
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 18 February 2022
Sec. Neurotrauma
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.826266
 Megan E. Cosgrove1
Megan E. Cosgrove1 Jordan R. Saadon1
Jordan R. Saadon1 Charles B. Mikell1
Charles B. Mikell1 Patricia L. Stefancin2
Patricia L. Stefancin2 Leor Alkadaa1
Leor Alkadaa1 Zhe Wang1
Zhe Wang1 Sabir Saluja1
Sabir Saluja1 John Servider1
John Servider1 Bayan Razzaq1
Bayan Razzaq1 Chuan Huang3,4
Chuan Huang3,4 Sima Mofakham1,5*
Sima Mofakham1,5*Recovery of consciousness after traumatic brain injury (TBI) is heterogeneous and difficult to predict. Structures such as the thalamus and prefrontal cortex are thought to be important in facilitating consciousness. We sought to investigate whether the integrity of thalamo-prefrontal circuits, assessed via diffusion tensor imaging (DTI), was associated with the return of goal-directed behavior after severe TBI. We classified a cohort of severe TBI patients (N = 25, 20 males) into Early and Late/Never outcome groups based on their ability to follow commands within 30 days post-injury. We assessed connectivity between whole thalamus, and mediodorsal thalamus (MD), to prefrontal cortex (PFC) subregions including dorsolateral PFC (dlPFC), medial PFC (mPFC), anterior cingulate (ACC), and orbitofrontal (OFC) cortices. We found that the integrity of thalamic projections to PFC subregions (L OFC, L and R ACC, and R mPFC) was significantly associated with Early command-following. This association persisted when the analysis was restricted to prefrontal-mediodorsal (MD) thalamus connectivity. In contrast, dlPFC connectivity to thalamus was not significantly associated with command-following. Using the integrity of thalamo-prefrontal connections, we created a linear regression model that demonstrated 72% accuracy in predicting command-following after a leave-one-out analysis. Together, these data support a role for thalamo-prefrontal connectivity in the return of goal-directed behavior following TBI.
Patients with severe traumatic brain injury (TBI), defined as initial Glasgow Coma Scale (GCS) < 8 and loss of consciousness for at least 24 h, recover consciousness at variable intervals (1, 2). The mechanisms underlying coma and recovery of consciousness in patients with TBI are not fully understood. The combined effects of both primary injury to brainstem arousal nuclei (3, 4) and corticothalamic circuits (5), and secondary injury due to ischemia (6), microvascular dysfunction (7), and edema (8), affect the level of consciousness and ultimately patient outcomes after TBI.
After the acute phase of injury has passed, ~70% of patients eventually regain consciousness (9). Clinical assessment of consciousness generally involves testing command-following ability and other indicators of brain function (10–12). While consciousness may precede the ability to follow commands, recovery of command-following is an important predictor of functional outcome (13, 14). Importantly, the time until recovery of command-following is also a robust predictor of outcome (15, 16). However, the underlying mechanisms that enable command-following are not well-understood.
Although consciousness is required for voluntary behavior, less is known about what brain circuits support the language-guided control needed to follow verbal commands. The absence of this voluntary behavior, however, is not a prerequisite for consciousness, as a subset of these patients may display cognitive-motor dissociation (17). Resting fMRI connectivity of frontal networks differentiated brain hemorrhage patients who followed commands from those who did not (18). Invasive and scalp recordings of the prefrontal cortex also revealed the emergence of complex cortical activity as patients recovered consciousness and eventually followed commands (19). Small randomized trials support a role for stimulation of prefrontal cortex to improve command-following behavior; a third of patients who responded to transcranial direct current stimulation (tDCS) of PFC regained command-following ability (20). The patients who responded to tDCS tended to have preserved PFC and thalamic gray matter (21).
The role of the frontal lobes in facilitating consciousness remains controversial (22, 23). The prefrontal cortex is thought to be part of a large-scale “ignition” network that is activated when stimuli become conscious (24–26). However, data from newer “no-report” paradigms has challenged this view (27), and investigators have suggested that prefrontal activation simply reflects the behavioral report of conscious awareness. Given the prefrontal cortex's role in encoding rule-guided behavior (28, 29), action monitoring (30, 31), and cognitive control (32), it seems likely that the prefrontal cortex is essential to the recovery of language-guided behavior after TBI.
How the thalamus facilitates PFC function is a subject of active investigation (33). Traditionally, the thalamus was known as a passive relay of sensory signals to the cortex. However, recent studies suggest that the thalamus, and thalamocortical connectivity specifically, are also critically important for consciousness (34, 35). Severe TBI results in injuries to gray matter in the cortex and thalamus as well as widespread damage to subcortical white matter (36–38). In a cohort of these patients, thalamic atrophy was predictive of prolonged unconsciousness (39). Furthermore, structural and functional connectivity between thalamic and prefrontal regions has been shown to correlate with the level of consciousness (19, 34, 40). Thalamic stimulation has also demonstrated promise in augmenting the level of consciousness in both animal models (41, 42) and TBI patients (43, 44).
Higher-order thalamic nuclei such as the mediodorsal nucleus (MD) are actively involved in sustaining and switching task-related cortical representations by gating cortico-cortical connectivity (45). MD is the largest thalamic output to PFC and is critical for several cognitive tasks such as working memory (46). Several studies have revealed that MD activity is the key to sustaining PFC information during the delay period of working memory tasks (45, 47). Moreover, MD lesions lead to deficits in cognitive control (48, 49). While MD's exact function is not yet clear, recent data support the view that MD signals the representing behavioral context and dynamically controls the gain of cortico-cortical connections to allow for the formation of transient neuronal ensembles (45) and suppress competing motor plans (50) in response to task demands.
We, therefore, hypothesized that thalamic input (in particular MD) to PFC is important for recovery of consciousness and command-following after severe TBI. Diffusion MRI (dMRI) is a modality that can characterize abnormalities to these thalamo-prefrontal projections by detecting the restricted diffusion of water in the brain. By reconstructing the white-matter tracts of interest, quantitative measurements can shed light on the differences in tissue microstructure that may prognostically predict the loss or recovery of consciousness among TBI patients (51). To test our hypothesis, we assessed the integrity of thalamocortical projections on dMRI of TBI patients and developed a model for predicting command-following based on tractography analysis of these connections.
This retrospective study was approved by the Stony Brook University Hospital (SBUH) Committee on Research Involving Human Subjects (CORIHS) with a waiver of consent (2019-00464).
All scans and clinical details (with identifying information removed) are available to interested investigators upon reasonable request to the corresponding author.
Adult TBI patients (age ≥ 18) were screened for severe injury by searching chart documentation of initial Glasgow Coma Scale (GCS) < 8 or decompensation to GCS < 8 during the initial admission. Data and imaging were collected retrospectively from all patients who met the study's criteria (N = 25). Clinical information collected from patients included the date of injury, date of initiation of command-following, initial GCS score, findings on imaging, and interventions performed (Table 1). We identified the first day on which patients followed a simple verbal command (i.e., toe wiggling or tongue protrusion). Patients were subsequently categorized into the Early group (command-following within 30 days) and Late/Never group (after 30 days or never/death).
Clinical teams obtained MRIs for clinical prognosis, with a median period of 10 days between injury and MRI. In one case, the days from injury to MRI was significantly longer (Table 1). All images were acquired on the same 3 Tesla Siemens Trio MRI scanner. Structural images were collected via a 3D MPRAGE T1-weighted sequence with an isotropic voxel size of 1 x 1 x 1 mm3, and TE/TR/TI = 2.272/2,300/915 ms, FA=8. Diffusion-weighted imaging (DWI) was collected with EPI sequence with a single b-value of 1,000, slice thickness of 4 mm, TE/TR = 90/5,400 ms, in-plane resolution of 2 x 2 mm2, and 30 diffusion directions.
We drew regions of interest (ROI) manually on each patient's T1-weighted structural scan in FMRIB Software Library (FSL), using neuroanatomical landmarks because anatomical distortion made automated segmentation impractical. Representative PFC ROIs are depicted in Figure 1. The first slice used in PFC ROIs was one slice anterior to the corpus callosum. The anterior commissure-posterior commissure (AC-PC) line was drawn to delineate the OFC from the rest of the PFC. The OFC was drawn below the AC-PC line, anterior to the corpus callosum. The first slice used for the ACC ROI was also just anterior to the corpus callosum and included all slices where the ACC was visible. The mPFC began just anterior to the ACC and had all subsequent anterior slices. Lastly, the dlPFC included all remaining PFC; its boundaries were the OFC and ACC/mPFC. Whole thalamus ROIs were drawn based on visual differentiation of gray and white matter. MD thalamus ROIs were drawn based on the Morel Stereotactic Atlas of the Human Thalamus (52).
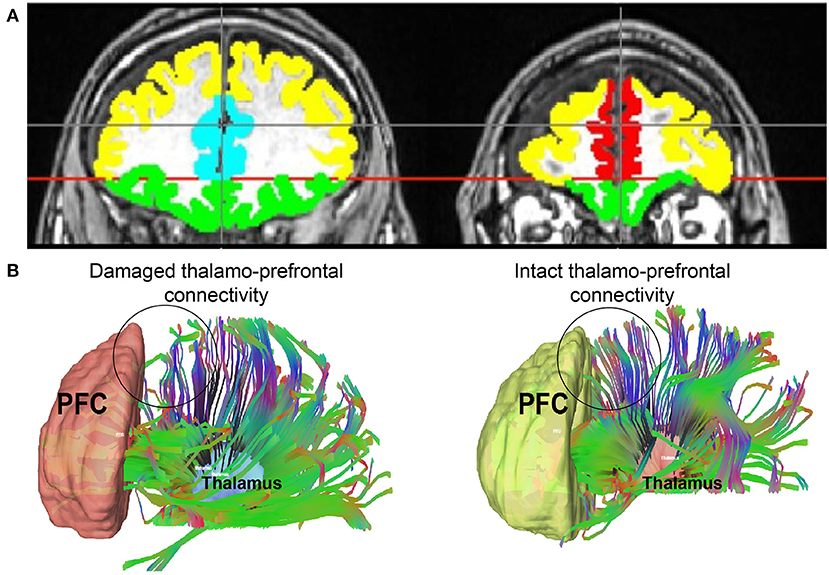
Figure 1. (A) An example of PFC regions of interest with colors representing each one. Yellow = dlPFC; green = OFC; teal = ACC; red = mPFC. (B) Thalamo-prefrontal tractography from two representative patients: one with damage to thalamo-prefrontal projections and one without. An area of decreased thalamo-prefrontal projections in the patient with damage to these connections is represented by a circle. This same area in the patient with intact projections is circled as well. dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex.
Diffusion MRI tractography was performed in order to characterize each patient's thalamo-prefrontal projections. Diffusion-weighted images were corrected for the off-resonance field produced by the susceptibility distribution of the subject's head using the FSL “topup” tool (53), and for the eddy currents produced by the rapid switching of the diffusion gradients using the FSL “eddy” tool (54). ROIs were merged to each patient's DWI using FSL “flirt” tool and confirmed for accuracy via visual inspection. A diffusion tensor model was fitted at each voxel in the brain-extracted DWI. The DTI model was used to generate fractional anisotropy (FA) maps for each subject. Values for FA were calculated and compared to patient clinical outcomes. Tractography was performed using the Quantitative Anisotropy (QA) algorithm (55), which augments deterministic tractography to correct for noisy fiber orientation distributions by incorporating anisotropic spinning along the fiber orientation. In separate experiments, the whole thalamus and MD thalamus in each hemisphere were used as seeds, and each ipsilateral prefrontal ROI was used as a target. Tractography was performed using the following tracking parameters: termination index = FA; threshold = random; angular threshold = 60°; maximum tracts = 10,000.
We analyzed each PFC region separately. We used a Student's t-test to compare the mean FA values between the Early and Late/Never groups. FA values were further adjusted for age and sex using a General Linear Model (GLM).
As a post-hoc analysis, we used linear regressions to assess clinical outcome predictability to further demonstrate the association between the thalamo-prefrontal connectivity and early command-following using FA values (log-transformed for normality), age, and sex. Stepwise regression was performed in R using the stepAIC function. The accuracy of the models was evaluated using a leave-one-out approach.
We retrospectively analyzed patients admitted with severe TBI who underwent DWI (N = 25, 20 males, mean age 50 ± 20.9; Table 1). This cohort is representative of the demographics of TBI on Long Island. Three patients never regained the ability to follow commands and eventually expired, though they survived for more than 30 days. The rest of the cohort regained the ability to follow commands, ranging from two days post-injury to 178 days post-injury, with a mean and standard deviation (mean ± SD) of 34 ± 50 and a median of 12.5 days. Sixteen (64%) patients had an early return of command-following (within 30 days of injury), and nine (36%) had late or no return of command-following.
We measured fractional anisotropy (FA) of white matter tracts between thalamus and dlPFC, mPFC, ACC, and OFC end regions (Figure 1). We performed right- and left-sided analyses separately for a total of eight separate tracts of interest. We split patients into two groups: (1) Early command-following group (command-following within 30 days of injury), and (2) Late/Never command-following group.
We found that FA, a measure of tract integrity, was significantly lower in the Late/Never group for whole thalamus connectivity to left and right ACC, right mPFC, and left OFC. The mean ± SD of significantly different subregions for Early and Late/Never groups, respectively, were as follows: left ACC, 0.356 ± 0.047 and 0.307 ± 0.051; right ACC, 0.373 ± 0.05 and 0.288 ± 0.042; right mPFC, 0.367 ± 0.031 and 0.302 ± 0.034; left OFC, 0.347 ± 0.043 and 0.298 ± 0.055. The results shown are adjusted for sex and age (Figure 2; Table 2) using a GLM. The FA values for connectivity of whole thalamus to left and right dlPFC also show the same trend, where these regions demonstrate higher FA values in the Early group and lower FA values in the Late/Never group. However, these differences were not statistically significant (P-values: 0.15 and 0.055 for left and right dlPFC connectivity, respectively) (Table 2).
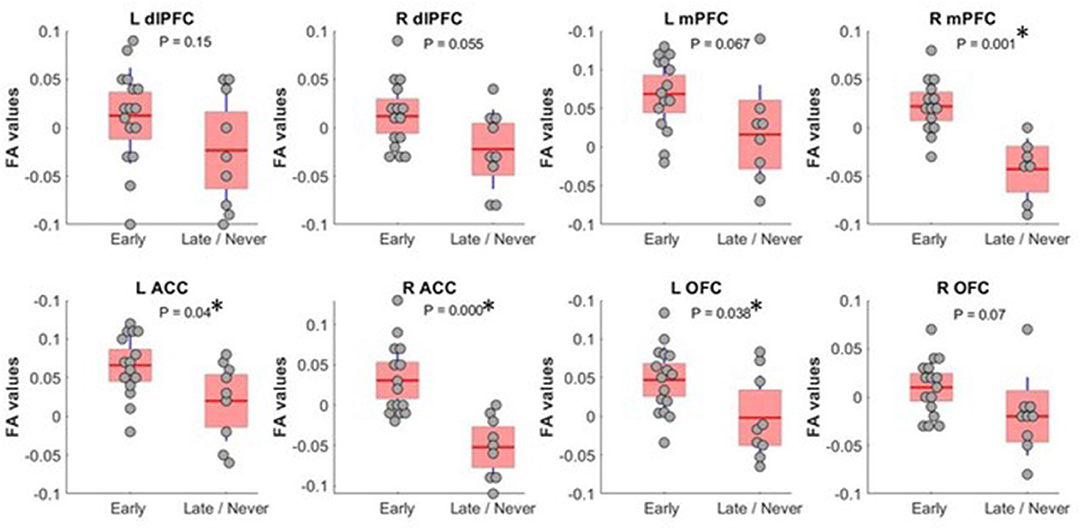
Figure 2. FA values (showing residual after adjusting for age and sex) between whole thalamus and PFC subregions. Subregions with significantly different FA between Early and Late/Never command-following groups are shown with an asterisk. A two-sample t-test was used to test residual of FA values after adjusting for age and sex using linear regression. R mPFC and R ACC survived a Bonferroni correction. dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; FA, fractional anisotropy.
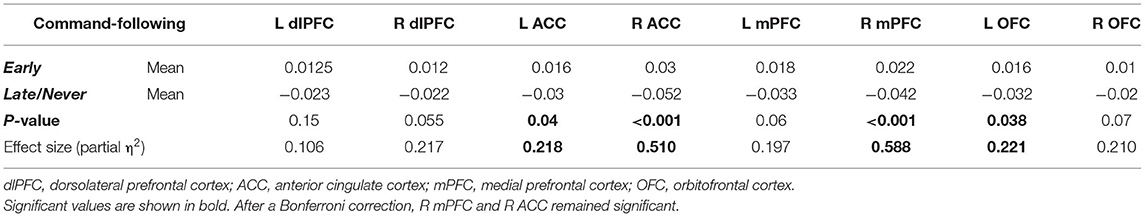
Table 2. Mean, P-values, and effect size of FA measurements (adjusted for age and sex) between whole thalamus and PFC regions in Early and Late/Never command-following groups using a two-sample t-test.
To shed light on MD's role in command-following, as MD has the largest reciprocal connectivity to PFC, we repeated the tractography analysis using MD as the seed instead of the whole thalamus. When we restricted the analysis to MD, FA values were significantly different between Early and Late/Never groups for connectivity to right mPFC, left and right ACC, and left and right OFC. The mean ± SD FA values for each significant sample in the Early and Late/Never groups, respectively, were as follows: right mPFC, 0.343 ± 0.048 and 0.267 ± 0.031; left ACC, 0.344 ± 0.047 and 0.284 ± 0.047; right ACC, 0.334 ± 0.051 and 0.273 ± 0.046; left OFC, 0.336 ± 0.050 and 0.266 ± 0.039; right OFC 0.337 ± 0.043 and 0.282 ± 0.035. Figure 3 shows that this result remains robust following age and sex correction using a GLM. The residuals and the P-values for each sample after GLM analysis are presented in Table 3. Similar to the whole thalamus data, connectivity from MD to left and right dlPFC, as measured by FA, was higher for the Early group than the Late/Never group, but this was not statistically significant (P-values: 0.15 and 0.13 for left and right dlPFC, respectively).
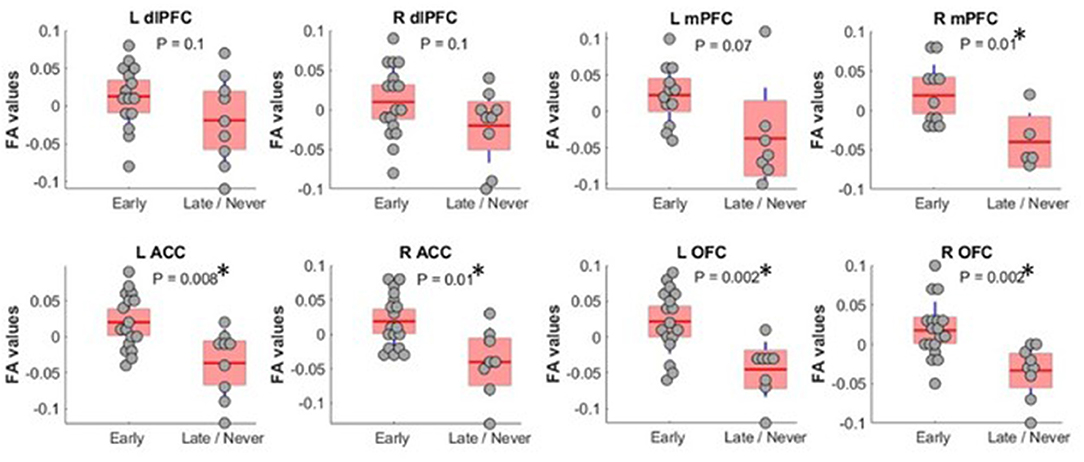
Figure 3. FA values (showing residual after adjusting for age and sex) between MD thalamus and PFC subregions. Subregions with significantly different FA between Early and Late/Never command-following groups are shown with an asterisk. A two-sample t-test was used to test residual of FA values after adjusting for age and sex using linear regression. L OFC and R OFC survived a Bonferroni correction. dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; FA, fractional anisotropy; MD, mediodorsal thalamic nucleus.
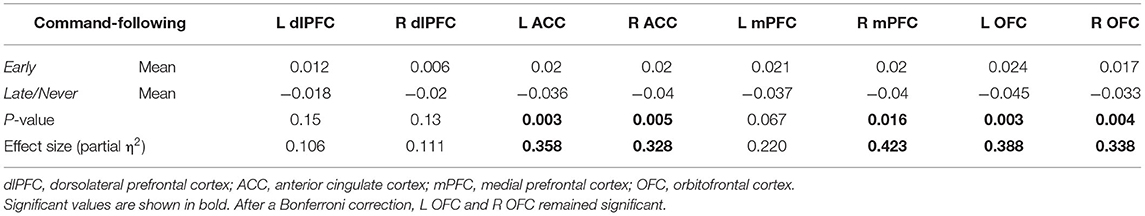
Table 3. Mean, P-values, and effect size of FA measurements (adjusted for age and sex) between MD thalamus and PFC regions in Early and Late/Never command-following groups using a two-sample t-test.
We used linear regression to develop a model to predict command-following within 30 days after TBI (Figure 4) to demonstrate the association between thalamo-prefrontal connectivity and early command-following. The model revealed that FA values of the frontal ROIs, along with patient sex, were predictive of patient clinical outcomes. Areas with the highest correlation in this model included left and right ACC, right mPFC, and left and right OFC.
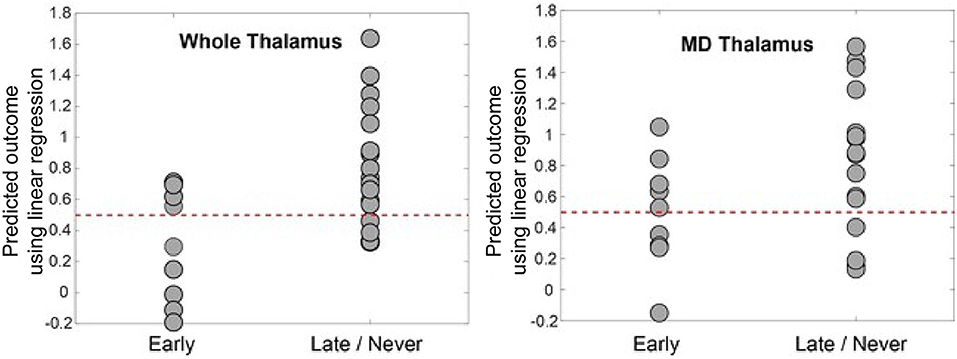
Figure 4. Predicted outcome by linear regression model vs. actual outcome. This was generated by leave-one-out cross-validation using log-transformed FA values between prefrontal regions and the whole thalamus (left panel) and MD (right panel). The dotted line at 0.5 represents the intuitive threshold used in this work to separate two different outcomes (represented by 0 and 1 in the regression). The prediction accuracy is 72% for the whole thalamus and 64% for MD. The model for whole thalamus selected left and right dlPFC, right mPFC, and right ACC as predictive variables. The model for MD selected left and right ACC, mPFC, OFC, and left dlPFC regions, as well as male sex as predictive variables. dlPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; FA, fractional anisotropy; MD, mediodorsal thalamic nucleus.
The final model for whole thalamus was:
Y ~ log(left dlPFC FA) + log(right dlPFC FA) + log(right ACC FA) + log(right mPFC FA).
The model for MD was:
Y ~ log(left dlPFC FA) + log(left ACC FA) + log(right ACC FA) + log(left mPFC FA) + log(right mPFC FA) + log(right OFC FA) + log(right OFC FA) + male sex.
The whole thalamus model was further evaluated using a leave-one-out approach. The model had 72% (18/25) accuracy for predicting the ability to follow commands within 30 days of injury, with a sensitivity of 81%, specificity of 56%, and F1 score of 75%. The coefficients for this linear regression model are shown in Table 4.
In this study, we investigated the role of the thalamo-prefrontal circuit in recovery of command-following, and thus goal-directed behavior, after TBI. We found that the integrity of thalamic connections with PFC subregions mPFC, ACC and OFC was significantly associated with early command-following. These data highlight a role for thalamo-prefrontal circuits in the recovery of goal-directed behavior after injury. Restricting our analysis to connections between MD and PFC subregions revealed similar results to the whole thalamus analysis, supporting previous research about its role in cognitively-based tasks (33, 45–48, 50). Our data stand in contrast to recently published studies in which thalamic injury was not correlated with unconsciousness (56, 57). However, these studies did not consider thalamocortical connectivity nor use the detailed anatomical analysis of our research. Moreover, although recently proposed models de-emphasize the frontal lobes' role in consciousness (58), our data show that PFC, more specifically its medial subregions, is important for recovery after TBI.
An important possible contributor to the observed findings is differences in laminar architecture between medial and lateral prefrontal cortex (59, 60). mPFC substantially lacks a granular layer, and markers of synaptic plasticity are inversely correlated to expression of granular neurons. Thus, mPFC is likely highly plastic, and thus potentially robust to disruption by mechanical injury (59). Further studies will use measures of cortical integrity to confirm this tantalizing possibility.
Another important finding of our study is the apparently critical role of medial portions of PFC (including mPFC and ACC). The mPFC is part of the default mode network (DMN), which governs the resting state of consciousness (61, 62). Previous studies have demonstrated that DMN connectivity is decreased at lower levels of consciousness such as the vegetative and minimally conscious states (63, 64). Another possible explanation is that medial PFC activity is correlated to task performance during periods when dlPFC activity is weak (65), as in the low-arousal state that characterizes the period after TBI. Moreover, task-related rules engage specific patterns of activity in PFC. Recent animal studies have shown that cortical representations needed for task performance require thalamic support (from higher-order thalamic nuclei such as MD) to be sustained (45). Thus, injuries that extend to the thalamus and thalamocortical projections likely hinder the formation and maintenance of these cortical representations associated with different goal-directed behaviors and delay command-following in TBI patients. In agreement with prior research (43, 44), our data suggest that augmenting activity in the thalamus, particularly MD, through techniques such as neurostimulation may improve goal-directed behavior after TBI.
Some limitations do affect our results. The study cohort was a convenience sample of severe TBI patients, which contributed some variability in demographics, injury patterns, and clinical management. However, given the heterogeneity of this population, it is not possible to make progress in TBI research without investigations of cohorts that reflect “real-world” TBI demographics. The number of patients in this study is small (N = 25) and may limit the generalizability of our results, as each TBI patient is unique. Our predictive model was created to demonstrate the potential utility of assessing thalamo-prefrontal connectivity in TBI patients. A more carefully conducted prediction study is needed to evaluate the accuracy of a prediction model such as using an independent validation set and/or multicenter data. Moreover, additional parcellation of the regions studied could provide further mechanistic information; the previously mentioned granularity gradients also exist in OFC, and future studies could investigate individual orbitofrontal subregions (66). Finally, the MRI imaging data in this study were acquired for clinical care, and were not fully optimized for research; nonetheless, we consider the imaging findings robust to multiple comparisons corrections. A prospective imaging study in a larger sample is warranted to confirm the findings in this study. Together, our data robustly highlight the role of thalamo-prefrontal connectivity in command-following.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Stony Brook University Committee on Research Involving Human Subjects. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SM and CM: study concept, design, and obtaining funding. CM, CH, and SM: study supervision and coordination. MC, JS, CM, SS, CH, and SM: drafting/revising the manuscript for content, including medical writing for content. PS and MC: acquisition of data. MC, PS, LA, ZW, CM, CH, and SM: analysis or interpretation of data. SM, CH, and PS: statistical analysis. All authors contributed to the article and approved the submitted version.
This work was funded by the Growing Convergence Research Program (NSF Award 2021002), a FUSION-TRO award (63845) from the Renaissance School of Medicine at Stony Brook University, as well as SEED grant funding from the Office of the Vice President for Research at Stony Brook University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Anthony Asencio, Susan Fiore, and Dr. Raphael Davis for their generous contributions to the conduct of the study. We also thank the Department of Neurosurgery at Stony Brook University Hospital for supporting this research.
1. Winans NJ, Liang JJ, Ashcroft B, Doyle S, Fry A, Fiore SM, et al. Modeling the return to consciousness after severe traumatic brain injury at a large academic level 1 trauma center. J Neurosurg. (2019) 133:1–9. doi: 10.3171/2019.2.JNS183568
2. Wang Z, Winans NJ, Zhao Z, Cosgrove ME, Gammel T, Saadon JR, et al. Agitation following severe traumatic brain injury is a clinical sign of recovery of consciousness. Front Surg. (2021) 8:627008. doi: 10.3389/fsurg.2021.627008
3. Edlow BL, Haynes RL, Takahashi E, Klein JP, Cummings P, Benner T, et al. Disconnection of the ascending arousal system in traumatic coma. J Neuropathol Exp Neurol. (2013) 72:505–23. doi: 10.1097/NEN.0b013e3182945bf6
4. Valko PO, Gavrilov YV, Yamamoto M, Noaín D, Reddy H, Haybaeck J, et al. Damage to arousal-promoting brainstem neurons with traumatic brain injury. Sleep. (2016) 39:1249–52. doi: 10.5665/sleep.5844
5. Forgacs PB, Frey H-P, Velazquez A, Thompson S, Brodie D, Moitra V, et al. Dynamic regimes of neocortical activity linked to corticothalamic integrity correlate with outcomes in acute anoxic brain injury after cardiac arrest. Ann Clin Transl Neurol. (2017) 4:119–29. doi: 10.1002/acn3.385
6. Stein SC, Graham DI, Chen X-H, Smith DH. Association between intravascular microthrombosis and cerebral ischemia in traumatic brain injury. Neurosurgery. (2004) 54:687–91. discussion: 691. doi: 10.1227/01.NEU.0000108641.98845.88
7. Glushakova OY, Johnson D, Hayes RL. Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood–brain barrier disruption, and progressive white matter damage. J Neurotrauma. (2014) 31:1180–93. doi: 10.1089/neu.2013.3080
8. Marmarou A, Signoretti S, Fatouros PP, Portella G, Aygok GA, Bullock MR. Predominance of cellular edema in traumatic brain swelling in patients with severe head injuries. J Neurosurg. (2006) 104:720–30. doi: 10.3171/jns.2006.104.5.720
9. Nakase-Richardson R, Whyte J, Giacino JT, Pavawalla S, Barnett SD, Yablon SA, et al. Longitudinal outcome of patients with disordered consciousness in the NIDRR TBI Model Systems Programs. J Neurotrauma. (2012) 29:59–65. doi: 10.1089/neu.2011.1829
10. Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. (1974) 2:81–4. doi: 10.1016/S0140-6736(74)91639-0
11. Giacino JT, Kalmar K, Whyte J. The JFK coma recovery scale-revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. (2004) 85:2020–9. doi: 10.1016/j.apmr.2004.02.033
12. Wijdicks EFM, Bamlet WR, Maramattom BV, Manno EM, McClelland RL. Validation of a new coma scale: the FOUR score. Ann Neurol. (2005) 58:585–93. doi: 10.1002/ana.20611
13. Suskauer SJ, Slomine BS, Inscore AB, Lewelt AJ, Kirk JW, Salorio CF. Injury severity variables as predictors of WeeFIM scores in pediatric TBI: time to follow commands is best. J Pediatr Rehabil Med. (2009) 2:297–307. doi: 10.3233/PRM-2009-0092
14. Greenwald BD, Hammond FM, Harrison-Felix C, Nakase-Richardson R, Howe LLS, Kreider S. Mortality following traumatic brain injury among individuals unable to follow commands at the time of rehabilitation admission: a National Institute on Disability and Rehabilitation Research traumatic brain injury model systems study. J Neurotrauma. (2015) 32:1883–92. doi: 10.1089/neu.2014.3454
15. Whyte J, Cifu D, Dikmen S, Temkin N. Prediction of functional outcomes after traumatic brain injury: a comparison of 2 measures of duration of unconsciousness. Arch Phys Med Rehabil. (2001) 82:1355–9. doi: 10.1053/apmr.2001.26091
16. Zhao Z, Liang JJ, Wang Z, Winans NJ, Morris M, Doyle S, et al. Cardiac arrest after severe traumatic brain injury can be survivable with good outcomes. Trauma Surg Acute Care Open. (2021) 6:e000638. doi: 10.1136/tsaco-2020-000638
17. Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. (2006) 313:1402. doi: 10.1126/science.1130197
18. Mikell CB, Banks GP, Frey H-P, Youngerman BE, Nelp TB, Karas PJ, et al. Frontal networks associated with command following after hemorrhagic stroke. Stroke. (2015) 46:49–57. doi: 10.1161/STROKEAHA.114.007645
19. Mofakham S, Liu Y, Hensley A, Saadon JR, Gammel T, Cosgrove ME, et al. Injury to thalamocortical projections following traumatic brain injury results in attractor dynamics for cortical networks. Prog Neurobiol. (2022) 210:102215. doi: 10.1016/j.pneurobio.2022.102215
20. Thibaut A, Bruno M-A, Ledoux D, Demertzi A, Laureys S. tDCS in patients with disorders of consciousness: sham-controlled randomized double-blind study. Neurology. (2014) 82:1112–8. doi: 10.1212/WNL.0000000000000260
21. Thibaut A, Di Perri C, Chatelle C, Bruno M-A, Bahri MA, Wannez S, et al. Clinical response to tDCS depends on residual brain metabolism and grey matter integrity in patients with minimally conscious state. Brain Stimul. (2015) 8:1116–23. doi: 10.1016/j.brs.2015.07.024
22. Boly M, Massimini M, Tsuchiya N, Postle BR, Koch C, Tononi G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J Neurosci. (2017) 37:9603–13. doi: 10.1523/JNEUROSCI.3218-16.2017
23. Odegaard B, Knight RT, Lau H. Should a few null findings falsify prefrontal theories of conscious perception? J Neurosci. (2017) 37:9593–602. doi: 10.1523/JNEUROSCI.3217-16.2017
24. Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, et al. Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci. (2001) 4:752–8. doi: 10.1038/89551
25. Melloni L, Molina C, Pena M, Torres D, Singer W, Rodriguez E. Synchronization of neural activity across cortical areas correlates with conscious perception. J Neurosci. (2007) 27:2858–65. doi: 10.1523/JNEUROSCI.4623-06.2007
26. Dehaene S, Changeux J-P. Experimental and theoretical approaches to conscious processing. Neuron. (2011) 70:200–27. doi: 10.1016/j.neuron.2011.03.018
27. Frässle S, Sommer J, Jansen A, Naber M, Einhäuser W. Binocular rivalry: frontal activity relates to introspection and action but not to perception. J Neurosci. (2014) 34:1738–47. doi: 10.1523/JNEUROSCI.4403-13.2014
28. Wallis JD, Anderson KC, Miller EK. Single neurons in prefrontal cortex encode abstract rules. Nature. (2001) 411:953–6. doi: 10.1038/35082081
29. Wallis JD, Miller EK. From rule to response: neuronal processes in the premotor and prefrontal cortex. J Neurophysiol. (2003) 90:1790–806. doi: 10.1152/jn.00086.2003
30. Gehring WJ, Knight RT. Prefrontal–cingulate interactions in action monitoring. Nat Neurosci. (2000) 3:516–20. doi: 10.1038/74899
31. Walton ME, Devlin JT, Rushworth MFS. Interactions between decision making and performance monitoring within prefrontal cortex. Nat Neurosci. (2004) 7:1259–65. doi: 10.1038/nn1339
32. Shenhav A, Cohen JD, Botvinick MM. Dorsal anterior cingulate cortex and the value of control. Nat Neurosci. (2016) 19:1286–91. doi: 10.1038/nn.4384
33. Parnaudeau S, Bolkan SS, Kellendonk C. The mediodorsal thalamus: an essential partner of the prefrontal cortex for cognition. Biol Psychiatry. (2018) 83:648–56. doi: 10.1016/j.biopsych.2017.11.008
34. Monti MM, Rosenberg M, Finoia P, Kamau E, Pickard JD, Owen AM. Thalamo-frontal connectivity mediates top-down cognitive functions in disorders of consciousness. Neurology. (2015) 84:167–73. doi: 10.1212/WNL.0000000000001123
35. Zheng ZS, Reggente N, Lutkenhoff E, Owen AM, Monti MM. Disentangling disorders of consciousness: insights from diffusion tensor imaging and machine learning. Hum Brain Mapp. (2017) 38:431–43. doi: 10.1002/hbm.23370
36. Anderson CV, Wood DM, Bigler ED, Blatter DD. Lesion volume, injury severity, and thalamic integrity following head injury. J Neurotrauma. (1996) 13:59–65. doi: 10.1089/neu.1996.13.59
37. Newcombe VFJ, Williams GB, Nortje J, Bradley PG, Harding SG, Smielewski P, et al. Analysis of acute traumatic axonal injury using diffusion tensor imaging. Br J Neurosurg. (2007) 21:340–8. doi: 10.1080/02688690701400882
38. Moen KG, Brezova V, Skandsen T, Håberg AK, Folvik M, Vik A. Traumatic axonal injury: the prognostic value of lesion load in corpus callosum, brain stem, and thalamus in different magnetic resonance imaging sequences. J Neurotrauma. (2014) 31:1486–96. doi: 10.1089/neu.2013.3258
39. Lutkenhoff ES, McArthur DL, Hua X, Thompson PM, Vespa PM, Monti MM. Thalamic atrophy in antero-medial and dorsal nuclei correlates with six-month outcome after severe brain injury. Neuroimage Clin. (2013) 3:396–404. doi: 10.1016/j.nicl.2013.09.010
40. Fernández-Espejo D, Bekinschtein T, Monti MM, Pickard JD, Junque C, Coleman MR, et al. Diffusion weighted imaging distinguishes the vegetative state from the minimally conscious state. Neuroimage. (2011) 54:103–12. doi: 10.1016/j.neuroimage.2010.08.035
41. Redinbaugh MJ, Phillips JM, Kambi NA, Mohanta S, Andryk S, Dooley GL, et al. Thalamus modulates consciousness via layer-specific control of cortex. Neuron. (2020) 106:66–75.e12. doi: 10.1016/j.neuron.2020.01.005
42. Bastos AM, Donoghue JA, Brincat SL, Mahnke M, Yanar J, Correa J, et al. Neural effects of propofol-induced unconsciousness and its reversal using thalamic stimulation. Elife. (2021) 10:e60824. doi: 10.7554/eLife.60824
43. Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, Gerber M, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. (2007) 448:600–3. doi: 10.1038/nature06041
44. Cain JA, Spivak NM, Coetzee JP, Crone JS, Johnson MA, Lutkenhoff ES, et al. Ultrasonic thalamic stimulation in chronic disorders of consciousness. Brain Stimul. (2021) 14:301–3. doi: 10.1016/j.brs.2021.01.008
45. Schmitt LI, Wimmer RD, Nakajima M, Happ M, Mofakham S, Halassa MM. Thalamic amplification of cortical connectivity sustains attentional control. Nature. (2017) 545:219–23. doi: 10.1038/nature22073
46. Peräkylä J, Sun L, Lehtimäki K, Peltola J, Öhman J, Möttönen T, et al. Causal evidence from humans for the role of mediodorsal nucleus of the thalamus in working memory. J Cogn Neurosci. (2017) 29:2090–102. doi: 10.1162/jocn_a_01176
47. Alexander GE, Fuster JM. Effects of cooling prefrontal cortex on cell firing in the nucleus medialis dorsalis. Brain Res. (1973) 61:93–105. doi: 10.1016/0006-8993(73)90518-0
48. Dolleman-van der Weel MJ, Morris RGM, Witter MP. Neurotoxic lesions of the thalamic reuniens or mediodorsal nucleus in rats affect non-mnemonic aspects of watermaze learning. Brain Struct Funct. (2009) 213:329–42. doi: 10.1007/s00429-008-0200-6
49. Parnaudeau S, O'Neill P-K, Bolkan SS, Ward RD, Abbas AI, Roth BL, et al. Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron. (2013) 77:1151–62. doi: 10.1016/j.neuron.2013.01.038
50. Rikhye RV, Gilra A, Halassa MM. Thalamic regulation of switching between cortical representations enables cognitive flexibility. Nat Neurosci. (2018) 21:1753–63. doi: 10.1038/s41593-018-0269-z
51. Bigler ED. Neuroimaging in mild traumatic brain injury. Psychol Inj Law. (2010) 3:36–49. doi: 10.1007/s12207-010-9064-1
52. Niemann K, Mennicken VR, Jeanmonod D, Morel A. The Morel stereotactic atlas of the human thalamus: atlas-to-MR registration of internally consistent canonical model. Neuroimage. (2000) 12:601–16. doi: 10.1006/nimg.2000.0650
53. Andersson JLR, Graham MS, Drobnjak I, Zhang H, Campbell J. Susceptibility-induced distortion that varies due to motion: correction in diffusion MR without acquiring additional data. Neuroimage. (2018) 171:277–95. doi: 10.1016/j.neuroimage.2017.12.040
54. Andersson JLR, Sotiropoulos SN. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage. (2016) 125:1063–78. doi: 10.1016/j.neuroimage.2015.10.019
55. Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE. (2013) 8:e80713. doi: 10.1371/journal.pone.0080713
56. Hindman J, Bowren MD, Bruss J, Wright B, Geerling JC, Boes AD. Thalamic strokes that severely impair arousal extend into the brainstem. Ann Neurol. (2018) 84:926–30. doi: 10.1002/ana.25377
57. Rohaut B, Doyle KW, Reynolds AS, Igwe K, Couch C, Matory A, et al. Deep structural brain lesions associated with consciousness impairment early after hemorrhagic stroke. Sci Rep. (2019) 9:4174. doi: 10.1038/s41598-019-41042-2
58. Koch C, Massimini M, Boly M, Tononi G. Neural correlates of consciousness: progress and problems. Nat Rev Neurosci. (2016) 17:307–21. doi: 10.1038/nrn.2016.22
59. García-Cabezas MÁ, Joyce MKP, John YJ, Zikopoulos B, Barbas H. Mirror trends of plasticity and stability indicators in primate prefrontal cortex. Eur J Neurosci. (2017) 46:2392–405. doi: 10.1111/ejn.13706
60. García-Cabezas MÁ, Hacker JL, Zikopoulos B A. Protocol for cortical type analysis of the human neocortex applied on histological samples, the atlas of Von Economo and Koskinas, and magnetic resonance imaging. Front Neuroanat. (2020) 14:576015. doi: 10.3389/fnana.2020.576015
61. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL, et al. Default mode of brain function. Proc Natl Acad Sci U S A. (2001) 98:676–82. doi: 10.1073/pnas.98.2.676
62. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. (2003) 100:253–8. doi: 10.1073/pnas.0135058100
63. Vanhaudenhuyse A, Noirhomme Q, Tshibanda LJ-F, Bruno M-A, Boveroux P, Schnakers C, et al. Default network connectivity reflects the level of consciousness in non-communicative brain-damaged patients. Brain. (2010) 133:161–71. doi: 10.1093/brain/awp313
64. Fernández-Espejo D, Soddu A, Cruse D, Palacios EM, Junque C, Vanhaudenhuyse A, et al. A role for the default mode network in the bases of disorders of consciousness. Ann Neurol. (2012) 72:335–43. doi: 10.1002/ana.23635
65. Silton RL, Heller W, Towers DN, Engels AS, Spielberg JM, Edgar JC, et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. (2010) 50:1292–302. doi: 10.1016/j.neuroimage.2009.12.061
Keywords: traumatic brain injury (TBI), thalamocortical connectivity, tractography, diffusion-weighted imaging, recovery of consciousness, goal-directed behavior
Citation: Cosgrove ME, Saadon JR, Mikell CB, Stefancin PL, Alkadaa L, Wang Z, Saluja S, Servider J, Razzaq B, Huang C and Mofakham S (2022) Thalamo-Prefrontal Connectivity Correlates With Early Command-Following After Severe Traumatic Brain Injury. Front. Neurol. 13:826266. doi: 10.3389/fneur.2022.826266
Received: 30 November 2021; Accepted: 25 January 2022;
Published: 18 February 2022.
Edited by:
Tony L. Strickland, Sports Concussion Institute, United StatesReviewed by:
Rafael Naime Ruggiero, University of São Paulo, BrazilCopyright © 2022 Cosgrove, Saadon, Mikell, Stefancin, Alkadaa, Wang, Saluja, Servider, Razzaq, Huang and Mofakham. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sima Mofakham, c2ltYS5tb2Zha2hhbUBzdG9ueWJyb29rbWVkaWNpbmUuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.