
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
STUDY PROTOCOL article
Front. Neurol. , 12 May 2022
Sec. Neuroepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.816147
 Satoshi Hosoki1
Satoshi Hosoki1 Yorito Hattori1*
Yorito Hattori1* Satoshi Saito1
Satoshi Saito1 Misa Takegami2
Misa Takegami2 Shuichi Tonomura1,3
Shuichi Tonomura1,3 Yumi Yamamoto4
Yumi Yamamoto4 Shuhei Ikeda1
Shuhei Ikeda1 Naohisa Hosomi5,6
Naohisa Hosomi5,6 Naoya Oishi7
Naoya Oishi7 Yoshiaki Morita8
Yoshiaki Morita8 Yoshihiro Miyamoto9
Yoshihiro Miyamoto9 Ryota Nomura10
Ryota Nomura10 Kazuhiko Nakano10
Kazuhiko Nakano10 Masafumi Ihara1*
Masafumi Ihara1*Introduction: The role of commensal microbiota in systemic diseases, including brain diseases, has attracted increasing attention. Oral infectious diseases, such as dental caries and periodontitis, are also involved in cerebrovascular diseases and cognitive impairment. Cerebral microbleeds (CMBs) and intracerebral hemorrhage due to small vessel disease (SVD), are presumably associated with a high risk of vascular cognitive impairment and stroke. We previously reported that Streptococcus mutans (S. mutans, the main pathogen of dental caries), harboring the cnm gene that encodes the collagen-binding protein Cnm, is associated with the development of hypertensive intracerebral hemorrhage and aggravation of CMBs. We also proposed a mechanism by which the circulating Cnm-expressing S. mutans causes intracerebral hemorrhage or CMBs; it binds to denuded basement membranes mainly composed of collagen IV through damaged tight junctions or it directly invades endothelial cells, resulting in blood-brain barrier injury. In November 2018, we initiated a multicenter, prospective cohort study (RAMESSES: Risk Assessment of Cnm-positive S. mutans in Stroke Survivors; UMIN Clinical Trials Registry: UMIN000045559) to explore the longitudinal association between Cnm-positive S. mutans and CMBs with comprehensive dental findings, which should determine the effect of Cnm-positive S. mutans in the oral cavity on the risk of CMB development and cognitive decline.
Methods: Fifteen domestic institutes will be enlisted to enroll 230 patients who have at least one CMB in the deep brain area and develop a stroke within the past year. The prevalence of Cnm-positive S. mutans based on oral specimens and dental hygiene will be examined. The primary outcome is the number of newly developed deep CMBs. The secondary outcomes include the new development of lobar, subtentorial, or any type of CMBs; symptomatic intracerebral hemorrhage or ischemic stroke; changes in cognitive function or frailty; major bleeding; all-cause mortality; and antibody titers against periodontal pathogens. The observation period will be 2 years.
Discussion: The 2-year longitudinal prospective cohort study is expected to establish the role of Cnm-positive S. mutans in SVD including CMBs and intracerebral hemorrhage from the perspective of the “brain-oral axis” and provide guidance for novel prophylactic strategies against Cnm-positive S. mutans-induced SVD.
Small vessel disease (SVD) refers to a variety of pathological, clinical, and neuroimaging changes that are caused by damage to the perforating cerebral arterioles, capillaries, and venules. Vascular pathologies underlying SVD include lipohyalinosis, fibrinoid necrosis, microatheroma, microaneurysms, and segmental arterial disorganization (1, 2). White matter hyperintensities (WMHs), lacunes, perivascular spaces, and cerebral microbleeds (CMBs) detected by brain magnetic resonance imaging (MRI) are major neuroimaging markers of SVD (3, 4). Among them, CMBs appear as small circular or elliptical lesions with a size of 10 mm or less that have a low signal intensity on gradient-echo T2*-weighted images (T2*WI) obtained by brain MRI (5–7). In addition, CMBs are an independent risk factor for cognitive impairment (8) and stroke (9), especially intracerebral hemorrhage (ICH) (10). It is well-known that the vascular risk factors, including hypertension, diabetes, dyslipidemia, and smoking, play important roles in developing and exacerbating SVD mediated by oxidative stress and vascular inflammation resulting in endothelial damage and disruption of the blood-brain barrier (BBB) (1). However, the effect of vascular risk factors on WMHs may be limited because WMHs have a substantial “non-vascular” or non-atheromatous etiology (4). This notion may be supported by the fact that the modification of vascular risk factors is not sufficient for the prevention and treatment of SVD.
Numerous studies have found an association between human microbiota and many pathological conditions, including cardiovascular diseases, diabetes, obesity, rheumatoid arthritis, Alzheimer's disease, and multiple sclerosis, which lead to the elucidation of the molecular mechanisms underlying this association (11). Specifically, the relationship between oral infections and cardiovascular diseases has been examined in several observational studies (12) and interventional studies (13). However, it remains to be fully elucidated which oral bacteria among more than 700 species are involved and how these causative agents exert the deleterious effects that induce systemic pathological changes (14). Streptococcus mutans (S. mutans) is a gram-positive bacterium and a major pathogen responsible for dental caries (15). Cnm, encoded by the cnm gene, is a cell-surface 120-kDa collagen-binding protein of S. mutans (16). Intravenous administration of Cnm-expressing S. mutans (Cnm-positive S. mutans) aggravates ICH in stroke-prone spontaneously hypertensive rats and cerebrovascular-endothelial-injured mice (17). We also reported that oral carriage of Cnm-positive S. mutans is associated with the prevalence of deep (basal ganglia and thalamus) CMBs and ICH (odds ratio: 4.5 vs. ischemic stroke) (18), and it is linked to an increased deep CMB incidence (incidence rate ratio: 13.9), based on cross-sectional studies (19), and to the severity of deep CMBs according to a longitudinal retrospective study (20). Cnm-positive S. mutans is also associated with cognitive impairment, accompanied by increased CMBs (19, 21).
Thus, cross-sectional and retrospective studies have identified strong associations of Cnm-positive S. mutans with ICH, deep CMBs, and cognitive dysfunction. However, the long-term effects of Cnm-positive S. mutans on SVD remain to be elucidated. In addition, the relationship between Cnm-positive S. mutans and oral hygiene and periodontal disease remains unknown. Therefore, in November 2018, we initiated a multicenter, prospective cohort study named RAMESSES (Risk Assessment of cnM-positivE S. mutans in StrokE Survivors) to explore the longitudinal association between Cnm-positive S. mutans and SVD, including CMBs, with comprehensive dental findings. In this article, we detail the protocol for the RAMESSES study (Figure 1).
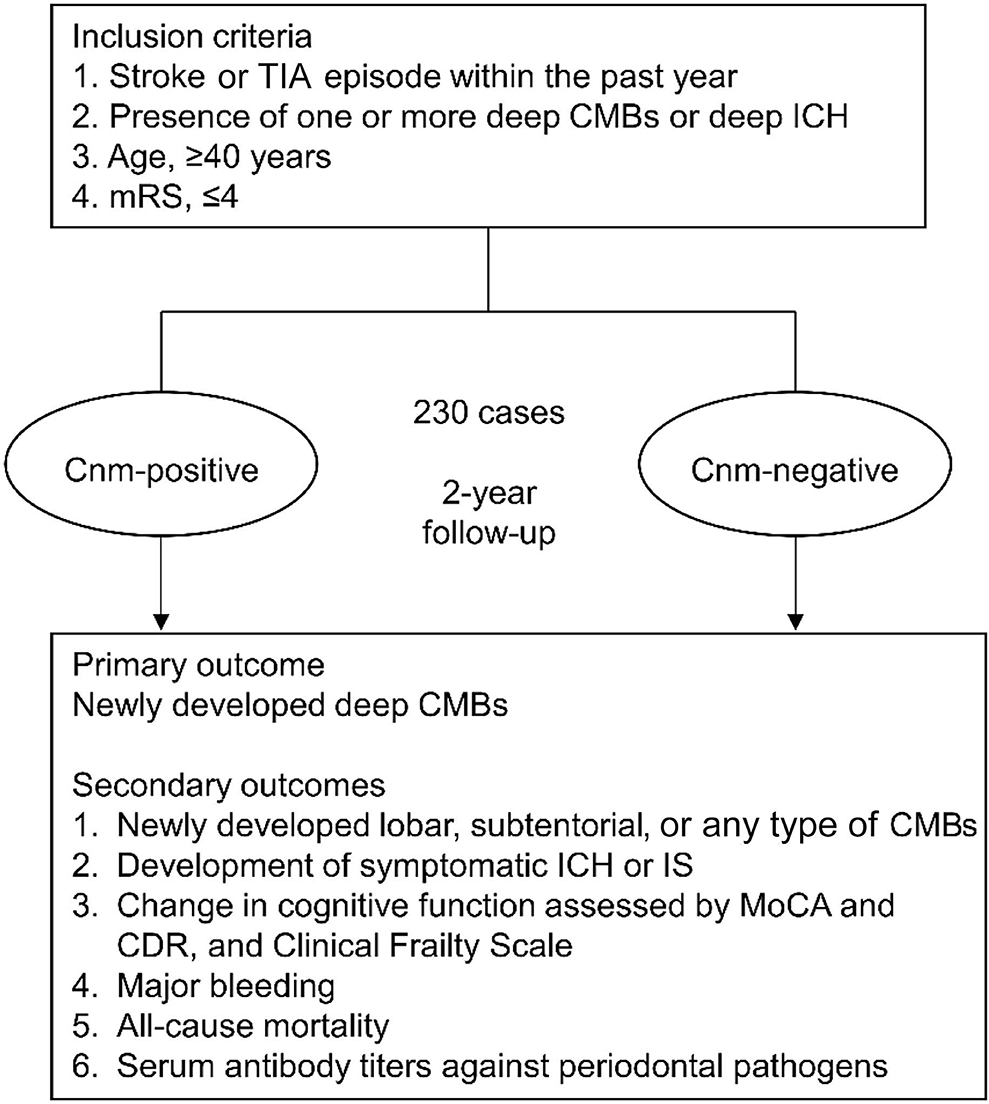
Figure 1. Research protocol of the RAMESSES study. The RAMESSES study is a multicenter, prospective, longitudinal, observational study to investigate the role of Cnm-positive Streptococcus mutans in the development of SVD and vascular cognitive impairment. Fifteen Japanese institutes participate in this study using a standardized MRI protocol. TIA, transient ischemic attack; CMBs, cerebral microbleeds; mRS, modified Rankin Scale; ICH, intracerebral hemorrhage; IS, ischemic stroke; MRI, magnetic resonance imaging; MoCA, Montreal Cognitive Assessment; CDR, clinical dementia rating.
The RAMESSES study is a multicenter longitudinal prospective cohort study. The purpose of the study is to investigate the relationship between Cnm-positive S. mutans and SVD. The study will involve 15 institutes, including the National Cerebral and Cardiovascular Center (NCVC) as the central coordinating center located in Japan. The study will register 230 patients who develop stroke within 1 year after onset and are diagnosed with at least one hemorrhagic lesion involving CMBs or ICH in the deep area detected on T2*WI. The primary and secondary outcomes will be assessed at baseline, 6, 12, 18, and 24 months after written informed consent is obtained. We will conduct the assessments according to Table 1 for 2 years. The study protocol has been approved by the Research Ethics Committee of each participating center (NCVC protocol number: M29-133), and this study has been registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry (UMIN-CTR: UMIN000045559). Each participant will be provided a detailed explanation of the purpose and potential risks of the study, and written informed consent will be obtained.
Inclusion criteria are defined as follows: (1) patients with stroke (cerebral infarction, ICH, or subarachnoid hemorrhage) or transient ischemic attack that developed within the past 365 days; (2) patients over 40 years of age; (3) patients whose modified Rankin Scale (mRS) score is 4 or less; (4) patients who have deep ICH or at least one deep CMB on T2*WI performed within the past 365 days; and (5) written informed consent has been obtained from the patients themselves or the proxies. Exclusion criteria are as follows: (1) patients who are contraindicated for MRI examination because of the presence of some metallic implants, such as pacemaker devices; (2) patients with bleeding diathesis; (3) patients who participate in other intervention trials; (4) patients who cannot conduct the Montreal Cognitive Assessment (MoCA) due to severe dementia, deafness, or visual impairment; (5) patients who have no remaining teeth; or (6) patients whose participation is judged as inappropriate by the principal investigator or sub-investigators if a patient is not deemed to have capacity to provide fully informed consent or is too unwell at the time of recruitment (Table 2).
All 3.0 Tesla MRI examinations will be performed using the following standardized MR imaging protocol. All slices will be taken parallel to the orbitomeatal line from the base of the skull to the vault. The imaging protocol includes T2*WI, fluid-attenuated inversion recovery (FLAIR), diffusion-weighted imaging (DWI), and apparent diffusion coefficient. The sequence parameters of T2*WI are as follows: voxel size, 0.7 × 0.7 × 4.0 mm; slice gap, 0 mm; flip angle, 20°; field of view, 230 mm; echo time, 12 ms; repetition time, 686 ms. The sequence parameters of FLAIR are as follows: voxel size, 0.7 × 0.7 × 5.0 mm; slice gap, 1 mm; flip angle, 150°; field of view, 230 mm; echo time, 114–117 ms; repetition time, 12,000 ms. The sequence parameters of DWI are not restricted. CMBs and symptomatic ICH will be evaluated using T2*WI-MRI. CMBs in deep or lobar areas indicated below will be defined and counted according to the Brain Observer MicroBleed Scale (5) or the Microbleed Anatomical Rating Scale (22). CMBs will be categorized into three groups: (1) deep CMBs defined elsewhere (5, 22), (2) lobar CMBs in the cortical gray or subcortical white matter, and (3) subtentorial CMBs in the cerebellum or brain stem. CMBs in any brain region (any CMB) will also be recorded. Newly developed CMBs will be recorded at follow-up after 1 and 2 years. Symptomatic ischemic stroke (IS) will be evaluated by DWI. Lacunes and WMHs will be evaluated using FLAIR images. A lacune is defined as a supratentorial hypointense lesion 3–15 mm in diameter with a hyperintense rim. Periventricular hyperintensities (PVHs) and deep white matter hyperintensities (DWMHs) will be scored using the Fazekas scale (23).
The primary outcome is the number of newly developed deep CMBs. The secondary outcomes include (1) new development of lobar, subtentorial, or any type of CMBs; (2) development of symptomatic ICH or IS; (3) change in cognitive function assessed by MoCA and clinical dementia rating (CDR), and in frailty according to the Clinical Frailty Scale version 2.0 (24); (4) major bleeding defined by the International Society on Thrombosis and Hemostasis criteria; (5) all-cause mortality; and (6) serum antibody titers against periodontal pathogens.
The sample size was determined by feasibility, but it was ensured to be substantial enough to perform the primary analysis. According to recent studies, the general prevalence rate of Cnm-positive S. mutans is 7–20% (19, 25–27). Our previous retrospective study revealed that patients with Cnm-positive S. mutans has an incidence rate ratio (IRR) of 13.9 (4.3–44.5) when compared with patients without (20). In this study, the minimum number is 223 subjects (27 subjects with Cnm-positive S. mutans and 196 without) based on Cnm-positive S. mutans prevalence of 12%, IRR of 4.3 (equivalent to the lower limit of the 95% confidence interval), a significance level of 5%, 90% power, and a follow-up period of 2 years. Therefore, we will recruit 230 subjects with considering a dropout rate of 10%.
Dental examinations will be conducted by well-trained dentists at each institute. Dental examinations will include (1) checking for the presence of dental caries; (2) dental radiography; (3) probing pocket depth assessed from the free gingival margin to the bottom of the sulcus or pocket; (4) full-mouth bleeding score, which is defined as the number of sites with gingival bleeding on probing divided by the total number of sites per mouth and multiplied by 100 (28); (5) the number of remaining and deficit teeth excluding the third molars; (6) the decayed, missing, and filled teeth (DMFT) index as an indicator of dental history or health (29). Sample collection and detection of Cnm-positive S. mutans will be performed using the following standardized protocol: oral saliva and dental plaque specimens will be collected from the patients using Seed-swab Type ⋎3 (Eiken Chemical, Tokyo, Japan). These samples will be inoculated on Mitis-Salivarius medium with bacitracin (MSB, 100 U/ml; Sigma-Aldrich, St. Louis, MO, USA) and 15% sucrose (MSB agar) and anaerobically incubated at 37°C for 48 h. The S. mutans colonies will be isolated and cultured in Brain Heart Infusion Broth (Difco Laboratories, Detroit, MI, USA) at 37°C for 24 h. Then, DNA from each strain will be extracted and analyzed by polymerase chain reaction using species-specific MKD primers to identify S. mutans isolates (27) and cnm primers to detect the cnm gene (16). A collagen-binding assay with type I collagen will be conducted to examine the collagen-binding activity of each isolated S. mutans strain, according to the method described elsewhere (30), with some modifications (27). The activity tests will be performed under fixed conditions using 1 mg of type I collagen and 1 × 1010 bacterial cells. The activity for each strain is expressed as a percentage compared with the positive control S. mutans TW871, which has 100% binding activity to type I collagen (27). These experiments will be conducted by a researcher who is blinded to the clinical information.
Serum samples will be collected during baseline evaluation to measure serum antibody titers against nine bacteria responsible for periodontal diseases by enzyme-linked immunosorbent assay, as previously reported (31). The nine bacteria are Porphyromonas gingivalis (P. gingivalis), Aggregatibacter actinomycetemcomitans, Eikenella corrodens, Fusobacterium nucleatum, Prevotella nigrescens, Prevotella intermedia, Treponema denticola, Tannerella forsythia, and Campylobacter rectus.
The cognitive function and mental status of the subjects will be evaluated in each institute by well-trained neurologists or psychologists who are familiar with CDR (at baseline and 2 years), MoCA (at baseline, 1 and 2 years), and geriatric depression scale (at baseline).
The frailty at baseline and 2 years (32) will be assessed in each institute by well-trained neurologists using the Clinical Frailty Scale version 2.0 (24).
An electronic data capture system, Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN, USA) (33), will be used for the collection and management of all data. Brain MRI data will be sent to the Central Evaluation Committee, and the data will be assessed by three neurologists belonging to the committee. Dental findings will be evaluated and stored by dentists in the Department of Pediatric Dentistry at Osaka University. All data will be collected and integrated at the NCVC. The data managers will perform quality control at each step of data handling to ensure the reliability of all data related to the study.
For the primary outcome, the incidence of newly developed CMBs will be compared between patients with and without Cnm-positive S. mutans using a quasi-Poisson regression model, adjusted for age, sex, risk factors, antithrombotic medication, history of heart disease, history of cerebrovascular diseases, and dental findings. For the secondary outcomes, which include the incidence of symptomatic ICH or IS, all-cause mortality, and total hemorrhagic events, the survival time curves will be derived for each group using the Kaplan-Meier method and the survival rates will be compared between the two groups using the log-rank test and Cox proportional hazard models. The changes of scores based on frailty scale scoring, the cognitive tests from baseline to 2 years, and the antibody titers against periodontal pathogens at baseline will be compared between patients with and without Cnm-positive S. mutans using generalized linear mixed models for repeated measurements based on data distributions. Statistical analysis will be conducted using SAS version 9.4 (SAS Institute, Cary, USA).
The study will be conducted at 15 Japanese institutes, including the NCVC, and is supported by funding from Grant-in-Aid for Early-Career Scientists, KAKEN (YH: 21K16961), and the BMS/Pfizer Japan Thrombosis Investigator Initiated Research Program (MI: CV185656).
The objective of the RAMESSES study, a multicenter longitudinal prospective cohort study, is to assess whether Cnm-positive S. mutans affects the development of deep CMBs, as the primary outcome. The secondary outcomes include assessments of the effects of Cnm-positive S. mutans on the new development of lobar, subtentorial, or any type of CMBs, symptomatic ICH or IS, the changes in cognitive function or frailty, major bleeding, all-cause mortality, and antibody titers against periodontal pathogens.
Cnm-positive S. mutans causes SVD, including CMBs and ICH, due to the collagen-binding activity, which mediates binding to type I collagen in teeth (27) and to type IV collagen (34) and laminin (35) in the basement membrane of cerebral blood vessels. Cnm-positive S. mutans binds strongly to the dentin tooth layer composed of type I collagen, which mediates the entry of S. mutans into the bloodstream by promoting the development of carious lesions in the periodontal space (34). After the induction of bacteremia, there are two plausible mechanisms by which Cnm is involved in BBB injury and subsequent development of CMBs. First, Cnm-positive S. mutans can adhere and attach to the denuded basement membrane via endothelial tight junction dehiscence, and the migration of circulating neutrophils to the lesion subsequently activates local inflammation with the secretion of matrix metalloproteinase (MMP)-9 that eventually exacerbates the BBB permeability (17). Second, Cnm-positive S. mutans has the ability to invade endothelial cells, which specifically requires Cnm for adherence and intracellular invasion (36–38). Once Cnm binding to circulating type IV collagen mediates attachment to the host endothelial surface, host cell signaling cascades are triggered that lead to the internalization of Cnm-positive S. mutans, the activation of pro-inflammatory responses, and eventually apoptosis of endothelial cells (36, 39) (Figure 2). BBB dysfunction not only induces red blood cell extravasation, forming CMBs and ICH, but also may aggravate the homeostasis of the internal environment in the brain with reduced delivery of glucose and other nutrients, along with impaired clearance of waste products and metabolites through efflux transporters, all of which can possibly lead to cognitive impairment (40).
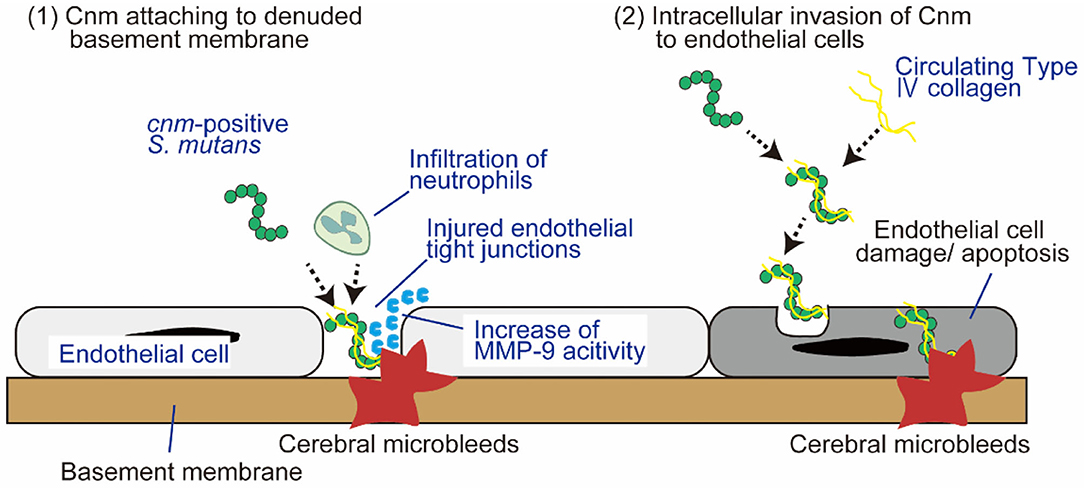
Figure 2. Two plausible mechanisms by which Cnm is involved in blood-brain barrier injury and subsequent development of CMBs. There are two plausible mechanisms by which Cnm is involved in BBB injury and subsequent development of CMBs: (1) Cnm-positive S. mutans attaches to denuded basement membrane facilitated by endothelial tight junction dehiscence. (2) Cnm-positive S. mutans directly invades endothelial cells. See text for details. BBB, blood-brain barrier; MMP-9, matrix metalloproteinase-9.
P. gingivalis is the main pathogen for periodontitis (41). Adherence of P. gingivalis to endothelial cells activates coagulation factors and protease-activated receptors 1 and 4 expressed on the surface of platelets (41). In addition, periodontal disease-induced disruption of the gingival epithelial barrier mediates bacteremia onset by facilitating the entry of oral bacteria, including S. mutans, into systemic circulation (42). Thus, oral carriage of P. gingivalis is possibly implicated in SVD pathology due to thrombotic occlusion and BBB disruption induced by endothelial damage and inflammation (43).
In conclusion, Cnm-positive S. mutans probably plays a critical role in the development of SVD, including CMBs and ICH, based on previous findings reported in basic and clinical retrospective observational studies. The RAMESSES study is expected to establish a firm understanding of the pathophysiology of Cnm-positive S. mutans for SVD as the “brain-oral axis”, which will guide the future development of novel prophylactic strategies against Cnm-positive S. mutans-induced SVD and vascular cognitive impairment.
The protocol and related documents have been approved by the Research Ethics Committee at the National Cerebral and Cardiovascular Center (approval number: M29-133). The patients/participants provided their written informed consent to participate in this study.
SH and YH wrote the manuscript and contributed to the study protocol. SS contributed to the study design and revised the manuscript. MT leads the statistical analysis. ST, YY, SI, and YMi discussed the study protocol. NO and YMo are responsible for the standardization of the MRI protocol. RN and KN contributed to dental examinations and expertise. MI conceived the study design, wrote the manuscript, and worked on revisions. All authors contributed to the article and approved the submitted version.
This study receives funding from Bristol-Myers Squibb (CV185656). The funder is not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
MI reports personal fees from Daiichi Sankyo, Eisai, and Bayer, and grants from Panasonic, Bristol-Myers Squibb, and Otsuka Pharmaceutical, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Iadecola C. The pathobiology of vascular dementia. Neuron. (2013) 80:844–66. doi: 10.1016/j.neuron.2013.10.008
2. Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. (2010) 9:689–701. doi: 10.1016/S1474-4422(10)70104-6
3. Shi Y, Wardlaw JM. Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc Neurol. (2016) 1:83–92. doi: 10.1136/svn-2016-000035
4. Wardlaw JM, Allerhand M, Doubal FN, Valdes Hernandez M, Morris Z, Gow AJ, et al. Vascular risk factors, large-artery atheroma, and brain white matter hyperintensities. Neurology. (2014) 82:1331–8. doi: 10.1212/WNL.0000000000000312
5. Cordonnier C, Potter GM, Jackson CA, Doubal F, Keir S, Sudlow CL, et al. improving interrater agreement about brain microbleeds: development of the Brain Observer MicroBleed Scale (BOMBS). Stroke. (2009) 40:94–9. doi: 10.1161/STROKEAHA.108.526996
6. Correa DG, Cruz Junior LC, Bahia PR, Gasparetto EL. Intracerebral microbleeds in sepsis: susceptibility-weighted MR imaging findings. Arq Neuropsiquiatr. (2012) 70:903–4. doi: 10.1590/S0004-282X2012001100017
7. Nandigam RN, Viswanathan A, Delgado P, Skehan ME, Smith EE, Rosand J, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. Am J Neuroradiol. (2009) 30:338–43. doi: 10.3174/ajnr.A1355
8. Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. (2016) 73:934–43. doi: 10.1001/jamaneurol.2016.1017
9. Pasi M, Cordonnier C. Clinical relevance of cerebral small vessel diseases. Stroke. (2020) 51:47–53. doi: 10.1161/STROKEAHA.119.024148
10. Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:81–94. doi: 10.1001/jamaneurol.2018.3122
11. Cani PD, Moens de Hase E, Van Hul M. Gut microbiota and host metabolism: from proof of concept to therapeutic intervention. Microorganisms. (2021) 9:1302. doi: 10.3390/microorganisms9061302
12. Zeng XT, Leng WD, Lam YY, Yan BP, Wei XM, Weng H, et al. Periodontal disease and carotid atherosclerosis: a meta-analysis of 17,330 participants. Int J Cardiol. (2016) 203:1044–51. doi: 10.1016/j.ijcard.2015.11.092
13. Kudo C, Shin WS, Sasaki N, Harai K, Kato K, Seino H, et al. Effects of periodontal treatment on carotid intima-media thickness in patients with lifestyle-related diseases: Japanese prospective multicentre observational study. Odontology. (2018) 106:316–27. doi: 10.1007/s10266-017-0331-4
14. Van Dyke TE, Starr JR. Unraveling the link between periodontitis and cardiovascular disease. J Am Heart Assoc. (2013) 2:e000657. doi: 10.1161/JAHA.113.000657
15. Ito S, Misaki T, Naka S, Wato K, Nagasawa Y, Nomura R, et al. Specific strains of Streptococcus mutans, a pathogen of dental caries, in the tonsils, are associated with IgA nephropathy. Sci Rep. (2019) 9:20130. doi: 10.1038/s41598-019-56679-2
16. Sato Y, Okamoto K, Kagami A, Yamamoto Y, Igarashi T, Kizaki H. Streptococcus mutans strains harboring collagen-binding adhesin. J Dent Res. (2004) 83:534–9. doi: 10.1177/154405910408300705
17. Nakano K, Hokamura K, Taniguchi N, Wada K, Kudo C, Nomura R, et al. The collagen-binding protein of Streptococcus mutans is involved in haemorrhagic stroke. Nat Commun. (2011) 2:485. doi: 10.1038/ncomms1491
18. Tonomura S, Ihara M, Kawano T, Tanaka T, Okuno Y, Saito S, et al. Intracerebral hemorrhage and deep microbleeds associated with cnm-positive Streptococcus mutans; a hospital cohort study. Sci Rep. (2016) 6:20074. doi: 10.1038/srep20074
19. Watanabe I, Kuriyama N, Miyatani F, Nomura R, Naka S, Nakano K, et al. Oral Cnm-positive Streptococcus mutans expressing collagen binding activity is a risk factor for cerebral microbleeds and cognitive impairment. Sci Rep. (2016) 6:38561. doi: 10.1038/srep38561
20. Hosoki S, Saito S, Tonomura S, Ishiyama H, Yoshimoto T, Ikeda S, et al. Oral carriage of Streptococcus mutans harboring the cnm gene relates to an increased incidence of cerebral microbleeds. Stroke. (2020) 51:3632–9. doi: 10.1161/STROKEAHA.120.029607
21. Miyatani F, Kuriyama N, Watanabe I, Nomura R, Nakano K, Matsui D, et al. Relationship between Cnm-positive Streptococcus mutans and cerebral microbleeds in humans. Oral Dis. (2015) 21:886–93. doi: 10.1111/odi.12360
22. Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jäger HR, et al. The microbleed anatomical rating scale (MARS): reliability of a tool to map brain microbleeds. Neurology. (2009) 73:1759–66. doi: 10.1212/WNL.0b013e3181c34a7d
23. Fazekas F, Chawluk JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am J Roentgenol. (1987) 149:351–6. doi: 10.2214/ajr.149.2.351
24. Rockwood K, Theou O. Using the clinical frailty scale in allocating scarce health care resources. Can Geriatr J. (2020) 23:210–5. doi: 10.5770/cgj.23.463
25. Lamba GS, Dufour D, Nainar SMH, Cioffi I, Levesque CM, Gong SG. Association of Streptococcus mutans collagen binding genes with severe childhood caries. Clin Oral Investig. (2020) 24:3467–75. doi: 10.1007/s00784-020-03217-4
26. Momeni SS, Ghazal T, Grenett H, Whiddon J, Moser SA, Childers NK. Streptococcus mutans serotypes and collagen-binding proteins Cnm/Cbm in children with caries analysed by PCR. Mol Oral Microbiol. (2019) 34:64–73. doi: 10.1111/omi.12254
27. Nomura R, Nakano K, Taniguchi N, Lapirattanakul J, Nemoto H, Gronroos L, et al. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol. (2009) 58:469–75. doi: 10.1099/jmm.0.007559-0
28. Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. Int Dent J. (1975) 25:229–35.
29. Schillinger T, Kluger W, Exner M, Mlekusch W, Sabeti S, Amighi J, et al. Dental and periodontal status and risk for progression of carotid atherosclerosis: the inflammation and carotid artery risk for atherosclerosis study dental substudy. Stroke. (2006) 37:2271–6. doi: 10.1161/01.STR.0000236495.82545.2e
30. Waterhouse JC, Russell RRB. Dispensable genes and foreign DNA in Streptococcus mutans. Microbiology. (2006) 152:1777–88. doi: 10.1099/mic.0.28647-0
31. Nakahara T, Hyogo H, Ono A, Nagaoki Y, Kawaoka T, Miki D, et al. Involvement of Porphyromonas gingivalis in the progression of non-alcoholic fatty liver disease. J Gastroenterol. (2018) 53:269–80. doi: 10.1007/s00535-017-1368-4
32. Morley JE, Vellas B, van Kan GA, Anker SD, Bauer JM, Bernabei R, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. (2013) 14:392–7. doi: 10.1016/j.jamda.2013.03.022
33. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
34. Nomura R, Naka S, Nemoto H, Otsugu M, Nakamura S, Ooshima T, et al. Potential high virulence for infective endocarditis in Streptococcus mutans strains with collagen-binding proteins but lacking PA expression. Arch Oral Biol. (2013) 58:1627–34. doi: 10.1016/j.archoralbio.2013.06.008
35. Sato Y, Okamoto K, Kagami A, Yamamoto Y, Ohta K, Igarashi T, et al. Application of in vitro mutagenesis to identify the gene responsible for cold agglutination phenotype of Streptococcus mutans. Microbiol Immunol. (2004) 48:449–56. doi: 10.1111/j.1348-0421.2004.tb03535.x
36. Abranches J, Miller JH, Martinez AR, Simpson-Haidaris PJ, Burne RA, Lemos JA. The collagen-binding protein Cnm is required for Streptococcus mutans adherence to and intracellular invasion of human coronary artery endothelial cells. Infect Immun. (2011) 79:2277–84. doi: 10.1128/IAI.00767-10
37. Abranches J, Zeng L, Bélanger M, Rodrigues PH, Simpson-Haidaris PJ, Akin D, et al. Invasion of human coronary artery endothelial cells by Streptococcus mutans OMZ175. Oral Microbiol Immunol. (2009) 24:141–5. doi: 10.1111/j.1399-302X.2008.00487.x
38. Avilés-Reyes A, Miller JH, Simpson-Haidaris PJ, Lemos JA, Abranches J. Cnm is a major virulence factor of invasive Streptococcus mutans and part of a conserved three-gene locus. Mol Oral Microbiol. (2014) 29:11–23. doi: 10.1111/omi.12041
39. Nomura R, Otsugu M, Hamada M, Matayoshi S, Teramoto N, Iwashita N, et al. Potential involvement of Streptococcus mutans possessing collagen binding protein Cnm in infective endocarditis. Sci Rep. (2020) 10:19118. doi: 10.1038/s41598-020-75933-6
40. Iadecola C. The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron. (2017) 96:17–42. doi: 10.1016/j.neuron.2017.07.030
41. Lourbakos A, Yuan YP, Jenkins AL, Travis J, Andrade-Gordon P, Santulli R, et al. Activation of protease-activated receptors by gingipains from Porphyromonas gingivalis leads to platelet aggregation: a new trait in microbial pathogenicity. Blood. (2001) 97:3790–7. doi: 10.1182/blood.V97.12.3790
42. Seymour RA, Lowry R, Whitworth JM, Martin MV. Infective endocarditis, dentistry and antibiotic prophylaxis; time for a rethink? Br Dent J. (2000) 189:610–6. doi: 10.1038/sj.bdj.4800845
Keywords: small vessel disease (SVD), cerebral microbleeds (CMBs), intracerebral hemorrhage (ICH), Cnm, Streptococcus mutans (S. mutans), dental caries, stroke
Citation: Hosoki S, Hattori Y, Saito S, Takegami M, Tonomura S, Yamamoto Y, Ikeda S, Hosomi N, Oishi N, Morita Y, Miyamoto Y, Nomura R, Nakano K and Ihara M (2022) Risk Assessment of Cnm-Positive Streptococcus mutans in Stroke Survivors (RAMESSES): Protocol for a Multicenter Prospective Cohort Study. Front. Neurol. 13:816147. doi: 10.3389/fneur.2022.816147
Received: 16 November 2021; Accepted: 19 April 2022;
Published: 12 May 2022.
Edited by:
Sreekanth Kumar Mallineni, Majmaah University, Saudi ArabiaReviewed by:
Yusuke Yakushiji, Kansai Medical University, JapanCopyright © 2022 Hosoki, Hattori, Saito, Takegami, Tonomura, Yamamoto, Ikeda, Hosomi, Oishi, Morita, Miyamoto, Nomura, Nakano and Ihara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yorito Hattori, eW9oMjAxOUBuY3ZjLmdvLmpw; Masafumi Ihara, aWhhcmFAbmN2Yy5nby5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.