
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 06 April 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.816216
 Huijuan Jin1†
Huijuan Jin1† Rentang Bi1†
Rentang Bi1† Jichuan Hu2†
Jichuan Hu2† Da Xu1
Da Xu1 Ying Su1
Ying Su1 Ming Huang3
Ming Huang3 Qiwei Peng1
Qiwei Peng1 Zhifang Li1
Zhifang Li1 Shengcai Chen1*
Shengcai Chen1* Bo Hu1*
Bo Hu1*Background and Purpose: Currently, acute ischemic stroke (AIS) is one of the most common and serious diseases in the world and is associated with very high mortality and morbidity even after thrombolysis therapy. This study aims to research the relationship between lactic dehydrogenase (LDH) and prognosis in AIS patients treated with intravenous rtPA.
Method: This study (a Multicenter Clinical Trial of Revascularization Treatment for Acute Ischemic Stroke, TRAIS) included 527 AIS patients in 5 cooperative medical institutions in China from January 2018 to February 2021. The primary outcome was major disability and death within 3 months (mRS score of 3–6), and the secondary outcomes were early neurological improvement (ENI), early neurological deterioration (END), moderate-severe cerebral edema (CE), and symptomatic intracranial hemorrhage (sICH).
Results: The mean age of the 527 patients was 65.6 ± 11.7 years, and the median baseline NIHSS score was 4 (interquartile range, 2–7). The median serum LDH level was 184 U/L (interquartile range, 163–212 U/L). In total, 287 (54.5%) patients acquired ENI, 68 (13.0%) patients suffered END, 53 (12.1%) patients were observed with moderate-severe CE, and 28 (6.2%) patients showed sICH. Within 3 months, 127 (25.15%) patients experienced the primary outcome and 42 (8.3%) patients died. Serum LDH levels before thrombolysis showed an independent association with the risk of primary outcome [adjusted odds ratio, 3.787; (95% CI, 1.525–9.404); P = 0.014]. When log-transformed LDH increased each standard deviation, the risk of primary outcome was raised by 80.1% (95% CI, 28.9–251.7%). A positive linear dependence between the risk of primary outcome and serum LDH levels (P of linearity = 0.0248, P of non-linearity = 0.8284) was shown in multivariable-adjusted spline regression models. Pre-thrombolysis LDH quartile also provided a conventional risk model and significant improvement of the prediction for clinical outcomes, with a net reclassification improvement index (NRI) = 41.86% (P < 0.001) and integrated discrimination improvement (IDI) = 4.68% (P < 0.001).
Conclusions: Elevated serum LDH levels predicted unfavorable clinical outcomes after intravenous thrombolysis in AIS patients.
Acute ischemic stroke (AIS) is currently one of the most disabling and lethal diseases in the world (1). Intravenous thrombolysis with recombinant tissue plasminogen activator (rt-PA) as well as endovascular therapy is the most effective primary modality of therapy (2, 3). However, a significant number of patients who receive intravenous rt-PA therapy still face the threat of complications such as hemorrhage transformation and cerebral edema (CE), with unfavorable recovery of neurological function (3, 4). Therefore, it is significant to explore novel prognostic biomarkers in AIS patients for clinical decision making.
Lactate dehydrogenase (LDH) is a critical enzyme of the anaerobic metabolic pathway and is mainly distributed in the cytoplasm and mitochondria of various tissues, including the brain, heart, liver, and lung under physiological conditions (5, 6). Once the tissue is injured, LDH is released to the extracellular space and leads to an increased serum LDH level. Thus, LDH has been regarded as a biomarker of both tissue injury and prognosis in many diseases, including acute myocardial infarction, acute hepatitis, and acute lung injury (7–10). Presumably, LDH gets rapidly upregulated in brain parenchyma in response to ischemia and hypoxia after AIS, and leaks into circulating blood with the aggravation of cerebral infarction and peripheral edema. LDH has been observed to be released from brain tissue in animal models of brain injury, including ischemic stroke, and has been applied as a marker of brain tissue injury in basial experiments (11–13). Clinically, LDH is found to be elevated in the serum and cerebrospinal fluid of patients with ischemic stroke and related to the occurrence of stroke (14).
The relationship between LDH levels and clinical outcomes in AIS patients has never been thoroughly studied (15, 16). This study aimed to analyze the correlation between serum LDH and clinical outcomes in patients receiving intravenous rt-PA treatment.
We conducted a retrospective study (Multicenter Clinical Trial of Revascularization Treatment for Acute Ischemic Stroke, TRAIS) using all consecutive AIS cases for thrombolysis in 5 transregional cooperative medical institutions in China, including Wuhan Union Hospital, the West Branch of Wuhan Union Hospital, Hefeng People's Hospital, the People's Hospital of Dongxihu District, and Yichang Central Hospital. Patients admitted from 1 January 2018 to 1 February 2021 were used in the analysis.
The ethics of this study are in line with the principles expressed in the Declaration of Helsinki. The local institutional review board approved all aspects of the study (ChiCTR2000033456).
Patients were selected according to the following criteria: (1) clinically confirmed AIS; (2) rt-PA injections consistent with the indications for thrombolytic therapy; and (3) patients aged 18 years or older. Exclusion criteria included: (1) AMI, severe infectious diseases, terminal cancer, hematological disease, hepatic or renal disease, recent major trauma or surgery; (2) mental disorders, severe cognitive dysfunction; and (3) incomplete clinical data. All available inpatient data, including history, clinical tests, laboratory tests, diagnostic tests, imaging studies, and discharge diagnoses, were used for the diagnosis of the above diseases.
All the patients received thrombolytic treatment in line with written institutional guidelines. The time window for thrombolysis was extended and limited up to 9 h guided by perfusion imaging (17, 18). Intravenous rt-PA (administered at a standard dose of 0.9 mg/kg body weight) was given according to the procedure recommended by the European Stroke organization guidelines in 2018 (19). In total, 10% of the total dose was given as the first dose and the remaining dose was given within the next hour. Continuous monitoring and evaluation were conducted during thrombolysis. After thrombolysis was completed, the patient was transferred to the Neurological ICU for intensive nursing.
Serum LDH levels were adopted from each patient both in the emergency ward before thrombolysis and within 1–3 days in the inpatient ward. We ensured that the serum sample did not develop hemolysis prior to testing.
We retrieved: (1) clinical assessment from the National Institute of Health stroke scale (NIHSS) score evaluated both on admission and 24 h after thrombolysis, baseline blood pressure, time from onset to treatment (OTT); (2) demographic information, including gender and age; (3) vascular risk factors such as alcohol drinking, cigarette smoking, hypertension, diabetes mellitus, coronary heart disease, and previous stroke; (5) auxiliary examination, including multimodal computerized tomography (CT), magnetic resonance imaging (MRI), cervical vascular ultrasound, and echocardiography of patients. All patients underwent the same level of standardized assessment prior to discharge and were given a personalized rehabilitation plan.
Participants were followed-up by modified Rankin Scale (mRS) scores at 3 months after intravenous thrombolysis by trained neurologists, who were unaware of treatment assignment. This study was conducted in January 2018, and follow-up work was completed in May 2021.
Clinical outcomes were determined as: (1) 3-month death or major disability (mRS, 3–6); (2) 3-month mortality (mRS score of 6); (3) cerebral edema, according to the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) criteria (20), we classified those with a swelling area >1/3 of hemicerebrum or midline deviation as moderate-severe cerebral edema, based on CT or MRI within 1–3 days after thrombolysis; (4) symptomatic intracranial hemorrhage (sICH), using the National Institute of Neurological Disorders and Stroke (NINDS) criteria (21); (5) early neurological improvement (ENI), defined as NIHSS score decrease of ≥ 4 or complete recovery within 24 h after thrombolysis; (6) early neurological deterioration (END), defined as NIHSS score increase of ≥ 4 within 24 h after thrombolysis.
In all processed data, variables that fit the normal distribution are recorded as the mean and standard deviation, while variables that are not normally distributed are recorded as the median, quartile, and distribution range. For dichotomous variables, we give the quantity and distribution ratio. According to the normality of the data distribution, independent samples 2-tailed T-test, Mann-Whitney U-test, or χ2 test are used for the dichotomous variables between groups.
The level of LDH was converted to a categorical variable according to the quartile to facilitate the comparison of differences between the two extreme groups. At the same time, LDH showed acceptable normality after natural logarithmic conversion. Concomitant variables in the multinomial logistic regression analysis include sex, age, OTT time, current cigarette smoking, alcohol drinking, history of stroke, hypertension, diabetes mellitus, dyslipidemia, and coronary heart disease and baseline NIHSS score. The statistical significance was set to the probability value < 0.05. All the above analyses are performed with SPSS 25.0 for Windows.
R (version 4.0.3) software, Net reclassification improvement (NRI), and comprehensive discriminant improvement (IDI) were used to evaluate the net benefit of reclassification for LDH survival and prediction of malignant edema. In addition, with MedCalc (version 20.0.3) software, we used receiver operating characteristic (ROC) curves to compare the overall discriminative ability between pre-thrombolysis and post-thrombolysis LDH for outcomes.
The data used for the analysis of the study results are available from the corresponding author on reasonable request.
As shown in Supplementary Figure 1, there was a total of 718 AIS patients treated with intravenous rt-PA in our medical centers, 55 patients were excluded for AMI, cancer, severe infection, or serious systemic disease, and 136 patients had a lack of clinical data, blood samples lost, or loss of follow-up. Finally, the data of 527 patients were applied to the subsequent analysis for this study. The mean age was 65.6 years (SD ± 11.7 years, range, 33–95 years), and 66.8% of patients were male. The median baseline NIHSS score was 4 (interquartile range, 2–7). The median serum LDH before thrombolysis was 190 U/L (interquartile range,163–212 U/L). All baseline characteristics among LDH quartiles are provided in Table 1. It was shown that higher serum LDH quartiles were associated with female patients, higher baseline NIHSS scores, and larger final infarct volume, while lower LDH levels are related to small-artery occlusion.
Within 3 months, 127 patients (25.1%) experienced primary outcomes (85 with severe disability and 42 with death; Table 2), and the cumulative rates for the four serum LDH quartiles (Q1 to Q4) were 15.7, 19.2, 18.3, and 48.4% (P < 0.001). After intravenous thrombolysis, 287 (54.5%) patients acquired ENI, 68 (13.0%) patients suffered END, 53 (12.1%) patients were found with moderate-severe CE, and 28 (6.2%) patients showed sICH.
After adjusting baseline NIHSS score and other potential confounders in model 2, when the highest and lowest quartiles (Q4 and Q1) were compared, serum LDH levels before thrombolysis showed independently associated with the risk of primary outcome (odds ratio, 3.753; 95% CI, 1.645–8.565; P for trend = 0.002). The patients in the highest quartile of LDH showed a 2.787-fold increased risk of death or disability compared with those in the lowest quartile. Moreover, when log-transformed LDH increased each standard deviation (SD), the risk of primary outcome got raised by 81.6% (95% CI, 32.9–248.1%) in model 2. Furthermore, a positive linear dose-response relationship between the risk of the primary outcome and pre-thrombolysis serum LDH was shown in multivariable-adjusted spline regression models (P of linearity = 0.0248, P of non-linearity = 0.8284; Figure 1).
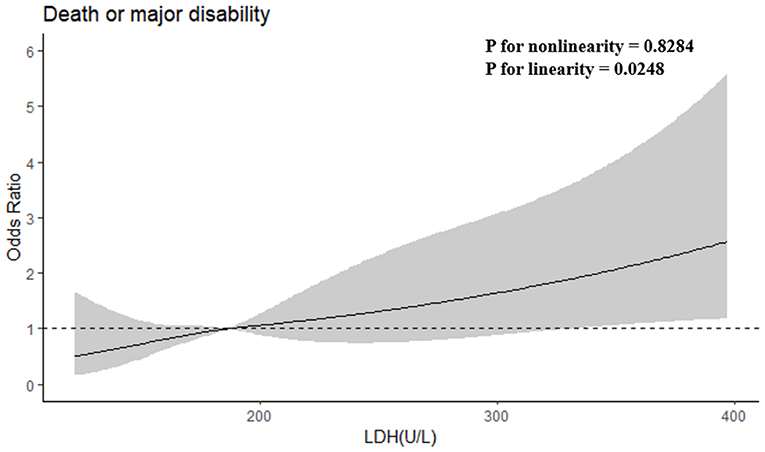
Figure 1. Adjusted odds ratios of primary outcome according to serum LDH. OR and 95% CI derived from restricted cubic spline regression, with knots placed at the 5th, 35th, 65th, and 95th percentiles of LDH. OR adjusted for the same variables as model 2 in Table 2.
We further examined the incremental predictive value of serum LDH before thrombolysis and the conventional model that includes all risk factors in Model 2 for the clinical outcomes of AIS patients treated with intravenous rt-PA. As shown in Table 3, adding pre-thrombolysis LDH quartile significantly improved the prediction for the risk of primary outcome of the conventional risk model, with NRI = 41.86% (P < 0.001) and IDI = 4.68% (P < 0.001). When log-transformed LDH was added to the model, the NRI and IDI for primary outcome were 41.14% (95%CI, 21.33–60.95%) and IDI of 3.08% (95%CI, 0.91–5.26%), respectively.
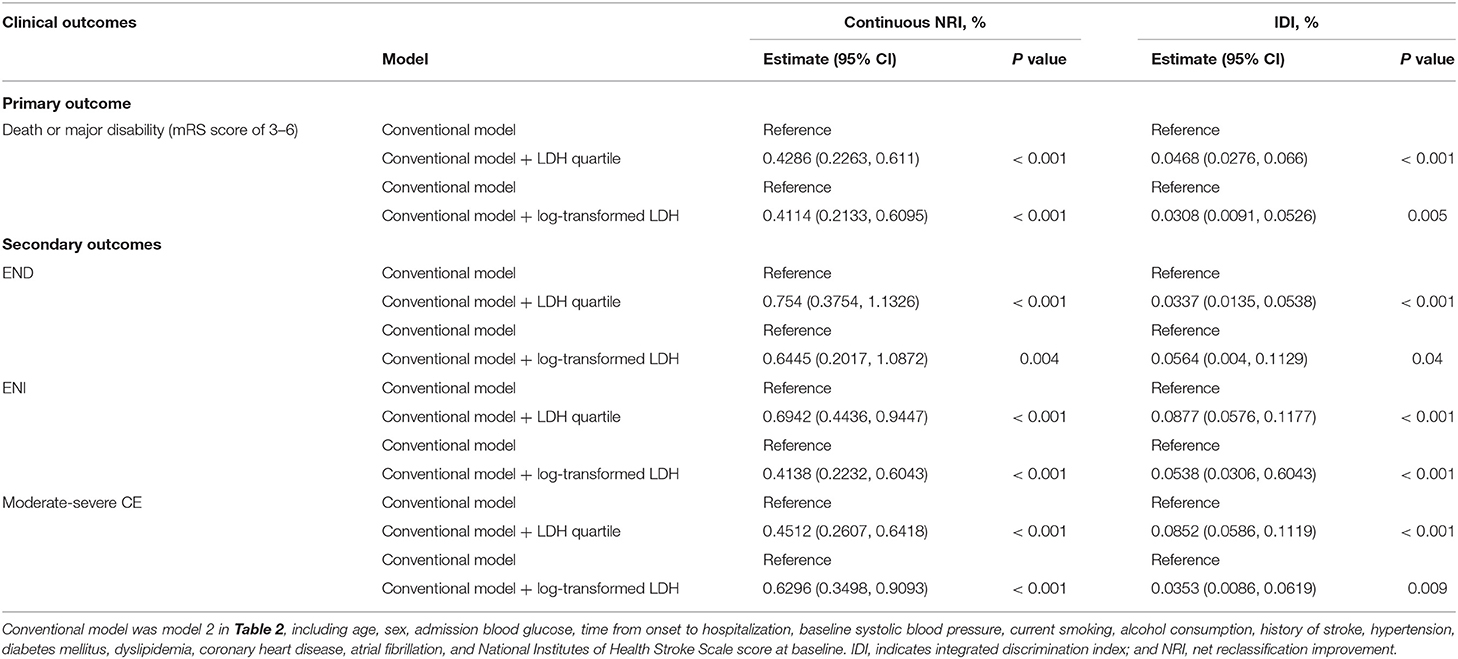
Table 3. Reclassification and discrimination Statistics for clinical outcomes by serum LDH before thrombolysis.
On the primary outcome, we conducted subgroup analyses to examine the potential modified effect of prespecified factors. Stratified by baseline systolic BP, age, gender, baseline NIHSS score, cigarette smoking, alcohol drinking, history of hypertension, and dyslipidemia, no statistically significant interaction between pre-thrombolysis serum LDH and these interesting factors were observed (all P values of interaction are >0.05; Table 4).
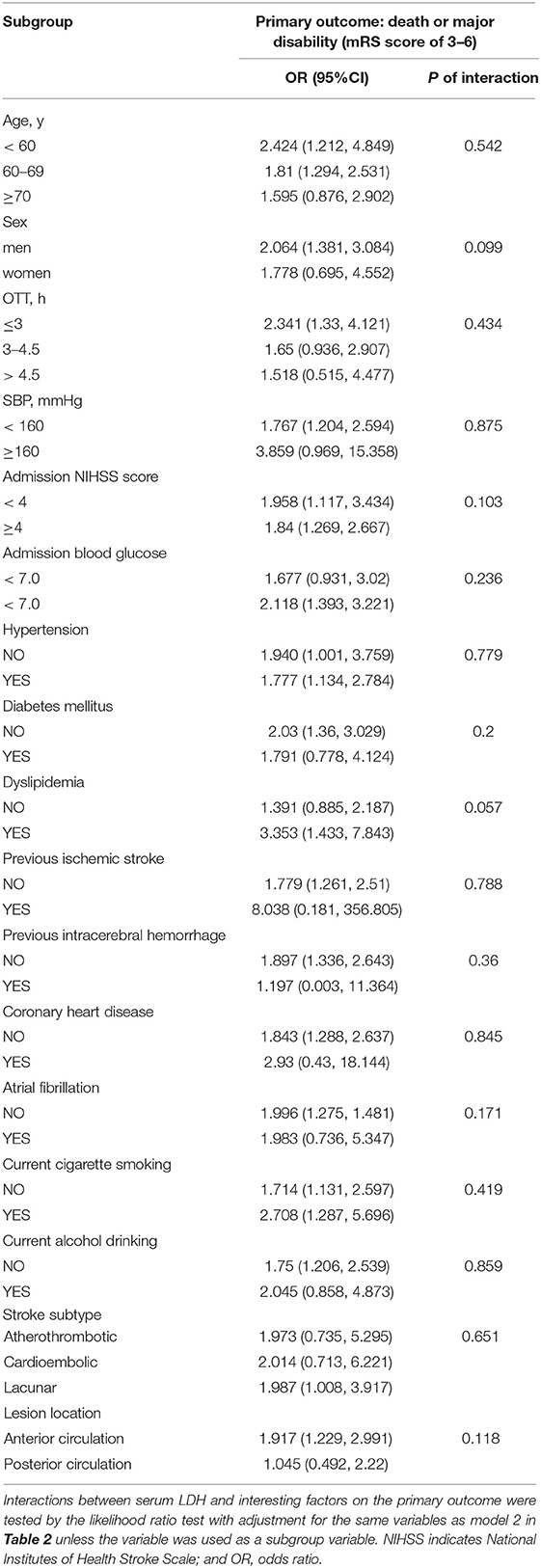
Table 4. Subgroup analysis of the association between LDH before thrombolysis and primary outcome (death or major disability).
The median LDH levels within 1–3 days after thrombolysis were 196 (quartiles, 173–230) U/L, significantly exceeding LDH levels before thrombolysis (P < 0.001). Therefore, we further analyzed the predictive power of LDH levels within 1–3 days on primary outcomes and 3-month mortality. In model 2, the OR of primary outcome for the Q4 vs. Q1 is 19.876 (95%CI, 4.626–85.397). Each SD increase in log-transformed LDH was associated with a 320.8% (121, 701.4%) increased risk of primary outcome (Supplementary Table 1). Furthermore, ROC curves were used to compare the overall discriminative ability between LDH before thrombolysis and within 1–3 days after thrombolysis for the 3-month outcome and to calculate optimal cut-off values which represent the highest sum of the specificity and sensitivity. Post-thrombolysis LDH had an obvious advantage in AUC relative to pre-thrombolysis LDH for predicting primary outcomes (Table 5).
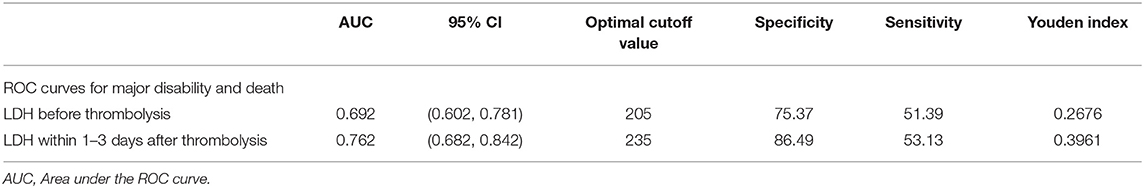
Table 5. AUC for serum LDH levels before and within 1–3 days after thrombolysis of 3-month prognosis.
Our study shows that (1) elevated serum LDH levels on admission were significantly associated with ENI, END, cerebral edema, and 3-month outcomes in AIS patients. (2) Serum LDH levels before thrombolysis have additional predictive incremental value for traditional models that include baseline NIHSS scores. (3) Serum LDH levels within 1–3 days after thrombolysis are more predictive of 3-month outcomes than baseline levels.
LDH, as an enzyme necessary for anaerobic metabolism, is localized and restricted intracellular unless local tissue is injured. The brain should be one of the sources of serum LDH after AIS. On one hand, intracellular LDH is upregulated for energy utilization and adaption to the ischemic and hypoxic environment results from occlusion of cerebral arteries (5), which may occur in all injured brain cells, including neurons, astrocytes, microglia, and so on. Elevated LDH was detected in the brain cells during hypoxia and reoxygenation (22). On the other hand, damage or death of brain cells allows LDH to be released into the extracellular space and then into the peripheral circulation through the damaged BBB. Furthermore, it has also been found that extracellular lactic acid, the catalytic products of LDH, stimulates vascular endothelial cells to express inflammatory factor IL-8 and vascular endothelial growth factor in tumor study (23–26), which possibly promote local inflammation and angiogenesis, contributing to the BBB destruction and cerebral edema in ischemic stroke. Thus, we hypothesized that the elevated serum LDH responsibly reflects the severity of brain tissue injury. In this study, we validated that serum LDH levels are associated with cerebral infarct size and cerebral edema and predicted neurological changes and 90-day outcomes.
During 1–3 days after thrombolysis, the cerebral infarct size may continue to expand with an acute progression of cerebral edema, leading to a continuous increasing LDH level. On the other hand, as the fact that BBB remains relatively intact within 24 h, the leakage of LDH to peripheral blood may be limited to a certain extent. Therefore, serum LDH levels within 1–3 days after thrombolysis possibly more accurately reflected cerebral damage after intravenous thrombolysis, thereby becoming a better predictor of 90-day outcomes, while serum LDH levels before thrombolysis may provide additional assistance in decision making for thrombolytic therapy.
Recently, elevated LDH levels were reported to be associated with 30-day mortality in AIS patients with COVID-19 (27). However, the elevated serum LDH levels likely resulted from pneumonia, which has been reported in other published articles (28). In contrast, we deliberately exclude other diseases that potentially increase serum LDH levels.
The limitation of this study is that (1) all the patients in this study were Chinese, so there is a lack of validation in non-Chinese patients; (2) the sample size is relatively small; (3) we did not record serum LDH levels on a continuous basis after intravenous thrombolysis.
In conclusion, serum LDH could be an extremely promising prognostic marker in AIS patients treated with intravenous rt-PA.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Huazhong University of Science and Technology. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
RB, SC, and JH conducted the data analysis and wrote the manuscript. JH, DX, YS, and MH helped with the data collection and literature search. BH together with HJ designed this study and directed the writing of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Key Research and Development Program of China (2018YFC1312200 to BH), the National Natural Science Foundation of China (Grants: 82090044 and 81820108010 to BH, and 81671147 to HJ), and Major Refractory Diseases Pilot Project of Clinical Collaboration with Chinese and Western Medicine (SATCM-20180339 to BH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We appreciate the data generously provided by Yichang Central Hospital and Hefeng People's Hospital.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.816216/full#supplementary-material
AIS, acute ischemic stroke; rt-PA, recombinant tissue plasminogen activator; CE, cerebral edema; LDH, lactate dehydrogenase; AMI, acute myocardial infarction; NIHSS, National Institute of Health stroke scale; OTT, time from onset to treatment (OTT); CT, computerized tomography; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; SITS-MOST, the Safe Implementation of Thrombolysis in Stroke-Monitoring Study; NINDS, National Institute of Neurological Disorders and Stroke; ENI, early neurological improvement; END, early neurological deterioration; OR, odds ratio; IDI, integrated discrimination improvement; NRI, net reclassification improvement; ROC, receiver operating characteristic.
1. Collaborators GBDS Global. regional, and national burden of stroke, 1990–2016: a systematic analysis for the global burden of disease study 2016. The Lancet Neurology. (2019) 18:439–458.
2. Ospel JM, Holodinsky JK, Goyal M. Management of acute ischemic stroke due to large-vessel occlusion: JACC focus seminar. J Am Coll Cardiol. (2020) 75:1832–43. doi: 10.1016/j.jacc.2019.10.034
3. Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. (2020) 368:l6983. doi: 10.1136/bmj.l6983
5. Farhana A, Lappin SL. “Biochemistry, lactate dehydrogenase,” In: StatPearls. (Treasure Island: StatPearls Publishing) (2021).
6. Klein R, Nagy O, Tóthová C, Chovanová F. Clinical and diagnostic significance of lactate dehydrogenase and its isoenzymes in animals. Veterin med int. (2020) 2020:5346483. doi: 10.1155/2020/5346483
7. Wang J, Li L, Dong BQ, Xu YJ, Zheng YD, Sun ZW, et al. Post-treatment serum lactic dehydrogenase as a predictive indicator for distant metastasis and survival of patients with nasopharyngeal carcinoma. Oncotarget. (2016) 7:27458–67. doi: 10.18632/oncotarget.8480
8. Thompson PW, Jones DD. Serum lactic dehydrogenase as a marker of joint damage in rheumatoid arthritis. Annal rheumatic dis. (1987) 46:263. doi: 10.1136/ard.46.3.263
9. Jin Y, Ye X, Shao L, Lin BC, He CX, Zhang BB, et al. Serum lactic dehydrogenase strongly predicts survival in metastatic nasopharyngeal carcinoma treated with palliative chemotherapy. Euro j cancer. (2013) 49:1619–26. doi: 10.1016/j.ejca.2012.11.032
10. Dumontet C, Drai J, Bienvenu J, Berard EN, Thieblemont C, Bouafia F, et al. Profiles and prognostic values of LDH isoenzymes in patients with non-hodgkin's lymphoma. Leukemia. (1999) 13:811–7. doi: 10.1038/sj.leu.2401417
11. Drews HJ, Yenkoyan K, Lourhmati A, Buadze M, Kabisch D, Verleysdonk S, et al. Intranasal losartan decreases perivascular beta amyloid, inflammation, and the decline of neurogenesis in hypertensive rats. Neurotherapeutics. (2019) 16:725–40. doi: 10.1007/s13311-019-00723-6
12. Wang M, Liang X, Cheng M, Yang L, Liu H, Wang X, et al. Homocysteine enhances neural stem cell autophagy in in vivo and in vitro model of ischemic stroke. Cell death dis. (2019) 10:561. doi: 10.1038/s41419-019-1798-4
13. Dai SH, Chen T, Li X, Yue KY, Luo P, Yang LK, et al. Sirt3 confers protection against neuronal ischemia by inducing autophagy: involvement of the ampk-mtor pathway. Free rad biol & med. (2017) 108:345–353. doi: 10.1016/j.freeradbiomed.2017.04.005
14. Chen MY, Wang XC, Lou DN, Hu ZX, Zhou ML, Lu XD. Association between the hydrogenase level and the occurrence of remote diffusion-weighted imaging lesions after spontaneous intracerebral hemorrhage. J clinic neurosci offic j Neurosurg Soc Austral. (2020) 77:49–54. doi: 10.1016/j.jocn.2020.05.045
15. Fahey M, Crayton E, Wolfe C, Douiri A. Clinical prediction models for mortality and functional outcome following ischemic stroke: a systematic review and meta-analysis. PloS One. (2018) 13:e0185402. doi: 10.1371/journal.pone.0185402
16. Whiteley W, Chong WL, Sengupta A, Sandercock P. Blood markers for the prognosis of ischemic stroke: a systematic review. Stroke. (2009) 40:e380–389. doi: 10.1161/STROKEAHA.108.528752
17. Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet. (London, England). (2010) 9727:1695–703. doi: 10.1016/S0140-6736(10)60491-6
18. Ma H, Campbell BCV, Parsons MW, Churilov L, Levi CR, Hsu C, et al. Thrombolysis guided by perfusion imaging up to 9 hours after onset of stroke. n Engl J Med. (2019) 380:1795–803. doi: 10.1056/NEJMoa1813046
19. Guidelines. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovascul dis. (2008) 25:457-507. doi: 10.1159/000131083
20. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in stroke-monitoring study. (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
21. Tissue plasminogen. activator for acute ischemic stroke. New Engl j med. (1995) 333:1581–87. doi: 10.1056/NEJM199512143332401
22. Xia Q, Mao M, Zeng Z, Luo Z, Zhao Y, Shi J, et al. Inhibition of SENP6 restrains cerebral ischemia-reperfusion injury by regulating annexin-A1 nuclear translocation-associated neuronal apoptosis. Theranostics. (2021) 11:7450–70. doi: 10.7150/thno.60277
23. Miyoshi N, Tanigawa T, Nishioka S, Maruyama K, Eguchi E, Tanaka K, et al. Association of salivary lactate dehydrogenase level with systemic inflammation in a Japanese population. J periodont res. (2018) 53:487–94. doi: 10.1111/jre.12537
24. Manerba M, Di Ianni L, Govoni M, Roberti M, Recanatini M, Di Stefano G. Lactate dehydrogenase inhibitors can reverse inflammation induced changes in colon cancer cells. Euro j pharmaceutic sci offic j Euro Federat Pharmaceutic Sci. (2017) 96:37–44. doi: 10.1016/j.ejps.2016.09.014
25. Augoff K, Hryniewicz-Jankowska A, Tabola R. Lactate dehydrogenase 5: an old friend and a new hope in the war on cancer. Cancer lett. (2015) 358:1–7. doi: 10.1016/j.canlet.2014.12.035
26. Ding J, Karp JE, Emadi A. Elevated lactate dehydrogenase. (LDH) can be a marker of immune suppression in cancer: interplay between hematologic and solid neoplastic clones and their microenvironments. Cancer biomark. (2017) 19:353–63. doi: 10.3233/CBM-160336
27. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J clinic investigat. (2020) 130:2620–9. doi: 10.1172/JCI137244
Keywords: ischemic stroke, lactic dehydrogenase, rt-PA, prognosis of prognosis, something, bio-marker
Citation: Jin H, Bi R, Hu J, Xu D, Su Y, Huang M, Peng Q, Li Z, Chen S and Hu B (2022) Elevated Serum Lactate Dehydrogenase Predicts Unfavorable Outcomes After rt-PA Thrombolysis in Ischemic Stroke Patients. Front. Neurol. 13:816216. doi: 10.3389/fneur.2022.816216
Received: 19 January 2022; Accepted: 28 February 2022;
Published: 06 April 2022.
Edited by:
Kittisak Sawanyawisuth, Khon Kaen University, ThailandReviewed by:
Liu Mingyong, Capital Medical University, ChinaCopyright © 2022 Jin, Bi, Hu, Xu, Su, Huang, Peng, Li, Chen and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shengcai Chen, Y2hlbnNoZW5nY2FpMjAwNkAxMjYuY29t; Bo Hu, aHVib0BtYWlsLmh1c3QuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.