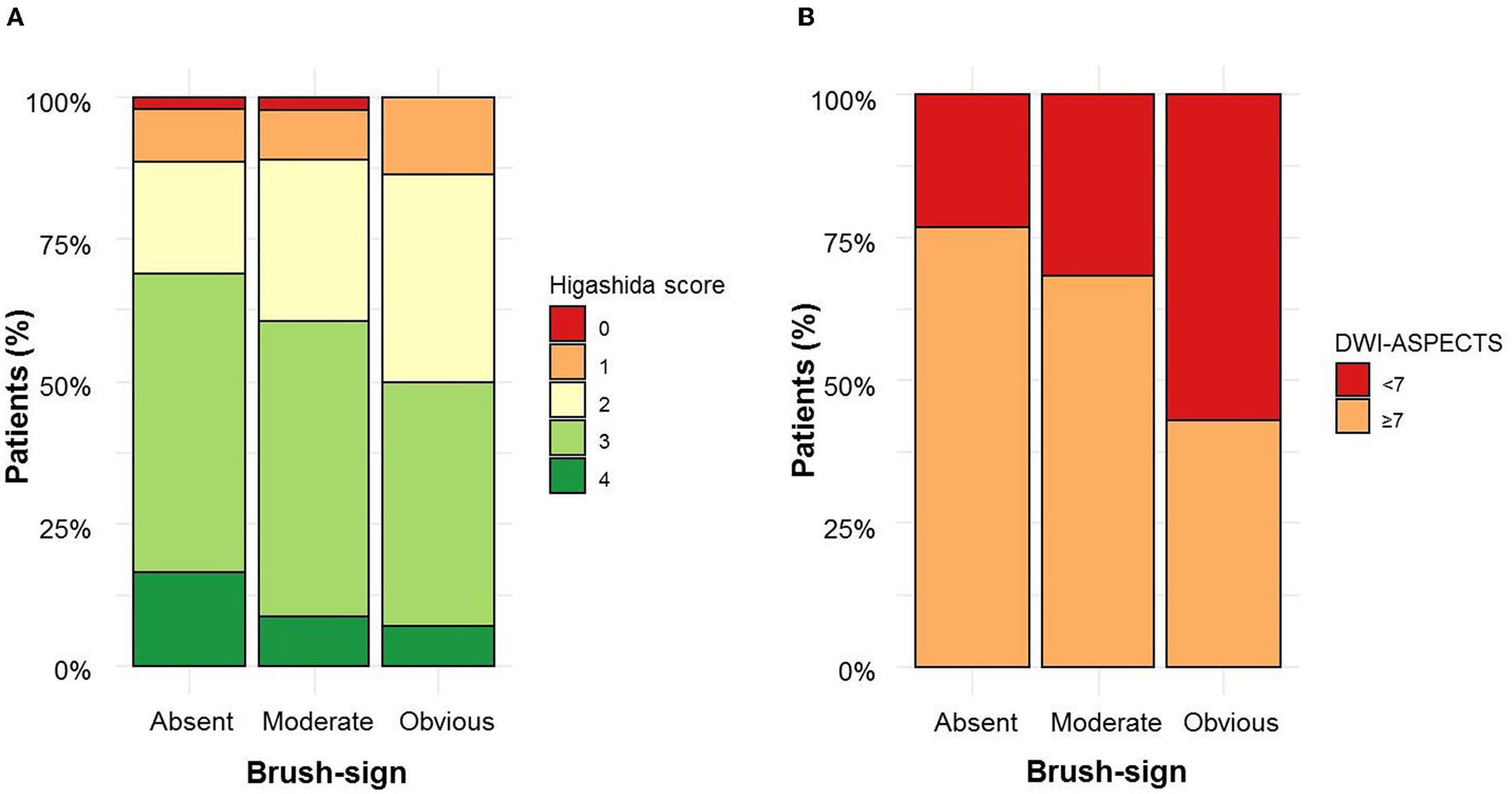
Brush sign severity according to collateral status (A) and diffusion-weighted imaging–Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) (B).
Factors Associated With DWI-ASPECTS in the Whole Study Population
On univariable analysis, a DWI-ASPECTS < 7 was associated with younger age, male sex, a higher NIHSS score, a poor collateral status, a brush-sign, M1 MCA segment, intracranial ICA, and tandem occlusion. Following multivariable analysis, after adjustment for age, sex, NIHSS score, intracranial occlusion site, tandem occlusion, and collateral status, the brush-sign was no longer associated with a DWI-ASPECTS < 7. Only a younger age, male sex, a higher NIHSS score, a poor collateral status, M1 MCA segment, and intracranial ICA occlusion remained associated with a DWI-ASPECTS < 7 (Table 2). We did not find any interaction between collateral status and the brush-sign, which means that the brush-sign presence did not affect the relationship between collateral status and DWI-ASPECTS as illustrated in Supplementary Figure 2.
Table 2
| Crude OR (95% CI) | p-value | Adjusted OR (95% CI)a | p-value | |
|---|---|---|---|---|
| Age | 0.98 (0.97–0.99) | <0.01 | 0.97 (0.96–0.99) | <0.01 |
| Male | 2.27 (1.53–3.39) | <0.01 | 1.79 (1.08–2.99) | 0.02 |
| NIHSS score | 1.15 (1.11–1.20) | <0.01 | 1.16 (1.10–1.21) | <0.01 |
| Brush-sign | 2.05 (1.37–3.06) | <0.01 | - | - |
| Poor collateral status | 9.05 (5.86–14.19) | <0.01 | 9.35 (5.59–16.02) | <0.01 |
| M1 MCA segment occlusionb | 1.81 (1.05–3.26) | 0.04 | 2.54 (1.25–5.38) | 0.01 |
| Intracranial ICA occlusionb | 3.93 (1.89–8.34) | <0.01 | 3.01 (1.16–8.00) | 0.02 |
| Tandem occlusion | 2.43 (1.30–4.53) | 0.01 | - | - |
Factors associated with diffusion-weighted imaging–Alberta Stroke Program Early Computed Tomography Score (DWI-ASPECTS) < 7 in univariable and multivariable analysis.
OR, odds ratio; NIHSS, national institute of health stroke score; MCA, middle cerebral artery; ICA, internal carotid artery.
Model was adjusted for age, sex, NIHSS score, collateral status, and intracranial occlusion site (brush-sign and tandem occlusion not retained by the backward selection).
vs. M2 middle cerebral artery segment occlusion.
Factors Associated With DWI-ASPECTS in Patients With M1 MCA Segment Occlusion
Among the 349 patients with M1 MCA segment occlusion, the univariable analysis showed that DWI-ASPECTS < 7 was associated with male sex, a higher NIHSS score, a brush-sign, a poor collateral status (Supplementary Table 1). On multivariable analysis, only male sex, a higher NIHSS score, and a poor collateral status remained associated with a DWI-ASPECTS < 7. Nor did we find any interaction between collateral status and the brush-sign to predict a DWI-ASPECTS < 7.
Discussion
Our study conducted on a large sample of patients with AIS along with LVO eligible for MT showed that the brush-sign was a marker of collateral status but did not predict independently a lower baseline DWI-ASPECTS.
In AIS, the increase of oxygen extraction fraction needed to maintain a constant cerebral metabolic rate of oxygen (CMRO2) leads to an increased level of deoxyhemoglobin within deep medullary veins (17, 18). This results in a signal drop on GRE T2*WI due to the paramagnetic properties of deoxyhemoglobin (3, 4). In addition, the dilatation of cerebral veins and brain acidosis may contribute to signal changes (19). Up to now, the relationship between the brush-sign and collateral status has not been addressed. We elaborated the hypothesis that a decreased collaterality supply may promote a low flow state leading to increased deoxygenation within the venous system.
Existing pieces of evidence on the relationship between veins' abnormal visibility and collateral status are often not focused on deep medullary veins analysis and offer divergent results (20–23). Xu et al. found a relationship between the presence of asymmetric deep medullary veins and a good leptomeningeal collateral circulation among 56 patients with AIS along with LVO (23). Park et al. showed an association between extensive prominent vessel signs (cortical and/or deep medullary vein) and a good collateral flow in 80 patients with LVO (20). However, in these two studies, assessment of collateral status using FLAIR imaging +/– post-contrast TOF MR angiography appears as a limitation (24). In addition, in the second study, MRI was performed within 3 days from stroke onset. Thus this delay may have concealed retrograde (or anterograde) flow due to extensive infarction and related edema (25). In contrast, Verma et al. observed that prominent cortical veins grade was associated with a poor leptomeningeal collateral status on DSA among 33 patients with acute occlusion of the M1 MCA segment (21). And a recent study conducted in 152 patients with AIS along with LVO found a correlation between prominent vessel sign (cortical and/or deep medullary vein) score and collateral status grade defined on the multiphase MR angiography collateral map (22). Focusing on deep medullary veins in a large sample of patients with AIS along with LVO, we found that brush-sign was associated with a poor collateral status assessed on pretreatment DSA. These latter observations suggest that the collateral status decides the ratio of deoxyhemoglobin–oxyhemoglobin in the venous system.
As the collateral status and hemodynamic impairment are closely linked to the baseline lesion volume, we further explored the relationship between brush-sign and DWI-ASPECTS. Guenego et al. have observed that the brush-sign may be uncoupled from collateral status but interacted with this latter to predict core infarct volume (26). Under favorable collateral blood-flow, patients with a brush-sign had the lowest core infarct volume. In contrast, under poor collateral blood-flow, the brush-sign was associated with the highest core infarct volume. In our study, the brush-sign did not appear as an independent predictor of a low DWI-ASPECTS in multivariable analysis, neither in the whole study population nor in the subset of patients with M1 MCA segment occlusion. Nor did we not observe any interaction between the brush-sign and collateral status to predict DWI-ASPECTS.
In the present study, about 1/3 of patients had a brush-sign according to the double reading of MRI. This frequency seems lower than those reported in most of the previous studies. Indeed, other authors, except Wang, et al. have reported a frequency of the brush-sign ranging from 47 to 96% (5–9, 20, 22, 23, 26). Several factors may explain this heterogeneity, such as the variable inclusion criteria (intracranial occlusion site, delay from stroke onset to MRI), the use of different sequences [T2-gradient-echo or susceptibility-weighted imaging (SWI)], or magnetic fields (1.5T or 3T MRI), as well as a combined analysis of deep medullary and cortical veins in some studies.
The strength of our study lies in the large sample of patients, allowing a robust analysis of the predictors of core infarct volume. In addition, we assessed collateral status with DSA that is considered as the “gold standard”. However, this method faces technical limits in patients with intracranial ACI occlusion. Among them, only patients with the persistence of residual anterograde flow or the use of contralateral catheterization had data about collateral status, thus leading to exclusion of a substantial proportion of patients from the analysis (14).
We acknowledge some limitations besides the monocentric and retrospective design. Merging patients with occlusion of intracranial ICA and/or M1 or M2 segment of the MCA may generate a certain level of heterogeneity although it has been widely used in previous studies (5–9, 20, 22, 23). However, subgroup analysis of patients with M1 MCA occlusion did not significantly modify the results and did not alter the interpretation of the data. Next, the sensitivity of the GRE T2* sequence is lower than SWI for the detection of the brush-sign (27). SWI, as a high-spatial-resolution 3-dimensional gradient-echo MR technique, is highly susceptible to paramagnetic substances and has demonstrated advantages over conventional GRE T2*WI (28, 29). Although delays and methods of image acquisition have improved, this method was previously considered time-consuming in an emergency setting. Furthermore, as the brush-sign was assessed through a qualitative approach, there may be bias in the interpretation of images. Quantitative susceptibility mapping is a development of SWI that can visualize veins and quantify blood oxygen saturation by measuring susceptibility values. This method may accurately identify hypointense vessels and provide more quantitative information about cerebral ischemia (30, 31). In addition, although DWI-ASPECTS is increasingly used in patients with AIS, this semi-quantitative analysis is less precise than a quantitative assessment of lesion volumes. It may overlook lesions within the striatocapsular region and only partially covers the MCA territory. This explains the wide range of true lesion volumes for a given DWI-ASPECTS (16). However, DWI-ASPECTS cut-off of 7 may be used as a reliable surrogate of DWI volume cut-off of 70 mL (16). Last, the use of either 1.5 T or 3 T magnetic fields related to MRI magnets availability may affect the frequency of the brush-sign (32). As 1.5T MRI has been performed in about 80% of the study population, our results can only be applied when 1.5 T MRI is used.
Summary/conclusions
In this large sample of patients with AIS along with LVO eligible for MT, the brush-sign appears as a marker of poor collateral status while it did not independently predict a low DWI-ASPECTS. Factors associated with the brush-sign as well as the prognostic value of the latter, not assessed here, deserve further exploration.
Funding
This work was supported by the RHU MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program Investissements d'Avenir operated by the French National Research Agency.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
Further anonymized data can be made available to qualified investigators on request to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Local Ethic Committee. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutionalrequirements.
Author contributions
LR, LM, NN, and CA designed the study, had a major role in the acquisition and interpretation of data, and drafted the manuscript for intellectual content. AB, MB, EO, JF, LD, and YB had a major role in the acquisition of data and in the revision of the manuscript for intellectual content. T-HC, OE, NM, and MH designed the study and had a major role in the acquisition of data and in the revision of the manuscript for intellectual content. CA, NM, and MB provide statistical analysis and improved methodology. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.828256/full#supplementary-material
References
1.
GröhnOHJKauppinenRA. Assessment of brain tissue viability in acute ischemic stroke by BOLD MRI: BOLD AND CEREBRAL ISCHEMIA. NMR Biomed. (2001) 14:432–40. 10.1002/nbm.739
2.
LeeJ-MVoKDAnHCelikALeeYHsuCYet al. Magnetic resonance cerebral metabolic rate of oxygen utilization in hyperacute stroke patients. Ann Neurol. (2003) 53:227–32. 10.1002/ana.10433
3.
RousselSAvan BruggenNKingMDGadianDG. Identification of collaterally perfused areas following focal cerebral ischemia in the rat by comparison of gradient echo and diffusion-weighted MRI. J Cereb Blood Flow Metab. (1995) 15:578–86. 10.1038/jcbfm.1995.71
4.
TamuraHHatazawaJToyoshimaHShimosegawaEOkuderaT. Detection of deoxygenation-related signal change in acute ischemic stroke patients by T2*-weighted magnetic resonance imaging. Stroke. (2002) 33:967–71. 10.1161/01.STR.0000013672.70986.E2
5.
HermierMNighoghossianNDerexLAdeleinePWiartMBerthezèneYet al. Hypointense transcerebral veins at T2*-weighted MRI: a marker of hemorrhagic transformation risk in patients treated with intravenous tissue plasminogen activator. J Cereb Blood Flow Metab. (2003) 23:1362–70. 10.1097/01.WCB.0000091764.61714.79
6.
MoritaNHaradaMUnoMMatsubaraSMatsudaTNagahiroSet al. Ischemic findings of T2*-weighted 3-tesla MRI in acute stroke patients. Cerebrovasc Dis. (2008) 26:367–75. 10.1159/000151640
7.
TerasawaYYamamotoNMorigakiRFujitaKIzumiYSatomiJet al. Brush sign on 3-T T2*-weighted MRI as a potential predictor of hemorrhagic transformation after tissue plasminogen activator therapy. Stroke. (2014) 45:274–6. 10.1161/STROKEAHA.113.002640
8.
MuckeJMöhlenbruchMKickingerederPKieslichPJBäumerPGumbingerCet al. Asymmetry of deep medullary veins on susceptibility weighted mri in patients with acute MCA stroke is associated with poor outcome. PLoS One. (2015) 10:e0120801. 10.1371/journal.pone.0120801
9.
WangYShiTChenBLinGXuYGengY. Prominent hypointense vessel sign on susceptibility-weighted imaging is associated with clinical outcome in acute ischaemic stroke. Eur Neurol. (2018) 79:231–9. 10.1159/000488587
10.
TaokaTFukusumiAMiyasakaTKawaiHNakaneTKichikawaKet al. Structure of the medullary veins of the cerebral hemisphere and related disorders. RadioGraphics. (2017) 37:281–97. 10.1148/rg.2017160061
11.
BarberPADemchukAMZhangJBuchanAM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. (2000) 355:1670–4. 10.1016/S0140-6736(00)02237-6
12.
FazekasFChawlukJAlaviAHurtigH. Zimmerman R. MR signal abnormalities at 15 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6. 10.2214/ajr.149.2.351
13.
GregoireSMChaudharyUJBrownMMYousryTAKallisCJagerHRet al. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology. (2009) 73:1759–66. 10.1212/WNL.0b013e3181c34a7d
14.
HigashidaRTFurlanAJ. Trial design and reporting standards for intra-arterial cerebral thrombolysis for acute ischemic stroke. Stroke. (2003) 34. 10.1161/01.STR.0000082721.62796.09
15.
MazyaMEgidoJAFordGALeesKRMikulikRToniDet al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe implementation of treatments in stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. (2012) 43:1524–31. 10.1161/STROKEAHA.111.644815
16.
de Margerie-MellonCTurcGTisserandMNaggaraOCalvetDLegrandLet al. Can DWI-ASPECTS substitute for lesion volume in acute stroke?Stroke. (2013) 44:3565–7. 10.1161/STROKEAHA.113.003047
17.
TsivgoulisG. Alexandrov AV. Cerebral hemodynamics in acute stroke: pathophysiology and clinical implications. J Vasc Interv Neurol. (2008) 1:65–9.
18.
BaronJ-CJonesT. Oxygen metabolism, oxygen extraction and positron emission tomography: Historical perspective and impact on basic and clinical neuroscience. Neuroimage. (2012) 61:492–504. 10.1016/j.neuroimage.2011.12.036
19.
SchilingAMBlankenburgFBBernardingJHeidenreichJOWolfKJ. Intracerebral pH affects the T2 relaxation time of brain tissue. Neuroradiology. (2002) 44:968–72. 10.1007/s00234-002-0873-0
20.
ParkM-GYangT-IOhS-JBaikSKKangYHParkK-P. Multiple hypointense vessels on susceptibility-weighted imaging in acute ischemic stroke: surrogate marker of oxygen extraction fraction in penumbra?Cerebrovasc Dis. (2014) 38:254–61. 10.1159/000367709
21.
VermaRKHsiehKGratzPPSchankathACMordasiniPZublerCet al. Leptomeningeal collateralization in acute ischemic stroke: Impact on prominent cortical veins in susceptibility-weighted imaging. Eur J Radiol. (2014) 83:1448–54. 10.1016/j.ejrad.2014.05.001
22.
LeeHJRohHGLeeSBJeonYSParkJJLeeT-Jet al. Collateral estimation by susceptibility-weighted imaging and prediction of functional outcomes after acute anterior circulation ischemic stroke. Sci Rep. (2021) 11:21370. 10.1038/s41598-021-00775-9
23.
XuZDuanYYangBHuangXPeiYLiX. Asymmetric deep medullary veins in patients with occlusion of a large cerebral artery: association with cortical veins, leptomeningeal collaterals, and prognosis. Front Neurol. (2019) 10:1292. 10.3389/fneur.2019.01292
24.
RaymondSBSchaeferPW. Imaging brain collaterals: quantification, scoring, and potential significance. Top Magn Reson Imaging. (2017) 26:67–75. 10.1097/RMR.0000000000000123
25.
CampbellBCChristensenSTressBMChurilovLDesmondPMParsonsMWet al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. (2013) 33:1168–72. 10.1038/jcbfm.2013.77
26.
GuenegoALeipzigMFahedRSussmanESFaizyTDMartinBWet al. Effect of oxygen extraction (Brush-Sign) on baseline core infarct depends on collaterals (HIR). Front Neurol. (2021) 11:618765. 10.3389/fneur.2020.618765
27.
Jensen-KonderingUBöhmR. Asymmetrically hypointense veins on T2*w imaging and susceptibility-weighted imaging in ischemic stroke. World J Radiol. (2013) 5:156. 10.4329/wjr.v5.i4.156
28.
HaackeEMMittalSWuZNeelavalliJChengY-CN. Susceptibility-weighted imaging: technical aspects and clinical applications, Part 1. Am J Neuroradiol. (2009) 30:19–30. 10.3174/ajnr.A1400
29.
HallerSHaackeEMThurnherMMBarkhofF. Susceptibility-weighted imaging: technical essentials and clinical neurologic applications. Radiology. (2021) 299:3–26. 10.1148/radiol.2021203071
30.
HaackeEMTangJNeelavalliJChengYNC. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. J Magn Reson Imaging. (2010) 32:663–76. 10.1002/jmri.22276
31.
VinayagamaniSSheelakumariRSabarishSSenthilvelanSRosRThomasBet al. Quantitative susceptibility mapping: technical considerations and clinical applications in neuroimaging. J Magn Reson Imaging. (2021) 53:23–37. 10.1002/jmri.27058
32.
ReichenbachJRBarthMHaackeEMKlarhöferMKaiserWA. Moser E. High-resolution MR venography at 30 Tesla. J Comput Assist Tomogr. (2000) 24:949–57. 10.1097/00004728-200011000-00023
Summary
Keywords
brush-sign, collaterality, lesion volume, thrombectomy, MRI, DWI-ASPECTS
Citation
Rascle L, Bani Sadr A, Amaz C, Mewton N, Buisson M, Hermier M, Ong E, Fontaine J, Derex L, Berthezène Y, Eker OF, Cho T-H, Nighoghossian N and Mechtouff L (2022) Does the Brush-Sign Reflect Collateral Status and DWI-ASPECTS in Large Vessel Occlusion?. Front. Neurol. 13:828256. doi: 10.3389/fneur.2022.828256
Received
03 December 2021
Accepted
28 January 2022
Published
02 March 2022
Volume
13 - 2022
Edited by
Jean-Marc Olivot, Centre Hospitalier Universitaire de Toulouse, France
Reviewed by
Adrien Guenego, Stanford Healthcare, United States; Alain Viguier, Hôpital Purpan, France
Updates
Copyright
© 2022 Rascle, Bani Sadr, Amaz, Mewton, Buisson, Hermier, Ong, Fontaine, Derex, Berthezène, Eker, Cho, Nighoghossian and Mechtouff.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Laura Mechtouff laura.mechtouff@chu-lyon.fr
†These authors have contributed equally to this work and share last authorship
This article was submitted to Stroke, a section of the journal Frontiers in Neurology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.