
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 14 February 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.737441
This article is part of the Research TopicIschemic Stroke Management: From Symptom Onset to Successful Reperfusion and BeyondView all 60 articles
 Sheng-Feng Lin1,2,3,4
Sheng-Feng Lin1,2,3,4 Han-Hwa Hu5,6,7*
Han-Hwa Hu5,6,7* Hai-Lun Chao8*
Hai-Lun Chao8* Bo-Lin Ho9,10
Bo-Lin Ho9,10 Chih-Hung Chen11,12
Chih-Hung Chen11,12 Lung Chan7
Lung Chan7 Huey-Juan Lin13
Huey-Juan Lin13 Yu Sun14
Yu Sun14 Yung-Yang Lin15
Yung-Yang Lin15 Po-Lin Chen16
Po-Lin Chen16 Shinn-Kuang Lin17
Shinn-Kuang Lin17 Cheng-Yu Wei18
Cheng-Yu Wei18 Yu-Te Lin19
Yu-Te Lin19 Jiunn-Tay Lee20
Jiunn-Tay Lee20 A-Ching Chao9,10* on behalf of the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) Study Group
A-Ching Chao9,10* on behalf of the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) Study GroupBackground: The triglyceride-glucose (TyG) index has recently been proposed as a reliable marker of insulin resistance. There is insufficient evidence to verify that the TyG index is correlated with functional outcomes and hemorrhagic transformation and in patients with stroke treated with intravenous thrombolysis (IVT).
Methods: We designed a multicenter cohort study, which enrolled patients with acute ischemic stroke treated with IVT between December 2004 and December 2016. The TyG index was divided into tertiles and calculated on a continuous scale. Unfavorable functional outcomes were defined by the modified Rankin Scale of 3–6 at 90 days and the incident rates of symptomatic intracranial hemorrhage (SICH) within 36 h of IVT onset were surveyed. Stroke severity was defined as mild (4–8), moderate (9–15), or high (≥16) based on the National Institutes of Health Stroke Scale (NIHSS) scores.
Results: Among 914 enrolled patients, the tertiles of the TyG index were 8.48 for T1, 8.48–9.04 for T2, and 9.04 for T3. T3 showed an increased risk of unfavorable functional outcomes at 90 days [odds ratio (OR): 1.76; P = 0.0132]. The TyG index was significantly associated with unfavorable functional outcomes at 90 days (OR: 1.32; P = 0.0431 per unit increase). No association was found between the TyG index and SICH. These findings were applicable for T3 with stroke of moderate (OR, 2.35; P = 0.0465) and high severity (OR: 2.57, P = 0.0440) patients with stroke.
Conclusion: This study supports the strong association between the increased TyG index and increased unfavorable functional outcomes at 90 days in patients with acute ischemic stroke treated with IVT. These findings were found to be robust in patients with moderate and high stroke severity.
Early detection of insulin resistance (IR) is crucial in preventing cardiovascular diseases (1, 2). Glucose clamp tests are performed in the two ways: (1) the hyperglycemic clamp technique for quantification of beta-cell sensitivity to glucose and (2) the euglycemic insulin clamp technique for quantification of peripheral cell sensitivity to insulin (3). These techniques are regarded as the gold standard for quantifying IR (4) and rely on constant insulin infusion and measurement of glucose disposal under steady-state conditions (3, 4). The limitations of the glucose clamp technique include labor-intensive and time-consuming procedures, which require experienced physicians to perform human studies. In 1985, a simple equation homeostasis model assessment-IR (HOMA-IR) (5) was developed to assess IR by calculating the product of fasting levels of insulin and glucose (6). Although widely used in the research field, the use of HOMA-IR is greatly limited in clinical practice because of the need for insulin measurement.
Recently, the triglyceride-glucose (TyG) index has been recognized as a reliable surrogate biomarker of IR (7) and has been proposed and validated to be highly correlated with HOMA-IR across all the ages and various ethnic groups (7–10). The TyG index was used for the first time in 2008 by Simental-Mendia et al. (11) to identify IR in an apparently healthy population and it was formally proposed by Guerrero-Romero et al. (12) in 2010. The rationale for the application of the TyG index is that IR is the most common cause of increased triglyceride (TG) and glucose levels in serum tests (13). Some studies showed high correlation between the TyG index and IR by glucose clamp tests (9, 14) and a study (9) showed that the TyG index exhibited higher diagnostic performance than HOMA-IR for IR in some studies. Furthermore, there has been increasing evidence supporting the correlation between the TyG index and acute adverse outcomes in patients with cardiovascular disease. Recent studies have suggested that the TyG index is highly associated with carotid atherosclerosis (15) and the outcomes and prevalence of coronary artery diseases (16, 17). By using HOMA-IR, previous studies have found that IR is associated with neurological worsening and functional status (18–20). In addition, a recent study (21) found that an increased TyG index was associated with a higher risk of stroke recurrence, functional worsening, and mortality. The above studies suggest that the TyG index is a marker for acute adverse effects in cardiovascular and cerebrovascular diseases.
Nonetheless, relevant investigations of the association between the TyG index and intravenous thrombolytic outcomes for stroke are lacking. The Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) registry contains a nationwide cohort of Taiwan and longitudinal follow-up data for patients with stroke treated with intravenous thrombolysis (IVT) (22, 23). The TTT-AIS registry comprehensively enrolls patients with acute ischemic stroke with all the levels of severity. The aim of this study was to investigate the relationship between the TyG index and outcomes of functional status and symptomatic intracranial hemorrhage among ethnic Chinese patients with acute ischemic stroke treated with IVT. The novelty and significance of this study are as follows: (1) evaluation of patients with acute ischemic stroke in different categories of the TyG index, (2) nationwide, multicenter cohort study design encompassing the representative population, and (3) evaluation of patients with acute ischemic stroke of all the levels of severity.
This study had a prospective cohort design and encompassed a multicenter study of 30 hospitals in Taiwan. Clinical data were collected prospectively and registered in the TTT-AIS registry. Baseline demographic information included age, sex, alcohol use, history of hypertension, diabetes mellitus, coronary artery disease, atrial fibrillation, blood pressure on arrival at the hospital, use of antiplatelet and anticoagulant medications, the National Institutes of Health Stroke Scale (NIHSS) score at baseline, and time from stroke onset to IVT was retrieved by the investigators. For patients with acute ischemic stroke arriving at the hospital within 3 h of stroke onset, IV alteplase was used for the thrombolytic regimen. Serum glucose levels and lipid profiles were obtained from each patient after an overnight fast ≥8 h within 24–48 h of stroke onset. A written informed consent was obtained from all the patients. This study was approved by the Institutional Review Board of the Kaohsiung Medical University Hospital (reference number: KMUH-IRB-20140305).
The TyG index was calculated by using the following formula: Ln [TG (mg/dl) × fasting glucose (mg/dl)/2]. According to the previous studies (24, 25), a simple cutoff of the TyG index ≥8.4 is sufficiently reliable to classify Asian individuals with IR. The inclusion criteria for eligible patients were as follows: (1) treatment with IVT for acute ischemic stroke adhering to the National Institute of Neurological Disorders and Stroke (NINDS) criteria (26) and (2) measurement of fasting glucose and lipid profiles in a fasting state during 24–72 h following the administration of IV alteplase. The exclusion criteria for IVT were based on the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) study criteria (27). All the enrolled patients with stroke underwent brain CT on arrival to the hospital and another routine brain CT was performed within 24–36 h post-IVT.
We evaluated the clinical outcomes of (1) unfavorable functional outcome status defined by the modified Rankin Scale (mRS) of 3–6 at 90 days; (2) mortality at 90 days; (3) symptomatic intracranial hemorrhage (SICH); (4) the NINDS standard as per which any intracranial hemorrhage deteriorated to the NIHSS score of ≥ 1 or led to death within 36 h (26); (5) the European Cooperative Acute Stroke Study (ECASS) II standard, as per which any intracranial hemorrhage deteriorated to the NIHSS score of ≥ 4 or led to death (28); and (6) the SITS-MOST standard for a type 2 parenchymal hemorrhage (a local or remote parenchymal intracranial hemorrhage exceeding 30% of the infarct) with clinical deterioration of the NIHSS score of ≥ 4 or death within 36 h (27). Patients who presented with the baseline NIHSS scores of 4–8, 9–15, and ≥ 16 were categorized into the mild, moderate, and high severity groups, respectively.
To compare the groups with and without IR, the Student's t-test was used for continuous variables and the Pearson's chi-squared test was used for categorical variables. We evaluated the relationship between the TyG index and lipid profiles by using two methods: (1) partitioning of the TyG index to the territorial scale and (2) examination of the TyG index on a continuous scale. The multiple logistic regression models were employed to determine the odds ratios (ORs) and their 95% CIs, the study outcomes were used as dependent variables, and the TyG index (either in tertile or in continuous scale) and the unbalanced covariates between the groups with and without IR were independent variables. T1 was used as the reference group for the models analyzed on a tertile scale. As a sensitivity analysis, we performed a stratified analysis according to the stroke severity of each patient. Statistical significance was defined as p < 0.05. All the analyses were performed by using SAS software (version 9.4; North Carolina, USA) and Stata software (version 15; Texas, USA).
At the time of designing this study, there had been no published study investigating the relationship between the TyG index and clinical outcomes in patients with acute ischemic stroke treated with IVT. The required sample size was estimated in a previous study (29), which explored the relationship between HOMA-IR and clinical outcomes in patients with stroke treated with IVT. In this study (29), HOMA-IR in the upper tertile (OR, 8.54, 95% CI, 1.67–43.55; P = 0.01) was associated with unfavorable functional outcome when compared with the lower tertile and HOMA-IR in the middle tertile (OR, 2.96, 95% CI, 0.61–14.40; P = 0.178) was not significantly associated with unfavorable functional outcome. Therefore, the required sample size should be 101; when we hypothesized that the parameters of effect size of OR were in a range from 2.96 to 8.54, the probability of exposure (the upper tertile) was 0.33 and significance level and power were 0.05 and 0.95, respectively.
From 1st December, 2004 to 31st December, 2016, a total of 914 patients with acute ischemic stroke who had completed IVT were enrolled and laboratory tests for glucose and lipid profile were performed in a fasting state following admission. Of these, 652 patients had IR (TyG index ≥ 8.4) and 262 patients did not had IR (TyG index < 8.4). In the groups without and with IR (Table 1), the average age was 69.8 ± 13.0 and 68.8 ± 11.9 years (P = 0.2941), the proportion of female sex was 36.6 and 36.2% (P = 0.8994), the average dose of alteplase was 0.78 ± 0.14 and 0.80 ± 0.14 (P = 0.0747), and the mean NIHSS score at onset was 14.2 ± 6.6 and 13.6 ± 7.4 (P = 0.2809), respectively; there was no significant difference in the antithrombotic medication usage. The systolic (161.6 ± 29.6 vs. 154.1 ± 29.1 mm Hg, P = 0.0011) and diastolic (91.4 ± 19.7 vs. 87.6 ± 18.3 mm Hg, P = 0.0078) blood pressures were noted for the group with IR. Medical comorbidities of hypertension and diabetes mellitus were higher in the group with IR than in the group without IR, while the prevalence of atrial fibrillation was higher in the group without IR.
The functional outcome distribution defined by the mRS at 90 days is shown in Figure 1. Patients with stroke in the group without IR showed higher proportions of favorable outcome (mRS, 0–2) at 90 days (47.9%) than those patients with stroke in the group with IR (41.7%). The cutoff value of the TyG index on the tertiles was T1 < 8.48, T2 ranging between 8.48 and 9.04, and T3 > 9.04, respectively. On classification by tertiles, the proportions of favorable functional outcomes at 90 days decreased with increasing tertiles (T1, 46.7%; T2, 44.5%; and T3, 38.8%).
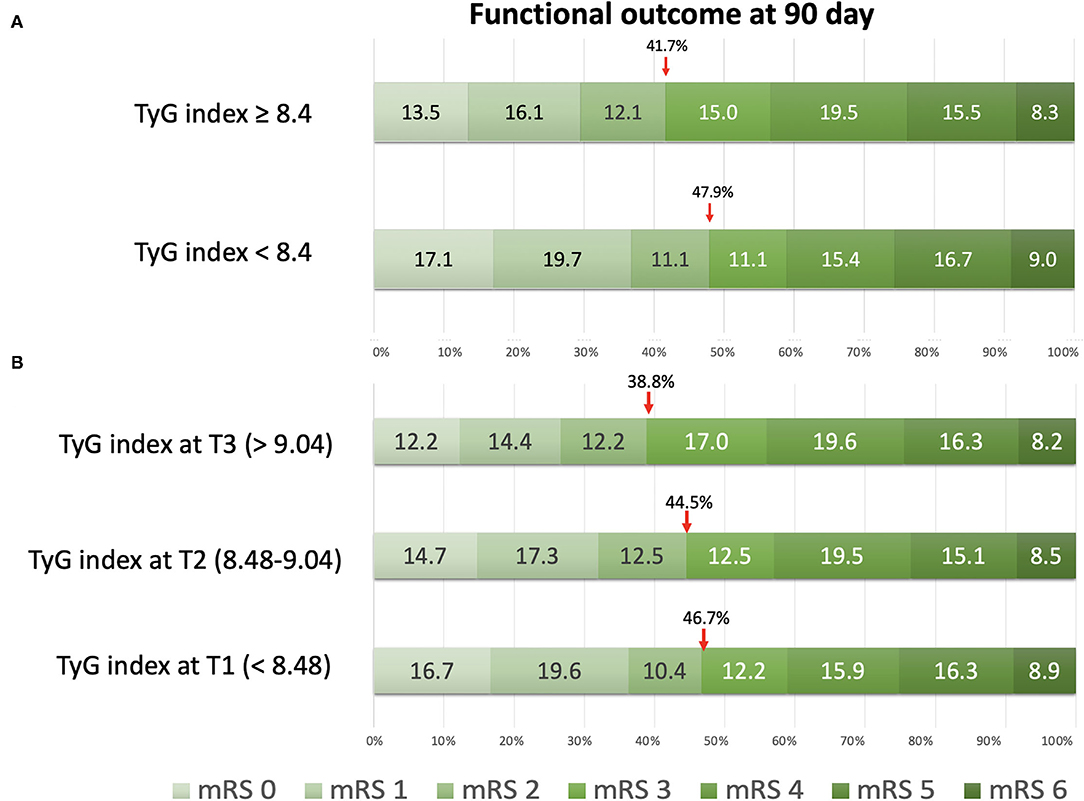
Figure 1. The distribution of functional outcomes at 90 days. (A) Classification by insulin resistance status with the triglyceride-glucose (TyG) index at 8.4. (B) Classification by tertiles of the TyG index.
Clinical outcomes that were investigated by categorizing patients with stroke into tertiles of the TyG index are shown in Figure 2. For unfavorable functional outcomes at 90 days, T3 had the highest event rate of 73.6%, compared to T1 (63.8%) and T2 (68.0%) (Table 2). After adjustment for age and sex, the logistic regression model showed a significant increase in unfavorable functional outcomes at 90 days for T3 (OR, 1.69; 95% CI, 1.16–2.46; P = 0.0059). In the multivariate-adjusted models, T3 showed a significantly increased risk of unfavorable functional outcomes at 90 days (OR, 1.76; 95% CI, 1.13–2.76; P = 0.0132). All the tertiles showed similar rates for mortality, ranging from 8.2 to 8.9%. Additionally, there was no significant difference in the rates of SICH according to the NINDS, the ECASS II, and the SITS-MOST criteria.
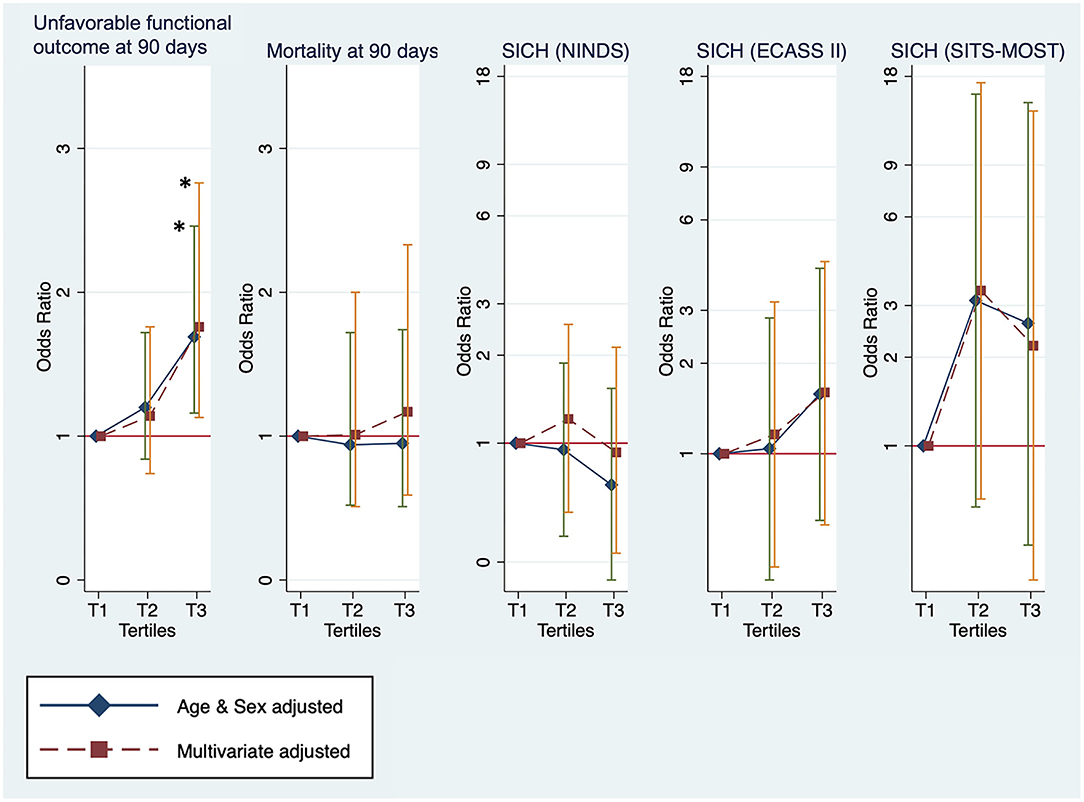
Figure 2. The outcomes measures for unfavorable functional outcome status and mortality at 90 days and symptomatic intracranial hemorrhage (SICH) by tertiles of the TyG index. *Statistical significance (p < 0.05).
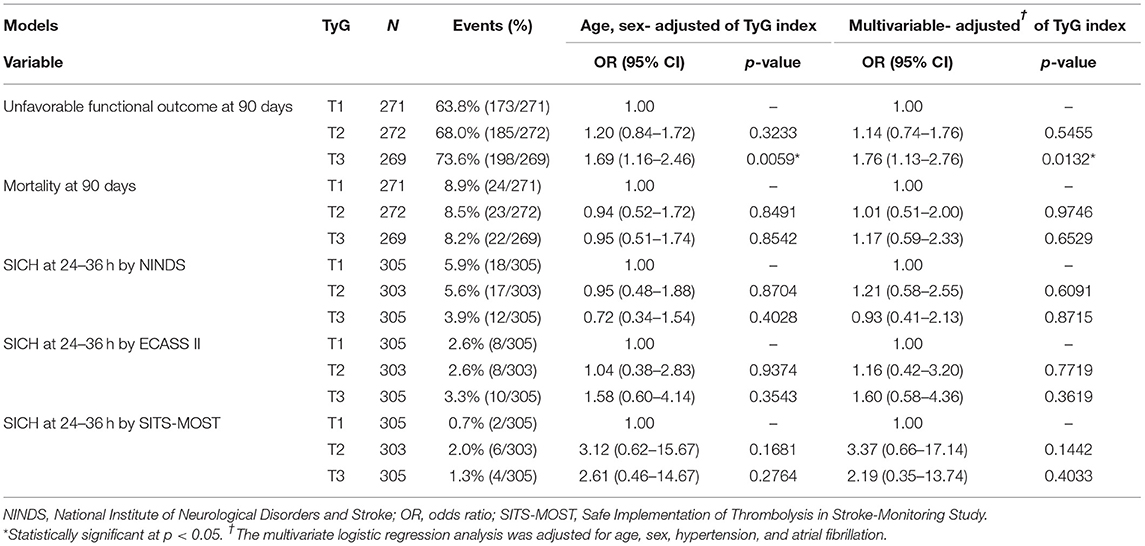
Table 2. Functional outcomes and symptomatic intracranial hemorrhage (SICH) by tertiles of the triglyceride-glucose (TyG) index.
The association between clinical outcomes and the TyG index was explored on a continuous scale (Table 3). The TyG index on a continuous scale showed a significantly increased risk of unfavorable functional outcomes at 90 days in the models of adjustment for age and sex (OR, 1.27, 95% CI, 1.02–1.58; P = 0.0361) and of multivariate adjustment (OR, 1.32, 95% CI, 1.01–1.73; P = 0.0431). Consistent with the results analyzed by using tertiles, the TyG index was not significantly associated with the outcomes of mortality and SICH within 36 h.
The stratification analysis according to stroke severity and tertiles of the TyG index are shown in Table 4. The analysis of unfavorable functional outcomes at 90 days after severe stroke is shown in Figure 3. For patients with mild stroke severity (NIHSS score of 4–8) treated with IVT, no significant association between the TyG index and unfavorable functional outcomes at 90 days was found. In contrast, the T3 category of patients with stroke with moderate severity (NIHSS score of 9–15) showed a 2-fold increased risk of unfavorable functional outcomes at 90 days in both the age and sex-adjusted (OR: 2.47, 95% CI 1.25–4.90, P = 0.0096) and multivariate-adjusted regression models (OR, 2.35; 95% CI, 1.01–5.44; P = 0.0465). The T3 category of patients with stroke with high stroke severity (NIHSS score of ≥16) showed a two-fold increased risk of unfavorable functional outcomes at 90 days in age and sex-adjusted (OR: 2.31, 95% CI 1.06–5.02, P = 0.0355) and multivariate-adjusted regression models (OR: 2.57, 95% CI 1.03–6.44, P = 0.0440). Similar to the previous analysis, no significant association was found between the TyG index and outcome measures of mortality and SICH. Lastly, the TyG index on a continuous scale for moderate and severe stroke severity consistently showed an increased risk of unfavorable functional outcomes at 90 days (Table 5).
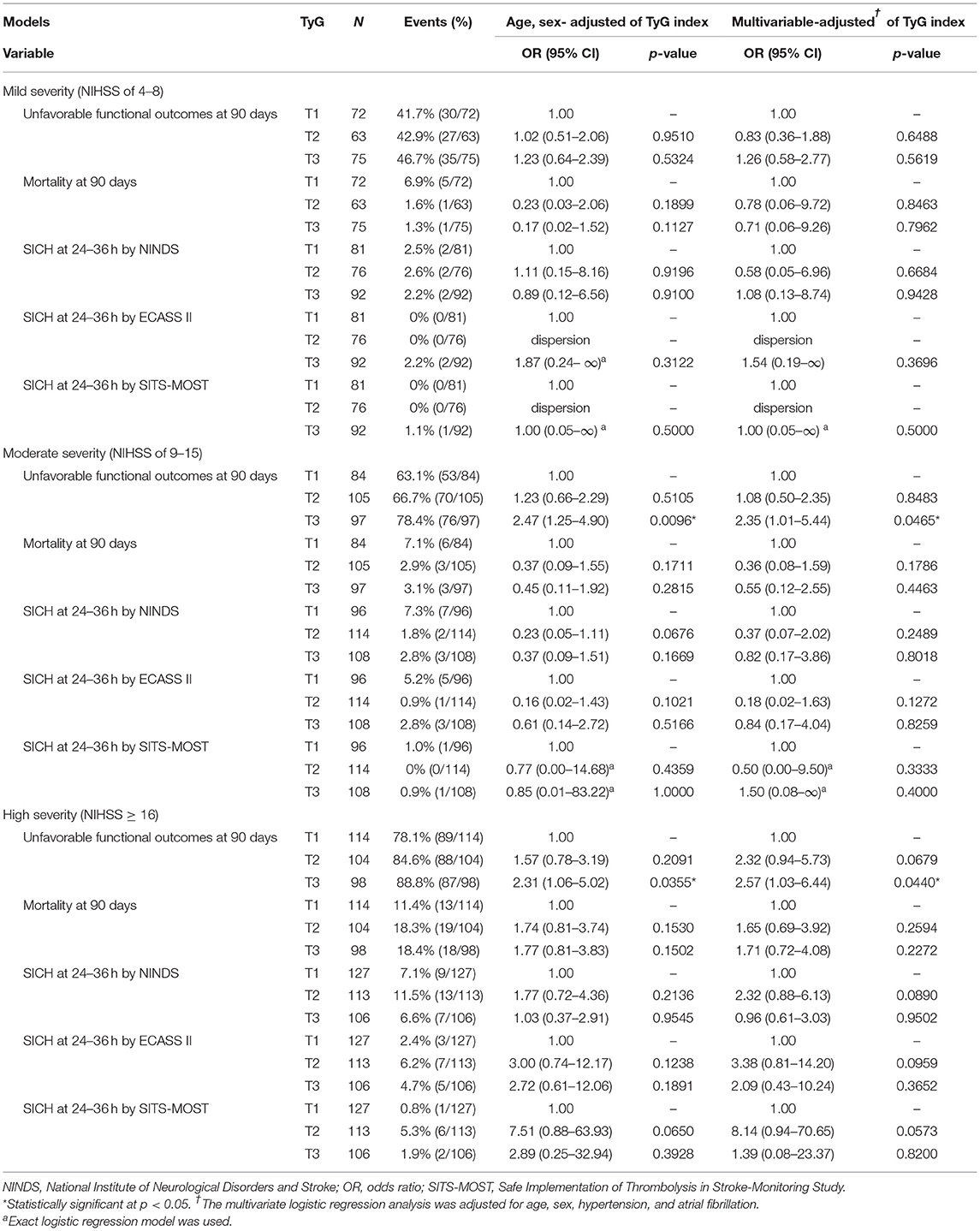
Table 4. Sensitivity analysis: functional outcomes and SICH by tertiles of the TyG index on different stroke severities.
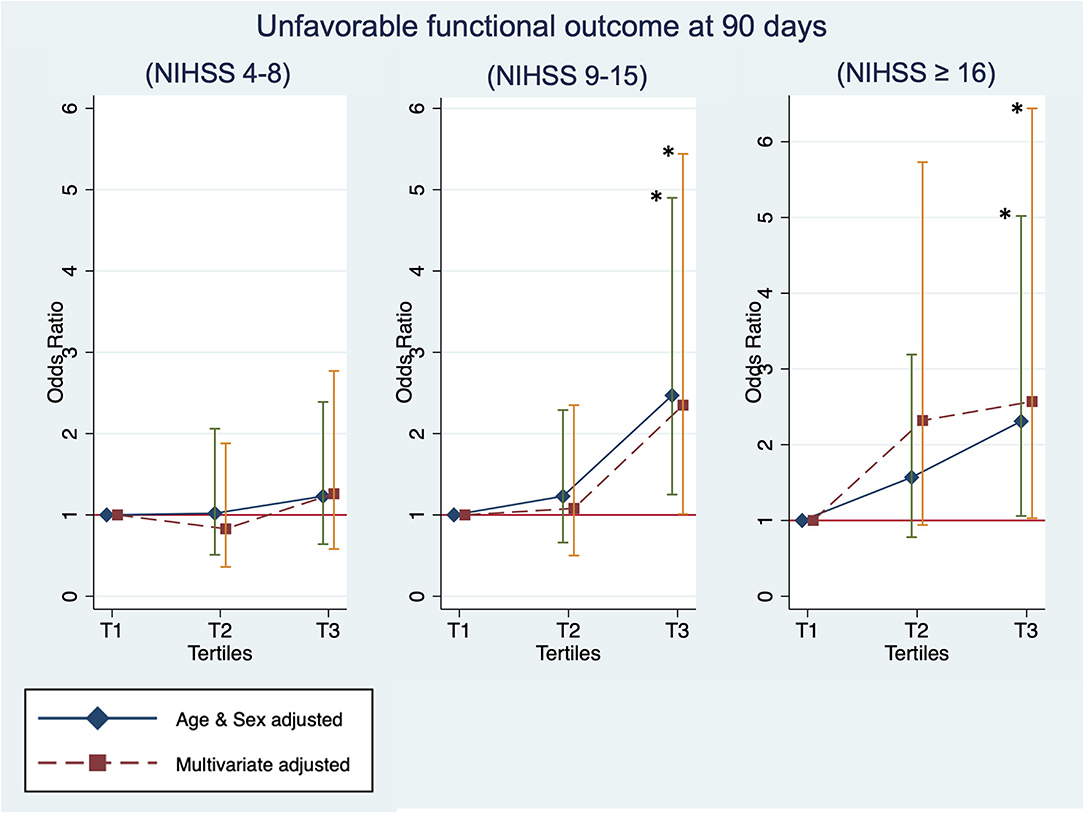
Figure 3. Sensitivity analysis: The unfavorable outcome at 90 days by tertiles of the TyG index and the baseline stroke severity of mild [National Institutes of Health Stroke Scale (NIHSS) of 4–8], moderate (NIHSS of 9–15), and high (NIHSS of ≥16). *Statistical significance (p < 0.05).
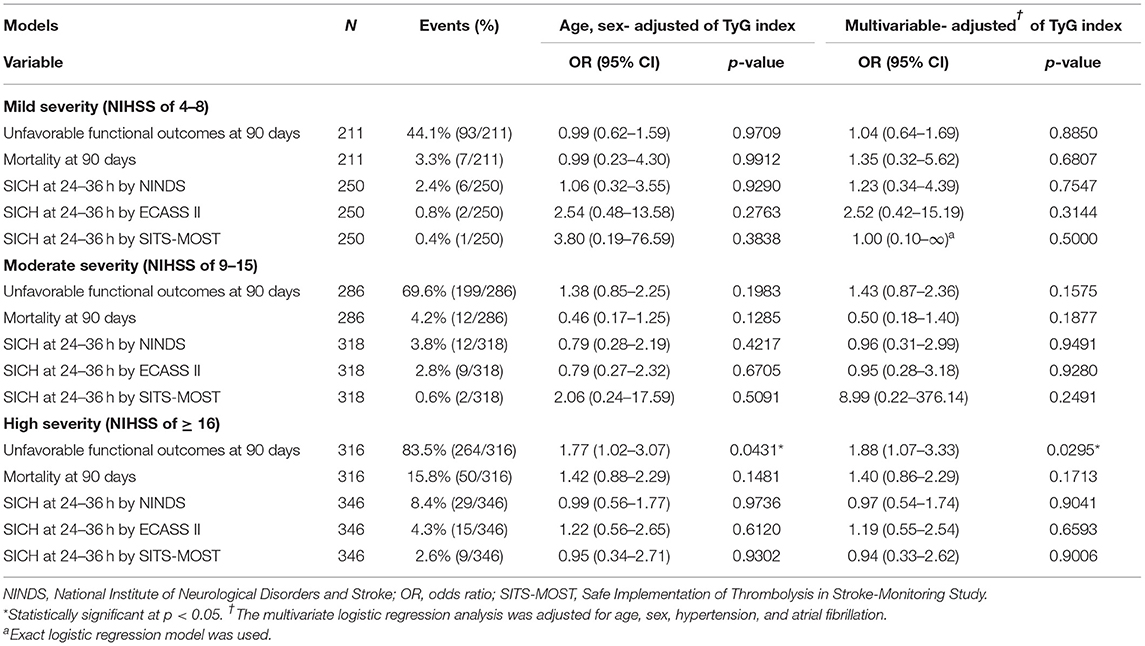
Table 5. Sensitivity analysis: functional outcomes and SICH by the TyG index on continuous scale (per unit) and different stroke severities.
Our results showed that the TyG index was associated with unfavorable functional outcomes at 90 days in patients with acute ischemic stroke treated with IVT. This association was more robust in patients with stroke presenting with moderate to high baseline severity. In addition, there was no significant association between the TyG index and outcomes of mortality or SICH within 36 h.
Our analysis employing the TyG index corroborated the results of previous studies. In an earlier investigation, higher resistance to IVT therapy and poor clot dissolution were observed in patients with stroke with metabolic syndrome under transcranial Doppler examination (30, 31). An earlier study showed that HOMA-IR in the upper tertile was associated with eight-fold increased risk of unfavorable functional outcome when compared with the lower tertile (29). In addition, a recent large-scale study that enrolled nondiabetic Chinese patients with stroke found that higher HOMA-IR was linked to poor stroke recovery and recurrence (19). These studies suggested that patients with stroke presenting with higher IR should be poor responders to IVT and more susceptible to unfavorable recovery. This study uncovers important implications that the TyG index, such as HOMA-IR, is an effective biomarker for selecting patients who benefit from aggressive strategies for vascular reperfusion. Unlike HOMA-IR, the TyG index is more practical for use in clinical conditions due to the lack of insulin measurement.
Recent studies have suggested that the TyG index is highly associated with carotid atherosclerosis (15), major adverse cardiovascular events in patients with non-ST-segment elevation acute coronary syndrome (ACS), and major adverse cardiovascular and cerebrovascular events in patients with ST-segment elevation ACS (16, 17). The above studies support that the TyG index is a marker for acute adverse effects in cardiovascular and cerebrovascular diseases. In terms of physiology, high values of the TyG index or IR should oppose IVT for ischemic stroke by several mechanisms. First, several studies reported that higher IR was associated with elevated levels of thrombin activatable fibrinolysis inhibitor and plasminogen activator inhibitor 1 (PAI-I) (32, 33), which attenuated fibrinolysis by IVT (33). Second, IR augments the density of blood clots and impairs the effect of IVT (34).
Our results showed no significant association between the TyG index and SICH outcomes and are compatible with our previous analysis of SICH outcomes on TG levels in the TTT-AIS study (35). This findings were consistent with an earlier study that used HOMA-IR for patients with stroke treated with IVT (29). Based on previous studies of TG, which did not show robust association with intracranial hemorrhage in patients with stroke treated without thrombolysis (36–40), we deduced that the TyG index was not a reliable biomarker for hemorrhagic transformation. Additionally, the TyG index showed no significant association with the outcome of mortality. The most reasonable explanation was that two-thirds of patients with stroke presented with moderate and high severity and these patients were more susceptible to SICH with IVT when compared to those with low severity or with the low NIHSS score (41–44). In a separate analysis, the mortality in our cases was also strongly attributable to SICH (Supplementary Table S1).
Our cutoff values for tertiles of the TyG index at 8.48 and 9.04 should be used as a standard. A recent study investigating patients with type 2 diabetes mellitus found that the TyG index >9.5 significantly increased macrovascular complications, including cerebrovascular diseases and albuminuria (45). Another study that enrolled patients with minor cases (the median NIHSS score, 4; quartile deviation, 2.5) reported that the TyG index of >9.2 increased neurological worsening (21). A cross-sectional study exploring silent brain infarcts reported increased multiple silent infarcts in the group with the median TyG index >8.5 (46). To the best of our knowledge, patients with acute ischemic stroke with the TyG index >9 should be cautious of worsening clinical neurological function.
We confirmed that the TyG index was robust in predicting unfavorable functional outcomes at 90 days for moderate and severe stroke severity. This was also the first study to explore the TyG index for different stroke severities. In terms of physiology, we proposed that the harder and longer segments of clots in patients with stroke with high severity would be aggravated by attenuated fibrinolysis due to increased IR. In addition, the distinctive strengths of this study are as follows: (1) a longitudinal cohort study design with a large sample size of patients with stroke treated with IVT, (2) determination of the extent to which a higher level of the TyG index contributes to unfavorable functional outcomes at 90 days, and (3) estimation of outcomes using the TyG index on tertile and continuous scales, with robust results in sensitivity analysis.
Overall, our results are consistent to earlier studies investigating the effect of IR in patients with acute ischemic stroke. Due to the need of insulin measurement, use of HOMA-IR in the real-world practice is limited. For patients with stroke, the lipid profile and blood glucose are routine laboratory tests. Therefore, physicians can easily monitor the effect of IR in patients with stroke with the TyG index. However, further study is warranted to evaluate whether controlling the TyG index <9.0 would improve the functional outcomes and accelerate neurological recovery. More evidence is needed to generalize the results for clinical practice in Asians and other ethnic population.
Stress hyperglycemia and IR are reported as the adaptive response that increase the chance of the patient to survive (47). Chronic hyperglycemia is known harmful with numerous complications (48, 49). Recently, acute hyperglycemia has been considered protective, since patients could have greater cellular resistance to ischemic and hypoxic injury (47, 50). In terms of pathophysiology, stress hyperglycemia is caused predominantly by excessive gluconeogenesis, glycogenolysis, and IR. While epinephrine and norepinephrine augment hepatic gluconeogenesis and glycogenolysis, inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and C-reactive protein, induce IR (51, 52). In addition, glucose is the primary energy source of the brain (53). Therefore, we consider that stress hyperglycemia should not be excluded, since it represents the physiological response to improve survival in patients with stroke.
This study had some limitations. First, the TTT-AIS registry (22, 23) has no clinical data on insulin levels. Further, HOMA-IR was not used in this study. Second, the TTT-AIS registry was initiated in 2004 at a time when thrombectomy had not been introduced in Taiwan. Third, although we have conducted the subgroup analysis in diabetic and nondiabetic patients according to medical history, no significant association between functional outcomes and the TyG index was found (Supplementary Table S2). Two factors should explain this: (1) history of diabetes mellitus reported by patients was imprecise and underdiagnosed and (2) the smaller sample size in subgroup analysis. However, a recently published study (54) showed that the TyG index was associated with early neurological deterioration (an increase of the NIHSS ≥2 or the NIHSS ≥1 in the motor dysfunction within 72 h) in patients with untreated diabetes (adjusted OR: 3.94, 95% CI, 1.47–10.53, P = 0.006). Accordingly, the TyG index should be applicable in predicting functional outcome in patients with stroke with diabetes mellitus. Fourth, hemoglobin A1c was not measured in this study. The prevalence of diabetes mellitus was possibly underdiagnosed in this study.
In conclusion, this study supports a strong association between higher levels of the TyG index and increased unfavorable functional outcomes at 90 days in patients with acute ischemic stroke treated with IVT. This association was robust in patients with moderate (NIHSS of 9–15) and high stroke severity (NIHSS ≥ 16). While HOMA-IR is not readily available, the TyG index would be a surrogate marker of IR. This study determines a cutoff value of TyG index >9.0 that is useful for predicting unfavorable functional outcomes in patients with acute ischemic stroke treated with IVT in Asians, but further study is needed to validate this cutoff value in other ethnic population.
The data analyzed in this study is subject to the following licenses/restrictions: The datasets presented in this article are not readily available because the Institutional Review Board of Kaohsiung Medical University has restricted their distribution. Requests to access these datasets should be directed to A-Ching Chao, YWNoY2hAY2Mua211LmVkdS50dw==.
The studies involving human participants were reviewed and approved by Kaohsiung Medical University Hospital (IRB: KMUH-IRB-20140305). The patients/participants provided their written informed consent to participate in this study.
S-FL wrote the first draft of the article. All authors contributed to the conception and design of the study, acquisition, analysis, and interpretation of data.
This study was supported by grants from the Ministry of Science and Technology (Reference Number: MOST 108-2314-B-037-038-MY3) and the Kaohsiung Medical University Hospital (Reference Number: KMUH110-0R65).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.737441/full#supplementary-material
1. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation. (2004) 110:380–5. doi: 10.1161/01.CIR.0000136581.59584.0E
2. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Wilson PW. Insulin resistance, the metabolic syndrome, and incident cardiovascular events in the Framingham Offspring Study. Diabetes. (2005) 54:3252–7. doi: 10.2337/diabetes.54.11.3252
3. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. (1979) 237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214
4. Kim JK. Hyperinsulinemic-euglycemic clamp to assess insulin sensitivity in vivo. Methods Mol Biol. (2009) 560:221–38. doi: 10.1007/978-1-59745-448-3_15
5. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. (1985) 28:412–9. doi: 10.1007/BF00280883
6. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. (2004) 27:1487–95. doi: 10.2337/diacare.27.6.1487
7. Lim J, Kim J, Koo SH, Kwon GC. Comparison of triglyceride glucose index, and related parameters to predict insulin resistance in Korean adults: An analysis of the 2007-2010 Korean National Health and Nutrition Examination Survey. PLoS ONE. (2019) 14:e0212963. doi: 10.1371/journal.pone.0212963
8. Locateli JC, Lopes WA, Simões CF, de Oliveira GH, Oltramari K, Bim RH, et al. Triglyceride/glucose index is a reliable alternative marker for insulin resistance in South American overweight and obese children and adolescents. J Pediatr Endocrinol Metab. (2019) 32:1163–70. doi: 10.1515/jpem-2019-0037
9. Vasques AC, Novaes FS., de Oliveira MaS, Souza JR, Yamanaka A, Pareja JC, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. (2011) 93:e98–e100. doi: 10.1016/j.diabres.2011.05.030
10. Du T, Yuan G, Zhang M, Zhou X, Sun X, Yu X. Clinical usefulness of lipid ratios, visceral adiposity indicators, and the triglycerides and glucose index as risk markers of insulin resistance. Cardiovasc Diabetol. (2014) 13:146. doi: 10.1186/s12933-014-0146-3
11. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. (2008) 6:299–304. doi: 10.1089/met.2008.0034
12. Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
13. Alizargar J, Bai CH, Hsieh NC, Wu SV. Use of the triglyceride-glucose index (TyG) in cardiovascular disease patients. Cardiovasc Diabetol. (2020) 19:8. doi: 10.1186/s12933-019-0982-2
14. Mohd Nor NS, Lee S, Bacha F, Tfayli H, Arslanian S. Triglyceride glucose index as a surrogate measure of insulin sensitivity in obese adolescents with normoglycemia, prediabetes, and type 2 diabetes mellitus: comparison with the hyperinsulinemic-euglycemic clamp. Pediatr Diabetes. (2016) 17:458–65. doi: 10.1111/pedi.12303
15. Irace C, Carallo C, Scavelli FB, De Franceschi MS, Esposito T, Tripolino C, et al. Markers of insulin resistance and carotid atherosclerosis. A comparison of the homeostasis model assessment and triglyceride glucose index. Int J Clin Pract. (2013) 67:665–72. doi: 10.1111/ijcp.12124
16. Mao Q, Zhou D, Li Y, Wang Y, Xu SC, Zhao XH. The triglyceride-glucose index predicts coronary artery disease severity and cardiovascular outcomes in patients with non-ST-segment elevation acute coronary syndrome. Dis Markers. (2019) 2019:6891537. doi: 10.1155/2019/6891537
17. da Silva A, Caldas APS, Hermsdorff HHM, Bersch-Ferreira Â, Torreglosa CR, Weber B, et al. Triglyceride-glucose index is associated with symptomatic coronary artery disease in patients in secondary care. Cardiovasc Diabetol. (2019) 18:89. doi: 10.1186/s12933-019-0893-2
18. Ago T, Matsuo R, Hata J, Wakisaka Y, Kuroda J, Kitazono T, et al. Insulin resistance and clinical outcomes after acute ischemic stroke. Neurology. (2018) 90:e1470–e7. doi: 10.1212/WNL.0000000000005358
19. Jing J, Pan Y, Zhao X, Zheng H, Jia Q, Mi D, et al. Insulin resistance and prognosis of nondiabetic patients with ischemic stroke: the ACROSS-China Study (Abnormal Glucose Regulation in Patients With Acute Stroke Across China). Stroke. (2017) 48:887–93. doi: 10.1161/STROKEAHA.116.015613
20. Pan Y, Jing J, Chen W, Zheng H, Jia Q, Mi D, et al. Post-glucose load measures of insulin resistance and prognosis of nondiabetic patients with ischemic stroke. J Am Heart Assoc. (2017) 6:e004990. doi: 10.1161/JAHA.116.004990
21. Zhou Y, Pan Y, Yan H, Wang Y, Li Z, Zhao X, et al. Triglyceride Glucose Index and Prognosis of Patients With Ischemic Stroke. Front Neurol. (2020) 11:456. doi: 10.3389/fneur.2020.00456
22. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MM, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) study. Stroke. (2010) 41:885–90. doi: 10.1161/STROKEAHA.109.575605
23. Chao AC, Liu CK, Chen CH, Lin HJ, Liu CH, Jeng JS, et al. Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke. (2014) 45:2359–65. doi: 10.1161/STROKEAHA.114.005245
24. Moon S, Park JS, Ahn Y. The Cut-off Values of Triglycerides and Glucose Index for Metabolic Syndrome in American and Korean Adolescents. J Korean Med Sci. (2017) 32:427–33. doi: 10.3346/jkms.2017.32.3.427
25. Navarro-González D, Sánchez-Íñigo L, Pastrana-Delgado J, Fernández-Montero A, Martinez JA. Triglyceride-glucose index (TyG index) in comparison with fasting plasma glucose improved diabetes prediction in patients with normal fasting glucose: the vascular-metabolic CUN cohort. Prev Med. (2016) 86:99–105. doi: 10.1016/j.ypmed.2016.01.022
26. Group NIoNDaSr-PSS. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
27. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. (2007) 369:275–82. doi: 10.1016/S0140-6736(07)60149-4
28. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
29. Calleja AI, García-Bermejo P, Cortijo E, Bustamante R, Rojo Martínez E, González Sarmiento E, et al. Insulin resistance is associated with a poor response to intravenous thrombolysis in acute ischemic stroke. Diabetes Care. (2011) 34:2413–7. doi: 10.2337/dc11-1242
30. Arenillas JF, Ispierto L, Millán M, Escudero D, Pérez de la Ossa N, Dorado L, et al. Metabolic syndrome and resistance to IV thrombolysis in middle cerebral artery ischemic stroke. Neurology. (2008) 71:190–5. doi: 10.1212/01.wnl.0000317092.21210.e6
31. Arenillas JF, Sandoval P, Pérez de la Ossa N, Millán M, Guerrero C, Escudero D, et al. The metabolic syndrome is associated with a higher resistance to intravenous thrombolysis for acute ischemic stroke in women than in men. Stroke. (2009) 40:344–9. doi: 10.1161/STROKEAHA.108.531079
32. Chen R, Yan J, Liu P, Wang Z, Wang C. Plasminogen activator inhibitor links obesity and thrombotic cerebrovascular diseases: The roles of PAI-1 and obesity on stroke. Metab Brain Dis. (2017) 32:667–73. doi: 10.1007/s11011-017-0007-3
33. Denorme F, Wyseure T, Peeters M, Vandeputte N, Gils A, Deckmyn H, et al. Inhibition of Thrombin-Activatable Fibrinolysis Inhibitor and Plasminogen Activator Inhibitor-1 Reduces Ischemic Brain Damage in Mice. Stroke. (2016) 47:2419–22. doi: 10.1161/STROKEAHA.116.014091
34. van Rooy MJ, Duim W, Ehlers R, Buys AV, Pretorius E. Platelet hyperactivity and fibrin clot structure in transient ischemic attack individuals in the presence of metabolic syndrome: a microscopy and thromboelastography study. Cardiovasc Diabetol. (2015) 14:86. doi: 10.1186/s12933-015-0249-5
35. Lin SF, Chao AC, Hu HH, Lin RT, Chen CH, Chan L, et al. Low Cholesterol Levels Increase Symptomatic Intracranial Hemorrhage Rates After Intravenous Thrombolysis: A Multicenter Cohort Validation Study. J Atheroscler Thromb. (2019) 26:513–27. doi: 10.5551/jat.46151
36. Bonaventure A, Kurth T, Pico F, Barberger-Gateau P, Ritchie K, Stapf C, et al. Triglycerides and risk of hemorrhagic stroke vs. ischemic vascular events: the three-city study. Therosclerosis. (2010) 210:243–8. doi: 10.1016/j.atherosclerosis.2009.10.043
37. Wieberdink RG, Poels MM, Vernooij MW, Koudstaal PJ, Hofman A, van der Lugt A, et al. Serum lipid levels and the risk of intracerebral hemorrhage: the Rotterdam Study. Arterioscler Thromb Vasc Biol. (2011) 31:2982–9. doi: 10.1161/ATVBAHA.111.234948
38. Sturgeon JD, Folsom AR, Longstreth WT, Shahar E, Rosamond WD, Cushman M. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. (2007) 38:2718–25. doi: 10.1161/STROKEAHA.107.487090
39. Zia E, Pessah-Rasmussen H, Khan FA, Norrving B, Janzon L, Berglund G, et al. Risk factors for primary intracerebral hemorrhage: a population-based nested case-control study. Cerebrovasc Dis. (2006) 21:18–25. doi: 10.1159/000089589
40. Zhou JF, Wang JY, Luo YE, Chen HH. Influence of hypertension, lipometabolism disorders, obesity and other lifestyles on spontaneous intracerebral hemorrhage. Biomed Environ Sci. (2003) 16:295–303.
41. Lee HJ, Lee JS, Choi JC, Cho YJ, Kim BJ, Bae HJ, et al. Simple estimates of symptomatic intracranial hemorrhage risk and outcome after intravenous thrombolysis using age and stroke severity. J Stroke. (2017) 19:229–31. doi: 10.5853/jos.2016.01109
42. Lou M, Safdar A, Mehdiratta M, Kumar S, Schlaug G, Caplan L, et al. The HAT Score: a simple grading scale for predicting hemorrhage after thrombolysis. Neurology. (2008) 71:1417–23. doi: 10.1212/01.wnl.0000330297.58334.dd
43. Mazya M, Egido JA, Ford GA, Lees KR, Mikulik R, Toni D, et al. Predicting the risk of symptomatic intracerebral hemorrhage in ischemic stroke treated with intravenous alteplase: safe Implementation of Treatments in Stroke (SITS) symptomatic intracerebral hemorrhage risk score. Stroke. (2012) 43:1524–31. doi: 10.1161/STROKEAHA.111.644815
44. Flint AC, Faigeles BS, Cullen SP, Kamel H, Rao VA, Gupta R, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke. (2013) 44:3365–9. doi: 10.1161/STROKEAHA.113.002794
45. Chiu H, Tsai HJ, Huang JC, Wu PY, Hsu WH, Lee MY, et al. Associations between triglyceride-glucose index and micro- and macro-angiopathies in type 2 diabetes mellitus. Nutrients. (2020) 12:328. doi: 10.3390/nu12020328
46. Nam KW, Kwon HM, Jeong HY, Park JH, Kwon H, Jeong SM. High triglyceride-glucose index is associated with subclinical cerebral small vessel disease in a healthy population: a cross-sectional study. Cardiovasc Diabetol. (2020) 19:53. doi: 10.1186/s12933-020-01031-6
47. Marik PE., Bellomo R. Stress hyperglycemia: an essential survival response! Crit Care. (2013) 17:305. doi: 10.1186/cc12514
48. Luitse MJ, Velthuis BK, Kappelle LJ, van der Graaf Y, Biessels GJ, Group DS. Chronic hyperglycemia is related to poor functional outcome after acute ischemic stroke. Int J Stroke. (2017) 12:180–6. doi: 10.1177/1747493016676619
49. Giri B, Dey S, Das T, Sarkar M, Banerjee J, Dash SK. Chronic hyperglycemia mediated physiological alteration and metabolic distortion leads to organ dysfunction, infection, cancer progression and other pathophysiological consequences: An update on glucose toxicity. Biomed Pharmacother. (2018) 107:306–28. doi: 10.1016/j.biopha.2018.07.157
50. Soeters MR, Soeters PB. The evolutionary benefit of insulin resistance. Clin Nutr. (2012) 31:1002–7. doi: 10.1016/j.clnu.2012.05.011
51. Dungan KM, Braithwaite SS, Preiser JC. Stress hyperglycaemia. Lancet. (2009) 373:1798–807. doi: 10.1016/S0140-6736(09)60553-5
52. Bar-Or D, Rael LT, Madayag RM, Banton KL, Tanner A, Acuna DL, et al. Stress hyperglycemia in critically ill patients: insight into possible molecular pathways. Front Med (Lausanne). (2019) 6:54. doi: 10.3389/fmed.2019.00054
53. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. (2013) 36:587–97. doi: 10.1016/j.tins.2013.07.001
Keywords: acute ischemic stroke, triglyceride, triglyceride-glucose index, intravenous thrombolysis, symptomatic intracranial hemorrhage
Citation: Lin S-F, Hu H-H, Chao H-L, Ho B-L, Chen C-H, Chan L, Lin H-J, Sun Y, Lin Y-Y, Chen P-L, Lin S-K, Wei C-Y, Lin Y-T, Lee J-T and Chao A-C (2022) Triglyceride-Glucose Index and Intravenous Thrombolysis Outcomes for Acute Ischemic Stroke: A Multicenter Prospective–Cohort Study. Front. Neurol. 13:737441. doi: 10.3389/fneur.2022.737441
Received: 07 July 2021; Accepted: 11 January 2022;
Published: 14 February 2022.
Edited by:
Peter Sporns, University Hospital of Basel, SwitzerlandReviewed by:
Sheng Zhang, Zhejiang Provincial People's Hospital, ChinaCopyright © 2022 Lin, Hu, Chao, Ho, Chen, Chan, Lin, Sun, Lin, Chen, Lin, Wei, Lin, Lee and Chao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Han-Hwa Hu, aGFuaHdhQGhvdG1haWwuY29t; Hai-Lun Chao, Y2hhby5oZWxlbkBnbWFpbC5jb20=; A-Ching Chao, YWNoY2hAY2Mua211LmVkdS50dw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.