
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 10 March 2022
Sec. Sleep Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.781054
This article is part of the Research TopicClinical Aspects of Obstructive Sleep Apnea and Cardiovascular ConsequencesView all 9 articles
Background: Obstructive sleep apnea (OSA) is a potential risk factor in cardiovascular diseases, including arrhythmia, coronary artery disease, and heart failure (HF). Continuous positive airway pressure (CPAP) therapy is an effective therapy for OSA and the underlying HF, partly through a 5–9% increase in the left ventricular ejection fraction (LVEF). However, the data on the factors associated with the efficacy of CPAP on LVEF in patients with HF complicated by OSA are scarce. This study aimed to investigate whether LVEF improves in patients with OSA and HF after 1 month of CPAP therapy, and to clarify which factors are associated with the degree of LVEF improvement.
Method: This was a prospective, single-arm, open-label study. We enrolled moderate-to-severe patients with OSA and HF who were being followed up at the cardiovascular center of Toranomon Hospital (Tokyo, Japan). The parameters of sleep study and LVEF were assessed at the baseline and after 1 month of CPAP. The multivariate regression analyses, with changes in LVEF as a dependent variable, were performed to determine the factors that were associated with the degree of LVEF improvement.
Results: We analyzed 55 consecutive patients with OSA and HF (mean age: 60.7 ± 12.2 years, mean LVEF value: 37.2 ± 9.8%). One month of CPAP treatment decreased the apnea-hypopnea index (AHI) from 45.3 ± 16.1 to 5.4 ± 4.1 per hour, and the LVEF improved from 37.2 ± 9.8 to 43.2 ± 11.7%. The multivariate regression analyses demonstrated that age and body mass index (BMI) were significant determinants of LVEF improvement.
Conclusion: The LVEF improved significantly after 1 month of CPAP therapy in Japanese patients with OSA and HF. Multivariate regression analyses indicated that an improvement in LVEF was likely to be observed in young patients with obesity.
The aging of the population and prolongation of the lives of patients with cardiovascular disease due to improvements in therapy have resulted in an increased incidence of HF (1). Despite advances in both pharmacologic and non-pharmacologic therapies, the mortality and the rate of repeat hospitalization due to exacerbation of HF remain high (2, 3). Therefore, further treatment strategies targeting conditions with potential HF risk are needed.
Obstructive sleep apnea (OSA), one of the most prevalent sleep-disordered breathing characterized by repeated episodes of upper airway obstruction during sleep, consequent chronic intermittent hypoxia, and sleep fragmentation, has been recognized as a potential risk factor in the development of cardiovascular diseases (4–6). The Sleep Heart Health Study showed an adjusted hazard ratio for incident HF of 1.58 for men with AHI of ≥30 compared with those with AHI of <5 (7). The prevalence of OSA in HF populations is reportedly high (4, 8, 9). An observational study reported a negative prognostic impact of untreated OSA in patients with HF (10).
Based on the results of clinical studies, CPAP therapy has been demonstrated as an effective therapy for OSA and the underlying HF (10, 11) through an increase in the LVEF (12, 13). The degree of improvement in LVEF varied from 5 to 9% in these studies (12, 13). However, no study has investigated the efficacy of CPAP for OSA and underlying HF among the Japanese population. Moreover, it is unclear which factors are associated with the degree of LVEF improvement by CPAP therapy. This study aimed to investigate whether LVEF in Japanese patients with OSA and HF improved after 1 month of CPAP therapy and to clarify which factors are associated with the degree of LVEF improvement.
We enrolled patients who were being followed up at the cardiovascular center in Toranomon Hospital (Tokyo, Japan) between January 1, 2001 and March 1, 2005, if they met the following inclusion criteria: (1) the presence of symptomatic HF with reduced LVEF, which was defined as an LVEF of <50% on echocardiography (14) within 1 month before the diagnostic sleep study, and with New York Heart Association (NYHA) Class II or above; (2) stable clinical status, defined as the absence of hospital admissions and obtainment of optimal medical therapy for at least 1 month before enrollment in the study; (3) having a diagnosis of moderate-to-severe sleep apnea from a sleep study, which was defined as ≥ 15 apnea or hypopnea events per hour of sleep (i.e., AHI); and (4) good adherence to CPAP (night usage of ≥ 4 h on 70% of the days during CPAP therapy) (15) during the initial month. The exclusion criteria were as follows: (1) age <20 or > 80 years, (2) the presence of known untreated neoplasms, (3) a history of stroke with neurologic deficit, and (4) a history of severe chronic pulmonary disease. Informed consent was obtained from all the patients who participated in the study. The study was conducted in compliance with the Declaration of Helsinki and in accordance with the ethics policies of the institutions involved.
All patients were diagnosed with sleep apnea based on the results of overnight polysomnography (PSG) using a digital polygraph (SomnoStarα Sleep System; SensorMedics Corp.; Yorba Linda, CA, USA) at our sleep laboratory. We used the definitions and scoring methods for sleep apnea (16, 17). We defined patients with predominantly central sleep apnea as having an AHI of ≥15 events/h of sleep, of which > 50% were central events. These patients were excluded from further analyses. The remaining patients who were diagnosed with moderate-to-severe OSA received CPAP therapy. The CPAP was titrated manually during a second overnight sleep study to determine the appropriate pressure level for each patient. Thereafter, a CPAP therapy without supplemental oxygen was initiated for the patients. The patients were instructed to use the device while sleeping at home.
Body mass index (BMI), systolic and diastolic blood pressure (BP), heart rate (HR), and subjective sleepiness assessed by the Epworth Sleepiness Scale (ESS) (18) were assessed at the baseline and 1 month later. Left ventricular systolic function was expressed as LVEF, and the plasma norepinephrine level and NYHA functional classes were evaluated at the baseline and 1 month later. The LVEF was measured by echocardiography using the modified Simpson's rule, and blood samples were obtained early in the morning after the sleep study.
All variables are shown as mean ± SD. The comparison between parameters in diagnostic PSG and CPAP was performed using a two-tailed paired t-test. Changes in cardiac function were assessed using a two-tailed paired t-test. Univariate regression analyses were performed to determine which factors were associated with the degree of LVEF improvement, in which changes in LVEF were included as a dependent variable. The variables included in the univariate analyses were included in a stepwise multivariate regression analysis. Statistical significance was set at p < 0.05. Statistical analyses were performed using the statistical package for social sciences (SPSS) (SPSS Inc., Chicago, IL, USA).
We analyzed 55 consecutive patients with HF and reduced LVEF. The baseline patient characteristics are shown in Table 1. The proportion of men was 94.5%. The mean age was 60.7 ± 12.2 years, and the mean BMI was 27.4 ± 5.4 kg/m (2). The mean LVEF was 37.2 ± 9.8%. The data of the sleep study on diagnosis and CPAP are shown in Table 2. The data on CPAP demonstrated considerable improvements in sleep apnea and sleep quality, which were expressed as sleep stages, percentage of slow-wave sleep, and rapid eye movement (REM) sleep. The CPAP therapy decreased AHI, as well as the arousal index from 45.3 ± 16.1 to 5.4 ± 4.1 per hour and 43.9 ± 19.6 to 15.7 ± 10.3 per hour, respectively. The LVEF improved from 37.2 ± 9.8 to 43.2 ± 11.7% after 1 month of CPAP treatment (Figure 1). Significant decreases in systolic and diastolic BP and HR (Figures 2A–C) after treatment were noted. The serum norepinephrine concentration decreased, and the ESS improved significantly after the treatment, while no significant changes were observed in BMI (Figures 3A–C).
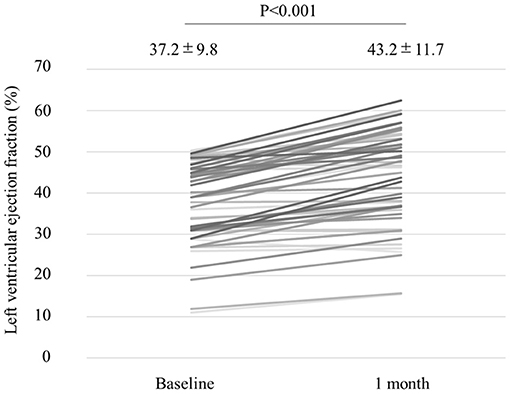
Figure 1. Left ventricular ejection fraction (LVEF) improved from 37.2 to 43.2%, with a statistical significance after 1 month of CPAP treatment.
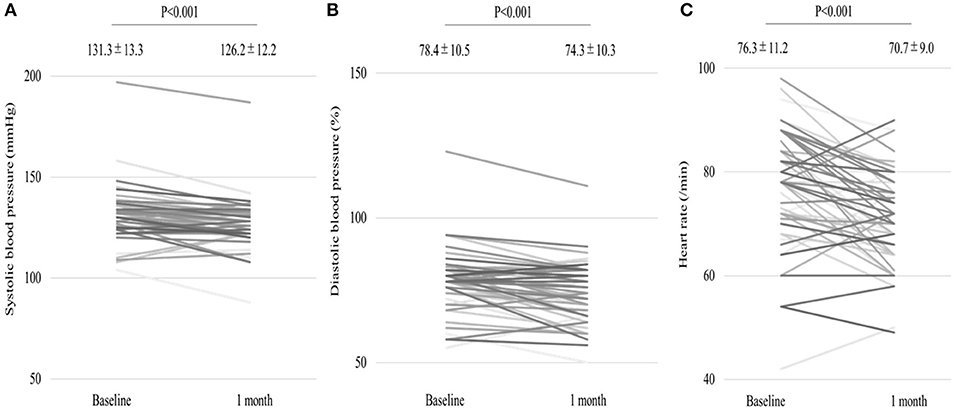
Figure 2. (A,B) Significant decrease in systolic and diastolic blood pressures (BP) as well as heart rate (C) after 1 month of continuous positive airway pressure (CPAP) treatment.
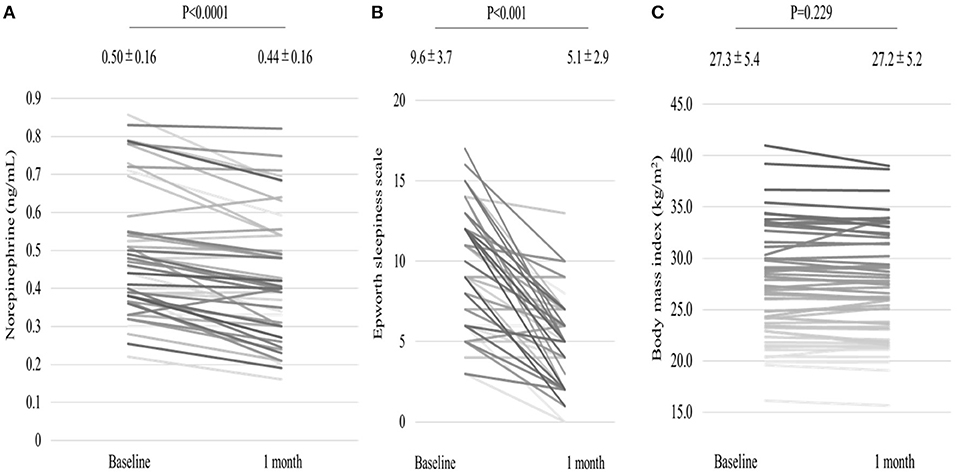
Figure 3. Serum norepinephrine concentration levels (A) decreased, and Epworth Sleepiness Scale (B) improved after 1 month of CPAP treatment, while no significant change in BMI (C) was observed.
Univariate regression analysis showed that age, BMI, atrial fibrillation, lowest SpO2, and pressure levels of CPAP were significantly associated with improvements in LVEF (Table 3), whereas the changes in variables after CPAP therapy, including BMI, BP, HR, norepinephrine concentration, and ESS, were not associated (Table 4). After adjusting for confounding variables, the age and BMI remained significant determinants of LVEF improvement (Table 5).
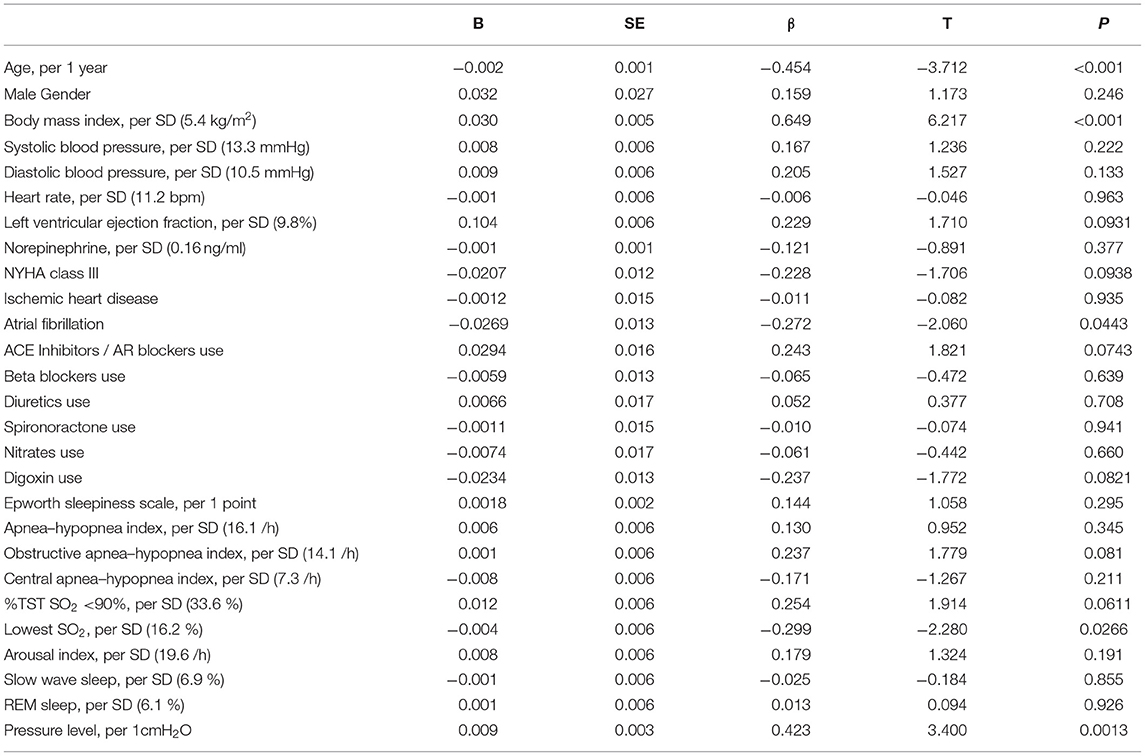
Table 3. Univariate regression analysis for baseline variables associated with the improvement of LVEF.
In this study, 1-month CPAP treatment for OSA significantly increased LVEF improvements in Japanese patients with HF complicated by OSA. The degree of improvement in the LVEF was 6% in this population. Multivariate regression analyses showed that age and BMI were determinants of LVEF improvement.
Previous studies have shown that the degree of LVEF improvement by CPAP therapy varies from 5 to 9% (12, 13), which is consistent with our results. Kaneko et al. (12) examined the effects of CPAP on cardiac function in patients with reduced LVEF and OSA. They reported that CPAP therapy improved OSA, reduced daytime systolic BP and HR, and increased LVEF by 9%.
To date, several underlying mechanisms of how CPAP improves LVEF have been described. First, the obstructive apnea due to upper airway obstruction activates the sympathetic nervous system in patients with HF via hypoxia, hypercapnia, decreased cardiac output, and arousal from sleep (19, 20). Additionally, the acute arousal from sleep evokes sympathetic activation and BP elevation, which are carried over into the daytime (21–23). The treatment of OSA with CPAP therapy lowers sympathetic nervous overactivation, resulting in decreased BP and HR (23). The mechanisms of these effects remain uncertain but may be related to the adaptation of chemoreceptor reflexes or central processes governing autonomic outflow. Second, the respiratory efforts during obstructive apnea decrease the intrathoracic pressure, leading to increased left ventricular transmural pressure, left ventricular afterload, and cardiac metabolic demand (24, 25). Furthermore, augmentation of venous return to the right ventricle results in a leftward septal shift and reduced left ventricular preload and stroke volume (26). The CPAP therapy for exaggerated intrathoracic negative pressure may decrease left ventricular transmural pressure and left ventricular afterload, potentially counteracting or minimizing the effects of respiratory efforts during obstructive apnea on left ventricular systolic function (27–29). Meanwhile, the CPAP therapy increases intrathoracic pressure, which decreases systemic venous return, while positive pressure ventilation increases pulmonary vascular resistance and right ventricular afterload, thus, reducing the right ventricular stroke volume (30, 31), left ventricular preload, and stroke volume (32–35). These ambivalent effects of CPAP on hemodynamics may depend on an individual's specific preload and afterload states (30).
To date, the factors associated with LVEF improvement remain unknown. The novelty of our study is that we demonstrated age and BMI as determinants of LVEF improvement by CPAP. The LVEF was more likely to improve in young patients with obesity after 1 month of CPAP therapy.
Several speculations can be made regarding the mechanisms of these results. In patients with obesity, the pharyngeal fat deposition facilitates upper airway narrowing and collapses by increasing the external peripharyngeal soft tissue pressure. In addition, fat deposition to the chest wall leads to a reduction in compliance and an increase in airway resistance (36–38). An improvement in exaggerated negative intrathoracic pressure by CPAP is speculated to have a positive effect on hemodynamics more in patients with obesity, which may partly explain our finding that LVEF was more likely to improve in patients with obesity. Age is a determinant of the prognosis of HF, as shown in previous reports (3, 39–41). Advancing age is accompanied by the loss of cardiomyocytes via apoptosis and necrosis (42–44) and decreased responses to both sympathetic and parasympathetic antagonists (45). Based on these data, the elderly patients with HF might be less likely to show LVEF improvement with CPAP therapy for OSA.
This was a single-center, non-randomized study with a relatively small study population. Our data should be interpreted carefully, and further studies with larger populations are required to confirm our data. Another limitation is that one of the inclusion criteria was good adherence to CPAP therapy. Therefore, our results may not be generalizable. Regarding the lowest SpO2 on CPAP therapy of 87.4%, the residual hypoxia may affect the changes in LVEF, although detailed data of the sleep study on CPAP were unavailable. The LVEF measurement might be inaccurate in patients with atrial fibrillation, which may have affected the results of changes in LVEF, although LVEF was measured with a conventional averaging of three beats. In addition, we could not demonstrate an association between obesity and intrathoracic negative pressure because the intrathoracic pressure in the study population was not measured.
Among patients with OSA and HF, the LVEF significantly improved after 1 month of CPAP therapy. The degree of LVEF improvement was significantly associated with age, BMI, atrial fibrillation, the severity of hypoxia, and the treatment pressure level of CPAP. After adjustment for baseline variables and changes in variables at 1 month, the LVEF was more likely to be improved in young patients with obesity.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
TK contributed to conception and design of the study. RN wrote the first draft of the manuscript. TD, HT, KN, and S-iM reviewed the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Grant to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group, from the Ministry of Health, Labor, and Welfare, Japan Grant/Award No: 20FC1027; JSPS KAKENHI, Grant/Award No: JP17K09527, 21K08116.
RN and TK are affiliated with a department endowed by Philips Respironics, ResMed, and Fukuda Denshi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. (2002) 347:1397–402. doi: 10.1056/NEJMoa020265
2. Packer M, Bristow MR, Cohn JN, Colucci WS, Fowler MB, Gilbert EM, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US carvedilol heart failure study group. N Engl J Med. (1996) 334:1349–55. doi: 10.1056/NEJM199605233342101
3. Jong P, Vowinckel E, Liu PP, Gong Y, Tu JV. Prognosis and determinants of survival in patients newly hospitalized for heart failure: a population-based study. Arch Intern Med. (2002) 162:1689–94. doi: 10.1001/archinte.162.15.1689
4. Chan J, Sanderson J, Chan W, Lai C, Choy D, Ho A, et al. Prevalence of sleep-disordered breathing in diastolic heart failure. Chest. (1997) 111:1488–93. doi: 10.1378/chest.111.6.1488
5. Peker Y, Kraiczi H, Hedner J, Löth S, Johansson A, Bende M. An independent association between obstructive sleep apnoea and coronary artery disease. Eur Respir J. (1999) 14:179–84. doi: 10.1034/j.1399-3003.1999.14a30.x
6. Mooe T, Franklin KA, Holmström K, Rabben T, Wiklund U. Sleep-disordered breathing and coronary artery disease: long-term prognosis. Am J Respir Crit Care Med. (2001) 164:1910–3. doi: 10.1164/ajrccm.164.10.2101072
7. Gottlieb DJ, Yenokyan G, Newman AB, O'Connor GT, Punjabi NM, Quan SF, et al. Prospective study of obstructive sleep apnea and incident coronary heart disease and heart failure: the sleep heart health study. Circulation. (2010) 122:352–60. doi: 10.1161/CIRCULATIONAHA.109.901801
8. Ferrier K, Campbell A, Yee B, Richards M, O'Meeghan T, Weatherall M, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. (2005) 128:2116–22. doi: 10.1378/chest.128.4.2116
9. Javaheri S. Sleep disorders in systolic heart failure: a prospective study of 100 male patients. The final report. Int J Cardiol. (2006) 106:21–8. doi: 10.1016/j.ijcard.2004.12.068
10. Wang H, Parker JD, Newton GE, Floras JS, Mak S, Chiu KL, et al. Influence of obstructive sleep apnea on mortality in patients with heart failure. J Am Coll Cardiol. (2007) 49:1625–31. doi: 10.1016/j.jacc.2006.12.046
11. Kasai T, Narui K, Dohi T, Yanagisawa N, Ishiwata S, Ohno M, et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest. (2008) 133:690–6. doi: 10.1378/chest.07-1901
12. Kaneko Y, Floras JS, Usui K, Plante J, Tkacova R, Kubo T, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. (2003) 348:1233–41. doi: 10.1056/NEJMoa022479
13. Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. Am J Respir Crit Care Med. (2004) 169:361–6. doi: 10.1164/rccm.200306-752OC
14. Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, et al. JCS/JHFS 2021 guideline focused update on diagnosis and treatment of acute and chronic heart failure. J Card Fail. (2021) 27:1404–44 doi: 10.1016/j.cardfail.2021.04.023
15. Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, et al. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. (1993) 147:887–95. doi: 10.1164/ajrccm/147.4.887
16. Redline S, Budhiraja R, Kapur V, Marcus CL, Mateika JH, Mehra R, et al. The scoring of respiratory events in sleep: reliability and validity. J Clin Sleep Med. (2007) 3:169–200. doi: 10.5664/jcsm.26818
17. Iber C, Ancoli-Israel S, Chesson A, Qua S. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine. (2007).
18. Johns MW. Sleepiness in different situations is measured by the Epworth Sleepiness Scale. Sleep. (1994) 17:703–10. doi: 10.1093/sleep/17.8.703
19. Bradley TD, Floras JS. Sleep apnea and heart failure: part I: obstructive sleep apnea. Circulation. (2003) 107:1671–8. doi: 10.1161/01.CIR.0000061757.12581.15
20. Bradley TD, Tkacova R, Hall MJ, Ando S, Floras JS. Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clin Sci (Lond). (2003) 104:231–8. doi: 10.1042/CS20020157
21. Arabi Y, Morgan BJ, Goodman B, Puleo DS, Xie A, Skatrud JB. Daytime blood pressure elevation after nocturnal hypoxia. J Appl Physiol. (1999) 87:689–98. doi: 10.1152/jappl.1999.87.2.689
22. Xie A, Skatrud JB, Puleo DS, Morgan BJ. Exposure to hypoxia produces long-lasting sympathetic activation in humans. J Appl Physiol. (2001) 91:1555–62. doi: 10.1152/jappl.2001.91.4.1555
23. Usui K, Bradley TD, Spaak J, Ryan CM, Kubo T, Kaneko Y, et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. J Am Coll Cardiol. (2005) 45:2008–11. doi: 10.1016/j.jacc.2004.12.080
24. Parker JD, Brooks D, Kozar LF, Render-Teixeira CL, Horner RL, Douglas Bradley T, et al. Acute and chronic effects of airway obstruction on canine left ventricular performance. Am J Respir Crit Care Med. (1999) 160:1888–96. doi: 10.1164/ajrccm.160.6.9807074
25. Hall MJ, Ando S, Floras JS, Bradley TD. Magnitude and time course of hemodynamic responses to Mueller maneuvers in patients with congestive heart failure. J Appl Physiol. (1985) 85:1476–84. doi: 10.1152/jappl.1998.85.4.1476
26. Shiomi T, Guilleminault C, Stoohs R, Schnittger I. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest. (1991) 100:894–902. doi: 10.1378/chest.100.4.894
27. Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation. (1998) 98:2269–75. doi: 10.1161/01.CIR.98.21.2269
28. Pinsky MR, Matuschak GM, Klain M. Determinants of cardiac augmentation by elevations in intrathoracic pressure. J Appl Physiol. (1985) 58:1189–98. doi: 10.1152/jappl.1985.58.4.1189
29. Deng F, Raza A, Guo J. Treating obstructive sleep apnea with continuous positive airway pressure reduces risk of recurrent atrial fibrillation after catheter ablation: a meta-analysis. Sleep Med. (2018) 46:5–11. doi: 10.1016/j.sleep.2018.02.013
30. Bradley TD, Holloway RM, McLaughlin PR, Ross BL, Walters J, Liu PP. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis. (1992) 145:377–82. doi: 10.1164/ajrccm/145.2_Pt_1.377
31. Jardin F, Farcot JC, Guéret P, Prost JF, Ozier Y, Bourdarias JP. Echocardiographic evaluation of ventricles during continuous positive airway pressure breathing. J Appl Physiol. (1984) 56:619–27. doi: 10.1152/jappl.1984.56.3.619
32. Johnston WE, Vinten-Johansen J, Santamore WP, Case LD, Little WC. Mechanism of reduced cardiac output during positive end-expiratory pressure in the dog. Am Rev Respir Dis. (1989) 140:1257–64. doi: 10.1164/ajrccm/140.5.1257
33. Montner PK, Greene ER, Murata GH, Stark DM, Timms M, Chick TW. Hemodynamic effects of nasal and face mask continuous positive airway pressure. Am J Respir Crit Care Med. (1994) 149:1614–8. doi: 10.1164/ajrccm.149.6.8004320
34. Jardin F, Farcot JC, Boisante L, Curien N, Margairaz A, Bourdarias JP. Influence of positive end-expiratory pressure on left ventricular performance. N Engl J Med. (1981) 304:387–92. doi: 10.1056/NEJM198102123040703
35. Jardin F, Delorme G, Hardy A, Auvert B, Beauchet A, Bourdarias JP. Reevaluation of hemodynamic consequences of positive pressure ventilation: emphasis on cyclic right ventricular afterloading by mechanical lung inflation. Anesthesiology. (1990) 72:966–70. doi: 10.1097/00000542-199006000-00003
36. Sharp JT, Henry JP, Sweany SK, Meadows WR, Pietras RJ. The total work of breathing in normal and obese men. J Clin Invest. (1964) 43:728–39. doi: 10.1172/JCI104957
37. Yap JC, Watson RA, Gilbey S, Pride NB. Effects of posture on respiratory mechanics in obesity. J Appl Physiol. (1995) 79:1199–205. doi: 10.1152/jappl.1995.79.4.1199
38. Ladosky W, Botelho MA, Albuquerque JP. Jr. Chest mechanics in morbidly obese non-hypoventilated patients. Respir Med. (2001) 95:281–6. doi: 10.1053/rmed.2001.1035
39. Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation. (1993) 88:107–15. doi: 10.1161/01.CIR.88.1.107
40. Pulignano G, Del Sindaco D, Tavazzi L, Lucci D, Gorini M, Leggio F, et al. Clinical features and outcomes of elderly outpatients with heart failure followed up in hospital cardiology units: data from a large nationwide cardiology database (IN-CHF Registry). Am Heart J. (2002) 143:45–55. doi: 10.1067/mhj.2002.119608
41. White HD, Aylward PE, Huang Z, Dalby AJ, Weaver WD, Barvik S, et al. Mortality and morbidity remain high despite captopril and/or valsartan therapy in elderly patients with left ventricular systolic dysfunction, heart failure, or both after acute myocardial infarction: results from the valsartan in acute myocardial infarction Trial (VALIANT). Circulation. (2005) 112:3391–9. doi: 10.1161/CIRCULATIONAHA.105.551143
42. Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, et al. Evidence for cardiomyocyte renewal in humans. Science. (2009) 324:98–102. doi: 10.1126/science.1164680
43. Zhang XP, Vatner SF, Shen YT, Rossi F, Tian Y, Peppas A, et al. Increased apoptosis and myocyte enlargement with decreased cardiac mass; distinctive features of the aging male, but not female, monkey heart. J Mol Cell Cardiol. (2007) 43:487–91. doi: 10.1016/j.yjmcc.2007.07.048
44. Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, et al. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol. (1996) 271:H1215–28. doi: 10.1152/ajpheart.1996.271.3.H1215
Keywords: CPAP, OSA (obstructive sleep apnea), short-term, HFrEF (heart failure with reduced ejection fraction), LVEF (left ventricular ejection fraction)
Citation: Naito R, Kasai T, Dohi T, Takaya H, Narui K and Momomura S-i (2022) Factors Associated With the Improvement of Left Ventricular Systolic Function by Continuous Positive Airway Pressure Therapy in Patients With Heart Failure With Reduced Ejection Fraction and Obstructive Sleep Apnea. Front. Neurol. 13:781054. doi: 10.3389/fneur.2022.781054
Received: 22 September 2021; Accepted: 14 February 2022;
Published: 10 March 2022.
Edited by:
Kittisak Sawanyawisuth, Khon Kaen University, ThailandReviewed by:
Tsai-Yu Wang, Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Naito, Kasai, Dohi, Takaya, Narui and Momomura. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takatoshi Kasai, a2FzYWktdEBteDYubmlzaXEubmV0
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.