
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 12 January 2023
Sec. Neuro-Otology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1102294
This article is part of the Research Topic Towards an Understanding of Tinnitus Heterogeneity, Volume II View all 27 articles
Introduction: This study was performed to assess identifiable abnormalities in tinnitus patients with normal hearing.
Methods: The medical records of subjective non-pulsatile tinnitus patients with normal hearing confirmed by conventional pure-tone audiometry who visited our tinnitus clinic between March 2020 and May 2022 were reviewed. The loudness discomfort level (LDL), extended high-frequency hearing loss (EHFHL), summating potential (SP)/action potential (AP) ratio, distortion product otoacoustic emission (DPOAE), thresholds of auditory brainstem response (ABR) wave V, somatic modulation, and psychiatric symptoms, such as anxiety, depression, and stress were evaluated by questionnaires.
Results: Decreased LDL (n = 48, 59.8%) was the most frequent finding, followed by EHFHL (n = 29, 35.4%), increased SP/AP ratio (n = 27, 32.9%), psychiatric symptoms (n = 24, 29.3%), decreased DPOAE (n = 17, 20.7%), somatic modulation (n = 8, 9.8%), and increased ABR threshold (n = 3, 3.7%); 75.6% of patients had one or more of these findings. The presence of psychiatric symptoms was independently associated with the Tinnitus Handicap Inventory (THI) score.
Conclusion: Tinnitus in patients with normal hearing may be accompanied by a combination of various subclinical abnormal audiological findings. However, the presence of psychiatric symptoms alone was independently associated with tinnitus distress.
Tinnitus is the conscious awareness of a tonal or composite noise without an identifiable cause (1). Changes in the central auditory pathway caused by peripheral auditory deafferentation due to age-related hearing loss or noise-induced hearing loss may explain the tinnitus percept in most situations because tinnitus usually occurs following hearing deterioration. The risk factors are similar for age-related hearing loss and tinnitus, with both aging and cardiovascular disease as prognostic factors (2).
However, some tinnitus patients show normal hearing in conventional audiometry and do not feel any subjective hearing loss or aggravation of hearing loss along with new-onset tinnitus. Despite conventional audiometry findings within the normal range, about 10–15% of subjects have self-report hearing loss (3). A cross-sectional study based on the National Health and Nutrition Examination Survey 1999–2002 reported that confusion/memory, self-reported hearing difficulty, pain/tingling in hands/feet, balance problems, and diabetes were common in patients with persistent tinnitus and normal audiometric threshold (3). With regard to risk factors associated with tinnitus in subjects with normal hearing, the Korea National Health and Nutrition Examination Survey (KNHANES) showed that tinnitus is associated with female sex, ischemic heart disease, dyslipidemia, noise exposure, and depression (4).
Tinnitus disorders are present in tinnitus patients with tinnitus and accompanying emotional, attentional, or cognitive problems (1). Tinnitus distress results from integrating various brain networks involving the limbic, auditory, hypothalamus, etc., (5). A recent review highlighted the role of the triple network in tinnitus distress (6). Tinnitus distress is also aggravated by accompanying hyperacusis (7). Taken together, these findings suggest that various changes in the auditory, emotional, and somatosensory systems may be involved in the generation and maintenance of tinnitus. These changes include decreased loudness discomfort level (LDL), extended high-frequency hearing loss (EHFHL), increased summating potential (SP)/action potential (AP) ratio, decreased distortion product otoacoustic emission (DPOAE), altered wave V thresholds of auditory brainstem response (ABR), somatic modulation, and accompanying psychiatric symptoms (4, 7–28). In addition, various subclinical auditory dysfunctions may be hidden. These have been reported in studies in subjects with normal hearing and tinnitus. On the other hand, as we gained more clinical experience in identifying auditory abnormalities in tinnitus patients with normal hearing, we observed that there always exists not just one auditory abnormality at one time in many patients with tinnitus with normal hearing. In many cases, several abnormal findings occurred simultaneously. However, to our knowledge, it remains unclear which characteristics are most common in these patients and whether they exist alone or simultaneously. In addition, insufficient information is available regarding which factors are most closely associated with tinnitus distress.
It is important to know which etiologies are more common in cases of normal hearing and tinnitus because, when these patients visit a tinnitus clinic, more successful treatment outcomes may be achieved by preparing for more common causes in advance and first addressing these causes. Therefore, this study was performed to assess the identifiable audiological abnormalities and psychiatric problems in tinnitus patients with normal hearing.
The medical records of tinnitus patients who visited a tinnitus clinic at a tertiary university hospital between March 2020 and May 2022 were screened and reviewed. The inclusion criteria were all pure-tone threshold not exceeding 25 dB from 250 to 8 kHz and non-pulsatile subjective tinnitus. Exclusion criteria were as follows: brain malignancies; and neurological deficits. Based on review of medical records, we documented the patients' age, sex, accompanying symptoms (aural fullness, headache, dizziness, attention problems, temporomandibular/neck pain, sleep disturbance, and history of exposure to loud noise), accompanying diabetes mellitus, and/or hypertension.
The Tinnitus Handicap Inventory (THI) score was determined for all patients. THI score ≥38 was considered to indicate the presence of moderate distress. Psychiatric symptoms were assessed using several questionnaires, including Beck's Depression Inventory (BDI), Beck's Anxiety Inventory (BAI), and the Brief Encounter Psychosocial Instrument (BEPSI). Anxiety was considered present in cases with BAI score ≥22. Depression was defined as BDI score ≥16. BEPSI score ≥1.8 was taken to indicate the presence of stress.
For audiological evaluation, pure-tone audiometry, speech audiometry, ABR, electrocochleography, and tinnitogram consisting of pitch (Hz), loudness (dB SL), and minimal masking levels (MMLs) were performed. The mean pure-tone hearing threshold was calculated as the arithmetic means of hearing at 0.5, 1, 2, and 4 Hz. For bilateral tinnitus, the hearing threshold of the right side was used to calculate the pure-tone threshold. DPOAE was measured using the Neuro-Audio system (Neurosoft, Ivanovo, Russia), and the presence of DPOAE was defined as a signal-to-noise-ratio > 6 dB at five of eight f2 frequencies up to 8 kHz. Click ABRs were recorded with Navigator Pro software (Biological Systems Co., Mundelein, IL, USA). The threshold of wave V and wave V amplitude at the 90-dB click stimulus were documented.
A threshold exceeding 30 dBnHL was defined as an increased threshold of wave V of ABR (29). Decreased LDL was defined as LDL ≤ 90 dB at two or more frequencies (7). Increased SP/AP ratio was defined as a ratio ≥0.4 (30). EHFHL was defined as hearing threshold >15 dB in patients in their 20 s, 50 dB in those in their 30 s, 55 dB in those in their 40 s, 65 dB in those in their 50 s, and 75 dB in those in their 60 s (31).
Somatic modulation test was performed as described previously (32). Patients were considered to have positive somatic modulation if their tinnitus was modulated by at least one of the neck or jaw maneuvers.
Descriptive analysis was conducted to evaluate patient characteristics. Numerical data were compared between groups using Student's t-test. Pearson's correlation analysis was performed to analyze correlations between pairs of numerical variables. Binary logistic regression analysis with the ENTER method was performed to determine which abnormal findings significantly affected THI and tinnitus loudness. All analyses were performed using SPSS for Windows ver. 27.0 (IBM Corp., Armonk, NY, USA). In all analyses, p < 0.05 was taken to indicate statistical significance.
Eighty-two patients consisting of 28 males (34.1%) and 54 females (65.9%) with a mean age of 37 years (range: 14–64 years) were included in the study. The symptom duration was 13.87 months (range: 0.5–240 months). The mean hearing levels were 7.78 ± 3.94 dB on the right (range: 2–17 dB) (Figure 1) and 7.71 ± 4.08 dB on the left (range: 2–18 dB). With regard to laterality, 47.5% (n = 39) had unilateral tinnitus, 43.9% (n = 36) had bilateral tinnitus, and the remaining patients (n = 7, 8.5%) complained of non-lateralized tinnitus. The most common accompanying symptom was aural fullness (n = 35, 42.7%), followed by headaches and hyperacusis (n = 25, 30.5%), sleep disturbance (n = 22, 26.8%), dizziness (n = 20, 24.4%), neck pain (n = 16, 19.5%), and attention difficulty (n = 9, 11.0%). Hypertension (n = 8, 9.8%), diabetes (n = 1, 1.2%), and thyroid disease (n = 5, 6.1%) were reported as comorbidities, and 12.2% (n = 11) of patients had a history of exacerbation after noise exposure. Based on the results of the questionnaires, 65.2% (n = 15/23) of the patients had a moderate or higher level of stress, while 26.1% (n = 6) and 22.0% (n = 18) had anxiety and depression, respectively.
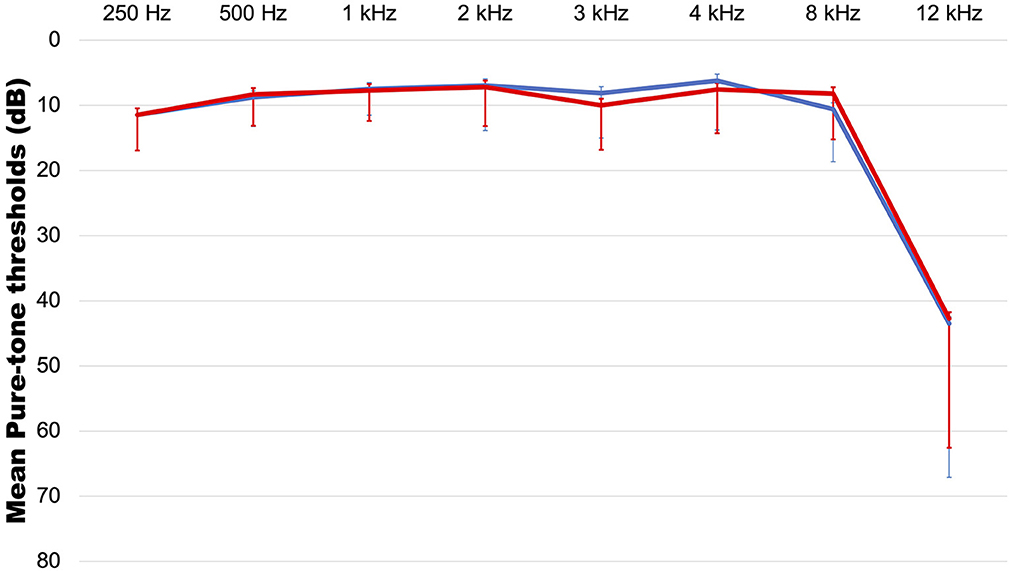
Figure 1. The average audiogram. red line: right side, blue line: left side. Error bar indicates standard deviation.
Decreased LDL (n = 49, 59.8%) was the most common possible etiology, followed by EHFHL (n = 29, 35.4%), increased SP/AP ratio (n = 27, 32.9%), psychiatric symptoms (n = 24, 29.3%), decreased DPOAE response (n = 17, 20.7%), somatic modulation (n = 8, 9.8%), and increased ABR threshold (n = 3, 3.7%) (Table 1).
Among the items evaluated, 24.4% (n = 20) of patients were positive for three abnormal findings, followed by 23.2% (n = 19) with one abnormal finding, 22.0% (n = 18) with two, 9.8% (n = 8) with four, and 2.4% (n = 2) had five abnormal findings (Table 1). There were no abnormal findings in 18.3% (n = 15) of cases.
The number of abnormal findings showed a weak positive correlation with tinnitus awareness (r = 0.341, p = 0.002) and effect of tinnitus on life (r = 0.231, p = 0.037). However, the THI showed no correlation with the number of abnormal findings (p > 0.05).
Regression analysis showed that only the presence of accompanying psychiatric symptoms was significantly associated with THI≥38 (Table 2). None of the etiological factors examined showed a significant association with tinnitus loudness (data not shown).
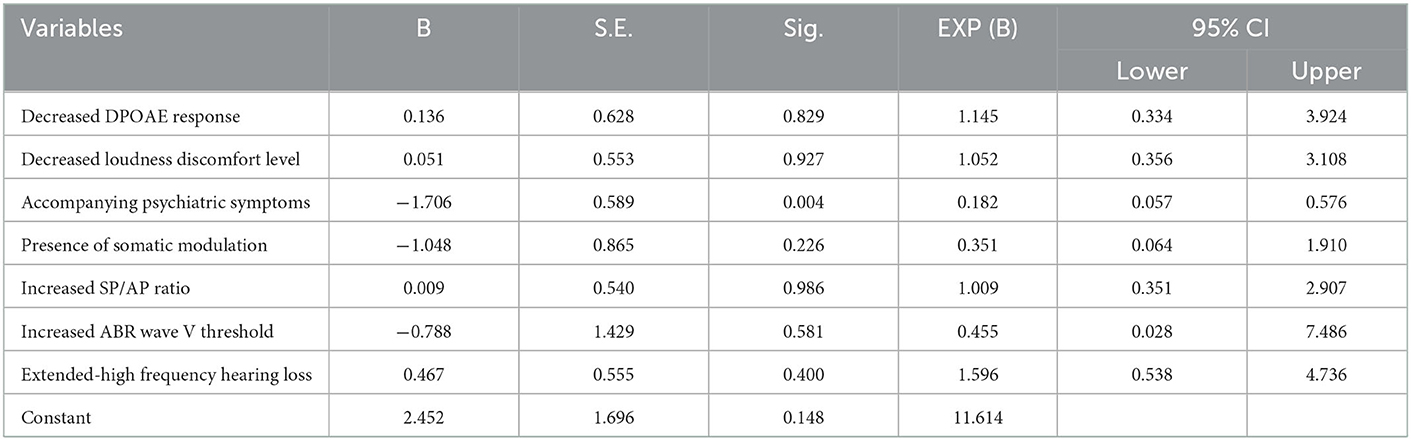
Table 2. Results of binary logistic regression analysis of the factors affecting Tinnitus Handicap Inventory in tinnitus patients with normal hearing.
Decreased LDL showed the highest incidence in this study (n = 48, 59.8%), followed by various audiological findings suggestive of subclinical auditory dysfunction in patients with normal hearing and tinnitus. In most cases, patients had multiple abnormal findings, while 18.3% of patients did not show any prominent abnormal findings. In addition, the presence of psychiatric symptoms was independently associated with THI. None of the audiological findings examined in this study showed a significant relation to tinnitus distress.
Patients with concomitant tinnitus and hyperacusis complained of more severe tinnitus distress, and a criterion for the co-occurrence of LDL ≤ 90 dB at two or more frequencies can be applied to predict accompanying hyperacusis (7). As described above, decreased LDL was the most common abnormal finding in this study. Similar to this study, in our previous study with 194 tinnitus patients, 26.3% had subjective symptoms, and 68.4% also showed lower LDL by the same criteria (7). Therefore, we assumed that hyperacusis is not a unique symptom observed only in tinnitus patients with normal hearing but seems to be a common symptom in tinnitus patients regardless of the hearing level.
On the other hand, decreased sound tolerance was persistent in adolescents with normal hearing and tinnitus who did not recover during 1-year follow-up (8). To diagnose decreased sound tolerance, thorough history taking, audiological assessment, and psychological evaluation are necessary to exclude the possibility of misophonia (9). In a recent study, where tinnitus patients and a control group of patients with normal or symmetric hearing loss were enrolled, patients with unilateral tinnitus had significantly lower LDLs than the control group (10). However, those with bilateral tinnitus showed no difference in LDLs compared to the control group. The authors interpreted that the decreased sound tolerance may reflect hidden cochlear damage, but it leads to unilateral tinnitus only and bilateral involvement depends on the hearing status (10). These authors discussed a previous study reporting differences in quantitative electroencephalography (qEEG) findings between unilateral tinnitus and bilateral tinnitus, with the former showing increased gamma-band activity in the contralateral parahippocampal and auditory cortex, and the latter showing an association with delta activity in the ventrolateral prefrontal cortex (11).
EHFHL is quite common, occurring in 64% of subjects aged 18–65 years, and can begin even in the early 20 s for males (12). Risk factors include noise exposure, drugs, infection, premature aging, heredity, and head trauma. EHFHL is associated with a high risk of future hearing loss and may also affect speech recognition (13). In addition, EHFHL is correlated with cognitive performance, regardless of tinnitus (14). EHFHL can also cause tinnitus in subjects with normal hearing and may appear normal in conventional audiometry, especially in young patients under 35 years old, and tinnitus patients were shown to have a worse extended high-frequency (EHF) threshold than controls (15). Worsening of EHFHL was observed during 1-year follow-up in patients with decreased sound tolerance and persistent tinnitus (8).
Auditory nerve fibers with high thresholds and low spontaneous firing rates are preferentially destructed in cochlear synaptopathy after aging or noise exposure (33). Reduced amplitude of ABR wave I and increased ABR V/I amplitude ratio are the most common predictors of cochlear synaptopathy in animals (16). However, ABR wave I is not often measured in humans, and the amplitude of wave V varies widely (16, 34)—these may limit the applicability of ABR to detect cochlear synaptopathy in humans. Various techniques have been tried to overcome the shortcomings of conventional ABR and increase the detection rate of synaptopathy in humans. As a result, a small latency shift of wave V in masked ABR was found to be a better indicator of cochlear synaptopathy in humans and mice (35). In addition, the quantification of envelope following responses (EFR) evoked by the application of rectangular amplitude modulation tone predicted word recognition better than conventional sinusoidal amplitude modulation (36). The other study by Vasilkov et al. also reported the optimal stimulation paradigms for this measurement method (37). In addition to ABR, electrocochleography (ECoG) is also available to detect hidden hearing loss. The results of ECoG are usually interpreted as audiological evidence of endolymphatic hydrops, and are also regarded as cochlear synaptopathy because of the similarity to ABR in that the summating potential comes from hair cells and the AP is equivalent to wave I of ABR (17).
A previous study showed a higher SP/AP ratio in patients with normal hearing and tinnitus than in those without tinnitus (18). Cochlear hydrops or cochlear synaptopathy may have been mixed etiologies in our patients.
Subclinical auditory dysfunction can also be assessed by DPOAE or ABR. Some reports provided evidence of increased latency of ABR wave I in subjects with normal hearing with tinnitus compared to those without tinnitus (19). Contrary to ABR wave I, which often shows an increased latency and decreased amplitude in tinnitus patients with normal hearing, alterations in waves III and V are inconsistent (38). Some reported the increased latencies of waves III and V (39). However, our previous study observed a shortening of latency in waves III and V in patients with bilateral tinnitus compared to the normal control (40). For abnormal DPOAE, reduced DPOAE amplitude is common in tinnitus patients with normal hearing, suggesting subclinical cochlear degeneration. These observations suggest that outer hair cells (OHCs) play an important role in tinnitus generation. However, others suggested that, although OHC dysfunction is associated with tinnitus, changes in OHCs do not always lead to the generation of tinnitus (20). That is, OHC dysfunction is not the only determinant of tinnitus (21). DPOAE and transient evoked OAE (TEOAE) did not differ according to the presence or absence of tinnitus in subjects with normal hearing (18). Another study similarly showed that DPOAE amplitudes did not differ according to the presence of tinnitus and/or hyperacusis, and were instead affected by EHF hearing thresholds (22). Reduced DPOAE is common in subjects with normal hearing regardless of accompanying tinnitus.
Some tinnitus patients may modulate their tinnitus by head and neck maneuvers or eye movement, regardless of hearing loss (23). Somatosensory tinnitus is a condition associated with head and neck pain or problems, such as temporomandibular joint disorders and bruxism (24). The following conditions indicate the presence of somatosensory influence: neck or jaw pain that appears simultaneously with tinnitus; neck/jaw symptoms that are simultaneously aggravated with tinnitus; head or neck trauma preceding tinnitus; varying pitch, loudness, and/or location; and discrepancies in audiogram and unilateral tinnitus (25). Disinhibition or unfamiliar somatosensory input to the dorsal cochlear nucleus may be regarded by the brain as changed auditory perception even in subjects with normal hearing (26). The percentage of patients with somatic modulation in this study was merely 9.8%, which was much lower than our previous study results (61.7%). We assumed that this substantial difference was due to differences in clinical settings or the experiences of audiologists (32).
Of the psychiatric symptoms, higher rates of depression were seen in tinnitus patients with normal hearing even after adjusting for other confounders, such as age, sex, past medical history, and noise exposure (4). These patients have a higher prevalence of depression and anxiety than those without tinnitus, and the severity of psychiatric symptoms is correlated with tinnitus distress (27). Although younger tinnitus patients tend to have normal hearing compared to older patients, the rates of self-reported depression and stress showed no differences according to age (28). In our study, about a quarter of subjects with normal hearing showed psychiatric symptoms, which was less than initially expected. However, this was the sole, independent prognostic factor for tinnitus distress, suggesting that control of psychiatric symptoms is the most important consideration for relieving tinnitus distress.
The auditory dysfunction may be addressed by correction of decreased sound tolerance. To our knowledge, there have been no randomized controlled trials of medications for treatment of hyperacusis. Therefore, gabapentin, anticonvulsants, anxiolytics, or antidepressants may be chosen empirically based on the clinician's clinical experience. We usually recommend that patients with hyperacusis alone avoid sound and use ear protection because of their heightened sensitivity to sound. For those with both tinnitus and decreased sound tolerance, listening to broadband noise, such as pink noise at a well-tolerated level that does not induce discomfort, may be appropriate (41). In addition, sound therapy is recommended to induce habituation to tinnitus. Total masking or partial masking based on tinnitus retraining therapy can be chosen based at the physician's discretion. Next, patients should be checked for recurrent vertigo or fluctuating hearing loss to exclude the possibility of endolymphatic hydrops. In addition, noise exposure history and ABR results should also be confirmed to avoid missing the possibility of cochlear synaptopathy. Due to the possibility of somatic tinnitus, various treatments, including physical therapy and muscle relaxants, may also be considered. It should be emphasized that tinnitus does not have a single cause, so treatment must be multifaceted. Of the various treatments available, the psychiatric symptoms should be treated first.
This study had several limitations. The relatively low number of 82 patients was too small to analyze the overall etiologies of tinnitus in patients with normal hearing. We checked EHF hearing loss at 12 kHz, but a more diverse analysis would have been possible if we had tested up to 16 kHz. However, it was impossible to acquire additional data because the audiometer in our hospital did not support frequencies in this range. In addition, no abnormal findings were observed among the items reviewed in 18.3% of the patients. These patients may have had subclinical abnormalities that could not be detected by questionnaires or audiological tests (42).
Various subclinical auditory abnormalities were observed in tinnitus patients with normal hearing, and most cases showed several abnormalities simultaneously. However, only the presence of psychiatric symptoms was independently associated with THI.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ewha Mokdong University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HL: conceptualization, data analysis and methodology, writing-original draft, and writing-review and editing. YP: writing-original draft, data analysis, and writing-review and editing. S-HS and SB: writing-review and editing. ZL: data curation, data analysis, and methodology. All authors contributed to the article and approved the submitted version.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI21C1574040021).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. De Ridder D, Schlee W, Vanneste S, Londero A, Weisz N, Kleinjung T, et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog Brain Res. (2021) 260:1–25. doi: 10.1016/bs.pbr.2020.12.002
2. Kim S, Park JM, Han JS, Seo JH, Han KD, Joo YH, et al. Age-related hearing loss in the Korea National Health and Nutrition Examination Survey. PLoS ONE. (2020) 15:e0243001. doi: 10.1371/journal.pone.0243001
3. Spankovich C, Gonzalez VB, Su D, Bishop CE. Self reported hearing difficulty, tinnitus, and normal audiometric thresholds, the National Health and Nutrition Examination Survey 1999–2002. Hear Res. (2018) 358:30–6. doi: 10.1016/j.heares.2017.12.001
4. Choi J, Lee CH, Kim SY. Association of tinnitus with depression in a normal hearing population. Medicina. (2021) 57:114. doi: 10.3390/medicina57020114
5. Granjeiro RC, Kehrle HM, de Oliveira TS, Sampaio AL, de Oliveira CA. Is the degree of discomfort caused by tinnitus in normal-hearing individuals correlated with psychiatric disorders? Otolaryngol Head Neck Surg. (2013) 148:658–63. doi: 10.1177/0194599812473554
6. De Ridder D, Vanneste S, Song JJ, Adhia D. Tinnitus and the triple network model: a perspective. Clin Exp Otorhinolaryngol. (2022) 15:205–12. doi: 10.21053/ceo.2022.00815
7. Shin SH, Byun SW, Lee ZY, Kim MJ, Kim EH, Lee HY. Clinical findings that differentiate co-occurrence of hyperacusis and tinnitus from tinnitus alone. Yonsei Med J. (2022) 63:1035–42. doi: 10.3349/ymj.2022.0274
8. Sanchez TG, Roberts LE. Total remission or persistence of tinnitus and decreased sound level tolerance in adolescents with normal audiograms: a follow-up study. Prog Brain Res. (2021) 260:253–68. doi: 10.1016/bs.pbr.2020.05.025
9. Raj-Koziak D, Gos E, Kutyba J, Skarzyński H, Skarzyński PH. Decreased sound tolerance in tinnitus patients. Life. (2021) 11:87. doi: 10.3390/life11020087
10. Tae Cho Y, An YH, Hyuk Jang D, Hyun Kim D, Yeon Kim N, Jeong Kim H, et al. The relation of sound level tolerance to tinnitus ears in human. J Int Adv Otol. (2022) 18:1–7. doi: 10.5152/iao.2022.21320
11. Vanneste S, Plazier M, van der Loo E, Van de Heyning P, De Ridder D. The difference between uni- and bilateral auditory phantom percept. Clin Neurophysiol. (2011) 122:578–87. doi: 10.1016/j.clinph.2010.07.022
12. Motlagh Zadeh L, Silbert NH, Sternasty K, Swanepoel W, Hunter LL, Moore DR. Extended high-frequency hearing enhances speech perception in noise. Proc Natl Acad Sci USA. (2019) 116:23753–9. doi: 10.1073/pnas.1903315116
13. Mishra SK, Saxena U, Rodrigo H. Extended high-frequency hearing impairment despite a normal audiogram: relation to early aging, speech-in-noise perception, cochlear function, and routine earphone use. Ear Hear. (2022) 43:822–35. doi: 10.1097/AUD.0000000000001140
14. Waechter S, Wilson WJ, Magnusson M, Brännström KJ. Extended high frequency hearing, but not tinnitus, is associated with every-day cognitive performance. Front Psychol. (2022) 13:913944. doi: 10.3389/fpsyg.2022.913944
15. Song Z, Wu Y, Tang D, Lu X, Qiao L, Wang J, et al. Tinnitus is associated with extended high-frequency hearing loss and hidden high-frequency damage in young patients. Otol Neurotol. (2021) 42:377–83. doi: 10.1097/MAO.0000000000002983
16. Guest H, Munro KJ, Prendergast G, Howe S, Plack CJ. Tinnitus with a normal audiogram: Relation to noise exposure but no evidence for cochlear synaptopathy. Hear Res. (2017) 344:265–74. doi: 10.1016/j.heares.2016.12.002
17. Stuermer KJ, Beutner D, Foerst A, Hahn M, Lang-Roth R, Walger M. Electrocochleography in children with auditory synaptopathy/neuropathy: diagnostic findings and characteristic parameters. Int J Pediatr Otorhinolaryngol. (2015) 79:139–45. doi: 10.1016/j.ijporl.2014.11.025
18. Kara E, Aydin K, Akbulut AA, Karakol SN, Durmaz S, Yener HM, et al. Assessment of Hidden Hearing Loss in Normal Hearing Individuals with and Without Tinnitus. J Int Adv Otol. (2020) 16:87–92. doi: 10.5152/iao.2020.7062
19. Dadoo S, Sharma R, Sharma V. Oto-acoustic emissions and brainstem evoked response audiometry in patients of tinnitus with normal hearing. Int Tinnitus J. (2019) 23:17–25. doi: 10.5935/0946-5448.20190004
20. Gentil F, Meireles S, Roza T, Santos C, Parente M, Almeida E, et al. Comparison of otoacoustic emissions in patients with tinnitus having normal hearing vs. mild hearing loss. Int Tinnitus J. (2015) 19:39–46. doi: 10.5935/0946-5448.20150007
21. Kehrle HM, Granjeiro RC, Sampaio ALL, Farias MS, Martins VS, Oliveira CACP. Ten years follow up of patients with tinnitus and normal hearing. Int Tinnitus J. (2022) 26:57–62. doi: 10.5935/0946-5448.20220008
22. Tai Y, Mertes IB, Chappell J, Jeon CB, Husain FT. Comparison of otoacoustic emissions in tinnitus and hyperacusis in adults with normal hearing sensitivity. Int J Audiol. (2022) 19:1–11. doi: 10.1080/14992027.2022.2052980
23. Haider HF, Hoare DJ, Costa RFP, Potgieter I, Kikidis D, Lapira A, et al. Pathophysiology, diagnosis and treatment of somatosensory tinnitus: a scoping review. Front Neurosci. (2017) 11:207. doi: 10.3389/fnins.2017.00207
24. Ralli M, Greco A, Turchetta R, Altissimi G, de Vincentiis M, Cianfrone G. Somatosensory tinnitus: current evidence and future perspectives. J Int Med Res. (2017) 45:933–47. doi: 10.1177/0300060517707673
25. Michiels S, Ganz Sanchez T, Oron Y, Gilles A, Haider HF, Erlandsson S, et al. Diagnostic criteria for somatosensory tinnitus: a delphi process and face-to-face meeting to establish consensus. Trends Hear. (2018) 22:2331216518796403. doi: 10.1177/2331216518796403
26. Sedley W. Tinnitus: does gain explain? Neuroscience. (2019) 407:213–28. doi: 10.1016/j.neuroscience.2019.01.027
27. Kehrle HM, Sampaio AL, Granjeiro RC, de Oliveira TS, Oliveira CA. Tinnitus annoyance in normal-hearing individuals: correlation with depression and anxiety. Ann Otol Rhinol Laryngol. (2016) 125:185–94. doi: 10.1177/0003489415606445
28. Park SY, Han JJ, Hwang JH, Whang ES, Yeo SW, Park SN. Comparison of tinnitus and psychological aspects between the younger and older adult patients with tinnitus. Auris Nasus Larynx. (2017) 44:147–51. doi: 10.1016/j.anl.2016.04.007
29. Lim HW, Kim EA, Chung JW. Audiological follow-up results after newborn hearing screening program. Clin Exp Otorhinolaryngol. (2012) 5:57–61. doi: 10.3342/ceo.2012.5.2.57
30. Orchik DJ, Shea JJ, Ge X. Transtympanic electrocochleography in Menière's disease using clicks and tone-bursts. Am J Otol. (1993) 14:290–4.
31. Wang M, Ai Y, Han Y, Fan Z, Shi P, Wang H. Extended high-frequency audiometry in healthy adults with different age groups. J Otolaryngol Head Neck Surg. (2021) 50:52. doi: 10.1186/s40463-021-00534-w
32. Lee HY, Kim SJ, Choi JY. Somatic modulation in tinnitus: clinical characteristics and treatment outcomes. J Int Adv Otol. (2020) 16:213–7. doi: 10.5152/iao.2020.8067
33. Schaette R, McAlpine D. Tinnitus with a normal audiogram: physiological evidence for hidden hearing loss and computational model. J Neurosci. (2011) 31:13452–7. doi: 10.1523/JNEUROSCI.2156-11.2011
34. Chen F, Zhao F, Mahafza N, Lu W. Detecting noise-induced cochlear synaptopathy by auditory brainstem response in tinnitus patients with normal hearing thresholds: a meta-analysis. Front Neurosci. (2021) 15:778197. doi: 10.3389/fnins.2021.778197
35. Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, et al. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci. (2016) 36:3755–64. doi: 10.1523/JNEUROSCI.4460-15.2016
36. Mepani AM, Verhulst S, Hancock KE, Garrett M, Vasilkov V, Bennett K, et al. Envelope following responses predict speech-in-noise performance in normal-hearing listeners. J Neurophysiol. (2021) 125:1213–22. doi: 10.1152/jn.00620.2020
37. Vasilkov V, Garrett M, Mauermann M, Verhulst S. Enhancing the sensitivity of the envelope-following response for cochlear synaptopathy screening in humans: the role of stimulus envelope. Hear Res. (2021) 400:108132. doi: 10.1016/j.heares.2020.108132
38. Milloy V, Fournier P, Benoit D, Noreña A, Koravand A. Auditory brainstem responses in tinnitus: a review of who, how, and what? Front Aging Neurosci. (2017) 9:237. doi: 10.3389/fnagi.2017.00237
39. Kehrle HM, Granjeiro RC, Sampaio AL, Bezerra R, Almeida VF, Oliveira CA. Comparison of auditory brainstem response results in normal-hearing patients with and without tinnitus. Arch Otolaryngol Head Neck Surg. (2008) 134:647–51. doi: 10.1001/archotol.134.6.647
40. Song K, Shin SA, Chang DS, Lee HY. Audiometric profiles in patients with normal hearing and bilateral or unilateral tinnitus. Otol Neurotol. (2018) 39:e416–21. doi: 10.1097/MAO.0000000000001849
41. Jastreboff PJ, Jastreboff MM. Decreased sound tolerance: hyperacusis, misophonia, diplacousis, and polyacousis. Handb Clin Neurol. (2015) 129:375–87. doi: 10.1016/B978-0-444-62630-1.00021-4
Keywords: tinnitus, normal hearing, tinnitus distress, sound intolerance, psychiatric symptom
Citation: Park Y, Shin S-H, Byun SW, Lee ZY and Lee HY (2023) Audiological and psychological assessment of tinnitus patients with normal hearing. Front. Neurol. 13:1102294. doi: 10.3389/fneur.2022.1102294
Received: 18 November 2022; Accepted: 29 December 2022;
Published: 12 January 2023.
Edited by:
Tobias Kleinjung, University of Zurich, SwitzerlandReviewed by:
Laura Jacxsens, Antwerp University Hospital, BelgiumCopyright © 2023 Park, Shin, Byun, Lee and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ho Yun Lee,  aG95dW4xMDA0QGdtYWlsLmNvbQ==
aG95dW4xMDA0QGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.