- 1Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu, China
- 2Department of Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, China
- 3Department of Radiation Oncolygy, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, China
Background: Traumatic brain injury (TBI) is a serious public health issue all over the world. This study was designed to evaluate the prognostic value of lactate to albumin ratio (LAR) on patients with moderate to severe TBI.
Methods: Clinical data of 273 moderate to severe TBI patients hospitalized in West China Hospital between May 2015 and January 2018 were collected. Multivariate logistic regression analyses were used to explore risk factors and construct a prognostic model of in-hospital mortality in this cohort. A receiver operating characteristic (ROC) curve was drawn to evaluate the discriminative ability of this model.
Results: Non-survivors had higher LAR than survivors (1.09 vs. 0.53, p < 0.001). Results of multivariate logistic regression analysis showed that Glasgow Coma Scale (GCS; odds ratio [OR] = 0.743, p = 0.001), blood glucose (OR = 1.132, p = 0.005), LAR (OR = 1.698, p = 0.022), subdural hematoma (SDH; OR = 2.889, p = 0.006), intraparenchymal hemorrhage (IPH; OR = 2.395, p = 0.014), and diffuse axonal injury (DAI; OR = 2.183, p = 0.041) were independent risk factors of in-hospital mortality in included patients. These six factors were utilized to construct the prognostic model. The area under the ROC curve (AUC) values of single lactate, albumin, and LAR were 0.733 (95% Cl; 0.673–0.794), 0.740 (95% Cl; 0.683–0.797), and 0.780 (95% Cl; 0.725–0.835), respectively. The AUC value of the prognostic model was 0.857 (95%Cl; 0.812–0.901), which was higher than that of LAR (Z = 2.1250, p < 0.05).
Conclusions: Lactate to albumin ratio is a readily available prognostic marker of moderate to severe TBI patients. A prognostic model incorporating LAR is beneficial for clinicians to evaluate possible progression and make treatment decisions in TBI patients.
Introduction
Traumatic brain injury (TBI), defined as an alteration of brain function or other evidence of brain pathology due to external force, is a serious public health problem worldwide (1). Over the past decade, the prognosis of TBI has been improved significantly, attributable to better implementation of pre-admission treatments, rapid CT examination, and high-standard critical care measures (2). However, the continuously increased incidence rate of TBI makes it still a serious public health issue. Consequently, predicting the possible prognosis of patients in the early stage and then making suitable treatment strategies are significant to improve the outcomes of TBI patients. The serum lactate level, a widely acknowledged indicator of tissue hypoperfusion, has been confirmed associated with organ failure and mortality in many clinical settings, such as sepsis, trauma, and pediatric critical Illness (3–7). In addition, several studies have been conducted to explore the prognostic value of serum lactate level in TBI patients (8–10). Most of these studies showed that higher serum lactate was associated with worse injury severity and poor outcome in TBI patients. As an important metabolic marker of the whole body, serum lactate level is actually influenced by many factors, such as hepatic and renal function.
In order to stabilize and improve the predictive value of serum lactate, the lactate to albumin ratio (LAR), a new marker which synthetically combines the clinical significance of lactate and albumin, is developed and practically tested in several groups of patients (11–13). Results of these studies showed that LAR might be superior to single lactate in predicting mortality of critically ill patients. Moreover, recent research concluded that the prognostic value of LAR was better than single lactate on predicting neurologic outcomes and survival to discharge after out-of-hospital cardiac arrest (14). Therefore, we make a reasonable assumption that LAR is similarly valuable in predicting mortality of moderate to severe TBI patients. This observational study was designed to verify our scientific hypothesis.
Materials and Methods
Patients
This study was performed in a West China Hospital. Patients diagnosed with moderate to severe TBI and transferred to our hospital within 4 h after injuries between May 2015 and January 2018 were eligible in this study. Diagnoses of TBI were confirmed according to findings of CT and MRI. Exclusion criteria were listed as below: (1) patients transferred from other hospital after suffering injuries; (2) patients hospitalized in our hospital <48 h; (3) patients complicated with severe hepatorenal, cardiovascular, or respiratory diseases, cancer, and other central nervous system diseases; and (4) patients lacked in complete laboratory results. A total of 273 patients were finally included in this study. The study was approved by the ethics committee of West China hospital, Sichuan University. Informed consent forms for joining observational research of each patient were legally obtained from themselves or their authorized families when they were admitted based on the research policy of our hospital.
Data Collection
Vital signs and Glasgow Coma Scale (GCS) were recorded once patients were admitted to the emergency department of our hospital. Injury Severity Scores (ISSs) of other regions except for the head were added. Sequential Organ Failure Assessment (SOFA) score on the first day of hospitalization was also recorded. The blood samples of patients in admission were taken for blood biochemistry, blood routine, and arterial gas analysis, such as lactate level. Laboratory results of the first blood sample on admission were recorded in this study for statistical analysis. Occurrence of hypoxia on admission was also collected and was divided into two severities, i.e., mild hypoxia (60 mmHg ≤ PaO2 <80 mmHg) and severe hypoxia (PaO2 <60 mmHg). The primary outcome of this study was in-hospital mortality.
Statistical Analysis
Kolmogorov-Smirnov test was performed to test the normality of variables. Normally distributed variables were presented as mean ± SD, and non-normally distributed variables were presented as median (interquartile range). Moreover, categorical variables were shown in the form of numbers (percentage). Independent Student's t-test and Mann-Whitney U-test were respectively performed to analyze differences between two groups of normally distributed and non-normally distributed variables. We performed a Chi-square test to examine the difference of categorical variables. The relationship between LAR and other factors was tested by Spearman rank correlation analysis. Univariate and multivariate logistic regression were sequentially used to explore the association between risk factors and mortality in this study cohort. In addition, independent risk factors were incorporated to construct a prognostic model by multivariate logistic regression. We drew the receiver operating characteristic (ROC) curves and evaluated the predictive value of LAR and the prognostic model by calculating the area under the ROC curves (AUC). Z test was utilized to compare the difference of AUC between LAR and the prognostic model.
A value of p < 0.05 was considered to be of statistical significance. SPSS 22.0 Windows software (SPSS, Inc., Chicago, IL, USA) was used for all statistical analyses and figure drawing.
Results
Baseline Characteristics of Survivors and Non-survivors in TBI Patients
There were 124 survivors and 149 non-survivors with a mortality rate of 54.6% in this study (Table 1). Age and male ratio did not differ between survivors and non-survivors (43 vs. 43, p = 0.707; 77.4 vs. 73.8%, p = 0.491). Motor vehicle crash and falling injury respectively ranked first and second among the injury causes with 66.3 and 19.8%. Initial vital signs, such as systolic and diastolic blood pressure, heart rate, did not differ between survivors and non-survivors (123 vs. 120, p = 0.287; 72 vs. 70, p = 0.094; 98 vs. 103, p = 0.184). However, the body temperature of non-survivors was significantly lower than survivors (36.7 vs. 36.8, p = 0.003). In addition, non-survivors had significantly lower GCS than survivors (5 vs. 7, p < 0.001). Results of laboratory tests showed that non-survivors had a significant higher level of glucose, lactate, LAR, blood urea nitrogen (BUN), serum creatinine, lactate dehydrogenase (LDH), and prothrombin time (PT). Whereas the level of platelet, hemoglobin, and albumin was significantly lower in non-survivors. The incidence of subarachnoid hemorrhage (SAH), subdural hematoma (SDH), intraparenchymal hemorrhage (IPH), diffuse axonal injury (DAI), and cerebral infarction were both higher in non-survivors than survivors. In addition, non-survivors had shorter length of intensive care unit (ICU) stay and length of hospital stay than survivors (p < 0.001).
Correlation Between Other Factors and LAR by Spearman Analysis in Included TBI Patients
Spearman correlation analysis showed that GCS (r = −0.347, p < 0.001) and platelet (r = −0.444, p < 0.001) were negatively and moderately associated with LAR level, while SOFA (r = 0.443, p < 0.001), glucose (r = 0.447, p < 0.001), serum creatinine (r = 0.340, p < 0.001), and PT (r = 0.420, p < 0.001) were positively and moderately related with LAR level (Table 2).
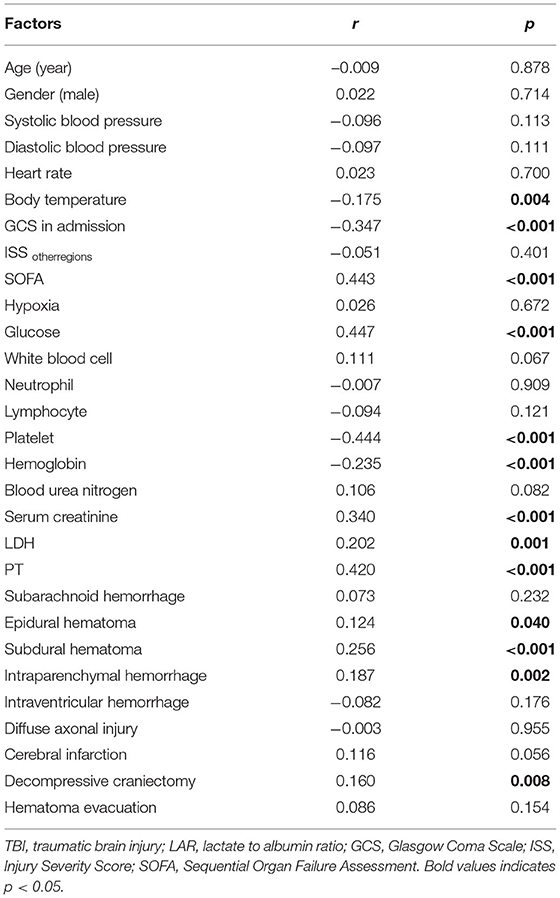
Table 2. Correlation between other factors and LAR by Spearman analysis in patients with moderate to severe TBI.
Univariate and Multivariate Analyses of Risk Factors for Mortality in Included TBI Patients
In univariate logistic regression analysis, we found that body temperature (OR = 0.674, p = 0.004), GCS (OR = 0.647, p < 0.001), platelet (OR = 0.993, p < 0.001), and hemoglobin (OR = 0.978, p < 0.001) were negatively correlated with outcomes in TBI patients (Table 3). Moreover, SOFA (OR = 1.475, p < 0.001), glucose (OR = 1.275, p < 0.001), LAR (OR = 3.611, p < 0.001), BUN (OR = 1.068, p = 0.030), serum creatinine (OR = 1.007, p = 0.003), LDH (OR = 1.002, p < 0.001), PT (OR = 1.292, p < 0.001), SAH (OR = 1.870, p = 0.011), SDH (OR = 2.490, p = 0.001), IPH (OR = 3.199, p < 0.001), and DAI (OR = 1.997, p = 0.010) were risk factors of mortality in the TBI patients. Furthermore, results of multivariate logistic regression analysis indicated that six factors, i.e., GCS (OR = 0.743, p = 0.001), glucose (OR = 1.132, p = 0.005), LAR (OR = 1.698, p = 0.022), SDH (OR = 2.889, p = 0.006), IPH (OR = 2.395, p = 0.014), and DAI (OR = 2.183, p = 0.041), were independently associated with mortality after adjusting confounders.
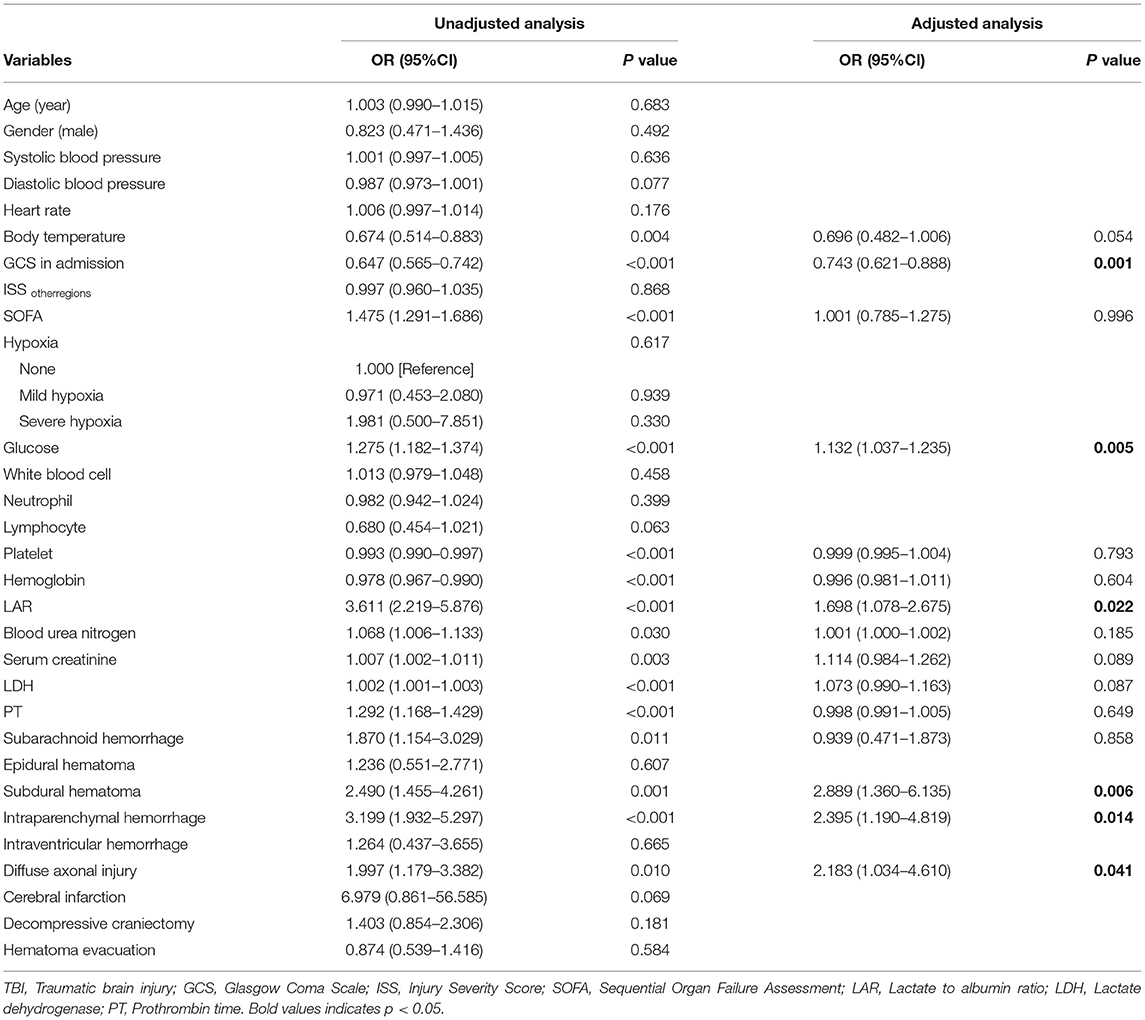
Table 3. Univariate and multivariate logistic regression analysis of risk factors for mortality in patients with moderate to severe TBI.
Predictive Value of LAR and Constructed Prognostic Model
Combining GCS, glucose, LAR, SDH, IPH, and DAI, we constructed a prognostic model for predicting mortality in the TBI patients by multivariate logistic regression analysis. As shown in Figure 1, the AUC values of single lactate, albumin, and LAR are 0.733 (95% Cl; 0.673–0.794), 0.740 (95% Cl; 0.683–0.797), and 0.780 (95% Cl; 0.725–0.835), respectively (Table 4). The AUC value of LAR and the prognostic model was 0.780 (95% Cl; 0.725–0.835) and 0.857 (95% Cl; 0.812–0.901), respectively. The AUC value of LAR was higher than that of GCS (Z = 1.221, p > 0.05), single lactate value (Z = 1.1251, p > 0.05), and single albumin (Z = 0.9923, p > 0.05) though without statistical significance. However, the prognostic model incorporating LAR had a significant higher AUC value than single LAR (Z = 2.1250, p < 0.05).
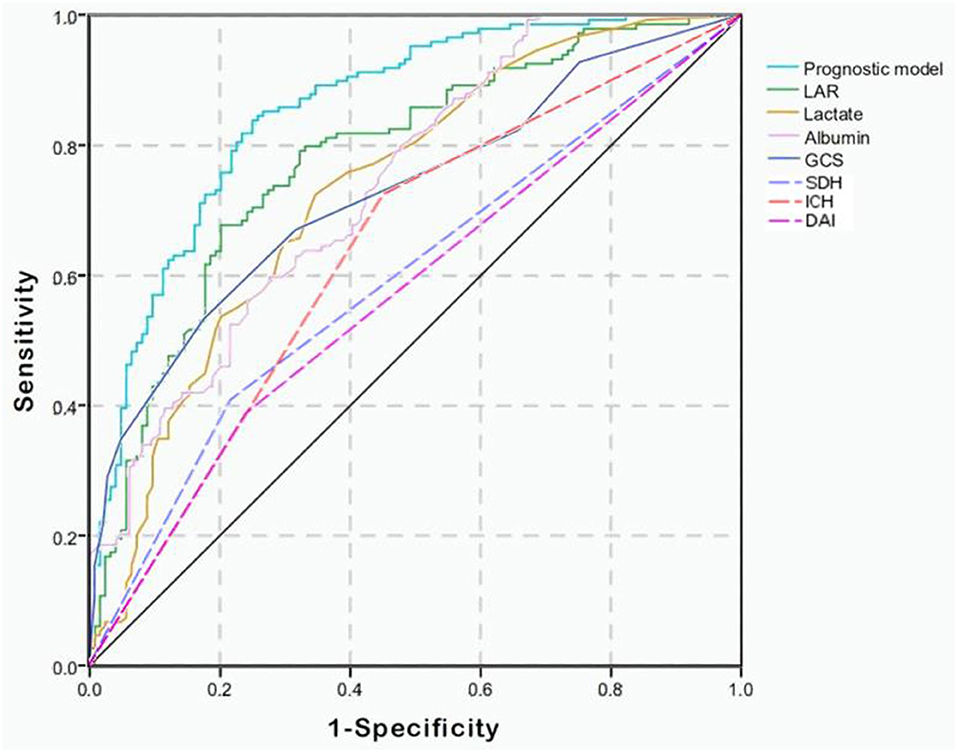
Figure 1. Receiver operating characteristic (ROC) curves of predictive factors and the prognostic model for predicting mortality in moderate to severe TBI patients. The area under the curve (AUC) of GCS, lactate, albumin, LAR, SDH, IPH, and DAI and the constructed prognostic model was 0.729, 0.733, 0.740, 0.780, 0.596, 0.637, 0.574, and 0.857, respectively.
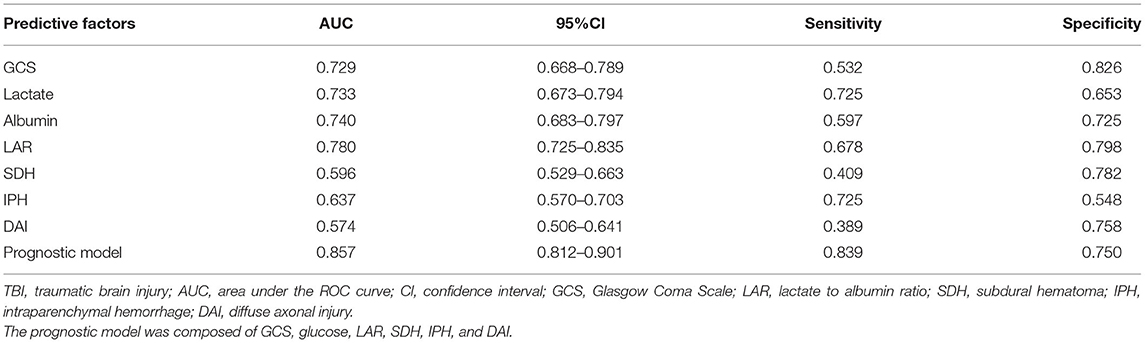
Table 4. Value of factors and constructed prognostic model on predicting mortality in patients with moderate to severe TBI.
Discussion
Previous studies have shown the added prognostic value of lactate value in trauma patients (6, 15, 16). In addition, several research studies have confirmed that LAR was associated with mortality and the development of multiple organ dysfunction syndrome (MODS) in generalized or pediatric sepsis patients (11–13, 17, 18). A recent study showed that LAR had a higher value than single lactate level on predicting neurologic outcomes and survival to discharge in patients suffering out-of-hospital cardiac arrest (14). We make a reasonable hypothesis that LAR would also be superior to single lactate in predicting mortality in TBI patients.
As a component of LAR, the serum lactate is widely acknowledged as an indicator of inadequate tissue perfusion. In addition, the correlation between serum lactate and mortality has been verified in many clinical settings, such as sepsis, shock, and trauma (19–22). However, several studies exploring the association between lactate and outcome in TBI patients showed different conclusions (9, 23–25). One of these studies even indicated that TBI patients whose serum lactate >5 mmol/L were likely to have better survival than those with relatively low lactate level (24). Furthermore, the exogenous supplement of lactate by infusing hypertonic sodium lactate has been verified beneficial for survival and neurologic outcome and cognitive recovery in TBI animal models and patients (26–32). In our study, serum lactate was higher in non-survivors than survivors and was useful in predicting mortality in moderate to severe TBI patients with an AUC of 0.733. The most key point we think to understand and discuss the relationship between blood lactate and the outcome of TBI patients is the different meaning of increased serum lactate between the initial pathophysiological state and exogenous supplement state.
A previous study found that serum lactate would still increase even in normotensive TBI patients (24). This fact indicated an initial increase of serum lactate after TBI could not only be caused by peripheral tissue hypoperfusion due to blood loss, but also the worsening tissue oxygenation due to complications, such as acute lung injury and neurogenic lung edema. The detailed mechanism of initially increased lactate after TBI deserves further exploration. Initially being put forward in 1994, the astrocyte–neuron lactate shuttle has changed the opinion that lactate is only an useless waste during the anaerobic metabolism process (33). It was illustrated that astrocyte would uptake glucose and metabolized it into lactate under the stimulation of much glutamate. The generated lactate would be transferred to neurons and enter the tricarboxylic acid cycle for energy demand of brain. The increased serum lactate directly penetrating the blood-brain-barrier would also accumulate in neuronal intercellular space and be utilized by neurons for energy production (34, 35). Moreover, one study discovered that brain uptake of lactate reflected by arterio-venous differences for lactate (AVDlac) was higher in more severe TBI patients and non-survivors (36). Therefore, a reasonable conjecture is that uptake of lactate from neuron after more severe TBI would decrease more serum levels of lactate. However, it was confirmed that the magnitude of absorbed lactate by brain was extremely small compared with the magnitude of serum lactate level (36). Therefore, the initial fluctuation of serum lactate level after TBI is mainly attributable to pathophysiological changes of the systemic body but not of single brain. This argument might be confirmed by the finding of the previous study that blood lactate levels were associated with SOFA score, which reflects systemic organ failure in unspecified ICU patients (37). In addition, blood lactate level was also verified inversely associated with GCS in isolated TBI patients (24). Although higher serum lactate is beneficial for brain energy supplements, the effect of poor pathophysiologic condition indicated by higher serum lactate on outcome could be greater than relatively transient and small effect of energy supplement. Generally, the initially increased serum lactate in the natural pathophysiologic condition is inversely associated with favorable outcome in TBI patients by reflecting the degree of systemic organ failure and initial brain injury severity.
On the contrary, the continuously increased serum lactate level during exogenous infusion of hypertonic sodium lactate could indicate better survival and recovery after TBI (30, 38, 39). Because increased serum lactate during exogenous supplement is not a reflection of initial tissue hypoperfusion and organ failure, but only means more alternative energy fuel for the injured brain. This is a key point to distinguish the meaning of increased serum lactate under the initial pathophysiological condition and exogenous supplement condition. The beneficial effects of hypertonic sodium lactate on the injured brain have been definitely recognized, i.e., improving cerebral perfusion and brain glucose availability, reversing impaired brain metabolism, and oxygenation. (26–28). In addition to the function of neuroenergetic material, lactate is actually a crucial signaling molecule, which could modulate the production of pentose phosphate, an important molecule to prevent oxidative stress injury in brain (40–43). It was testified that lactate would provide 60% of the energy source for cerebral metabolism as blood lactate increases to 5 mmol/L (44, 45), To sum up, the increased serum lactate level during exogenous supplement of lactate is beneficial for neurologic and survival outcome and cognitive recovery after TBI.
The albumin level of non-survivors was significantly lower than survivors in this study. Produced by hepatocytes, albumin works in multiple ways to maintain the physiologic function of the healthy body, such as constituting plasma osmotic pressure, transporting insoluble small organic molecules, and combining heavy metal ions to eliminate their toxic effects. In addition, low albumin level is also considered as an efficient marker of malnutrition. The cause of hypoalbuminemia after TBI is diversified, i.e., initial blood loss due to injury, consumption by secondary oxidative stress injury, and physiological hypoalbuminemia resulted from massive crystal liquid infusion. The reduction of serum albumin and its association with mortality after TBI have been confirmed in previous studies (46–49). The correlation between hypoalbuminemia and poor outcome of TBI patients could be explained by the brain edema and subsequent increased intracranial pressure resulted from insufficient intravascular osmolality. In addition, a lower level of albumin could indicate more severe degree of the systemic inflammatory response, which was discovered correlated with poor outcome of TBI patients (50, 51). In our study, the AUC value of single lactate was 0.733. After the incorporation of albumin, the AUC value of LAR was increased to 0.780. This result indicated that LAR, calculated by the value of lactate and albumin, could more comprehensively reflect tissue injury severity and systemic organ function of TBI patients. The prognostic model constructed by us, which consisted of GCS, glucose, LAR, SDH, IPH, and DAI, is useful in predicting mortality of moderate to severe TBI patients with high discriminative ability and sensitivity.
This study had several limitations. Firstly, this observational study was performed in a single center so that the selection bias was inevitable. A further prospective study with a larger sample size in other centers should be conducted to externally validate the predictive value of our prognostic model. Secondly, the long-term neurologic outcome and recovery status were not followed up and the specific causes of death were not recorded so that we could not explore the correlation between LAR and them. Thirdly, the drugs and operations of prehospital emergency medical care which could influence the serum lactate level were not recorded by us. Our results might be confounded by these factors.
Conclusion
The LAR is an effective and readily available marker of outcome in moderate to severe TBI patients. The prognostic model incorporating LAR with high predictive value is beneficial for clinicians to evaluate possible progression and make treatment decisions in moderate to severe TBI patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of West China hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
RW: conception and design. MH: collection and assembly of data. RW and MH: data analysis and interpretation. RW and FQ: manuscript writing. JZ and JX: manuscript proofread and revision. All the authors take responsibility for the final manuscript and approve it for publication.
Funding
This work was supported by the 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (ZYJC18007) and the Key Research and Development Project of Science and Technology Department of Sichuan Province (2019YFS0392).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Archiv Physic Med Rehabil. (2010) 91:1637–40. doi: 10.1016/j.apmr.2010.05.017
2. Lu J, Marmarou A, Choi S, Maas A, Murray G, Steyerberg EW. Mortality from traumatic brain injury. Acta neurochirurgica Supplemen. (2005) 95:281–5. doi: 10.1007/3-211-32318-X_58
3. Thanachartwet V, Desakorn V, Sahassananda D, Jittmittraphap A, Oer-Areemitr N, Osothsomboon S, et al. Serum procalcitonin and peripheral venous lactate for predicting dengue shock and/or organ failure: a prospective observational study. PLoS Neglecte Tropic Dis. (2016) 10:e0004961. doi: 10.1371/journal.pntd.0004961
4. Sobhian B, Kröpfl A, Hölzenbein T, Khadem A, Redl H, Bahrami S. Increased circulating D-lactate levels predict risk of mortality after hemorrhage and surgical trauma in baboons. Shock (Augusta, Ga). (2012) 37:473–7. doi: 10.1097/SHK.0b013e318249cb96
5. Mikkelsen ME, Miltiades AN, Gaieski DF, Goyal M, Fuchs BD, Shah CV, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Critic Care Mee. (2009) 37:1670–7. doi: 10.1097/CCM.0b013e31819fcf68
6. Guyette F, Suffoletto B, Castillo JL, Quintero J, Callaway C, Puyana JC. Prehospital serum lactate as a predictor of outcomes in trauma patients: a retrospective observational study. J Trauma. (2011) 70:782–6. doi: 10.1097/TA.0b013e318210f5c9
7. Bai Z, Zhu X, Li M, Hua J, Li Y, Pan J, et al. Effectiveness of predicting in-hospital mortality in critically ill children by assessing blood lactate levels at admission. BMC Pediatr. (2014) 14:83. doi: 10.1186/1471-2431-14-83
8. Stefani MA, Modkovski R, Hansel G, Zimmer ER, Kopczynski A, Muller AP, et al. Elevated glutamate and lactate predict brain death after severe head trauma. Annal Clinic Translat Neurol. (2017) 4:392–402. doi: 10.1002/acn3.416
9. Fu YQ, Bai K, Liu CJ. The impact of admission serum lactate on children with moderate to severe traumatic brain injury. PloS One. (2019) 14:e0222591. doi: 10.1371/journal.pone.0222591
10. Dübendorfer C, Billeter AT, Seifert B, Keel M, Turina M. Serial lactate and admission SOFA scores in trauma: an analysis of predictive value in 724 patients with and without traumatic brain injury. Euro J Trauma Emerg Surg Offic Publicat Euro Trauma Soc. (2013) 39:25–34. doi: 10.1007/s00068-012-0212-z
11. Shin J, Hwang SY, Jo IJ, Kim WY, Ryoo SM, Kang GH, et al. Prognostic value of the lactate/albumin ratio for predicting 28-day mortality in critically ill sepsis patients. Shock (Augusta, Ga). (2018) 50:545–50. doi: 10.1097/SHK.0000000000001128
12. Wang B, Chen G, Cao Y, Xue J, Li J, Wu Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Critic Care. (2015) 30:271–5. doi: 10.1016/j.jcrc.2014.10.030
13. Lichtenauer M, Wernly B, Ohnewein B, Franz M, Kabisch B, Muessig J, et al. The lactate/albumin ratio: a valuable tool for risk stratification in septic patients admitted to ICU. Int J Mol Sci. (2017) 18:9. doi: 10.3390/ijms18091893
14. Kong T, Chung SP, Lee HS, Kim S, Lee J, Hwang SO, et al. The prognostic usefulness of the lactate/albumin ratio for predicting clinical outcomes in out-of-hospital cardiac arrest: a prospective, multicenter observational study (koCARC) Study. Shock (Augusta, Ga). (2020) 53:442–51. doi: 10.1097/SHK.0000000000001405
15. Freitas AD, Franzon O. Lactate as predictor of mortality in polytrauma. Arquivos brasileiros de cirurgia digestiva. (2015) 28:163–6. doi: 10.1590/S0102-67202015000300004
16. Kong VY, Weale RD, Laing GL, Bruce JL, Oosthuizen GV, Sartorius B, et al. A raised serum lactate level is an independent predictor of in-hospital mortality in patients with isolated cerebral gunshot wounds. Suid-Afrikaanse tydskrif vir geneeskunde. (2018) 108:413–7. doi: 10.7196/SAMJ.2018.v108i5.12837
17. Choi SJ, Ha EJ, Jhang WK, Park SJ. Association between the lactate/albumin ratio and mortality in pediatric septic shock patients with underlying chronic disease: retrospective pilot study. Minerva pediatrica. (2016) 16:24. doi: 10.23736/S2724-5276.16.04551-5
18. Moustafa AA, Antonios MA, Abdellatif EM, Hussain AH. Association of lactate/albumin ratio level to organ failure and mortality in severe sepsis in a pediatric intensive care unit in Egypt. Turk J Pediatr. (2018) 60:691–701. doi: 10.24953/turkjped.2018.06.010
19. Ryoo SM, Lee J, Lee Y-S, Lee JH, Lim KS, Huh JW, et al. Lactate level versus lactate clearance for predicting mortality in patients with septic shock defined by sepsis-3. Critic Care Med. (2018) 46:e489–e95. doi: 10.1097/CCM.0000000000003030
20. Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, et al. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Inten Care Med. (2016) 42:202–10. doi: 10.1007/s00134-015-4127-0
21. Raux M, Le Manach Y, Gauss T, Baumgarten R, Hamada S, Harrois A, et al. Comparison of the prognostic significance of initial blood lactate and base deficit in trauma patients. Anesthesiology. (2017) 126:522–33. doi: 10.1097/ALN.0000000000001490
22. Johnson MC, Alarhayem A, Convertino V, Carter R, Chung K, Stewart R, et al. Comparison of compensatory reserve and arterial lactate as markers of shock and resuscitation. J Trauma Acute Care Surg. (2017) 83:603–8. doi: 10.1097/TA.0000000000001595
23. Dekker SE, de Vries HM, Lubbers WD, van de Ven PM, Toor EJ, Bloemers FW, et al. Lactate clearance metrics are not superior to initial lactate in predicting mortality in trauma. Euro J Trauma Emerg Surg Offic Publicat Euro Trauma Soc. (2017) 43:841–51. doi: 10.1007/s00068-016-0733-y
24. Cureton EL, Kwan RO, Dozier KC, Sadjadi J, Pal JD, Victorino GP. A different view of lactate in trauma patients: protecting the injured brain. J Surg Res. (2010) 159:468–73. doi: 10.1016/j.jss.2009.04.020
25. Zehtabchi S, Sinert R, Soghoian S, Liu Y, Carmody K, Shah L, et al. Identifying traumatic brain injury in patients with isolated head trauma: are arterial lactate and base deficit as helpful as in polytrauma? Emerg Med J: EMJ. (2007) 24:333–5. doi: 10.1136/emj.2006.044578
26. Arifianto MR, Ma'ruf AZ, Ibrahim A, Bajamal AH. Role of hypertonic sodium lactate in traumatic brain injury management. Asian J Neurosurg. (2018) 13:971–5. doi: 10.4103/ajns.AJNS_10_17
27. Carteron L, Solari D, Patet C, Quintard H, Miroz JP, Bloch J, et al. Hypertonic lactate to improve cerebral perfusion and glucose availability after acute brain injury. Critic Care Med. (2018) 46:1649–55. doi: 10.1097/CCM.0000000000003274
28. Millet A, Cuisinier A, Bouzat P, Batandier C, Lemasson B, Stupar V, et al. Hypertonic sodium lactate reverses brain oxygenation and metabolism dysfunction after traumatic brain injury. British journal of anaesthesia. (2018) 120:1295–303. doi: 10.1016/j.bja.2018.01.025
29. Bouzat P, Sala N, Suys T, Zerlauth J-B, Marques-Vidal P, Feihl F, et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive care Med. (2014) 40:412–21. doi: 10.1007/s00134-013-3203-6
30. Mannino C, Glenn TC, Hovda DA, Vespa PM, McArthur DL, Van Horn JD, et al. Acute glucose and lactate metabolism are associated with cognitive recovery following traumatic brain injury. J Neurosci Res. (2018) 96:696–701. doi: 10.1002/jnr.24097
31. Rice AC, Zsoldos R, Chen T, Wilson MS, Alessandri B, Hamm RJ, et al. Lactate administration attenuates cognitive deficits following traumatic brain injury. Brain Res. (2002) 928:156–9. doi: 10.1016/S0006-8993(01)03299-1
32. Alessandri B, Schwandt E, Kamada Y, Nagata M, Heimann A, Kempski O. The neuroprotective effect of lactate is not due to improved glutamate uptake after controlled cortical impact in rats. J Neurotrauma. (2012) 29:2181–91. doi: 10.1089/neu.2011.2067
33. Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proceed Nat Acad Sci United States Am. (1994) 91:10625–9. doi: 10.1073/pnas.91.22.10625
34. Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cerebr Blood Flow Metabol Offic J Int Soc Cerebr Blood Flow Metabol. (2007) 27:1766–91. doi: 10.1038/sj.jcbfm.9600521
35. Chen T, Qian YZ, Di X, Zhu JP, Bullock R. Evidence for lactate uptake after rat fluid percussion brain injury. Acta neurochirurgica Supplement. (2000) 76:359–64. doi: 10.1007/978-3-7091-6346-7_75
36. Glenn TC, Kelly DF, Boscardin WJ, McArthur DL, Vespa P, Oertel M, et al. Energy dysfunction as a predictor of outcome after moderate or severe head injury: indices of oxygen, glucose, and lactate metabolism. J Cerebr Blood Flow Metabol Offic J Int Soc Cerebr Blood Flow Metabol. (2003) 23:1239–50. doi: 10.1097/01.WCB.0000089833.23606.7F
37. Jansen TC, van Bommel J, Woodward R, Mulder PGH, Bakker J. Association between blood lactate levels, Sequential Organ Failure Assessment subscores, and 28-day mortality during early and late intensive care unit stay: a retrospective observational study. Critic Care Med. (2009) 37:2369–74. doi: 10.1097/CCM.0b013e3181a0f919
38. Ichai C, Armando G, Orban J-C, Berthier F, Rami L, Samat-Long C, et al. Sodium lactate versus mannitol in the treatment of intracranial hypertensive episodes in severe traumatic brain-injured patients. Intens Care Med. (2009) 35:471–9. doi: 10.1007/s00134-008-1283-5
39. Bisri T, Utomo BA, Fuadi I. Exogenous lactate infusion improved neurocognitive function of patients with mild traumatic brain injury. Asian J Neurosurg. (2016) 11:151–9. doi: 10.4103/1793-5482.145375
40. Dusick JR, Glenn TC, Lee WNP, Vespa PM, Kelly DF, Lee SM, et al. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. J Cerebr Blood Flow Metabol. (2007) 27:1593–602. doi: 10.1038/sj.jcbfm.9600458
41. Mosienko V, Teschemacher AG, Kasparov S. Is L-lactate a novel signaling molecule in the brain? J Cerebr Blood Flow Metabol. (2015) 35:1069–75. doi: 10.1038/jcbfm.2015.77
42. Castillo X, Rosafio K, Wyss MT, Drandarov K, Buck A, Pellerin L, et al. A probable dual mode of action for both L- and D-lactate neuroprotection in cerebral ischemia. J Cerebr Blood Flow Metabol. (2015) 35:1561–9. doi: 10.1038/jcbfm.2015.115
43. Sotelo-Hitschfeld T, Niemeyer MI, Mächler P, Ruminot I, Lerchundi R, Wyss MT, et al. Channel-mediated lactate release by K+-stimulated astrocytes. J Neurosci Offic J Soc Neurosci. (2015) 35:4168–78. doi: 10.1523/JNEUROSCI.5036-14.2015
44. Boumezbeur F, Petersen KF, Cline GW, Mason GF, Behar KL, Shulman GI, et al. The contribution of blood lactate to brain energy metabolism in humans measured by dynamic 13C nuclear magnetic resonance spectroscopy. J Neurosci Offic J Soc Neurosci. (2010) 30:13983–91. doi: 10.1523/JNEUROSCI.2040-10.2010
45. Quistorff B, Secher NH, Van Lieshout JJ. Lactate fuels the human brain during exercise. FASEB J Offic Publicat Federat Am Soc Experiment Biol. (2008) 22:3443–9. doi: 10.1096/fj.08-106104
46. Montalcini T, Moraca M, Ferro Y, Romeo S, Serra S, Raso MG, et al. Nutritional parameters predicting pressure ulcers and short-term mortality in patients with minimal conscious state as a result of traumatic and non-traumatic acquired brain injury. J Translat Med. (2015) 13:305. doi: 10.1186/s12967-015-0660-4
47. Chen D, Bao L, Lu SQ, Xu F. Serum albumin and prealbumin predict the poor outcome of traumatic brain injury. PloS One. (2014) 9:e93167. doi: 10.1371/journal.pone.0093167
48. Luo HC, Fu YQ, You CY, Liu CJ, Xu F. Comparison of admission serum albumin and hemoglobin as predictors of outcome in children with moderate to severe traumatic brain injury: a retrospective study. Medicine. (2019) 98:e17806. doi: 10.1097/MD.0000000000017806
49. Baltazar GA, Pate AJ, Panigrahi B, LaBoy S, Prosniak R, Mody A, et al. Malnutrition as measured by albumin and prealbumin on admission is associated with poor outcomes after severe traumatic brain injury. Am Surgeon. (2015) 81:E61–3. doi: 10.1177/000313481508100208
50. Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutri Clinic Pract Offic Publicat Am Soc Parent Enter Nutri. (2005) 20:369–76. doi: 10.1177/0115426505020004369
Keywords: traumatic brain injury, lactate, albumin, lactate to albumin ratio, prognosis
Citation: Wang R, He M, Qu F, Zhang J and Xu J (2022) Lactate Albumin Ratio Is Associated With Mortality in Patients With Moderate to Severe Traumatic Brain Injury. Front. Neurol. 13:662385. doi: 10.3389/fneur.2022.662385
Received: 01 February 2021; Accepted: 03 February 2022;
Published: 01 April 2022.
Edited by:
Alfonso Lagares, University Hospital October 12, SpainReviewed by:
Marco Fidel Avila-Rodriguez, Universidad del Tolima, ColombiaDhaval Shukla, National Institute of Mental Health and Neurosciences (NIMHANS), India
Copyright © 2022 Wang, He, Qu, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min He, aGVtaW4xOTkxMDMwNkB3Y2hzY3UuY24=; Jianguo Xu, eHVqZ0BzY3UuZWR1LmNu
 Ruoran Wang
Ruoran Wang Min He
Min He Fengyi Qu3
Fengyi Qu3 Jianguo Xu
Jianguo Xu