
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 08 March 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.823494
This article is part of the Research Topic Cerebral Autoregulation and Neurovascular Coupling in Brain Disorders View all 25 articles
Background: Studies exploring the relationship between blood pressure (BP) fluctuations and outcome in acute ischemic stroke (AIS) patients treated with intravenous thrombolysis (IVT) are limited. We aimed to investigate the influence of blood pressure variability (BPV) during the first 24 h after IVT on early neurological deterioration (END) and 3-month outcome after IVT in terms of different stroke subtypes.
Methods: Clinical data from consecutive AIS patients who received IVT were retrospectively analyzed. The hourly systolic BP of all patients were recorded during the first 24 h following IVT. We calculated three systolic BPV parameters, including coefficient of variability (CV), standard deviation of mean BP (SD) and successive variation (SV), within the first 6, 12, and 24 h after IVT. END was defined as neurological deterioration with an increase in the National Institutes of Health Stroke Scale (NIHSS) score ≥ 4 points within the first 72 h after admission. Follow-up was performed at 90 days after onset, and favorable and poor outcomes were defined as a modified Rankin Scale scores (mRS) of ≤1 or ≥2, respectively.
Results: A total of 339 patients, which were divided into those with (intracranial artery stenosis or occlusion group, SIASO group) and without (non-SIASO group) SIASO, were included. Among them, 110 patients (32.4%) were with SIASO. Patients in SIASO group had higher NIHSS on admission and difference in term of mRS at 90 days compared with non-SIASO group (P < 0.001). In SIASO group, patients in favorable outcome group were younger and had lower NIHSS on admission, lower SV-24 h (14.5 ± 4.3 vs. 11.8 ± 3.2, respectively) and lower SD-24 h (12.7 ± 3.8 vs. 10.9 ± 3.3, respectively), compared with patients with poor outcome (all P < 0.05). In the multivariable logistic regression analysis, compared with the lowest SV (SV < 25% quartile), SV50−75% [odds ratio (OR) = 4.449, 95% confidence interval (CI) = 1.231–16.075, P = 0.023] and SV>75% (OR = 8.676, 95% CI = 1.892–39.775, P = 0.005) were significantly associated with poor outcome at 3 months in patients with SIASO, adjusted for age, NIHSS on admission and atrial fibrillation. No BPV parameters were associated with END in SIASO group. In non-SIASO group, there were no significant association between BPV patterns and END or 90-day outcome.
Conclusions: SV-24 h had a negative relationship with 3-month outcome in AIS patients with SIASO treated with IVT, indicating that BPV may affect the outcome of AIS.
Globally, stroke is the second most common cause of death and the third most common disabling disease (1, 2). Intravenous thrombolysis therapy (IVT) can significantly improve functional outcomes. However, previous studies have reported that IVT outcomes are associated with various factors (3–5), including age (6), Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (7–10), National Institutes of Health Stroke Scale (NIHSS) score on admission, and systolic blood pressure (SBP) on admission (11).
Previous studies have confirmed that racial difference in intracranial atherosclerosis stenosis (ICAS), and non-Caucasian population are at higher risk of ICAS (12–16). The incidence of ICAS is up to 46.6% in Chinese with ischemic (17). ICAS is an important risk factor for poor prognosis after acute ischemic stroke (AIS) (18, 19).
Blood pressure variability (BPV) is the fluctuation of blood pressure (BP) over a certain period. BP fluctuations are a process of constant adjustment in response to various environmental factors, both inside and outside the body, to better adapt to the needs of the body. Fluctuations of BP are related to a range of factors, such as mood, temperature, and circadian rhythms. These factors ultimately influence BP by affecting nerve and humoral regulation (20). Recently, higher BPV within 24 h after stroke was reported to be associated with poor outcome after IVT (11, 19, 21–23). However, ICAS, which is identified to be associated with poor outcome after AIS as we mentioned before, was not analyzed in these previous studies. Hemodynamic change is one of the possible mechanisms for cerebral infarction caused by ICAS. The hemodynamic status in the ICAS territory can be easily altered by BP fluctuation (24, 25). Under normal condition, cerebral autoregulation occurs to maintain the cerebral blood flow against the effect of hypoperfusion or hyperperfusion (26). However, cerebral autoregulation is impaired after ischemic stroke, especially for patients with ICAS (24). The fluctuation of peripheral blood pressure can significantly affect cerebral perfusion pressure in these patients. A rapid drop of BP may easily lead to a rapidly decreasing perfusion, especially in patients with ICAS, while a rapid rise of BP may potentially lead to intracranial hemorrhage, which might result in early neurological deterioration (END) and poorer outcome (24, 25). Nevertheless, studies focusing on the relationship between BPV and outcome of AIS patients with symptomatic ICAS are still limited. In the present study, we aimed to explore the influence of BPV during the first 24 h after IVT on END and 3-month outcome in patients who had intracranial artery stenosis or occlusion (SIASO) and received IVT.
Patients with AIS who were treated with recombinant tissue plasminogen activator (r-tPA) IVT and admitted to Dongguan People's Hospital between 1 January 2017 and 31 December 2019 were consecutively recruited. The inclusion criteria were as follows: (1) aged >18 years; (2) AIS was confirmed by magnetic resonance imaging (MRI) during hospitalization; (3) onset of ischemic stroke symptoms was within 4.5 h of treatment with r-tPA; (4) clinical features and BP were recorded at baseline and then hourly for 24 h after IVT; (5) prestroke modified Rankin Scale (mRS) score ≤1; and (6) follow-up by face-to-face consultation or by phone at 3 months, with complete documentation. The exclusion criteria were as follows: (1) additional endovascular therapy after IVT; (2) incomplete clinical data or lost to follow-up. This study was approved by the ethics committee of Dongguan People's Hospital (approval number: KYKT2018-002). The study was considered exempt from requiring informed consent from patients because of its retrospective nature.
SIASO was defined as no <50% stenosis of the intracranial artery that was responsible for the AIS (27). Symptomatic intracranial artery occlusion (SIAO) was defined as signal loss of distal blood flow ipsilateral to the infarction (27). Patients who didn't meet the criteria of SIAO were those with symptomatic intracranial artery stenosis (SIAS) in SIASO group. The degree of stenosis was measured using three-dimensional time-of-flight magnetic resonance angiography [the MRI parameters are detailed in our previous article (28)] by comparing the vessel diameter at the site of stenosis with the normal vessel diameter distal to the stenosis (29).
Demographic data including age, sex, and a history of hypertension, diabetes mellitus, smoking, atrial fibrillation (AF), and previous stroke were collected. Stroke subtypes were classified according to the TOAST criteria (30), and the NIHSS on admission, onset-to-treatment time, SBP on admission, and hourly SBP for 24 h after IVT were also collected.
The hourly SBPs of all patients were recorded during the first 24 h after IVT using a cuff-type BP monitor. According to the guidelines (31), intravenous urapidil was administered to reduce BP levels to <185/110 mmHg just before r-tPA administration, and to maintain BP levels at <180/105 mmHg during the first 24 h after IVT. The BPV was calculated using the following equation (19):
(1) Standard deviation of mean BP (SD): ,
(2) Coefficient of variability (CV [%]): SD/BPmean × 100,
(3) Successive variation (SV): (19).
In our study, the definition of END referred to neurological deterioration, with an increase in NIHSS score ≥4 points in the first 72 h after admission (32).
We followed up these patients and assessed their mRS scores at 90 days after onset. Favorable and poor outcomes were defined as mRS ≤1 and ≥2, respectively. Incidents of stroke recurrence and death within the follow-up period were also recorded.
BPVs within 6 h (SV-6 h, SD-6 h, CV-6 h), 12 h (SV-12 h, SD-12 h, CV-12 h), and 24 h (SV-24 h, SD-24 h, CV-24 h) after IVT was calculated.SV was a continuous variable and SV-24 h was divided into four grades based on the quartiles. We assessed the relationship between SV and END, so did the relationship between SV and the 3-month outcome. We also assessed the relationship between each interval of SV-24 h and END, as well as the relationship between each interval of SV-24 h and the 3-month outcome.
Statistical analyses were conducted using SPSS for Windows (v.20.0; IBM Corp., Armonk, NY, USA). Continuous variables with a normal distribution were reported as the mean ± SD and non-normally distributed variables as the median and interquartile range. Univariate analyses were performed using the t-test/Mann–Whitney U-test (for two groups) or one-way analysis of variance/Kruskal–Wallis H-test (for three or more groups) for continuous variables, and the χ2 test for categorical variables. Variables with P < 0.05 in the univariate analysis were included in further binary multivariate logistic regressions. Statistical significance was defined as P < 0.05 (two-sided). Statistical graphs were drawn using GraphPad Prism (v.9.0; GraphPad Software, San Diego, CA, USA).
Three hundred and sixty-two AIS patients who were treated with r-tPA IVT in our stroke unit were collected consecutively. Among these 362 patients, 23 patients were excluded for the following reasons: baseline mRS ≥ 2 (n = 7), underwent additional endovascular therapy (n = 4), died as a result of acute myocardial infarction (n = 2), and lost to follow-up (n = 10). Therefore, 339 patients were enrolled in this study and among them, 110 patients were with SIASO (Figure 1).
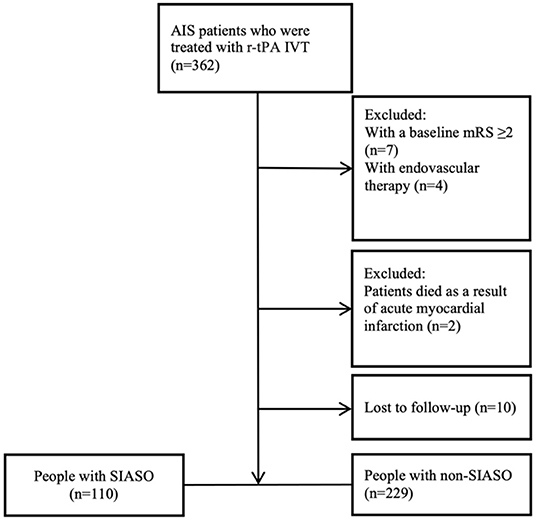
Figure 1. Flowchart of the selection process. AIS, acute ischemic stroke; IVT, intravenous thrombolysis; mRS, modified rankin scale; SIASO, symptomatic intracranial artery stenosis or occlusion.
These 339 patients had a mean age of 61.9 ± 12.1 years. Among them, 248 patients (73.2%) were male, and urapidil was used to lower BP in 34 patients (10.0%) before IVT. Forty-two patients (12.4%) had END and 195 patients (57.5%) had a poor outcome at the 3-month follow-up (Table 1).
We divided these 339 patients into two groups (SIASO group and non-SIASO group) based on whether they were with SIASO. There is no difference between these two groups in terms of age (62.0 ± 12.5 vs. 61.8 ± 12.0 years, P = 0.864), male (78.2 vs. 70.7%, P = 0.148), hypertension (66.4 vs. 72.5%, P = 0.247), diabetes mellitus (26.4 vs. 27.1%, P = 0.890), smokers/ex-smokers (43.6 vs. 34.1%, P = 0.088), AF (20.9 vs. 21.8%, P = 0.846), previous stroke (20.9 vs. 14.4%, P = 0.131), onset to treatment time (OTT, 194.2 ± 54.8 vs. 195.7 ± 56.6 min, P = 0.823) or symptomatic hemorrhagic transformation (sHT, 5.5 vs. 2.2%, P = 0.112). Patients in the SIASO group had a higher NIHSS on admission [9.0 (5.8–14.0) vs. 6.0 (4.0–9.0), respectively], a higher mRS at 90 days and a higher rate of hemorrhagic transformation (HT) compared with the non-SIASO group (P < 0.05, respectively). In the SIASO group, there were 26 patients (23.6%) with SIAS and 84 patients (76.4%) with SIAO. The respective characteristics of patients are shown in Table 1.
In SIASO group, there were significant differences among these four groups of SV quartiles in terms of age, hypertension, use of antihypertensive therapy before IVT, AF, and mRS score at 90 days. In the non-SIASO group, there were significant differences among the quartiles of SV in terms of hypertension, smokers/ex-smokers, systolic blood pressure on admission, OTT and NIHSS on admission (Table 2). In the univariable analysis of risk factors for END, there were no differences in age, AF, SBP on admission, OTT, NIHSS on admission, or medical history between the SIASO and non-SIASO groups (Table 3; Supplementary Table 1).
In SIASO group, compared with those with favorable outcome, patients with poor outcome were older, with higher NIHSS scores on admission and were more likely to have AF (Table 3). In non-SIASO group, those with favorable outcome were older, with higher NIHSS scores on admission as well as longer OTT and were more likely to have AF compared with people in poor outcome group (Supplementary Table 1).
Compared with patients in the favorable outcome group, SV-24 h and SD-24 h were significantly higher in the poor outcome group (all P < 0.05) in SIASO group, while CV-24 h did not differ between the two groups (Table 3). However, SV-24 h, SD-24 h, and CV-24 h were not related with 3-month poor outcome in non-SIASO group (Supplementary Table 1).
Independent variables in SIASO group that were significantly different between the favorable and poor outcome groups in the univariable analysis (p < 0.05) were then entered into the subsequent logistic regression models (Table 4). We set two models for logistic regression. In Model 1, age, NIHSS score on admission, AF, SD-24 h, and overall SV-24 h were entered using a backward Wald method. NIHSS score on admission [odds ratio (OR) = 1.206, 95% confidence interval (CI) = 1.084–1.342, P = 0.001] and overall SV (OR = 1.182, 95% CI = 1.035–1.348, P = 0.013) were significantly associated with poor outcome at 3 months. In Model 2, patients were divided into four groups based on systolic SV value quartiles (SV <25% <10.84, SV25−50% = 10.84–13.25, SV50−75% = 13.26–16.00 and SV>75% > 16.00), and these were entered into the model along with the other variables used in Model 1. NIHSS score on admission (OR = 1.227, 95% CI = 1.099–1.369, P < 0.001), SV50−75% (SV <25% as reference; OR = 4.449, 95% CI = 1.231–16.075, P = 0.023), and SV>75% (SV <25% as reference; OR = 8.676, 95% CI = 1.892–39.775, P = 0.005) were significant predictors of poor outcome at 3 months (Table 4). The univariable analysis and logistic regression for non-SIASO patients were shown in Supplementary Table 2. The mRS score distribution in SIASO group according to SV quartiles is shown in Figure 2.
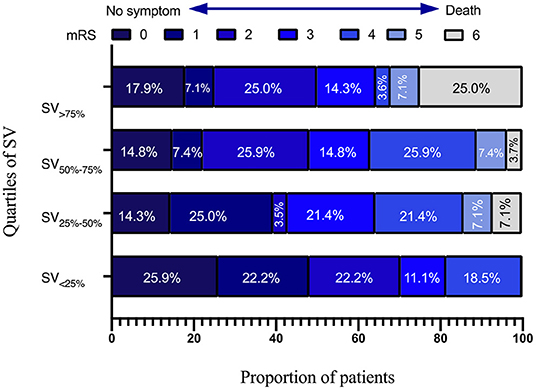
Figure 2. Modified Rankin Scale scores distribution in SIASO group according to quartiles of systolic SV-24 h.
The interaction analysis of age, AF, hypertension and SV-24 h showed only age and SV-24 h (OR = 1.161, 95% CI = 1.031–1.308, P = 0.014) were significantly associated with poor outcome at 3 months in SIASO group, while SV-24 h by age (OR = 0.996, 95% CI = 0.986–1.006, P = 0.389), SV-24 h by AF (OR = 1.059, 95% CI = 0.959–1.168, P = 0.257) or SV-24 h by hypertension (OR = 1.011, 95% CI = 0.941–1.087, P = 0.760) was not significantly associated with poor outcome at 3 months in SIASO group (Supplementary Table 3). Interaction analysis of BPV and SIASO on the 3-month poor outcome in all patients with IVT showed NIHSS on admission and SV-24 h by SIASO (interaction variable between SV-24 h and SIASO) (OR = 1.104, 95% CI = 1.060–1.149, P = <0.001), rather than SV-24 h (OR = 0.992, 95% CI = 0.937–1.050, P = <0.774) or SIASO (OR = 0.388, 95% CI = 0.067–2.247, P = 0.291), were significantly associated with poor outcome at 3 months (Supplementary Table 4).
In the univariate analysis, compared with patients in the favorable outcome group, SV-6 h, SV-12 h, and SD-12 h were significantly higher in the poor outcome group (all P < 0.05) in SIASO group, while SD-6 h, CV-6 h, and CV-12 h did not differ between the two groups (Supplementary Table 5). Age, NIHSS score on admission, AF, SV-6 h, SD-12 h, and SV-12 h were entered into the subsequent logistic regression models (Supplementary Table 6). SV-6 h (P = 0.130), SD-12 h (P = 0.960), and SV-12 h (P = 0.066) were not significantly associated with poor outcome at 3 months (Supplementary Table 6).
In SIASO group, although SV-24 h, SD-24 h, and CV-24 h all appeared to be higher in END group than in without-END group, there were no significant differences between the two groups (Table 3). In non-SIASO group, SV-24 h, SD-24 h, and CV-24 h were also not significantly different between END and without-END groups (Supplementary Table 1).
No relationship was found between the 3 BPV patterns (SV-24 h, SD-24 h, and CV-24 h) and all HT or sHT neither in SIASO group or in non-SIASO group, (Supplementary Tables 7, 8).
In the present study, higher systolic SV-24 was associated with poorer 3-month outcome in patients with SIASO who were treated with IVT. An increase in systolic SV-24 h may thus predict a higher risk of poor outcome at 3 months. However, there was no clear relationship between any pattern of BPV and 3-month outcome in patients without SIASO. There was not relationship between BPV and END in neither of the two groups. Our findings may help physicians to identify patients with a relatively high risk of poor outcome at 3 months.
SV is an important index of BPV, and in our study SV-24 h was associated with 3-month outcome in AIS patients after IVT in patients with SIASO, while it was not associated with 3-month outcome in patients without SIASO. A study by Yong and Kaste (33) in the European Cooperative Acute Stroke Study II trial revealed that high SBP variability is associated with poor outcome. Furthermore, an analysis from the Third International Stroke Trial (IST-3) reported an association between higher BPV and adverse events, the occurrence of symptomatic intracranial hemorrhage, and poor 6-month outcome (34). However, patients with and without SIASO were not assessed separately in those studies. In the IST-3 study, BP was measured only at three timepoints after IVT (34), while we measured BP hourly for at least 24 h after IVT. Thus, the BPV in our study was likely more accurate than in other studies that measured BP less frequently. Additionally, we studied the effect of BPV within different times after IVT on 3 months poor outcome and we found that SV-24 h, rather than BPV patterns within the first 6 or 12 h after IVT, was associated with poor outcomes at 3 months, which was rarely reported in previous studies. Therefore, it is important to avoid excessive fluctuations in BP within 24 h after IVT in patients with SIASO. We also analyzed the 3-month poor outcome of all IVT patients by the interaction of SV-24 h and SIASO, and it showed that the interaction did have a significant effect on 3 months poor outcome, which also confirms the finding that SV-24 h affect the 3-month outcome only in patients with SIASO.
Our study indicated that higher SV-24 h was associated with worse outcome at 3 months after IVT in patients with SIASO rather than in those without SIASO. Although there were some studies on the relationship between BPV and prognosis of AIS after IVT, they did not focus on the patients with SIASO. As ischemic stroke is a clinical syndrome with various pathogenesis, including large artery atherosclerosis, small arterial occlusion, and cardiogenic embolism, and BP fluctuations have different effects on ischemic stroke caused by different mechanisms (35), our research will be more targeted. In addition, 33–55% of ischemic stroke in Chinese is caused by intracranial arterial stenosis or occlusion (IASO) (35). Therefore, IASO should be taken into consideration when studying the relationship between BPV and ischemic stroke outcome. In our study, we evaluated intracranial arteries by 3D-TOF-MRA. Due to the limitations of MRA for assessing the degree of stenosis of cerebral arteries, it was difficult to distinguish SIAO from SIAS accurately. Therefore, it would be reasonable to combine SIAO and SIAS into the category of SIASO.
Recent studies have reported that many patients have autonomic dysfunction after ischemic stroke (36–38). BP fluctuation is a manifestation of autonomic dysfunction. During the early stage of ischemic stroke, a rapid increase in BP may cause intracranial pressure to rise and edema or bleeding to occur at the infarction site, which can lead to death or poor functional outcome (25). However, a large decrease in BP may lead to low perfusion in the infarct area; this effect might be much clearer in AIS patients with large-artery stenosis because patients with SIASO are very sensitive to BP fluctuations, especially in the first few hours after AIS (25, 26).
Cerebral autoregulation (CA) is the ability of the brain to regulate its own blood supply, which maintains an adequate and stable cerebral blood flow despite changes in cerebral perfusion pressure. However, CA is impaired after ischemic stroke, and this autoregulation is damaged in patients with SIASO; furthermore, patients with more severe stenosis tend to have more severe dysautoregulation (35). One study reported that CA impairment ipsilateral to the AIS is associated with a larger infarction and poorer clinical outcome compared with patients with unimpaired CA ipsilateral to the AIS (35, 39). With CA impairment, BP fluctuations have an important effect on cerebral blood flow. Moreover, when BPV increases to levels beyond the regulation of CA, it can lead to hypoperfusion or hyperperfusion in the infarction area (35, 39).
In the present study, high SV-24 h after IVT among patients with SIASO increased the risk of poor outcome at 3 months. This finding may help physicians to screen the most high-risk patients with poor prognosis from other high-risk patients at the early stage of AIS, and thus provide more appropriate interventions. Theoretically, since BP fluctuation in early stage of ischemic stroke may lead to hypoperfusion or intracerebral hemorrhage (24), BPV should be associated with END. However, we did not find any correlation between BPV and END. One possible reason is that in our study, there were only 20 patients (18.2%) with END in SIASO group which may not be powerful to detect the difference of BPV between with END and without END groups. Nevertheless, we found a tendency of association between high BPV and the occurrence of END. Besides, previous studies by other researchers have also failed to find a link between short-term outcome (2-week outcome and in-hospital outcome) and BPV during the acute phase of ischemic stroke (11, 39, 40).
The accuracy of BP measurement for patients with AF might be controversial. In theory, irregular ventricular rate in patients with AF may lead to more pronounced fluctuation in BP than in patients with sinus heart rate. However, we didn't exclude patients with AF in our study. Olson et al. analyzed 42 patients with AF in the condition of AF and sinus rhythm, and the result showed they had similar BPV in these two conditions (41). Maria et al. studied 13,827 patients and found no significant difference in BPV between patients with AF and patients with sinus rhythm (42). Besides, Guideline from American Heart Association in 2009 also specified that oscillographic blood pressure monitors were reliable in patients with AF (43).
Commonly-used BPV parameters consist of SV, SD, and CV. In our study, we found that a high SV-24 h, rather than SD-24 h or CV-24 h, may predict 3-month poor outcome in SIASO group. SD represents the dispersion of values around the mean but does not reflect the effect of the measuring sequence of BP. Since CV is calculated from SD, it does not reflect the aforementioned effect, either. SV estimates the variation in successive measurements and thus takes the sequence into account (44). Therefore, SV is more commonly used in studies because it better reflects the time-series variability of BP. In contrast, SD and CV couldn't reflect the temporal changes in the data, which can result in the same SD or CV in individuals with different clinical characteristics (45).
In our study, SV-24 h was higher in patients who were older and more likely to have hypertension or AF. However, an interaction term analysis was performed (Supplementary Table 7) and it demonstrated that SV-24 h was a risk factor of poor outcome independent of age and hypertension, which was similar to a previous review (46).
There were several strengths to our study. First, BPV was calculated by recording hourly BP within 24 h after thrombolysis, which was far more frequently than in other studies and led to more accurate BPV results [11, 14]. Second, to the best of our knowledge, ours was one of the few studies to have focused on AIS patients after IVT and consider the effects of SIASO on prognosis. However, there were also several limitations in our study. First, the sample size was relatively small. Second, our study was a retrospective study. Third, magnetic resonance angiography was used to evaluate arterial stenosis in this study; however, this method is not as accurate as digital subtraction angiography for evaluating arterial stenosis and might overestimate SIASO. Forth, only four stroke patients received EVT during our research from 2017 to 2019 as we were still developing emergent endovascular therapy in our hospital, thus the finding of our study may be limited. Lastly, intermittent cuff measurement was used to monitor BP in the present study, while continuous cuff monitoring or invasive arterial BP are more accurate.
Systolic SV had a negative relationship with 3-month outcome in AIS patients with SIASO who were treated with IVT, which indicates that BPV may affect the outcome of AIS. Further multi-center prospective studies with larger sample sizes are warranted.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Dongguan People's Hospital (approval number: KYKT2018-002). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Y-KC and Y-LL designed the work and analyzed the data. M-XY, W-CZ, D-HQ, W-CZ, and H-PY collected the clinical data. M-XY and Y-LL wrote the manuscript. M-XY and J-HZ wrote the diagrams. All authors read and approved the final manuscript, contributed toward data analysis, drafted and revised the paper, and agreed to be accountable for all aspects of the work.
This study was funded by the Guangdong Sci-Tech Commissioner Project, Grant/Award (Number: 20201800500572). The funding body had no role in the design of the study, collection, analysis, and interpretation of the data, and in writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.823494/full#supplementary-material
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2016) 380:2095–128. doi: 10.1016/S0140-6736(12)61728-0
2. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the global burden of disease study 2010. Lancet. (2012) 380:2197–223. doi: 10.1016/S0140-6736(12)61689-4
3. Liu YL, Xiao WM, Lu JK, Wang YZ, Lu ZH, Zhong HH, et al. Asymmetrical cortical vessel sign predicts prognosis after acute ischemic stroke. Brain Behav. (2020) 10:e01657. doi: 10.1002/brb3.1657
4. Marler JR, Tilley BC, Lu M, Brott TG, Lyden PC, Grotta JC, et al. Early stroke treatment associated with better outcome: The NINDS rt-PA stroke study. Neurology. (2000) 55:1649–55. doi: 10.1212/WNL.55.11.1649
5. Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. (2010) 375:1695–703. doi: 10.1016/S0140-6736(10)60491-6
6. Broderick JP, Phillips SJ, O'Fallon WM, Frye RL, Whisnant JP. Relationship of cardiac disease to stroke occurrence, recurrence, and mortality. Stroke. (1992) 23:1250–6. doi: 10.1161/01.STR.23.9.1250
7. Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. (2008) 84:412–7. doi: 10.1136/pgmj.2007.066118
8. Simonsen CZ, Schmitz ML, Madsen MH, Mikkelsen IK, Chandra RV, Leslie-Mazwi T, et al. Early neurological deterioration after thrombolysis: clinical and imaging predictors. Int J Stroke. (2016) 11:776–82. doi: 10.1177/1747493016650454
9. Kim JM, Moon J, Ahn SW, Shin HW, Park KY. The etiologies of early neurological deterioration after thrombolysis and risk factors of ischemia progression. J Stroke Cerebrovasc Dis. (2016) 25:383–8. doi: 10.1016/j.jstrokecerebrovasdis.2015.10.010
10. Ray BK, Hazra A, Ghosal M, Banerjee T, Chaudhuri A, Singh V, et al. Early and delayed fatality of stroke in Kolkata, India: Results from a 7-year longitudinal population-based study. J Stroke Cerebrovasc Dis. (2013) 22:281–9. doi: 10.1016/j.jstrokecerebrovasdis.2011.09.002
11. Kellert L, Hametner C, Ahmed N, Rauch G, MacLeod MJ, Perini F, et al. Reciprocal interaction of 24-hour blood pressure variability and systolic blood pressure on outcome in stroke thrombolysis. Stroke. (2017) 48:1827–34. doi: 10.1161/STROKEAHA.117.016876
12. Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. (1986) 17:648–55. doi: 10.1161/01.STR.17.4.648
13. Wong K, Li H, Chan Y, Ahuja A, Lam W, Wong A, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke. (2000) 31:2641–7. doi: 10.1161/01.STR.31.11.2641
14. Cruz-Flores S, Mazighi M, Ducrocq X, Bracard S, Houdart E, Woimant F. Prospective study of symptomatic atherothrombotic intracranial stenoses: the GESICA study. Neurology. (2006) 66:1187–91. doi: 10.1212/01.wnl.0000208404.94585.b2
15. Huang YN, Gao S, Li SW, Huang Y, Li JF, Wong KS, et al. Vascular lesions in Chinese patients with transient ischemic attacks. Neurology. (1997) 48:524–5. doi: 10.1212/WNL.48.2.524
16. Lee SJ, Cho S-J, Moon H-S, Shon YM, Chung CS. Combined extracranial and intracranial atherosclerosis in Korean patients. Arch Neurol. (2003) 60:1561–4. doi: 10.1001/archneur.60.11.1561
17. Wang Y, Zhao X, Liu L, Soo Y, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) Study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEAHA.113.003508
18. Famakin BM, Chimowitz MI, Lynn MJ, Stern BJ, George MG. Causes and severity of ischemic stroke in patients with symptomatic intracranial arterial stenosis. Stroke. (2009) 40:1999–2003. doi: 10.1161/STROKEAHA.108.546150
19. Barnett HJ, Gunton RW, Eliasziw M, Fleming L, Sharpe B, Gates P, et al. Causes and severity of ischemic stroke in patients with internal carotid artery stenosis. JAMA. (2000) 283:1429–36. doi: 10.1001/jama.283.11.1429
20. Sayk F, Teckentrup C, Becker C, Heutling D, Wellhöner P, Lehnert H, et al. Effects of selective slow-wave sleep deprivation on nocturnal blood pressure dipping and daytime blood pressure regulation. Am J Physiol Regul. (2010) 67:R191–7. doi: 10.1152/ajpregu.00368.2009
21. Endo K, Kario K, Koga M, Nakagawara J, Shiokawa Y, Yamagami H, et al. Impact of early blood pressure variability on stroke outcomes after thrombolysis the SAMURAI rt-PA registry. Stroke. (2013) 44:816–8. doi: 10.1161/STROKEAHA.112.681007
22. Kellert L, Sykora M, Gumbinger C, Herrmann O, Ringleb PA. Blood pressure variability after intravenous thrombolysis in acute stroke does not predict intracerebral hemorrhage but poor outcome. Cerebrovasc Dis. (2021) 33:135–40. doi: 10.1159/000334186
23. Castillo J, Leira R, García MM, Serena J, Blanco M, Dávalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. (2004) 35:520–6. doi: 10.1161/01.STR.0000109769.22917.B0
24. Ko Y, Park JH, Yang MH, Ko SB, Han MK, Oh CW, et al. The significance of blood pressure variability for the development of hemorrhagic transformation in acute ischemic stroke. Stroke. (2010) 41:2512–8. doi: 10.1161/STROKEAHA.110.595561
25. Powers WJ. Acute hypertension after stroke: the scientific basis for treatment decisions. Neurology. (1993) 43:461–7. doi: 10.1212/WNL.43.3_Part_1.461
26. Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. (2001) 124(Pt 3):457–67. doi: 10.1093/brain/124.3.457
27. Chimowitz MI, Strong J, Brown MB, Perkins A, Fayad PB. Prognosis of patients with symptomatic vertebral or basilar artery stenosis. Stroke. (1998) 29:1389–92. doi: 10.1161/01.STR.29.7.1389
28. Yin HP, Qiu DH, Qu JF, Lu ZH, Wang F, Liang MQ. Multiple hypointense vessels on susceptibility-weighted imaging predict early neurological deterioration in acute ischaemic stroke patients with severe intracranial large artery stenosis or occlusion receiving intravenous thrombolysis. Stroke Vasc Neurol. (2020) 5:361–7. doi: 10.1136/svn-2020-000343
29. Buchan AM. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade carotid stenosis. N Engl J Med. (1991) 325:445–53. doi: 10.1056/NEJM199108153250701
30. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
31. Shinohara Y, Yanagihara T, Abe K, Yoshimine T, Fujinaka T, Chuma T, et al. II. Cerebral infarction/transient ischemic attack (TIA). J Stroke Cerebrovasc Dis. (2011) 20:S31–73. doi: 10.1016/j.jstrokecerebrovasdis.2011.05.004
32. Siegler JE, Schild SM. Early Neurological seterioration (END) after stroke: the END depends on the definition. Int J Stroke. (2011) 6:211–2. doi: 10.1111/j.1747-4949.2011.00596.x
33. Yong M, Kaste M. Association of characteristics of blood pressure profiles and stroke outcomes in the ECASS-II trial. Stroke. (2008) 39:366–72. doi: 10.1161/STROKEAHA.107.492330
34. Berge E, Cohen G, Lindley RI, Sandercock P, Wardlaw JM, Sandset EC, et al. Effects of blood pressure and blood pressure-lowering treatment during the first 24 hours among patients in the Third International Stroke Trial of Thrombolytic treatment for acute ischemic stroke. Stroke. (2015) 46:3362–9. doi: 10.1161/STROKEAHA.115.010319
35. Chen J, Liu J, Xu WH, Xu R, Hou B, Cui LY, et al. Impaired dynamic cerebral autoregulation and cerebrovascular reactivity in middle cerebral artery stenosis. PLoS ONE. (2014) 9:e88232. doi: 10.1371/journal.pone.0088232
36. Tang S, Xiong L, Fan Y, Mok V, Leung TW. Stroke outcome prediction by blood pressure variability, heart rate variability, and baroreflex sensitivity. Stroke. (2020) 51:1317–20. doi: 10.1161/STROKEAHA.119.027981
37. Dütsch M, Burger M, Dörfler C, Schwab S, Hilz MJ. Cardiovascular autonomic function in poststroke patients. Neurology. (2007) 69:2249–55. doi: 10.1212/01.wnl.0000286946.06639.a7
38. Li X, Ge T, Leung H, Soo Y, Leung T. Autonomic dysfunction predicts clinical outcomes after acute ischemic stroke: a prospective observational study. Stroke. (2018) 49:215–8. doi: 10.1161/STROKEAHA.117.019312
39. Tziomalos K, Giampatzis V, Bouziana SD, Spanou M, Kostaki S, Papadopoulou M, et al. No association observed between blood pressure variability during the acute phase of ischemic stroke and in-hospital outcomes. Am J Hypertens. (2016) 29:841–6. doi: 10.1093/ajh/hpv191
40. Manning LS, Mistri AK, Potter J, Rothwell PM, Robinson TG. Short-term blood pressure variability in acute stroke: post hoc analysis of the controlling hypertension and hypotension immediately post stroke and continue or stop post-stroke antihypertensives collaborative study trials. Stroke. (2015) 46:1518–24. doi: 10.1161/STROKEAHA.115.009078
41. Olsen R, Amlie A, Omvik P. Twenty-four-hour ambulatory blood pressure monitoring in atrial fibrillation. Blood Press Monit. (2002) 7:149–56. doi: 10.1097/00126097-200206000-00002
42. Mehlum MH, Liestøl K, Wyller TB, Hua TA, Rostrup M, Berge E. Blood pressure variability in hypertensive patients with atrial fibrillation in the VALUE trial. Blood Press. (2019) 28:1–7. doi: 10.1080/08037051.2018.1524707
43. Muntner P, Shimbo D, Carey RM, Charleston JB. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. (2019) 73:e35–66. doi: 10.1161/HYP.0000000000000087
44. Divani AA, Liu X, Napoli MD, Lattanzi S, Ziai Z, James MJA, et al. Blood pressure variability predicts poor in-hospital outcome in spontaneous intracerebral hemorrhage. Stroke. (2019) 50:2023–9. doi: 10.1161/STROKEAHA.119.025514
45. Xu B, Ji Q, Zhang Y, Shen L, Cao M, Cai K. Postoperative blood pressure variability exerts an influence on clinical outcome after coil embolization of ruptured intracranial aneurysms. Neurol Res. (2017) 39:813–8. doi: 10.1080/01616412.2017.1348653
Keywords: blood pressure variability, symptomatic intracranial artery stenosis or occlusion, intravenous thrombolysis, acute ischemic stroke, prognosis
Citation: Yao M-X, Qiu D-H, Zheng W-C, Zhao J-H, Yin H-P, Liu Y-L and Chen Y-K (2022) Effects of Early-Stage Blood Pressure Variability on the Functional Outcome in Acute Ischemic Stroke Patients With Symptomatic Intracranial Artery Stenosis or Occlusion Receiving Intravenous Thrombolysis. Front. Neurol. 13:823494. doi: 10.3389/fneur.2022.823494
Received: 27 November 2021; Accepted: 08 February 2022;
Published: 08 March 2022.
Edited by:
Xiuyun Liu, Johns Hopkins University, United StatesReviewed by:
Zhen-Ni Guo, First Affiliated Hospital of Jilin University, ChinaCopyright © 2022 Yao, Qiu, Zheng, Zhao, Yin, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Lin Liu, bHlfdHdpbnNAMTI2LmNvbQ==; Yang-Kun Chen, Y3lrdW43OEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.