- 1Department of Magnetic Resonance Imaging, Shaanxi Provincial People's Hospital, Xi'an, China
- 2Philips Healthcare, Xi'an, China
Purpose: To determine how intracranial vascular wall and atherosclerosis plaque characteristics differ between young and old adults with sICAS.
Methods: Eighty-four consecutive patients with sICAS who underwent high-resolution magnetic resonance imaging (HRMRI) from December 2017 to July 2020 were retrospectively collected. These participants were divided into young adult group (18–50 years, n = 28) and old adult group (>50 years, n = 56). Reviewers were blinded to any clinical information and HRMRI scans were analyzed for qualitative and quantitative indicators of vascular walls and plaque at the maximal lumen narrowing site using the independent-sample t-test, Mann–Whitney U-test, chi-square test or Fisher exact test, and logistic regression analysis.
Results: Young patients with sICAS had significantly smaller maximum wall thickness (1.45 ± 0.38 vs.1.75 ± 0.51 mm2, P = 0.003), higher prevalence of positive remodeling (53.57 vs. 21.43%, P = 0.003), and lower prevalence of diabetes mellitus (14.29 vs. 35.71%, P = 0.04) than old patients. Plaque burden and other plaque features were comparable between young and old patients.
Conclusion: Young patients with sICAS have smaller maximum wall thickness and greater ability to reconstruct, and are more likely to show positive remodeling, which may lead to some atherosclerotic lesions being missed. Young patients with evidence of vessel narrowing should be carefully examined for presence of high-risk atherosclerotic plaque.
Introduction
Symptomatic intracranial atherosclerotic stenosis (sICAS) is an important cause of ischemic stroke worldwide (1, 2). In the past, sICAS was generally reported in older individuals, but more and more young adults are being affected nowadays due to earlier appearance of traditional vascular risk factors (e.g., hypertension, diabetes, hyperlipidemia) as a result of rapid improvements in living standards (3, 4). In a recent large study from China, atherosclerosis accounted for 43.7% of ischemic strokes in young adults (5). In the young, the high risk of recurrence after a stroke and the many ensuing years of disability and loss of productivity places a huge burden on family, society, and the economy; therefore, sICAS in the young is receiving increasing attention (6–9). However, current guidelines from the American Heart and Stroke Association and the Royal College of Physicians still have few specific recommendations for the management of stroke in young adults (10–12).
Atherosclerosis—including the formation of the atherosclerotic plaque, artery stenosis, and rupture of unstable plaque—is an important cause of ischemic stroke. Early interventions to limit the occurrence and development of atherosclerotic plaque can help prevent end-stage cerebrovascular events (13). Traditional arterial imaging techniques (such as CTA, MRA, DSA) can reveal the degree of stenosis of the affected vessels, but cannot evaluate the characteristics of atherosclerotic plaque. High-resolution MRI (HR-MRI) is increasingly being recognized as a useful modality for evaluating the characteristics of vascular wall lesions and the morphological and quantitative characteristics of plaques (14, 15). However, due to paucity of research on the topic, it is not clear whether there are differences in the characteristics of atherosclerotic plaque between young and old adults (6, 16–18). Therefore, the aim of this study was to determine whether the characteristics of intracranial vascular wall and atherosclerotic plaque differ between young and old adults and to establish the value of HR-MRI for evaluation of atheromatous plaque.
Materials and Methods
Ethics
The study was approved by the Ethics Committee of our institution and was performed in accordance with the tenets of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Patients
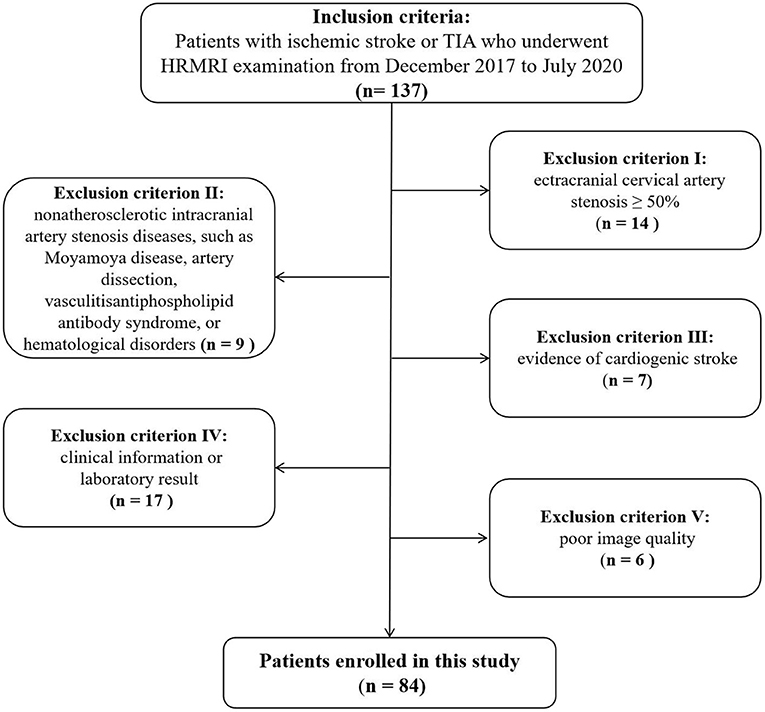
The data of consecutive of patients with cerebrovascular symptoms who underwent HR-MRI at our hospital between December 2017 and July 2020 were collected from the hospital database and retrospectively analyzed. The inclusion criteria were (1) age 18–80 years; (2) clinical symptoms of classic transient ischemic attack (TIA) or ischemic stroke; (3) HR-MRI performed within 2 weeks of symptom onset; (4) at least one intracranial atherosclerotic plaque identified on HR-MRI; and (5) The clinical symptoms of TIA or ischemic stroke was attributed to plaques of atherosclerotic stenosis of intracranial portion. Classic TIA was defined as distinct focal neurologic dysfunction or monocular blindness lasting <24 h. Complete ischemic stroke was defined as one or more minor (non-disabling) completed strokes with persistence of symptoms or signs for more than 24 h (19). The exclusion criteria were (1) non-atherosclerotic intracranial artery stenosis (e.g., due to Moyamoya disease, arterial dissection, vasculitis, reversible cerebral vasoconstriction syndrome, antiphospholipid antibody syndrome, or hematological disorders.); 2) extracranial carotid artery stenosis ≥50%; (3) cardiogenic stroke; (4) incomplete clinical information or laboratory results; and (5) poor image quality. The 84 patients that met these criteria were divided into two groups according to age: a young group (18–50 years old; n = 28) and an old group (9) (≥50 years old; n = 56).
MRI Protocol
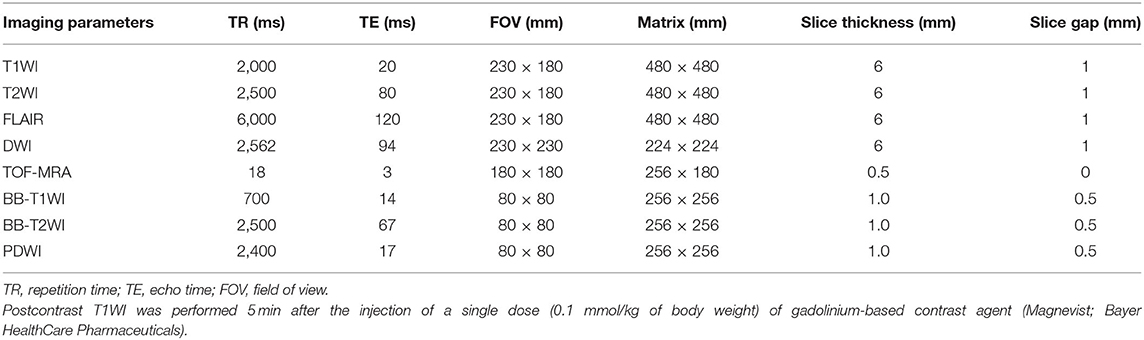
On admission, all patients underwent MRI (DWI, T1WI, T2WI, and FLAIR) on a 3.0T MR scanner (Ingenia CX, Philips Healthcare, The Netherlands) with a 32-channel neurovascular coil. HR-MRI scan was performed within 2 weeks of symptom onset. Scan included TOF-MRA angiography, BB-T1WI imaging, BB-T2WI imaging, PDWI Vista imaging, and postcontrast T1WI. The total scanning time was about 50 min. Table 1 lists the MRI sequences and parameters.
All MRI images were transformed to semi-automatic software (tsimaging.net) and RadiAnt DICOM Viewer (version 2020.2; http://www.radiantviewer.com) for analysis. Morphology and quantitative index of intracranial plaque at the site of maximum lumen narrowing (MLN) were analyzed. First, multiplanar reformations tool in postprocessing software was used to reconstruct the postcontrast T1WI images in both long and short axes, according to the vascular orientation at the MLN site. Traditional measurements and analysis were performed on a workstation by two experienced neuroradiologists (with 12 and 6 years of experience, respectively) who were blinded to the clinical data. The inner lumen and outer wall were manually outlined at the MLN site on reconstructed postcontrast T1WI images of each patient. The reference site was the nearest plaque-free segments proximal or distal to the MLN site. Maximum wall thickness (MWT), total vessel area (TVA), and lumen area (LA) were each measured thrice, and the values were averaged. Then, wall area (WA), plaque area (PA), plaque burden (PB), degree of stenosis (DS), remodeling index (RI), and remodeling type were calculated using the following formulas:
RI ≥ 1.05 was considered as positive remodeling (PR), and RI < 1.05 as non-positive remodeling (20–23).
Intraplaque hemorrhage (IPH) was considered to be present if the T1WI signal within the plaque was ≥150% of the T1WI signal of adjacent muscle or pons (24). Eccentric stenosis was considered present if the thinnest part of the wall was <50% of the thickest point on at least one T1WI slice. Concentric stenosis was diagnosed if the thinnest part of the wall was estimated to be no <50% of the thickest point on all image slices or a stenosis without wall thickening (22). Plaque enhancement on postcontrast T1WI was classified as “strong” if enhancement was equal to that of pituitary parenchymal enhancement or as “not strong” if enhancement was less than that of pituitary parenchymal enhancement (25). The irregular plaque surface was defined as the surface of uneven fluctuation (26) or ulceration that was identified on multicontrast MR vessel wall images with published criteria (27). Atherosclerotic plaque in the middle cerebral artery was defined as anterior circulation plaque and in the basal artery as posterior circulation plaque. The qualitative characteristics of intracranial arterial plaque were determined independently by two neuroradiologists who were blinded to the clinical data; disagreements were resolved by consensus.
Clinical Data Collection
Data were collected on age; sex; smoker; hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg); diabetes mellitus [fasting glucose ≥7.0 mmol/L, random glucose ≥11.1 mmol/L, or hemoglobin A1c (HbA1c) ≥7%]; HbA1c; hyperlipidemia [total cholesterol ≥5.18 mmol/L, triglycerides ≥1.7 mmol/L, low-density lipoprotein cholesterol (LDL) ≥ 3.37 mmol/L, high-density lipoprotein cholesterol (HDL) ≤1.04 mmol/L]; hyperhomocysteinemia (homocysteine ≥15 μmol/L); history of coronary artery disease; and family history of cardiovascular disease.
Statistical Analysis
Intraclass correlation coefficient (ICC) was used to evaluate interobserver agreement in measurement of MWT, TVA, and LA, and was classified as very good (r = 0.81–1.00), good (r = 0.61–0.80), moderate (r = 0.41–0.60), fair (r = 0.21–0.40), or poor (r < 0.20).
Normally distributed continuous variables were expressed as means ± standard deviation and non-normally distributed continuous variables as medians (25th−75th percentiles); comparison between groups was performed using the independent sample t-test or the Mann–Whitney U-test, respectively. Categorical variables were summarized as counts and percentages and compared using the chi-square or Fisher exact test. Logistic regression (binary variable) analysis was performed to assess the differences in plaque features between young and old adults after adjusting for confounding factors, and the results were expressed by the regression slope (β) or odds ratio (OR) and corresponding 95% confidence intervals (CIs). Clinical risk factors were considered to be potential confounders when the P-value was <0.1 during comparison analysis between young and old adult. For all other tests, statistical significance was at P < 0.05. Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA).
Results
Demographic Data
The flow diagram in Figure 1 summarizes the patient selection process. A total of 84 patients (70 men; mean age, 56.6 ± 16.1 years) with sICAS were included in this study. While 51 (60.71%) patients had complete ischemic stroke (on DWI), the other 33 (39.28%) had TIA. The plaque was in the anterior circulation in 38 (45.24%) patients, and in the posterior circulation in 46 (54.76%) patients.
Of the 84 patients, 28 (33.33%) were assigned to the young adult group (22 men; mean age, 38 ± 8 years) and 56 (66.67%) patients to the old adult group (29 men; mean age, 64 ± 9 years). Table 2 shows the characteristics of the two groups. The young group had significantly lower prevalence of hypertension (53.57 vs. 78.57%, P = 0.018) and diabetes mellitus (14.29 vs. 35.71%, P = 0.04); significantly lower mean systolic blood pressure (135.25 ± 19.86 mmHg vs. 147.57 ± 25.83 mmHg, P = 0.038) and HbA1c (5.96 ± 1.52% vs. 6.17 ± 1.17%, P = 0.023); significantly higher prevalence of hyperhomocysteinemia (67.86 vs. 42.86%, P = 0.031); and significantly higher mean homocysteine level (31.28 ± 23.73 μmol/L vs. 16.90 ± 6.38 μmol/L, P = 0.023). Prevalence of smoking, hyperlipidemia, and coronary artery disease, were similar in the two groups; mean diastolic blood pressure was also comparable between the two groups.
Plaque Characteristics
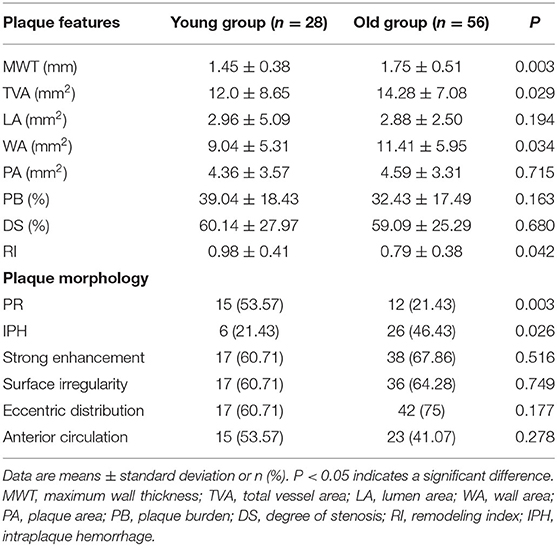
Figure 2 shows measurement of vessel plaque characteristics. Interobserver agreement in measurements of MWT, TVA, and LA was very good, with r values of 0.969 (95% CI, 0.952–980), 0.989 (95% CI, 0.982–0.993), and 0.919 (95% CI, 0.877–0.947), respectively. Table 3 presents a comparison of plaque characteristics in the two groups. Mean MWT (1.45 ± 0.38 mm2 vs.1.75 ± 0.51 mm2, P < 0.003), TVA (12.0 ± 8.65 mm2 vs. 14.28 ± 7.08 mm2, P < 0.029), and WA (9.04 ± 5.31 mm2 vs. 11.41 ± 5.95 mm2, P < 0.034) were significantly lower in the young group than in the old group. Prevalence of PR was significantly higher in the young group (53.57 vs. 21.43%, P < 0.003). Prevalence of IPH was significantly lower in the young group (21.43 vs. 46.43%, P < 0.026).
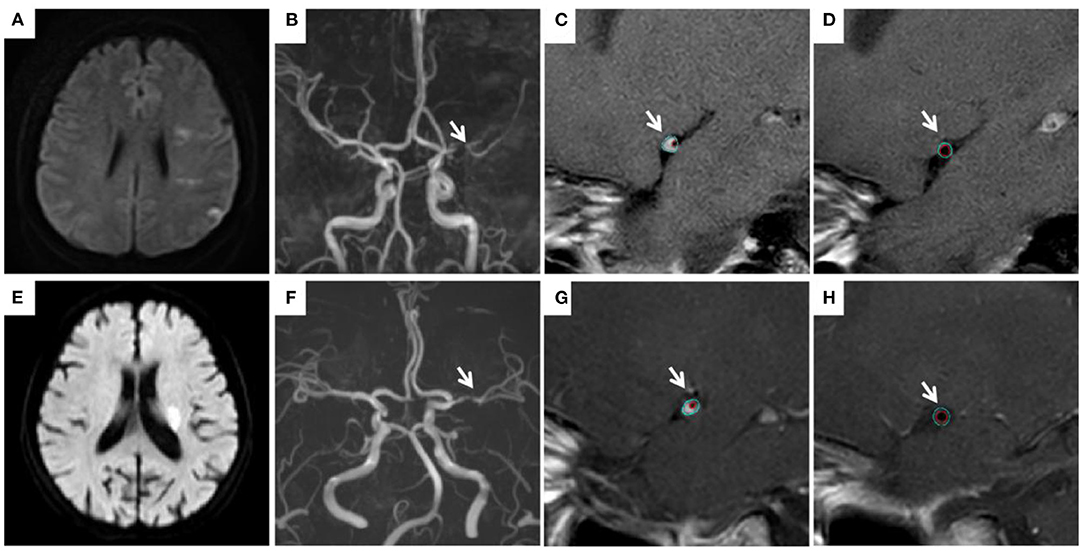
Figure 2. Measurement of vessel plaque characteristics. In a young patient (31 years old) with an infarction in the left hemisphere (A), left MCA stenosis (B) was observed. TVA was 7.81 mm2 at the MLN site (C) vs. 7.10 mm2 at the reference site (D). RI is 1.1. In a old patient (68 years old) with an infarction in the left basal ganglia (E), left MCA stenosis (F) was observed. TVA was 7.46 mm2 at the MLN site (G) vs. 8.12 mm2 at the reference site (H). RI is 0.92. MCA, middle cerebral artery; MLN, maximum lumen narrowing; TVA, total vessel area; RI, remodeling index.
Multivariable regression analysis revealed that MWT, PR, and prevalence of diabetes remained significantly different between young and old adult groups after adjusting for clinical confounding factors (both P < 0.05). MWT remained significantly lower (OR = 0.38; 95% CI: 0.002–0.635; P = 0.023), the prevalence of diabetes mellitus also remained significantly lower (OR = 0.173; 95% CI: 0.034–0.889; P = 0.036), while PR was significantly higher (OR = 5.416; 95% CI: 1.480–19.829; P = 0.011) in young group compared with old group. Prevalence of IPH (P = 0.102), hypertension (P = 0.062), and hyperhomocysteinemia (P = 0.137) were comparable between the two groups (Table 4).
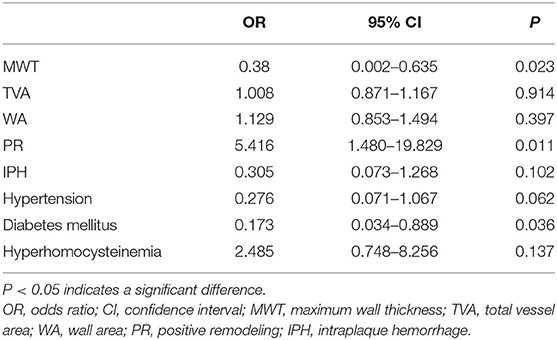
Table 4. Results of multivariable regression analysis showing factors associated with sICAS in the young individual.
Discussion
This study compared intracranial atherosclerotic plaque characteristics (identified with HR-MRI) and clinical factors between the young and old adult patients with sICAS and found significant differences between the two groups. Young individuals with sICAS had smaller MWT and were more likely to have PR; other plaque characteristics were similar in the two groups. Among clinical factors, diabetes mellitus was significantly less likely to be present in young patients than in old patients; other clinical factors were not significantly different between the two groups.
In this study, MWT, TVA, and WA were smaller in young patients with sICAS than in old patients but, after adjusting for potential confounders in multivariable analysis, only MWT remained significantly associated with sICAS in the young. Previous studies have also reported smaller MWT in younger patients. Cogswell et al. used 3T HR-MRI to measure internal carotid wall thickness and vessel outer wall diameter in healthy participants and found that both increase significantly with age (28). A study by de Freitas et al. (29) also showed that aging is independently related to increase in carotid intima-media thickness. The increase of MWT with age may represent the progression of atherosclerosis with age. Furthermore, instability and healing constantly alternate in atherosclerotic plaque, the repeated re-endothelialization of plaque surface and fibrosis leads to increase in MWT over time (13).
In this study, we found that PR was more likely in young patients with sICAS than in old patients. This suggests that the type of remodeling may be related to the plaque formation process. The duration of atherosclerotic plaque formation will be shorter in young patients than in old patients; because plaque development will still be in the early stage, the vessel will be more inclined to PR. However, the development of vessel tends to negative remodeling with the expand of life. Our result is consistent with reports of plaque remodeling in previous studies (30). Vessel wall compliance is better in young patients (as reflected by the lower systolic blood pressure) and so, when lumen stenosis occurs, vascular remodeling occurs more readily (31). This suggests that young patients with no stenosis or only mild stenosis, but having risk factors for early onset of large artery atherosclerosis, deserve special attention as PR may mask the presence of high-risk plaque. HR-MRI can be beneficial in such patients. In addition, PR is known to be a marker of plaque vulnerability (34), and our results showed that PR rate of young patients was higher than that of old patients, indicating that such patients had a higher incidence of vulnerable plaques, a higher risk of subsequent plaque rupture, and a higher possibility of cerebrovascular events eventually. Therefore, more attention should be paid to these patients clinically.
In the present study, young patients were found to be less likely to have diabetes mellitus than old patients; mean HbA1c was also significantly lower in young patients. It may suggests thatdiabetes mellitus has less effect on plaque' s development in young groups which has sICAS. A previous meta-analysis of 23 studies found that patients with type 2 diabetes mellitus and impaired glucose tolerance had greater carotid intima-media thickness than control patients (32). Further, Sun et al. showed that patients with high HbA1c had larger plaque burden (percent wall volume, max wall thickness) (33). This is consistent with our finding that diabetes and large MWT were less likely in young patients than in old patients.
Limitations
Our study has several limitations. First, this was a cross-sectional study with a small sample size. Second, we did not include severely disabled patients or those with severe cardiovascular disease treated with stents, pacemakers, or implantable cardioverter defibrillators; the selection bias might have affected our results. Future longitudinal studies on large samples are warranted to clarify the impact of age on plaque characteristics. Third, measurement of quantitative plaque indices is prone to subjective errors; however, we found excellent inter-reader consistency.
Conclusions
In conclusion, young patients with sICAS have smaller MWT and a greater ability for positive remodeling. Special attention is necessary to avoid missing the presence of high-risk plaque in these patients. HR-MRI appears to be a useful and reliable tool for evaluation of plaque characteristics.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
Ethical review and approval was not required for the current study in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Author Contributions
LL and MT drafted the manuscript and designed the study. LL performed the statistical analysis. XY and JG contributed to conducting the study and revised the manuscript. MT and KA provided technical support. LL, NM, XS, YN, and YW collected the data. XZ and XL helped design the study and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81270416), the Key Research and Development Program of Shaanxi Province of China (2018ZDXM-SF-038), and the Social Development Science and Technology Research Project of Shaanxi Province of China (2021SF-064).
Conflict of Interest
KA was employed by Philips Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all patients and their families for their cooperation.
Abbreviations
CI, confidence interval; DS, degree of stenosis; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein cholesterol; HR-MRI, high-resolution MRI; ICC, intraclass correlation coefficient; IPH, intraplaque hemorrhage; LA, lumen area; LDL, low-density lipoprotein cholesterol; MLN, maximal lumen narrowing; MWT, maximum wall thickness; OR, odds ratio; PA, plaque area; PB, plaque burden; RI, remodeling index; sICAS, symptomatic intracranial atherosclerotic stenosis; TIA, transient ischemic attack; TVA, total vessel area; WA, wall area.
References
1. Arenillas JF. Intracranial atherosclerosis: current concepts. Stroke. (2011) 42:S20–3. doi: 10.1161/STROKEAHA.110.597278
2. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. (2019) 18:394–405. doi: 10.1016/S1474-4422(18)30500-3
3. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. (2016) 15:913–24. doi: 10.1016/S1474-4422(16)30073-4
4. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995-2008. Ann Neurol. (2011) 70:713–21. doi: 10.1002/ana.22539
5. Li F, Yang L, Yang R, Xu W, Chen FP, Li N, et al. Ischemic stroke in young adults of Northern China: characteristics and risk factors for recurrence. Eur Neurol. (2017) 77:115–22. doi: 10.1159/000455093
6. Ekker MS, Boot EM, Singhal AB, Tan KS, Debette S, Tuladhar AM. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. (2018) 17:790–801. doi: 10.1016/S1474-4422(18)30233-3
7. Maaijwee NA, Rutten-Jacobs LC, Arntz RM, Schaapsmeerders P, Schoonderwaldt HC, van Dijk EJ, et al. Long-term increased risk of unemployment after young stroke: a long-term follow-up study. Neurology. (2014) 83:1132–8. doi: 10.1212/WNL.0000000000000817
8. Maaijwee NA, Rutten-Jacobs LC, Schaapsmeerders P, van Dijk EJ, de Leeuw FE. Ischaemic stroke in young adults: risk factors and long-term consequences. Nat Rev Neurol. (2014) 10:315–25. doi: 10.1038/nrneurol.2014.72
9. Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. (2013) 309:1136–44. doi: 10.1001/jama.2013.842
10. European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. (2008) 25:457–507. doi: 10.1159/000131083
11. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2014) 45:2160–236. doi: 10.1161/STR.0000000000000024
12. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000158
13. Vergallo R, Crea F. Atherosclerotic plaque healing. N Engl J Med. (2020) 383:846–57. doi: 10.1056/NEJMra2000317
14. Lindenholz A, van der Kolk AG, Zwanenburg JJM, Hendrikse J. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology. (2018) 286:12–28. doi: 10.1148/radiol.2017162096
15. Mandell DM, Mossa-Basha M, Qiao Y, Hess CP, Hui F, Matouk C, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol. (2017) 38:218–29. doi: 10.3174/ajnr.A4893
16. Ahn SH, Lee J, Kim YJ, Kwon SU, Lee D, Jung SC, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke. (2015) 46:697–703. doi: 10.1161/STROKEAHA.114.008181
17. Niu JW, Gao S, Cui LY, Peng B, Zhu YC, Ni J, et al. Intracranial atherosclerosis in Chinese young adult stroke patients. J Stroke Cerebrovasc Dis. (2014) 23:1519–23. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.030
18. Xu YY, Li ML, Gao S, Jin ZY, Sun ZY, Chen J, et al. Etiology of intracranial stenosis in young patients: a high-resolution magnetic resonance imaging study. Ann Transl Med. (2017) 5:319. doi: 10.21037/atm.2017.06.31
19. Zhao X, Hippe DS, Li R, Canton GM, Sui B, Song Y, et al. Prevalence and characteristics of carotid artery high-risk atherosclerotic plaques in chinese patients with cerebrovascular symptoms: a Chinese atherosclerosis risk evaluation II study. J Am Heart Assoc. (2017) 6:e005831. doi: 10.1161/JAHA.117.005831
20. Ma N, Jiang WJ, Lou X, Ma L, Du B, Cai JF, et al. Arterial remodeling of advanced basilar atherosclerosis: a 3-tesla MRI study. Neurology. (2010) 75:253–258. doi: 10.1212/WNL.0b013e3181e8e714
21. Varnava AM, Mills PG, Davies MJ. Relationship between coronary artery remodeling and plaque vulnerability. Circulation. (2002) 105:939–43. doi: 10.1161/hc0802.104327
22. Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis. (2010) 212:507–11. doi: 10.1016/j.atherosclerosis.2010.06.035
23. Zhang R, Zhang Q, Ji A, Lv P, Zhang J, Fu C, et al. Identification of high-risk carotid plaque with MRI-based radiomics and machine learning. Eur Radiol. (2021) 31:3116–26. doi: 10.1007/s00330-020-07361-z
24. Turan TN, Bonilha L, Morgan PS, Adams RJ, Chimowitz MI. Intraplaque hemorrhage in symptomatic intracranial atherosclerotic disease. J Neuroimaging. (2011) 21:e159–61. doi: 10.1111/j.1552-6569.2009.00442.x
25. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. Am J Neuroradiol. (2013) 34:299–304. doi: 10.3174/ajnr.A3209
26. Saba L, Anzidei M, Marincola BC, Piga M, Raz E, Bassareo PP, et al. Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol. (2014) 37:572–85. doi: 10.1007/s00270-013-0711-2
27. Yuan C, Zhang SX, Polissar NL, Echelard D, Ortiz G, Davis JW, et al. Identification of fibrous cap rupture with magnetic resonance imaging is highly associated with recent transient ischemic attack or stroke. Circulation. (2002) 105:181–5. doi: 10.1161/hc0202.102121
28. Cogswell PM, Lants SK, Davis LT, Donahue MJ. Vessel wall and lumen characteristics with age in healthy participants using 3T intracranial vessel wall magnetic resonance imaging. J Magn Reson Imaging. (2019) 50:1452–60. doi: 10.1002/jmri.26750
29. de Freitas EV, Brandao AA, Pozzan R, Magalhies ME, Castier M, Brandao AP. Study of the intima-media thickening in carotid arteries of healthy elderly with high blood pressure and elderly with high blood pressure and dyslipidemia. Clin Interv Aging. (2008) 3:525–34. doi: 10.2147/CIA.S213
30. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. (1987) 316:1371–5. doi: 10.1056/NEJM198705283162204
31. Smulyan H, Safar ME. Systolic blood pressure revisited. J Am Coll Cardiol. (1997) 29:1407–13. doi: 10.1016/S0735-1097(97)00081-8
32. Brohall G, Oden A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. (2006) 23:609–16. doi: 10.1111/j.1464-5491.2005.01725.x
33. Sun B, Zhao H, Liu X, Lu Q, Zhao X, Pu J, et al. Elevated hemoglobin A1c is associated with carotid plaque vulnerability: novel findings from magnetic resonance imaging study in hypertensive stroke patients. Sci Rep. (2016) 6:33246. doi: 10.1038/srep33246
Keywords: symptomatic intracranial atherosclerotic stenosis, high-resolution magnetic resonance imaging, ischemic stroke, young adult, plaque
Citation: Li L, Tang M, Yan X, Gao J, Ma N, Shi X, Niu Y, Wen Y, Ai K, Lei X and Zhang X (2022) Plaque Characteristics in Young Adults With Symptomatic Intracranial Atherosclerotic Stenosis: A Preliminary Study. Front. Neurol. 13:825503. doi: 10.3389/fneur.2022.825503
Received: 30 November 2021; Accepted: 19 January 2022;
Published: 10 February 2022.
Edited by:
Osama O. Zaidat, Northeast Ohio Medical University, United StatesReviewed by:
Nabil Kitchener, General Organization for Teaching Hospitals and Institutes, EgyptSeong-Joon Lee, Ajou University, South Korea
Copyright © 2022 Li, Tang, Yan, Gao, Ma, Shi, Niu, Wen, Ai, Lei and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Lei, bGVpeGlhb3lhbjE5NzFAMTYzLmNvbQ==; Xiaoling Zhang, enhsLjgyMkAxNjMuY29t
†ORCID: Ling Li orcid.org/0000-0001-7169-9531
 Ling Li
Ling Li Min Tang
Min Tang Xuejiao Yan1
Xuejiao Yan1 Jie Gao
Jie Gao