
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 13 April 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.740656
Objective: To investigate the correlation between prognosis and intracranial carotid artery calcification (ICAC) in patients with acute ischemic stroke (AIS) who receive intravenous thrombolysis (IVT).
Methods: A total of 156 AIS patients who received IVT from March 2019 to March 2020 were enrolled. The modified Woodcock visual score was used to evaluate ICAC in nonenhanced head CT scans. Patients were divided into high calcification burden (HCB; score ≥3) and low calcification burden (LCB; score <3) groups. Demographic, laboratory, imaging and clinical data were compared between the two groups, and whether HCB was a prognostic factor was evaluated.
Results: Compared with the LCB group, the HCB group had a higher incidence of atrial fibrillation (49.2 vs.22.1%, P < 0.001) and coronary heart disease (24.6 vs. 10.0%, P = 0.019) and higher serum homocysteine [15.31 (12.15, 17.50) vs. 14.40 (11.20, 16.20), P = 0.036] and hemoglobin A1c (6.93 ± 1.77 vs. 6.37 ± 0.74, P = 0.023) levels. Binary logistic regression analysis showed that atrial fibrillation (OR = 3.031, 95% CI: 1.312–7.006, P = 0.009) and HbA1c (OR = 1.488, 95% CI: 1.050–2.109, P = 0.026) were independent risk factors for ICAC. After adjusting for other risk factors, symptomatic-side and bilateral ICACs were independent risk factors for poor prognosis (OR = 1.969, 95% CI: 1.220–3.178, P = 0.006), (OR = 1.354, 95% CI: 1.065–1.722, P = 0.013) and mortality (OR = 4.245, 95% CI: 1.114–16.171, P = 0.034), (OR = 2.414, 95% CI = 1.152–5.060, P = 0.020) in patients with AIS who received IVT.
Conclusion: ICAC is closely related to the prognosis of acute ischemic stroke after intravenous thrombolysis.
Thrombolysis is known to improve outcomes following acute ischemic stroke. Intravenous thrombolysis using recombinant tissue-type plasminogen activator (rt-PA) is still considered the first line of treatment (1). However, the effect of thrombolysis is influenced by many factors.
Intracranial carotid artery calcification (ICAC) was first observed with radiographic pathology in the early 1960s (2). Later studies found that 69.4% of Chinese patients (3) and 82.2% of Dutch patients who were >55 years had ICAC on conventional head computed tomography (CT) (4). Vascular calcification is part of the atherosclerotic process and may indicates severe stenosis (5). Atherosclerotic calcification occurs in the form of hydroxyapatite deposits that resemble bone mineralization (6). The more advanced stages consist of calcified plaques. Confirmation of the coronary calcium score as a predictor of future cardiac events has spurred researchers' interest in ICAC. Recent clinical studies have demonstrated the risk factors for intracranial arterial calcification and its clinical significance (7). Studies have shown that intracranial vascular calcification is related to a higher risk of stroke independent of cardiovascular risk factors (8, 9). However, in contrast to the coronary vasculature, few studies have investigated the relationship between ICACs and the prognosis of patients with acute ischemic stroke (AIS) who receive intravenous thrombolysis (IVT) (10–13). And the conclusions of these studies are controversial. The question of whether atherosclerotic calcification might have distinct prognostic effects on stroke patients and therefore warrant specific treatment strategies still needs to be investigated.
In this context, we examined the relationship of the burden of ICAC with poor outcome, complications and mortality in patients treated with IVT for AIS. Studying the effect of ICAC on the prognosis of IVT in AIS may not only help to understand the relationship between ICAC and stroke, but also modify the acute management strategy, because ICAC information is usually available before treatment decision-making (13).
Medical records of the patients registered in China Cerebrovascular Disease Registration Platform with discharged diagnosis of “AIS” between March 2019 to March 2020 in the stroke center of our hospital were reviewed.
Inclusion criteria included the following: (1) 18 years of age or older; (2) diagnosis of AIS based on the diagnostic criteria of acute cerebral infarction formulated by the Compilation Committee of Chinese Cerebrovascular Disease Prevention Guidelines (14); (3) hospital admission within 4.5 h of ictus; and (4) receipt of standard thrombolytic treatment.
In order to increase the safety, feasibility and rationality of the study and increase the homogeneity of the study object to a certain extent, the following cases are excluded: (1) posterior circulation infarction; (2) pre-stroke Modified Rankin Scale (mRS) score > 2; (3) standard contraindications for intravenous rt-PA therapy; (4) Patients receiving endovascular treatment; and (5) patients with malignant tumors, severe liver and kidney dysfunction or drug/alcohol abuse.
The study was approved by the ethics committee of our hospital.
All AIS patients were treated with rt-PA at a dose of 0.9 mg/kg according to internationally recognized guidelines. Routine monitoring of neurological function scores and blood pressure was conducted. Cranial CT was repeated 24 h after thrombolysis to determine whether there was intracranial hemorrhage. If there was no intracranial hemorrhage, antithrombotic therapy and statin therapy were administered.
The following information was collected from all patients: sex, age, and medical history, including hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, coronary heart disease, and history of smoking or alcohol intake. Definition of hypertension: systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥90 mmHg (measured at least three times at different times and in the same way at rest), or normal blood pressure when taking antihypertensive drugs; Definition of diabetes: dry mouth, polyuria, emaciation and other diabetes symptoms and random blood glucose ≥11.1 mmol/L, or fasting blood glucose ≥7.0 mmol/L, or oral glucose tolerance test (OGTT) 2 h blood glucose ≥11.1 mmol/L, (at least two times); Definition of dyslipidemia (serum triglycerides >1.7 mmol/L, low-density lipoprotein >3.4 mmol/L, total cholesterol >5.2 mmol/L or by the use of lipid-lowering agents); Atrial fibrillation and coronary heart disease were determined according to previous medical history and ECG, or was being treated for it. Smoking history: smokers who smoke more than 1 cigarette a day for more than 1 year; those who quit smoking for more than 15 years are equivalent to non-smoking; Definition of drinking: The conversion method of alcohol consumption and the standard of excessive drinking refer to the research of Kehui Liu et al. (15). Patient baseline characteristics were recorded: systolic and diastolic blood pressure, TOAST classification, onset to needle time (ONT) (min), door to needle time (DNT) (min), NIHSS while admitted, mRS score while admitted, and NIHSS on the seventh day. “Improvement” was defined as a decrease in the NIHSS score by ≥ 4 points at the 7th day after treatment. “Deterioration” was described as an increase of at least four points in NIHSS at the end of the 7th day. Complications of symptomatic intracranial hemorrhage (sICH) were registered. We investigated laboratory data on admission to our hospital, including data on glucose, total cholesterol, low-density lipoprotein, hemoglobin A1c (HbA1c), creatinine, C-reactive protein, homocysteine, and fibrinogen levels.
All included patients underwent a non-contrast cranial CT axial scan (Discovery CT750HD CT Scanner; GE Healthcare, USA) at admission, all images were reconstructed at 0.625 mm axial sections. The ICAC scores were evaluated by a radiology specialist and a neurologist who were blinded to the clinical information, using the modified version of the Woodcock visual scoring (16). They had received standardized training before CT images evaluation. Bone window CT scans (modified window level: approximately 350 HU; width: 1,000 HU) through the skull base were used to calculate total carotid siphon calcification (TCSC) and symptomatic side calcification in the carotid siphon. The interrater agreement was excellent, the intraclass correlation coefficient from the two observers was 0.863 (P < 0.001). If the result was inconsistent, it should be decided by both parties through consultation. The calcification score of siphon segment represents the severity of internal carotid artery calcification. Absence or near absence of calcifications was recorded as one point; thin and discontinuous calcification was scored as two points; thin and continuous, or thick and discontinuous calcification was scored as three points; and thick and continuous calcification was recorded as four points (Figure 1). The calcification score was dichotomized into high calcification burden (HCB; score ≥3) and low calcification burden (LCB; score <3) groups (17, 18).

Figure 1. Intracranial internal carotid artery calcification score: (A), Absence or near absence of calcifications, score = 1; (B), thin and discontinuous calcification, score = 2; (C), thick and discontinuous calcification, score = 3; (D), thick and continuous calcification, score = 4.
Patient neurological function was evaluated at 3 months after discharge using the mRS score by trained neurologists blinded to other clinical data. The patients who were unable to show up for the follow-up visits were subjected to the mRS score questionnaire via multiple phone calls.
The mRS score was dichotomized into favorable (mRS score 0–2 [good recovery, no significant disability, slight disability]) or unfavorable [mRS score 3–6 (moderate disability, moderately severe disability, severe disability, or death)].
SPSS for Windows, version 22.0 (IBM Corporation, New York, USA) was used for statistical analysis. Continuous variables were presented as the mean ± SD or median (interquartile range), and categorical variables were presented as percentages. The chi-square test was used for categorical data. The normally distributed variables were compared with independent t-tests, and the Mann-Whitney U-test was used for continuous non-normally distributed variables. Logistic regression was used for the multivariate analysis of baseline characteristics as risk factors for ICAC. The factors with statistical significance (P < 0.1) in univariate analysis were taken as independent variables and entered into logistic regression equation for statistical analysis. An enter method was used to design the final model. To further evaluate the interactive effect of atrial fibrillation and HbA1c, a new term (atrial fibrillation × HbA1c) was calculated and included in the relevant regression models as an additional variable. Multivariate logistic regression analyses were used to analyze whether ICAC was an independent risk factor for intracranial hemorrhage, poor prognosis at 3 months, and mortality after thrombolysis adjusted for potential influencing factors selected based on univariate analyses, P < 0.05 was taken as the cut-off for inclusion. P < 0.05 and 95% CIs of ORs (logistic regression) were considered statistically significant.
A total of 156 patients were included in the study. A total of 61 patients (39.1%) had a high calcification burden (HCB). A comparison of the baseline characteristics of HCB patients and LCB patients was summarized in Table 1. Compared with patients in the LCB group, patients in the HCB group had a higher incidence of atrial fibrillation (49.2 vs. 22.1%, P < 0.001) and coronary heart disease (24.6 vs. 10.0%, P = 0.019), and higher HbA1c (6.93 ± 1.77 vs. 6.37 ± 0.74, P = 0.023) and higher serum homocysteine levels (15.31 [12.15, 17.50] vs. 14.40 [11.20, 16.20], P = 0.036). Stroke etiology, admission clinical parameters and clinical outcomes of HCB patients and LCB groups were compared. The NIHSS score while admitted to the HCB group was significantly higher than that of the LCB group [8.00 (3.00, 16.50) vs. 5.00 (2.00, 10.00), P = 0.004]. The NIHSS score on the 7th day was significantly higher in the HCB group than the LCB group [5.00 (0.50, 12.50) vs. 1.00 (0, 4.00), P < 0.001], and the proportion of deterioration (deterioration in NIHSS ≥4) in the HCB group was higher than that in the LCB group (11.5 vs. 2.1%, P = 0.029). The mRS score of the HCB group was higher than that of the LCB group at 3 months [3.00 (0, 4.50) vs. 1.00 (0, 3.00), P < 0.001] (Table 2). The potential risk factors for ICAC were listed in Figure 2. After correction for other risk factors, atrial fibrillation (OR = 3.031, 95% CI: 1.312–7.006, P = 0.009) and HbA1c (OR = 1.488, 95% CI: 1.050–2.109, P = 0.026) were independent risk factors for ICAC. The interaction term model showed that the interaction between atrial fibrillation and HbA1c was not significant (OR = 1.459, 95% CI: 0.503–4.440, P = 0.469). Clinical characteristics associated with poor outcome, sICH, mortality were shown in Table 3. Univariate analysis showed that symptomatic side and total modified Woodcock score were associated with poor outcome [1.00 (0, 2.00) vs. 2.00 (1.00, 3.00), P < 0.001], [2.00 (0, 4.00) vs. 4.00 (2.00, 6.00), P < 0.001], sICH [1.00 (0, 2.00) vs. 2.00 (1.00, 3.00), P = 0.007], [2.00 (0, 4.00) vs. 4.00 (2.00, 6.00), P = 0.009] and mortality [1.00 (1.00, 2.00) vs. 3.00 (2.50, 3.00), P < 0.001], [2.00 (1.00, 4.00) vs. 6.00 (4.00, 6.00), P < 0.001] (Figure 3). In the multivariate logistic regression analysis, symptomatic side and total modified Woodcock score were independently associated with poor outcome (OR = 1.969, 95% CI: 1.220–3.178, P = 0.006 and OR = 1.354, 95% CI: 1.065-1.722, P = 0.013) and death (OR = 4.245, 95% CI: 1.114–16.171, P = 0.034 and OR = 2.414, 95% CI: 1.152–5.060, P = 0.020), but not with sICH (OR = 1.200, 95% CI: 0.700–2.057, P = 0.508 and OR = 1.093, 95% CI: 0.833–1.435, P = 0.521), as shown in Table 4.
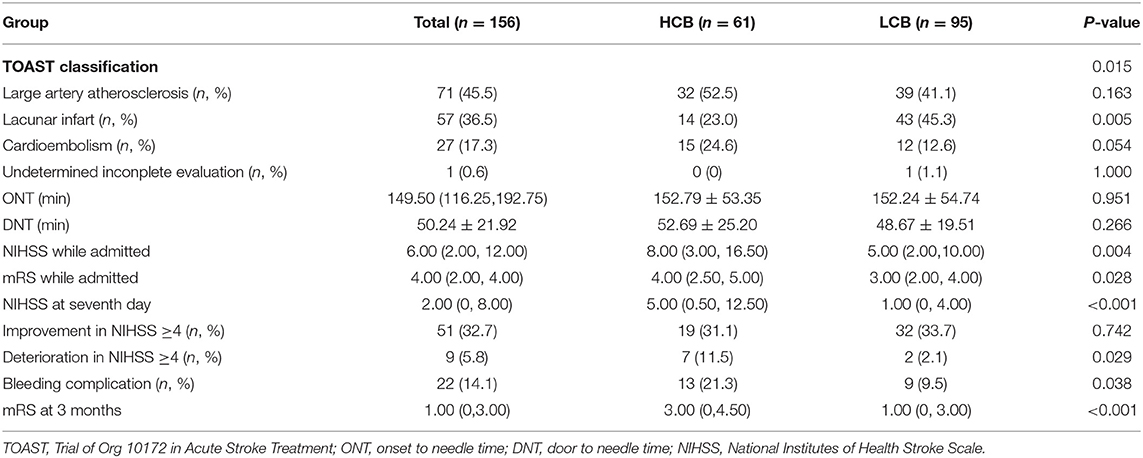
Table 2. Comparation of stroke etiology, admission clinical parameters and clinical outcomes of HCB and LCB groups.
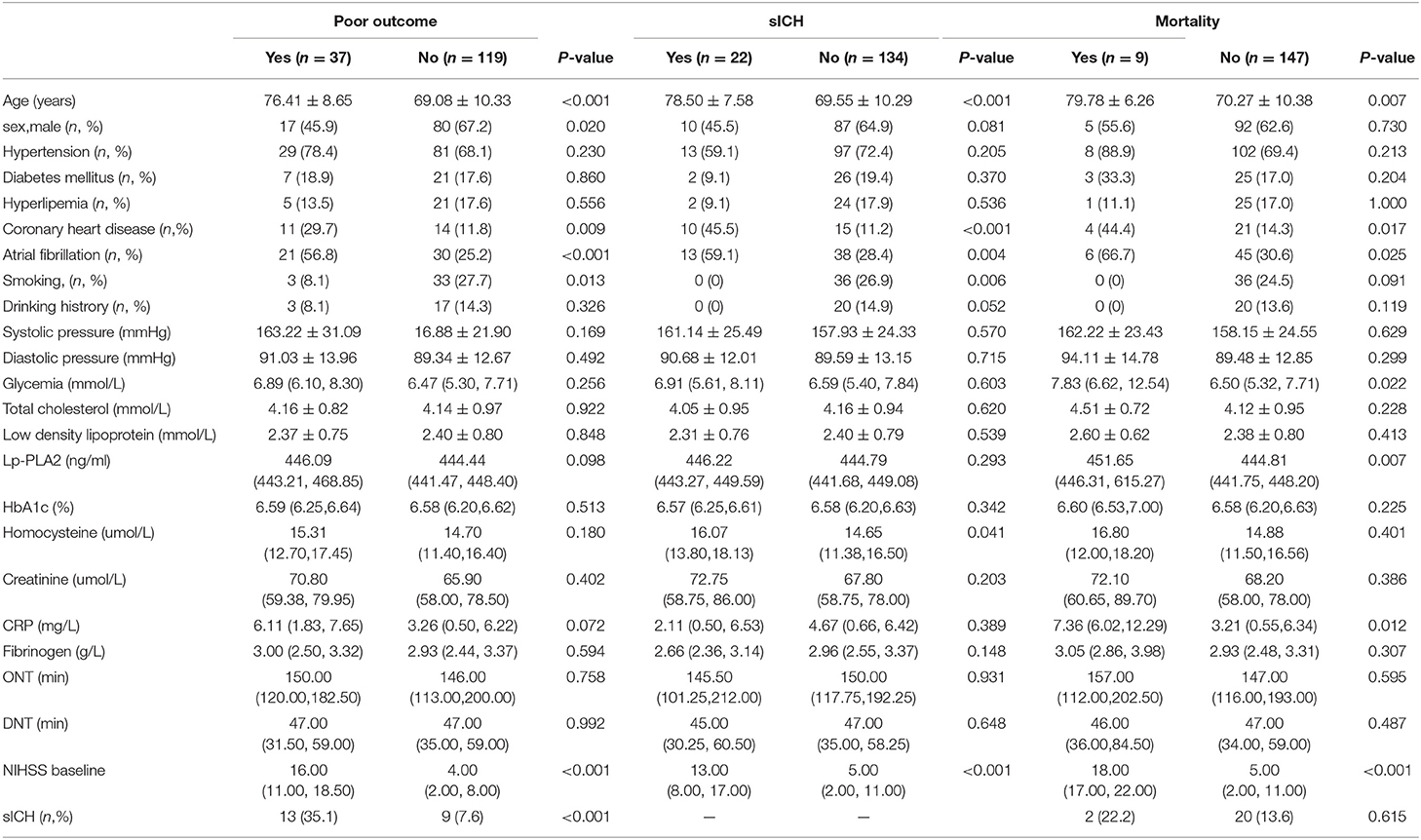
Table 3. Univariate analysis: clinical characteristics associated with poor outcome, sICH, mortality.
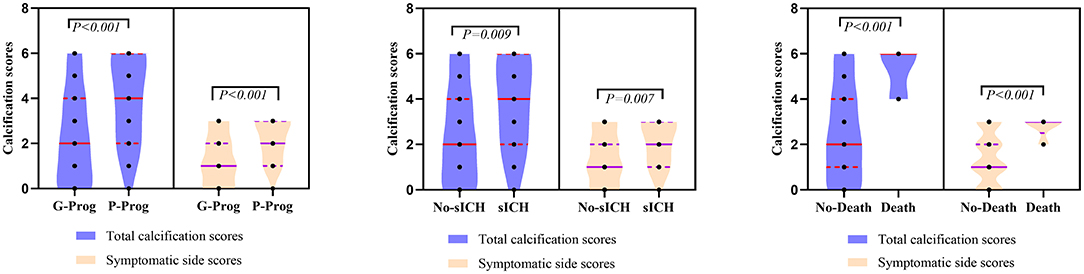
Figure 3. Comparation of symptomatic side and total modified Woodcock score of poor outcome, sICH and death.
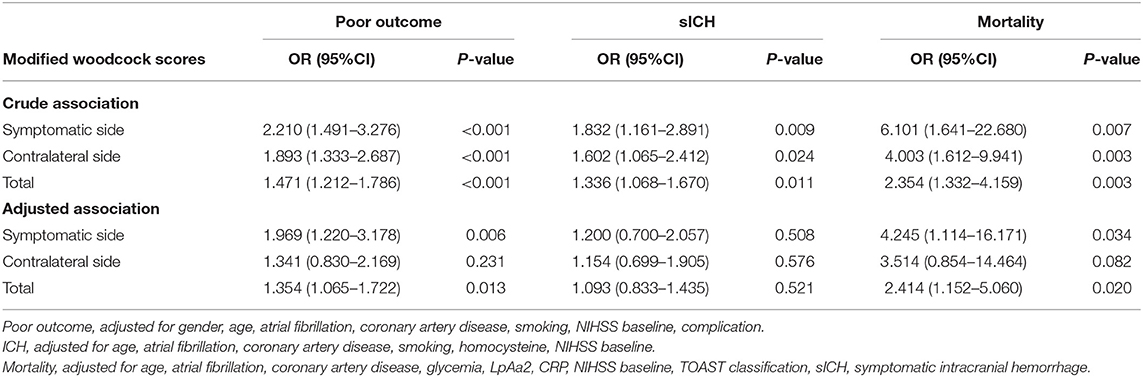
Table 4. Univariate and multivariate regression models for the relationship between ICAC and poor outcome, sICH, and mortality.
The retrospective patient cohort study including 156 acute ischemic stroke patients who received intravenous thrombolysis analyses risk factors for ICAC and the prognostic value of high ICAC for functional decline, intracranial hemorrhage and all-cause mortality during 3 months follow-up. The main findings of the study were that atrial fibrillation and HbA1c were independent risk factors for ICAC and that total ICAC score and ICAC score of the symptomatic side predicted the outcomes of functional decline, intracranial hemorrhage and all-cause mortality.
Although our study found that ICAC was closely related to atrial fibrillation, whether there was a causal relationship between ICAC and atrial fibrillation was uncertain. Atrial fibrillation may be a downstream reaction of ICAC. It is generally believed that most patients with ICAC have coronary artery calcification, which is a risk factor for atrial fibrillation (19–21).
Previous studies have found that diabetes mellitus or random blood glucose are independent risk factors for ICAC (11, 22, 23). Although our study did not find a correlation between immediate blood glucose and ICAC, we found that elevated glycosylated hemoglobin was an independent risk factor for ICAC. Glycosylated hemoglobin is a biomarker of average glucose levels that can be used to follow hyperglycemia over the long term (24). Levels of glycosylated hemoglobin are a biomarker for vascular health (25). The relationships between high HbA1c and ICAC might be explained by the negative effect of poor glycemic control. Artery calcification may be caused by the accumulation of advanced glycation end products (26). The important driving factors of atherosclerotic calcification in diabetes include endothelial dysfunction, oxidative stress, changes in mineral metabolism, increased production of inflammatory cytokines and release of osteoprogenitor cells from bone marrow to circulation (27).
ICAC is a manifestation of vascular aging and a risk factor for acute cerebrovascular disease. However, there are few studies on the effect of thrombolysis and the prognosis of acute ischemic stroke. Vascular calcification can be divided into two different forms in morphology, depending on the location, namely, in the intima or media (28). The effect of carotid artery calcification on vascular remodeling and intimal injury is still unclear (29). Some studies have shown that ICAC can lead to vascular stenosis, thus affecting the pass rate and success rate of intra-arterial mechanical thrombectomy (30).
The effect of ICAC on intravenous thrombolysis is still unclear. Xin et al. (10) found that after correcting other risk factors, ICAC had no effect on the efficacy and prognosis of intravenous thrombolysis. However, Wu et al.'s (31) study suggested that ICAC may form microemboli after intravenous thrombolysis to embolize distant small vessels, resulting in a poor thrombolytic effect or aggravation of symptoms. Our study found that patients with HCB in the siphon segment of the internal carotid artery had a worse response to thrombolysis than patients with LCB. The proportion of patients with HCB whose NIHSS score increased more than 4 points after thrombolysis was higher than that of patients with LCB. This result is consistent with the studies of Lee et al. and Tábuas-Pereira et al. (11, 22).
Considering that calcification is a sign of more severe atherosclerosis, we can reasonably expect that increased arterial calcification would impair autoregulation and make blood vessels more likely to rupture. However, whether ICAC is a risk factor for intracerebral hemorrhage after thrombolysis is still controversial. Lin et al. (17) asserted that ICAC was a risk factor for intracranial hemorrhage after thrombolysis. However, studies by Lee et al. and Tábuas-Pereira et al. (11, 22) suggested that there was no correlation between ICAC and symptomatic intracranial hemorrhage. In our study, after correcting for other confounding factors, ICAC was not an independent risk factor for post-thrombolytic hemorrhage. The reasons for these differences may include the following: (1). The research methods used in each study had different calcification scoring standards; (2). Selection bias and the coexistence of other covariates were not considered in other studies; (3). The research found that patients with HCB have a higher proportion of atrial fibrillation, and cardiogenic cerebral embolism caused by atrial fibrillation was prone to postinfarction hemorrhage, and therefore, the existence of these confounding factors led to the deviation of the results; and (4). Another contributor may be the type of calcification, which was not distinguished in any of these studies.
The cycle of repeated rupture and healing of the plaque, accompanied by inflammation of the core and fibrous cap, leads to the presence of heterogeneity and calcified carotid plaque (32). In animal models, vascular calcification is related to the severity of arteriosclerosis. Vascular calcification and decreased vascular elasticity can make organs more susceptible to the impact of high blood flow and systolic pressure fluctuations, resulting in decreased vascular perfusion (33–35). ICAC can reflect systemic vascular calcification. The higher mortality rate of patients with HCB after thrombolysis may be related to the higher rate of cardiovascular calcification, including aortic calcification. High coincident calcification may be associated with ischemic event recurrence, including stroke and acute coronary syndrome recurrence. In our study, there was significant difference in the prevalence of coronary heart disease between the two groups, and the effect on recurrent coronary heart disease after stroke was uncertain. In our study, after adjusting for other confounders, ICAC was an independent risk factor for mortality after thrombolysis in stroke. ICAC was associated with all-cause mortality in our cohort.
Lee et al. (22) found that the proportion of patients with HCB whose mRS score was higher than two points at 90 days was higher, while some other studies (10–12) posited that complicated with ICAC was not related to the three-month prognosis. After correcting for confounding factors such as sex, age, atrial fibrillation, coronary artery disease, smoking, NIHSS baseline, and complications, we found that ICAC was still an independent risk factor for poor prognosis of acute ischemic stroke at 3 months after thrombolysis. In our study, we found that HCB was positively related to the basic NIHSS score, which is an independent risk factor for poor prognosis (36–39). However, after correction of the basic NIHSS score, HCB was still an independent risk factor for poor prognosis at 3 months.
An important strength is that only few studies have investigated the relationship between ICAC and the prognosis of patients with acute ischemic stroke who receive intravenous thrombolysis. Yet, our study has several limitations. First, we investigated the prognosis of patients at only 3 months after discharge; longer-term follow-up studies are needed in the future. Second, this was a single-center study, and there was inevitably selection bias. And the sample size is relatively small, especially the sample of sICH and mortality, which leads to the imbalance of sample size. This could have affected the meaningfulness of results obtained. All of these limitations will be addressed in future studies by using a larger sample size. Third, patients who underwent revascularization treatment or received conventional conservative treatment within 4.5 h of onset were not included in the study. We would consider including such patients in the next study. Fourth, undoubtly, the un-calcified plaques were also identified as risk factor to their prognosis. However, in the original design of the project, only calcified plaques were studied, and the number and size of un-calcified plaques were not counted. The interaction between those morphologic characteristics of plaques will be added in future studies. And the types of calcification were not distinguished in our study.
Our study showed that atrial fibrillation and glycosylated hemoglobin were independently associated with the prevalence of ICAC. ICAC was an independent risk factor for poor prognosis and mortality of cerebral infarction after intravenous thrombolysis. CT scans coupled with modeling algorithms will provide an objective marker for the prognosis of intravenous thrombolysis in acute cerebral infarction. Intervention of atrial fibrillation or control of high glycosylated hemoglobin may improve the prognosis of acute cerebral infarction after thrombolysis from another perspective.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Yancheng Third People's Hospital. The patients/participants provided their written informed consent to participate in this study.
YS and ZD drafted the manuscript. YS, PP, and ZC participated in the literature collection. YS and GX analyzed the MRI features. HS and JZ reviewed and revised the manuscript. All authors read and approved the final manuscript.
This work was supported by the Research Project Fund of Clinical College of Jiangsu Medical Vocational College (20209127).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhou Y, Yan S, Song X, Gong Y, Li W, Wang M, et al. Intravenous thrombolytic therapy for acute ischemic stroke in Hubei, China: a survey of thrombolysis rate and barriers. BMC Neurol. (2019) 19:202. doi: 10.1186/s12883-019-1418-z
2. Ratinov G. Extradural intracranial portion of carotid artery, a clinicopathologic. Study. Arch Neurol. (1964) 10:66–73. doi: 10.1001/archneur.1964.00460130070010
3. Chen XY, Lam WW, Ng HK, Fan YH, Wong KS. The frequency and determinants of calcification in intracranial arteries in Chinese patients who underwent computed tomography examinations. Cerebrovas Dis. (2006) 21:91–7. doi: 10.1159/000090206
4. Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, et al. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. (2012) 43:1878–84. doi: 10.1161/strokeaha.111.648667
5. Chen XY, Lam WW, Ng HK, Fan YH, Wong KS. Intracranial artery calcification: a newly identified risk factor of ischemic stroke. J Neuroimag: Off J Am Soc Neuroimagg. (2007) 17:300–3. doi: 10.1111/j.1552-6569.2007.00158.x
6. Demer LL, Tintut Y. Mineral exploration: search for the mechanism of vascular calcification and beyond: the 2003 Jeffrey M. Hoeg award lecture. Arterioscler Thromb Vasc Bio. (2003) 23:1739–739:1739–43. doi: 10.1161/01.atv.0000093547.63630.0f
7. Wu XH, Chen XY, Wang LJ, Wong KS. Intracranial Artery Calcification and Its Clinical Significance. J Clin Neurol. (2016) 12:253–61. doi: 10.3988/jcn.2016.12.3.253
8. Bugnicourt JM, Leclercq C, Chillon JM, Diouf M, Deramond H, Canaple S, et al. Presence of intracranial artery calcification is associated with mortality and vascular events in patients with ischemic stroke after hospital discharge: a cohort study. Stroke. (2011) 42:3447–447:3447–53. doi: 10.1161/strokeaha.111.618652
9. Bos D, Portegies ML, van der Lugt A, Bos MJ, Koudstaal PJ, Hofman A, et al. Intracranial carotid artery atherosclerosis and the risk of stroke in whites: the Rotterdam study. JAMA Neurol. (2014) 71:405–11. doi: 10.1001/jamaneurol.2013.6223
10. He XW, Zhao R, Li GF, Zheng B, Wu YL, Shi YH, et al. Lack of correlation between intracranial carotid artery modified woodcock calcification score and prognosis of patients with acute ischemic stroke after intravenous thrombolysis. Front Neurol. (2019) 10:696. doi: 10.3389/fneur.2019.00696
11. Tábuas-Pereira M, Sargento-Freitas J, Silva F, Parra J, Mendes P, Seara V, et al. Intracranial carotid artery modified woodcock calcifi, et al. Intracranial internal carotid artery wall calcification in ischemic strokes treated with thrombolysis. Eur Neurol. (2018) 79:21–6. doi: 10.1159/000477901
12. Haussen DC, Gaynor BG, Johnson JN, Peterson EC, Elhammady MS, Aziz-Sultan MA, et al. Carotid siphon calcification impact on revascularization and outcome in stroke intervention. Clin Neurol Neurosurg. (2014) 120:73–7. doi: 10.1016/j.clineuro.2014.02.021
13. Gocmen R, Arsava EM, Oguz KK, Topcuoglu MA. Atherosclerotic intracranial internal carotid artery calcification and intravenous thrombolytic therapy for acute ischemic stroke. Atherosclerosis. (2018) 270:89–94. doi: 10.1016/j.atherosclerosis.2018.01.035
14. Liu L, Chen W, Zhou H, Duan W, Li S, Huo X, et al. Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. (2020) 5:159–76. doi: 10.1136/svn-2020-000378
15. Liu K, Ding Y, Lu X, Wang Z. Trends and socioeconomic factors in smoking and alcohol consumption among Chinese people: evidence from the 2008–2018 national health service surveys in Jiangsu Province. Arch Public Health. (2021) 79:127. doi: 10.1186/s13690-021-00646-9
16. Subedi D, Zishan US, Chappell F, Gregoriades ML, Sudlow C, Sellar R, et al. Intracranial carotid calcification on cranial computed tomography: visual scoring methods, semiautomated scores, and volume measurements in patients with stroke. Stroke. (2015) 46:2504–504:2504–9. doi: 10.1161/strokeaha.115.009716
17. Lin TC, Chao TH, Shieh Y, Lee TH, Chang YJ, Lee JD, et al. The impact of intracranial carotid artery calcification on the development of thrombolysis-induced intracerebral hemorrhage. J stroke Cerebrovasc Dis: off J National Stroke Ass. (2013) 22:e455–462. doi: 10.1016/j.jstrokecerebrovasdis.2013.05.008
18. Erbay S, Han R, Baccei S, Krakov W, Zou KH, Bhadelia R, et al. Intracranial carotid artery calcification on head CT and its association with ischemic changes on brain MRI in patients presenting with stroke-like symptoms: retrospective analysis. Neuroradiology. (2007) 49:27–33. doi: 10.1007/s00234-006-0159-z
19. Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R, et al. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET-EHRA consensus conference 'research perspectives in AF'. Euro Heart J. (2009) 30:2969–77c. doi: 10.1093/eurheartj/ehp235
20. Schoonderwoerd BA, Van Gelder IC, Crijns HJ. Left ventricular ischemia due to coronary stenosis as an unexpected treatable cause of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. (1999) 10:224–8. doi: 10.1111/j.1540-8167.1999.tb00664.x
21. Nishida K, Qi XY, Wakili R, Comtois P, Chartier D, Harada M, et al. Mechanisms of atrial tachyarrhythmias associated with coronary artery occlusion in a chronic canine model. Circulation. (2011) 123:137–46. doi: 10.1161/circulationaha.110.972778
22. Lee SJ, Hong JM, Lee M, Huh K, Choi JW, Lee JS. Cerebral arterial calcification is an imaging prognostic marker for revascularization treatment of acute middle cerebral arterial occlusion. J stroke. (2015) 17:67–75. doi: 10.5853/jos.2015.17.1.67
23. Yilmaz A, Akpinar E, Topcuoglu MA, Arsava EM. Clinical and imaging features associated with intracranial internal carotid artery calcifications in patients with ischemic stroke. Neuroradiology. (2015) 57:501–6. doi: 10.1007/s00234-015-1494-8
24. Brantley JN, Verla TD. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Advances Wound Care. (2015) 4:545–59. doi: 10.1089/wound.2015.0634
25. Willette AA, Bendlin BB, Colman RJ, Kastman EK, Field AS, Alexander AL, et al. Calorie restriction reduces the influence of glucoregulatory dysfunction on regional brain volume in aged rhesus monkeys. Diabetes. (2012) 61:1036–42. doi: 10.2337/db11-1187
26. Lee HY, Oh BH. Aging and arterial stiffness. Circ J: off J Japanese Circ Soc. (2010) 74:2257–22262. doi: 10.1253/circj.cj-10-0910
27. Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, et al. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. (2017) 37:191–204. doi: 10.1161/atvbaha.116.306256
28. Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol: CJASN. (2008) 3:1599–605. doi: 10.2215/cjn.02120508
29. Laugesen E, Høyem P, Thrysoe S, Hansen ESS, Mikkelsen AFS, Kerwin WS, et al. Negative carotid artery remodeling in early type 2 diabetes mellitus and increased carotid plaque vulnerability in obesity as assessed by magnetic resonance imaging. J Am Heart Assoc. (2018) 7:e008677. doi: 10.1161/jaha.118.008677
30. Hernández-Pérez M, Bos D, Dorado L, Pellikaan K, Vernooij MW, López-Cancio E, et al. A Intracranial carotid artery calcification relates to recanalization and clinical outcome after mechanical thrombectomy. Stroke. (2017) 48:342–7. doi: 10.1161/strokeaha.116.015166
31. Wu XH, Chen XY, Fan YH, Leung TW, Wong KS. High extent of intracranial carotid artery calcification is associated with downstream microemboli in stroke patients. J Stroke Cerebrovasc Dis: off J National Stroke Ass. (2017) 26:442–7. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.007
32. Thompson T, Shields KJ, Barinas-Mitchell E, Newman A, Sutton-Tyrrell K. Calcified carotid artery plaques predict cardiovascular outcomes in the elderly. J Hyperten. (2015) 33:810–7. doi: 10.1097/hjh.0000000000000488
33. Niederhoffer N, Lartaud-Idjouadiene I, Giummelly P, Duvivier C, Peslin R, Atkinson J. Calcification of medial elastic fibers and aortic elasticity. Hypertension. (1997) 29:999–1006. doi: 10.1161/01.hyp.29.4.999
34. Edmonds ME. Medial arterial calcification and diabetes mellitus. Z Kardiol. (2000) 89(Suppl 2):101–4. doi: 10.1007/s003920070107
35. O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. (2005) 46:200–4. doi: 10.1161/01.hyp.0000168052.00426.65
36. Adams HP Jr., Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH stroke scale score strongly predicts outcome after stroke: a report of the trial of org 10,172 in acute stroke treatment (TOAST). Neurology. (1999) 53:126–31. doi: 10.1212/wnl.53.1.126
37. Almekhlafi MA, Davalos A, Bonafe A, Chapot R, Gralla J, Pereira VM, et al. Impact of age and baseline NIHSS scores on clinical outcomes in the mechanical thrombectomy using solitaire FR in acute ischemic stroke study. AJNR Am J Neuroradiol. (2014) 35:1337–40. doi: 10.3174/ajnr.A3855
38. Li J, Zhang P, Wu S, Yi X, Wang C, Liu M. Factors associated with favourable outcome in large hemispheric infarctions. BMC Neurol. (2018) 18:152. doi: 10.1186/s12883-018-1148-7
Keywords: acute stroke, calcification, prognosis, carotid artery, intravenous thrombolysis
Citation: Shen Y, Dong Z, Xu G, Zhong J, Pan P, Chen Z and Shi H (2022) Correlation Between Intracranial Carotid Artery Calcification and Prognosis of Acute Ischemic Stroke After Intravenous Thrombolysis. Front. Neurol. 13:740656. doi: 10.3389/fneur.2022.740656
Received: 13 July 2021; Accepted: 08 March 2022;
Published: 13 April 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Mehmet Akif Topçuoglu, Hacettepe University, TurkeyCopyright © 2022 Shen, Dong, Xu, Zhong, Pan, Chen and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haicun Shi, c2hpaGFpY3VuNDFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.