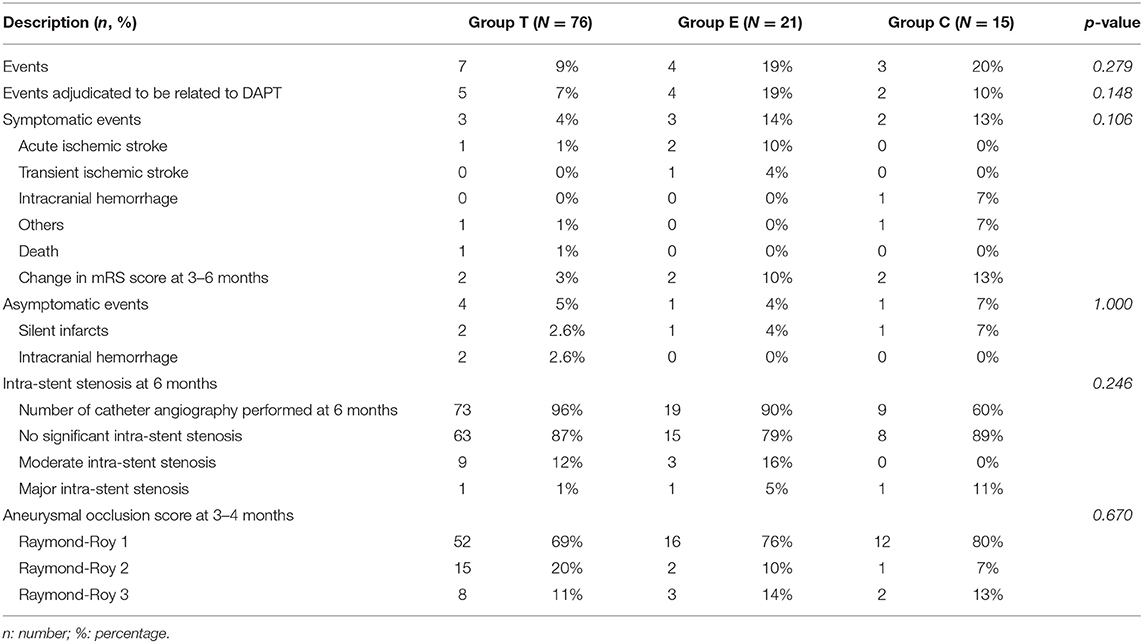
- 1Neuroradiology Unit, Department of Radiology, University Hospital of Brest, Brest, France
- 2Department of Neurosurgery, University Hospital of Brest, Brest, France
- 3Laboratory of Medical Information Processing—LaTIM INSERM UMR 1101, Brest, France
- 4Western Brittany Thrombosis Study Group GETBO EA3878, Brest, France
Introduction: Thromboembolic events represent the most frequent complications of endovascular treatment of unruptured intracranial aneurysm using stent-assisted coilling or flow diverter stents. Dual antiplatelet therapy has become the standard to prevent these but remains unstandardized. We present here a single center experience of 3 standardized antiplatelet regimens during brain aneurysm treatment, while emphasizing the use of the Cangrelor.
Method: We retrospectively reviewed data from patients treated using stent-assisted coilling or flow diverter stents from 2016 to 2021. We collected and compared safety and efficacy data within 6 months of three groups of patients corresponding to three antiplatelet standardized regimens: group T with Ticagrelor, with preprocedural preparation; group E with Eptifibatide, injected during procedure; group C with Cangrelor, injected during procedure.
Results: Data of 112 patients were analyzed and 76 belonged to group T, 21 to group E, and 15 to group C. Eleven events over the 14 recorded were adjudicated to be related to antiplatelets, their repartition did not differ between the 3 groups (p = 0.43). All symptomatic events (N = 8) were not distributed significantly differently between the 3 groups (p = 0.11) and asymptomatic events were also balanced (p = 1.00). Of these, 6 subjects had a change in the mRS score at 3–6 months. Thrombo-embolic events represented the most encountered events in the sample: 2 acute ischemic strokes were recorded in group E and 1 in group T; 1 transient ischemic stroke was noted in group E; 4 silent infarcts were found on control MRI (2 belonged to group T, 1 to group E and 1 to group C). Among 3 intracranial bleeding events, 1 was symptomatic in group C, 2 were asymptomatic in group T. On the control evaluation performed at 6 months, there was no significant difference on aneurysmal occlusion (p = 0.67).
Conclusion: This single-center retrospective study compared 3 antiplatelet regimens, finding no significant difference in the safety and efficacy in the context of endovascular treatments of unruptured aneurysm using stent or flow diverters. This study adds data for the Cangrelor use and suggests its usefulness in the field of neuro-endovascular intervention. Randomized controlled studies are warranted to confirm these results.
Introduction
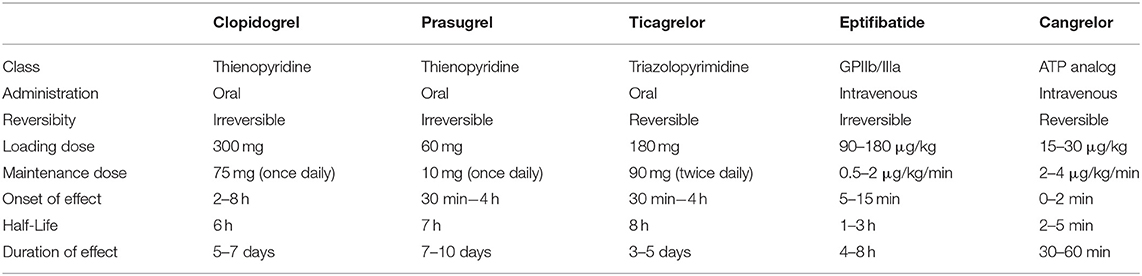
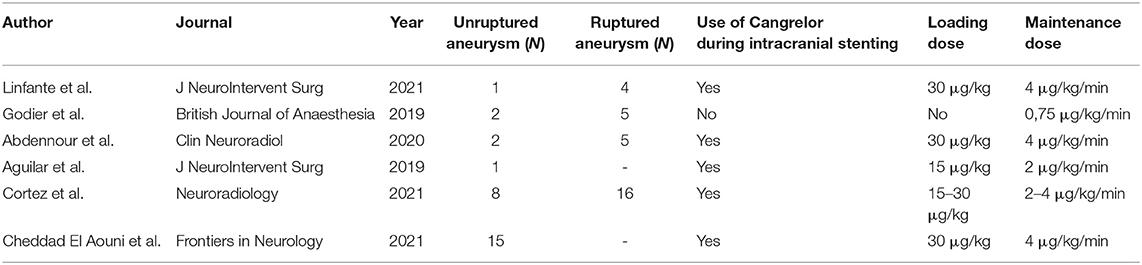
Stent-assisted endovascular therapies of intracranial aneurysms may be complicated by thromboembolic events (1, 2). Dual antiplatelet therapy (DAPT) has become a standard regimen to prevent them (3). Actually, there is no standardized DAPT protocol in interventional neuroradiology, which leads to divergent drugs use (4). The use of aspirin and a P2Y12 receptor antagonist is inferred from the cardiology literature (5). Historically, Clopidogrel was the first P2Y12 inhibitor to be used. Clopidogrel has the disadvantage of a wide response variability (6) and a duration of effect of more than 5 days (7). Ticagrelor and Prasugrel are faster acting (30 min−4 h) than Clopidogrel [2–8 h (8)] and they also present less interpatient response variability (7). These three P2Y12 inhibitors can only be administered orally. According to the cardiology literature (9–11), Eptifibatide has the advantage of being used intravenously, with a short onset (15 min) and offset of action (within 4–8 h) (12). Quite similar in structure to Ticagrelor, Cangrelor also allows intravenous administration (13) and short on/offsets (1 min) with a half-life of 3–5 min. Table 1 summarizes the pharmacokinetic and pharmacodynamic properties of these antiplatelet agents. In 2015, Cangrelor was approved by the U.S. Food and Drug Administration as adjunctive therapy for percutaneous coronary intervention as it was effective in reducing intra-stent thrombosis and the risk of peri-procedural myocardial infarction (14). Table 2 summarizes primary studies that investigated the use of Cangrelor in interventional neuroradiology (15–19). These studies used different doses of Cangrelor either for bolus (15–30 μg/kg) or maintenance (0.75–4 μg/kg/min), furthermore these studies reported various procedures from mechanical thrombectomy to ruptured aneurysm treatment.
In this study, we aimed to analyse the clinical safety and efficacy of three standardized antiplatelet regimens used during scheduled endovascular procedures for unruptured aneurysms using stent-assisted coilling (SAC) or flow diverter stents (FD), while emphasizing the place of Cangrelor.
Method
Population
Data from all patients treated for intracranial aneurysm from February 2016 to March 2021 at the University Hospital were retrospectively collected. Patients treated by simple coilling and ruptured aneurysms were excluded. Three groups of patients were constituted according to their initial antiplatelet regimen that included Ticagrelor, Eptifibatide or Cangrelor. Patients for which another molecule was used were not considered.
We retrospectively collected basic patient demographics, comorbidity, diagnosis, target aneurysm characteristics and procedure-related outcomes and complications from patient medical records included in a prospectively maintained database of all the interventional neuroradiology procedures performed in the center.
Procedures
Every indication for endovascular treatment was previously validated during a multidisciplinary meeting. The initial treatment strategy was recorded. According to the center habits, antiplatelet regimen was decided before the procedure. For the treatments with anticipated high risk of bleeding (as turning inside the aneurysm, difficult catheterization of recurrent branches, associated microaneurysms, etc.), intravenous antiplatelets were preferred (without P2Y12 loading dose before the procedure). Treatment related details were recorded.
Anti-platelet Regimen Groups
No platelet aggregation test was performed. All patients received a dose of 75 mg Aspirin 1 day prior to the procedure and on the day of the procedure. Systemic anticoagulation was achieved using an intravenous bolus injection of heparin (50 IU/kg, 3,000–5,000 IU, range) after the femoral/radial artery access followed by an additional 1,000–2,000 IU/h according to the results of the activated clotting time.
Group T used a loading dose of 180 mg Ticagrelor 1 day prior to the procedure, and 90 mg Ticagrelor on the day of the procedure.
Group E used a loading dose of 180 μg/kg Eptifibatide during the procedure. If used, the maintenance dose of Eptifibatide was administered at 2 μg/kg/min, within 2 h.
Group C used a loading dose of 30 μg/kg Cangrelor during the procedure, followed by a maintenance dose at 4 μg/kg/min.
Decision of an oral maintenance therapy of 90 mg Ticagrelor twice a day for group T was made after the procedure as soon as a clinical evaluation was possible. Decision of an oral switching for group E and C was made after the procedure as soon as a clinical evaluation was possible, via a loading dose of an oral P2Y12 receptor inhibitor (180 mg Ticagrelor or 60 mg Prasugrel), administered 2 h after discontinuation of Eptifibatide, or 30 min before Cangrelor infusion was stopped. All patients received a daily dose of 75 mg Aspirin as a maintenance therapy after the decision of oral DAPT.
Safety and Efficacy
Main outcome of interest was the presence of an event directly related to DAPT. Adjudication of such accountability was consensually made (by all investigators) on the standardized WHO-UMC system for case causality assessment by taking into account the events which corresponded to the terms “Certain” and “Probable / Likely” (20), with access to all data.
Other secondary items of safety were recorded mainly as symptomatic/asymptomatic hemorrhage/ischemic or other procedure-related events.
Symptomatic events included: intracranial hemorrhage, ischemic stroke, other neurologic symptoms unrelated to ischemia nor hemorrhage, arterial access site complication requiring surgical management and death from any cause within 3 months. Neurological symptoms were also classified as permanent or transient.
Asymptomatic events included transient ischemic attack and silent ischemia/hemorrhage were assessed on the basis of the control MRI at 3–4 months.
Additional relevant outcomes were shift and description of functional conditions from baseline to 3–6 months using the modified Rankin Scale (mRS) (21).
Endovascular treatment efficacy was passed on the basis of both control catheter angiography and MRI at 3–6 months. Eventual intrastent stenosis was recorded in three categories: unsignificant (<20% of the lumen), moderate (20–39%) and substantial (>40%); and aneurysmal occlusion using the Raymond Roy classification (22). The review of all imaging points were made consensually by the neuro-interventionists, JCG – JO – MA – MCEA, blinded to the clinical data.
Average Cost of Antiplatelets Regimen
The cost of antiplatelet drugs was reported using the following actual hospital market price: 50 mg Cangrelor powder for concentrate for solution for injection/infusion preparation USD 403.3; 10cc vial (2 mg/ml) Eptifibatide USD 36.87; 100cc vial (0.75 mg/ml) Eptifibatide USD 76.48; 90 mg Ticagrelor USD 1.15; 75 mg Aspegic USD 0.045.
The calculation of the cost was based on the actual consumption of antiplatelet drugs for each patient, extracted from the anesthetic record, taking into account the antiplatelet preparation before the procedure and during the procedure, and without taking into account the cost of the maintenance dose. If an infusion was maintained after the procedure without immediate oral switch, this was also taken into account in the cost. This global cost was then given by the average of this consumption for each regimen group.
Statistics
Data were analyzed using STATA/MP 16 (Statacorp LP, USA) with the aim to compare the safety and efficacy endpoints between the three groups of antiplatelet regimens. A parsimonious analysis was carried out: when they were more adequate, descriptive statistics were preferred. Quantitative variables were described by median an interquartile range (IQR) and compared using a Kruskal-Wallis test. A Chi-squared or Fisher's exact test was used when comparing frequencies between groups for qualitative variables. A value of p < 0.05 was considered significant.
Ethical Statement
The use of Cangrelor was off-label in this study. In all cases no final alternative was found to intracranial stenting and the choice to treat these patients under Cangrelor was made multidisciplinary. Patients were informed before each treatment of the initial strategy and possible alternatives and gave verbal consent. The study and need for patient informed consent was conducted in accordance with actual laws and ethics and with the Helsinki Declaration and its revisions: as a non-interventional retrospective study, a commitment to compliance (Reference Methodology MR-3) was declared to the French national information science and liberties commission (CNIL), in respect to the General Data Protection Regulation. NCT04504695.
Results
Population and Procedure
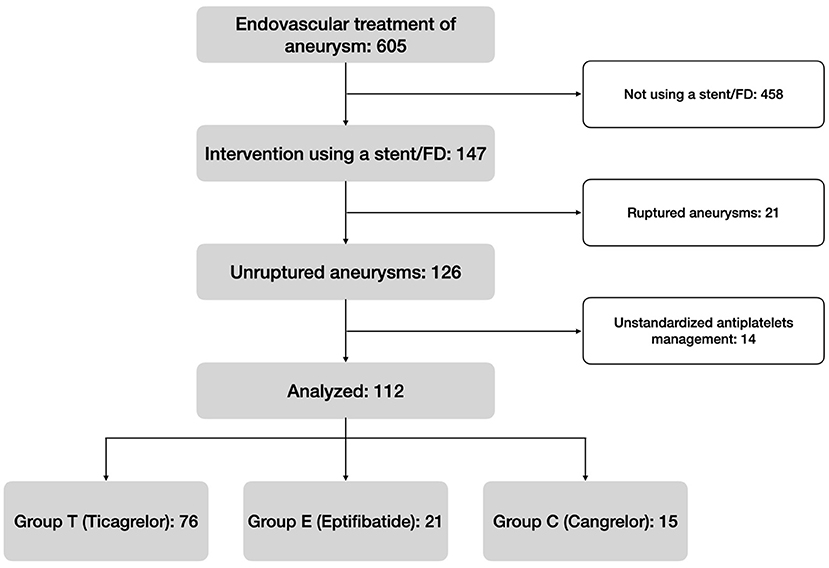
From February 2016 to March 2021, 605 patients who underwent endovascular intervention for the treatment of an aneurysm were screened. One hundred and forty seven of these were treated by SAC or FD, and 126 were scheduled. Fourteen cases were finally ruled out because the patients did not initially receive neither Ticagrelor, Eptifibatide, nor Cangrelor. Figure 1 summarizes the flow chart.
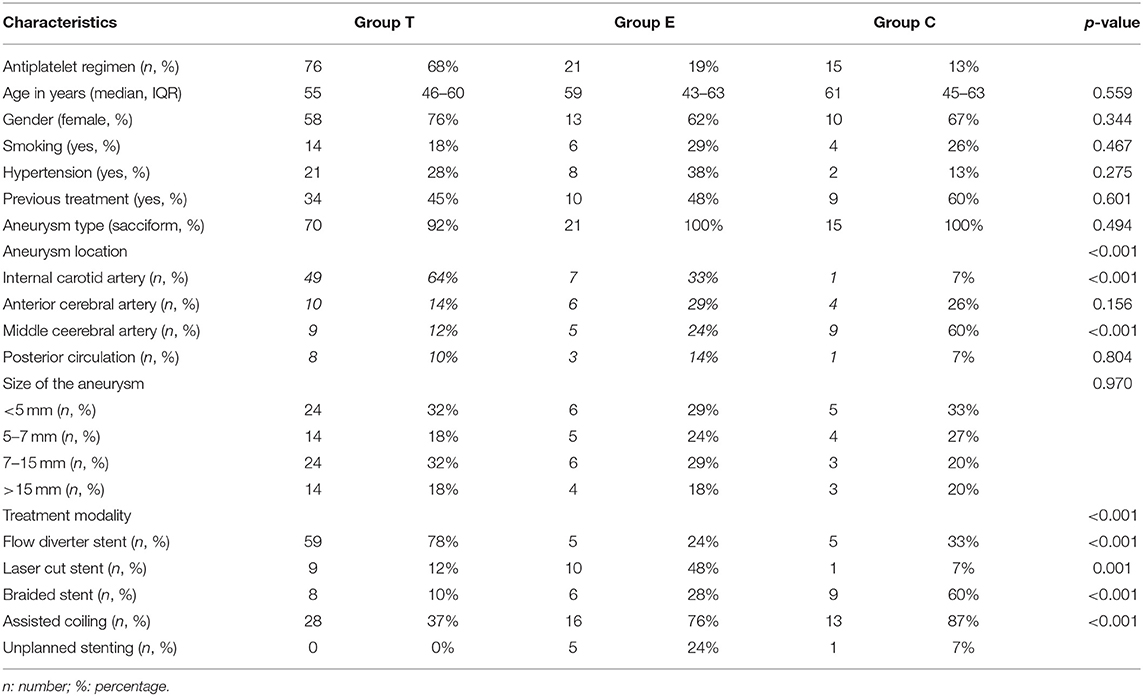
A total of 112 patients were retrospectively analyzed and 76 belonged to group T (68%), 21 to group E (19%), and 15 to group C (13%). Eighty-one (72%) were women and the median age was 55 years (45–63 years, IQR). No patients had renal failure. Only one stent was used for each procedure. There were no significant differences between the three groups concerning the patients' baseline-characteristics (p>0.05), neither regarding aneurysm shape (p = 0.494) or size (p = 0.970). Table 3 summarizes the general characteristics of the population.
Half (N = 57) of the intervention consisted in SAC and there were significantly less SAC in group T (p < 0.001; 37 vs. 76% in group E and 87% in group C). Seven stent placements (6%) were not initially planned. Considering the type of stents used, 20 (18%) were open-cell stents (Neuroform Atlas, Stryker, USA), 23 (20%) were braided stents (Leo+ and Leo+ baby, Balt, USA; Lvis Evo, Microvention, USA), and 69 (62%) were FD (Pipeline Embolization Device, Medtronic, USA; Silk Vista and Silk Vista Baby, Balt, USA; Surpass Evolve, Stryker, USA). More use of FD were significantly depicted in group T (p < 0.001; 78 vs. 24% in group E and 33% in group C). In group T, stents were preferentially placed in the internal carotid artery (51%, p = 0.001), in group E indifferently (p = 0.125), in group C in the middle cerebral artery (60%, p < 0.001).
Safety and Efficacy
All the patients' DAPT were switched to oral DAPT maintenance regimen; except for one of the patients in the group C for which antiplatelets were definitely stopped after a vessel rupture. All patients in groups T and E had a relay with Ticagrelor as a maintenance regimen. In group C, 13 patients were switched to Ticagrelor, and 1 to Prasugrel because of respiratory insufficiency.
The overall results concerning safety and efficacy are summarized in Table 4.
Eleven events over the 14 recorded were adjudicated (i.e., certainly or likely) to be related to DAPT (10% of events related to DAPT in the total sample), their repartition did not differ between the 3 groups (p = 0.432; 7% in group T, 15% in group E, 13% in group C).
All symptomatic events (N = 8) were not distributed significantly differently between the 3 groups (p = 0.106; 4% of the group T, 14% in group E and 13% in group C) and asymptomatic events were also balanced (p = 1.000; 5% in group T, 4% in group E and 7% in group C). Of these 8 symptomatic events, 6 subjects had a change in the mRS score at 3–6 months: 2 in group T (from 5 to 6 and from 0 to 2), 2 in group E (from 1 to 3 and from 0 to 1), 2 in group C (from 0 to 1 and from 3 to 4).
Death occurred in one patient in group T 2 months after the intervention and was related to aspiration pneumonia linked to general condition deterioration caused by a mass effect of a giant carotid aneurysm.
Thrombo-embolic events represented the most encountered events in the sample (8/14). Two acute ischemic strokes were recorded in group E and one in group T. One transient ischemic stroke was noted in group E. Four silent infarcts were found on control MRI (two belonged to group T, one to group E and one to group C). No delayed acute ischemic presentations were reported.
Among three intracranial hemorrhage events, one was symptomatic and occurred in group C (vessel rupture), two were asymptomatic and occurred in group T. These cases are illustrated in the Figure 2.
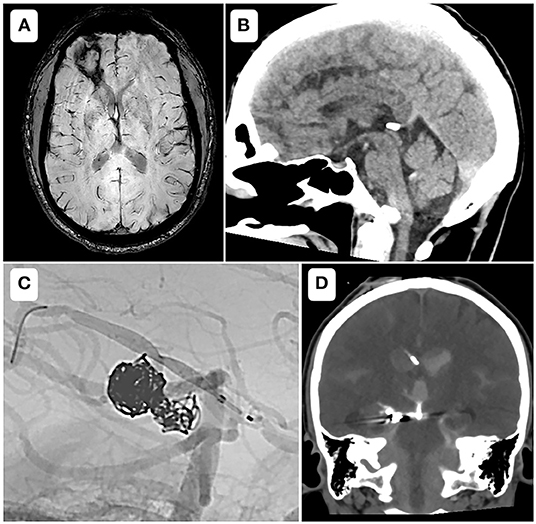
Figure 2. Bleeding events. (A) Axial susceptibility weighted imaging, showing a parenchymal bleeding sequelae at 4 months of a stent assisted coiling of the recanalization of a previously ruptured anterior communicating artery aneurysm. The patient belonged to group T and did not present any neurologic deficit. (B) Sagittal reformat of a day 1 control CT-scanner, after a placement of a flow diverter to treat an unruptured fusiform aneurysm of the right vertebral artery, showing an isolated intraventricular hemorrhage in the fourth ventricle. The patient belonged to group T and did not present any neurologic deficit. (C) Case of a 33-year-old female, modified Rankin Scale 3, with a history of aneurysmal rupture, who underwent a scheduled treatment for an early recanalization of a right posterior cerebral artery aneurysm. After coilling through a microcatheter from the right vertebral artery, a low profile flow diverter was placed through a microcatheter from the right posterior communicating artery. An intra-stent balloon angioplasty provoked a vessel rupture that was jugulated with glue. Heparin was reversed and Cangrelor infusion was stopped. The neurosurgeon was immediately notified and a ventricular derivation was performed within 30 min (D). The patient kept left hemiparesis, with a modified Rankin Scale of 4.
Two other miscellaneous events were recorded: one pseudo-aneurysm of the superficial femoral artery that required surgical intervention in the group C, and one contrast-induced encephalopathy that occurred in group T.
One hundred and one patients (90%) underwent a follow-up catheter angiography. There was no significant difference in the repartition of intra-stent stenosis between the 3 groups (p = 0.246), neither considering the aneurysmal occlusion rates (p = 0.670). The occlusion of the target aneurysm was complete at the control imaging (3–6 months) according to Raymond Roy's classification in 69% in group T, 76% in group E and 80% in group C.
Average Cost
In the group T, all the patients were given previously describe medication, for an average cost of USD 3.54 per patient.
In the group C, two patients required an additional vial of cangrelor due to their weight, for an average cost of USD 457.16 per patient.
In group E, two patients were continued on Eptifibatide for 24 h and one patient for 72 h due to three declared ischemic events, for a mean cost of USD 217.77 per patient.
Discussion
Safety and Efficacy
Facing the heterogeneity of practices and studies, the optimal DAPT regimen(s) for interventional neuroradiology cases remain unclear. This study did not depict a significant difference in the repartition of DAPT related events between the three groups of antiplatelet regimens, neither in the rate of all symptomatic (or a symptomatic) events. Thus, our study support the growing literature on the pending demonstration that Cangrelor could be an effective antiplatelet agent for preventing thromboembolic events in situation of stenting, and a safe agent regarding bleeding risk and possibility to reverse its effect rapidly.
The only event that occurred in the group that used Cangrelor that was considered as a thromboembolic complication was a silent infarction on the control MRI. This event did not modify the functional outcome of the patient nor the latter management of DAPT. Cangrelor was found to be useful when decision was made to proceed without DAPT regimen upfront because the need of stenting was not anticipated (6% of the sample), and these results are encouraging in terms of efficacy of the chosen antiplatelet management. Also, the percentage of symptomatic ischemic complications (3%) found in this study is in agreement with the literature: a meta-analysis of symptomatic ischemic complications with the use of a flow diverter by O'Kelly et al.found a rate of 4% (23) and a second meta-analysis with the use of a stent-assisted coiling by Phan et al. found a rate of symptomatic ischemia of 4.5% (1).
The only bleeding event using Cangrelor was symptomatic (Figure 2), and occurred while treating a previously ruptured P2-P3 aneurysm by a low profile FD. The operator decided to inflate the balloon in the stent and experienced an arterial rupture. The heparin was immediately reversed and the infusion of Cangrelor stopped, followed by an occlusion the posterior communicating axis. The neurosurgery team was immediately notified and an external ventricular derivation was performed within 30 min. At 3 months persisted a left hemiparesis, with a mRS score of 4 (for an initial mRS of 3). This case perfectly emphasizes that the rapid offset of Cangrelor allows prompt management during per procedural bleeding complications (i.e., aneurysm rupture, vessel perforation), or need of a surgical intervention (8).
The last event that occurred in patients under Cangrelor was a femoral puncture site false aneurysm, requiring surgical management. The mRS score of this patient was modified at 3 months (from 0 to 1).
The DAPT regimen did not affect aneurysm occlusion at 3–4 months and intra-stent stenosis on arteriography at 6 months.
Cangrelor Use
A loading dose of Cangrelor of 30 μg/kg and a maintenance dose of 4 μg/kg/min were used in our study. This dosage was derived from the cardiology literature (24). Preliminary studies have investigated Cangrelor at half dose, with a loading dose of 15 μg/kg and a maintenance dose of 2 μg/kg/min (18, 19). These studies did not show an increased ischemic risk. In our center, the choice of Cangrelor was preferred to Ticagrelor when there was a risk of bleeding during the procedure. Further studies are needed to assess the optimal dosage of Cangrelor for endovascular neuro-interventions regarding its safety and efficacy.
The use of Cangrelor requires careful planning with the anesthesia team. The maintenance infusion should be delivered immediately after the loading bolus. We prepared at the same time all syringes and operators had to wait 10 min after the bolus to place the stent and had to paid attention to the injection site patency until the oral relay.
Ticagrelor is the best choice for oral relay because it binds to the P2Y12 receptor in a different way than Cangrelor (25). In our study one subject in Group C was given Prasugrel as a relay because of pre-existing respiratory failure, limiting the theoretical use Ticagrelor.
According to the cardiologic literature, Ticagrelor can be given at any time during the infusion of Cangrelor (25). In vitro studies have shown interaction of Cangrelor with Prasugrel and Clopidogrel (26). In order to limit these interactions, it is recommended for the relay with Prasugrel to be performed 30 min before the end of the Cangrelor infusion (27). Clopidogrel should only be delivered after the end of the Cangrelor infusion (28). In our practice, we favor a relay with Ticagrelor because of less drug-drug interaction with Cangrelor, and less interpatient response variability.
One should not forget that Cangrelor is still an expensive drug and that cost-effectiveness analysis may be welcome in further studies, similar to that made with Ticagrelor (29).
Limitation
This was a retrospective, non-randomized, single-center study. Authors acknowledge an obvious selection bias: patients with anticipated procedure-related hemorrhage risk were preferentially treated using Eptifibatide or Cangrelor. Furthermore, the group using a Ticagrelor preparation used more FD without coiling. Similarly, at-risk locations, such as the middle cerebral artery because of the presence of recurrent divisions, were more represented in the groups that received intravenous antiplatelets. These groups (E and C) also included a greater proportion of patients for whom stenting was not initially planned, secondary to coil protrusion or, conversely, to avoid protrusion of a risky coil. Another limitation could be the relative diversity of procedures and devices used, as new medical implant devices with surface modifications are currently being developed and the safety and efficacy profiles of antiplatelets management in these cases should be studied (30, 31).
Our study included 15 patients treated using Cangrelor. To our knowledge, this is the largest current cohort of unruptured aneurysms with the use of Cangrelor (Table 2). Results of safety profile of Cangrelor was compared to two other standardized DAPT regiment in our center, but not compared to a proper control group. Also, different dosing protocols are described in the literature and have not been compared. Further prospective comparative studies with larger cohorts are needed to confirm our results and clarify the best protocols and real comparative safety profiles. Studies of Cangrelor in interventional neuroradiology are still rare. This medication has a rapid onset and offset of action, owing to its short half-life, that fit the demand for neuro-intervention procedures. This preliminary study paves the way for a randomized analysis to confirm its potential for routine use.
Conclusion
This single-center, retrospective study over 4 years and 3 months compared three DAPT regimens in the context of aneurysm treatment requiring a stent/FD, through the report of their safety and efficacy. From the results, Cangrelor allows for a secure transition to long-term DAPT and secured surgery in cases of unexpected complications. The studies on Cangrelor are still rare with few patients. Randomized controlled studies are warranted to confirm the results of our study.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MN, JG, and EM contributed to conception and design of the study. MC and MA organized the database. JO and MC performed the statistical analysis. MC, JO, and MA wrote the first draft of the manuscript. MC, EM, MA, MN, JG, and JO wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SAC, stent-assisted coiling; FD, flow diverter stents; DAPT, Dual antiplatelet therapy; MRI, Magnetic Resonance Imaging; mRS, modified Rankin Scale.
References
1. Phan K, Huo YR, Jia F, Phan S, Rao PJ, Mobbs RJ, et al. Meta-analysis of stent-assisted coiling versus coiling-only for the treatment of intracranial aneurysms. J Clin Neurosci. (2016) 31:15–22. doi: 10.1016/j.jocn.2016.01.035
2. Adeeb N, Griessenauer CJ, Foreman PM, Moore JM, Shallwani H, Motiei-Langroudi R, et al. Use of platelet function testing before pipeline embolization device placement: a multicenter cohort study. Stroke. (2017) 48:1322–30. doi: 10.1161/STROKEAHA.116.015308
3. Kim KS, Fraser JF, Grupke S, Cook AM. Management of antiplatelet therapy in patients undergoing neuroendovascular procedures. J Neurosurg. (2018) 129:890–905. doi: 10.3171/2017.5.JNS162307
4. Martínez M, Onal Y, Cohen JE, Kalousek V, Rivera R, Sordo JG, et al. First multicenter experience using the Silk Vista flow diverter in 60 consecutive intracranial aneurysms: technical aspects. J Neurointervent Surg. (2021) 13:1145–51. doi: 10.1136/neurintsurg-2021-017421
5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. (2009) 361:1045–57. doi: 10.1056/NEJMoa0904327
6. Flechtenmacher N, Kämmerer F, Dittmer R, Budde U, Michels P, Röther J, et al. Clopidogrel resistance in neurovascular stenting: correlations between light transmission aggregometry, verify now, and the multiplate. AJNR Am J Neuroradiol. (2015) 36:1953–8. doi: 10.3174/ajnr.A4388
7. Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, et al. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. (2010) 121:1188–99. doi: 10.1161/CIRCULATIONAHA.109.919456
8. Qamar A, Bhatt DL. Current status of data on cangrelor. Pharmacol Therapeutics. (2016) 159:102–9. doi: 10.1016/j.pharmthera.2016.01.004
9. Wang TY, White JA, Tricoci P, Giugliano RP, Zeymer U, Harrington RA, et al. Upstream clopidogrel use and the efficacy and safety of early eptifibatide treatment in patients with acute coronary syndrome: an analysis from the early glycoprotein IIb/IIIa inhibition in patients with non–ST-segment elevation acute coronary syndrome (EARLY ACS) trial. Circulation. (2011) 123:722–30. doi: 10.1161/CIRCULATIONAHA.110.958041
10. Harrington RA. Design and methodology of the PURSUIT trial: evaluating Eptifibatide for acute ischemic coronary syndromes. Am J Cardiol. (1997) 80:34B−8B. doi: 10.1016/S0002-9149(97)00575-4
11. Granada JF, Kleiman NS. Therapeutic use of intravenous eptifibatide in patients undergoing percutaneous coronary intervention: acute coronary syndromes and elective stenting. Am J Cardiovasc Drugs. (2004) 4:31–41. doi: 10.2165/00129784-200404010-00004
12. Dornbos D, Katz JS, Youssef P, Powers CJ, Nimjee SM. Glycoprotein IIb/IIIa inhibitors in prevention and rescue treatment of thromboembolic complications during endovascular embolization of intracranial aneurysms. Neurosurgery. (2018) 82:268–77. doi: 10.1093/neuros/nyx170
13. Norgard NB. Cangrelor: a novel P2Y 12 receptor antagonist. Expert Opin Investig Drugs. (2009) 18:1219–30. doi: 10.1517/13543780903136708
14. Bhatt DL, Stone GW, Mahaffey KW, Gibson CM, Steg PG, Hamm CW, et al. Effect of platelet inhibition with cangrelor during PCI on ischemic events. N Engl J Med. (2013) 368:1303–13. doi: 10.1056/NEJMoa1300815
15. Linfante I, Ravipati K, Starosciak AK, Reyes D, Dabus G. Intravenous cangrelor and oral ticagrelor as an alternative to clopidogrel in acute intervention. J NeuroIntervent Surg. (2021) 13:30–2. doi: 10.1136/neurintsurg-2020-015841
16. Godier A, Mesnil M, De Mesmay M, Dagois S, Thion L-A, Dupont C, et al. Bridging antiplatelet therapy with cangrelor in patients with recent intracranial stenting undergoing invasive procedures: a prospective case series. Br J Anaesthesia. (2019) 123:e2–e5. doi: 10.1016/j.bja.2019.03.019
17. Abdennour L, Sourour N, Drir M, Premat K, Shotar E, Taylor G, et al. Preliminary experience with cangrelor for endovascular treatment of challenging intracranial aneurysms. Clin Neuroradiol. (2020) 30:453–61. doi: 10.1007/s00062-019-00811-2
18. Aguilar-Salinas P, Agnoletto GJ, Brasiliense LBC, Santos R, Granja MF, Gonsales D, et al. Safety and efficacy of cangrelor in acute stenting for the treatment of cerebrovascular pathology: preliminary experience in a single-center pilot study. J NeuroIntervent Surg. (2019) 11:347–51. doi: 10.1136/neurintsurg-2018-014396
19. Cortez GM, Monteiro A, Sourour N, Clarençon Clarençon F, Elhorany M, Grigoryan M, et al. The use of cangrelor in neurovascular interventions: a multicenter experience. Neuroradiology. (2021) 63:925–34. doi: 10.1007/s00234-020-02599-2
20. Meyboom RHB, Hekster YA, Egberts ACG, Gribnau FWJ, Edwards IR. Causal or casual?: the role of causality assessment in pharmacovigilance. Drug Safety. (1997) 17:374–89. doi: 10.2165/00002018-199717060-00004
21. Broderick JP, Adeoye O, Elm J. Evolution of the modified rankin scale and its use in future stroke trials. Stroke. (2017) 48:2007–12. doi: 10.1161/STROKEAHA.117.017866
22. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. (2001) 32:1998–2004. doi: 10.1161/hs0901.095600
23. O'Kelly CJ, Spears J, Chow M, Wong J, Boulton M, Weill A, et al. Canadian experience with the pipeline embolization device for repair of unruptured intracranial aneurysms. AJNR Am J Neuroradiol. (2013) 34:381–7. doi: 10.3174/ajnr.A3224
24. Marino M, Rizzotti D, Leonardi S. Cangrelor: review of the drug and the CHAMPION programme (including PHOENIX). Curr Cardiol Rep. (2014) 16:493. doi: 10.1007/s11886-014-0493-4
25. Schneider DJ, Agarwal Z, Seecheran N, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and ticagrelor. JACC Cardiovasc Interv. (2014) 7:435–42. doi: 10.1016/j.jcin.2013.08.017
26. Judge HM, Buckland RJ, Jakubowski JA, Storey RF. Cangrelor inhibits the binding of the active metabolites of clopidogrel and prasugrel to P2Y12 receptors in vitro. Platelets. (2016) 27:191–5. doi: 10.3109/09537104.2015.1069809
27. Schneider DJ, Seecheran N, Raza SS, Keating FK, Gogo P. Pharmacodynamic effects during the transition between cangrelor and prasugrel. Coronary Artery Dis. (2015) 26:42–8. doi: 10.1097/MCA.0000000000000158
28. Schneider DJ, Agarwal Z, Seecheran N, Gogo P. Pharmacodynamic effects when clopidogrel is given before cangrelor discontinuation: transition from cangrelor to clopidogrel. J Interv Cardiol. (2015) 28:415–9. doi: 10.1111/joic.12229
29. Zhao YJ, Khoo AL, Lin L, Teng M, Wu TS, Chan MY, et al. Cost-effectiveness analysis of ticagrelor and prasugrel for the treatment of acute coronary syndrome. Value Health Reg Issues. (2016) 9:22–7. doi: 10.1016/j.vhri.2015.07.001
30. Borchert RJ, Simonato D, R Hickman C, Fuschi M, Thibault L, Henkes H, et al. P2Y12 inhibitors for the neurointerventionalist. Interv Neuroradiol. (2021). doi: 10.1177/15910199211015042. [Epub ahead of print].
Keywords: aneurysm, unruptured, Cangrelor, antiplatelets, stent-assisted coilling, flow diverter
Citation: Cheddad El Aouni M, Magro E, Abdelrady M, Nonent M, Gentric JC and Ognard J (2022) Safety and Efficacy of Cangrelor Among Three Antiplatelet Regimens During Stent-Assisted Endovascular Treatment of Unruptured Intracranial Aneurysm: A Single-Center Retrospective Study. Front. Neurol. 13:727026. doi: 10.3389/fneur.2022.727026
Received: 17 June 2021; Accepted: 02 February 2022;
Published: 04 March 2022.
Edited by:
Alexander Sirakov, University Hospital St. Ivan Rilski, BulgariaReviewed by:
Ahmed Mohamed Elhfnawy, Uniklinikum Giessen und Marburg, GermanyPervinder Bhogal, The Royal London Hospital, United Kingdom
Copyright © 2022 Cheddad El Aouni, Magro, Abdelrady, Nonent, Gentric and Ognard. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mourad Cheddad El Aouni, bW91cmFkLmNoZWRkYWRlbGFvdW5pQGNodS1icmVzdC5mcg==
 Mourad Cheddad El Aouni
Mourad Cheddad El Aouni Elsa Magro2,3
Elsa Magro2,3 Mohamed Abdelrady
Mohamed Abdelrady Julien Ognard
Julien Ognard