- 1Department of Neurology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
- 2Central Research Laboratory, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Background: The anterior (AcomA) and posterior communicating arteries (PcomA) are two of the most frequent sites for intracranial aneurysms. Anatomical variations in the Circle of Willis (COW) are frequently observed in patients with AcomA and PcomA aneurysms. Strong evidence is needed to determine the pooled estimate of the effect of COW variations on the formation and rupture of these aneurysms.
Aim: This systematic review and meta-analysis aimed to establish the effect of COW variations on the formation and rupture of AcomA and PcomA aneurysms using available studies.
Summary of review: PubMed, Embase, and Web of Science databases were systematically searched for studies published in English before September 21, 2022. Studies investigating AcomA aneurysms and the hypoplastic/aplastic A1 segment of the anterior cerebral artery and PcomA aneurysms and hypoplastic/aplastic PcomA or fetal-type posterior cerebral artery (FTP) were included. The heterogeneity of the studies was assessed using Cochran Q-test and I2 statistic. Pooled estimate was assessed using either a random- or fixed-effects model based on the heterogeneity of the studies. Among the 4,932 studies, 21 were eligible and included in the analysis. The presence of hypoplastic/aplastic A1 was significantly correlated with the formation [OR (95% confidence interval [CI]) = 7.97 (5.58, 11.39), P < 0.001] and rupture [OR (95%CI) = 1.87 (1.29, 2.72), P < 0.001] of AcomA aneurysms. Significant associations between FTP and both the formation [OR (95%CI) = 2.15 (1.41, 3.30), P < 0.001] and rupture [OR (95%CI) = 1.72 (1.26, 2.36), P < 0.001] of PcomA aneurysms were observed.
Conclusions: Significant associations were observed between COW variations and both the formation and rupture of AcomA and PcomA aneurysms. This can help in determining interventions for patients with aneurysms.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=225149, identifier: CRD42021225149.
Introduction
Intracranial aneurysms are acquired abnormal vascular dilations that occur in 1–2% of the population (1). The most dangerous complications of intracranial aneurysms are their rupture, which accounts for 80–85% of non-traumatic subarachnoid hemorrhages with high mortality and disability rates (1). Various risk factors have been identified for the rupture of
intracranial aneurysms, including age, aneurysm size, and location (2–4). The anterior (AcomA) and posterior communicating arteries (PcomA) are two of the most frequent sites for intracranial aneurysms (3), where ruptures are more likely to occur than at other sites (5).
Hemodynamics plays an important role in the growth and rupture of intracranial aneurysms (6). The Circle of Willis (COW) is a vascular network located at the base of the brain with a high variation rate (7). COW variations can cause hemodynamic changes by influencing cerebral blood flow; moreover, they are proposed to be contributors to the development of intracranial aneurysms (8, 9).
Hypoplastic/aplastic A1 and fetal-type posterior cerebral artery (FTP) are commonly observed in patients with AcomA and PcomA aneurysms, respectively. A series of studies have assessed the association between hypoplastic/aplastic A1 and the formation of AcomA aneurysms (10–13); meanwhile, the evidence on FTP and the formation of PcomA aneurysms is insufficient (14–16). In addition, there is a lack of consistency in the association between COW variations and rupture of AcomA and PcomA aneurysms (17–20).
Strong evidence is needed to determine the pooled estimate of the effect of COW variations on the formation and rupture of site-specific aneurysms. This will serve as evidence of the effect of hemodynamic factors in the development of intracranial aneurysms and may facilitate the prediction of rupture risk of observed aneurysms. Thus, this systematic review and meta-analysis aimed to establish the effect of COW variations on the formation and rupture of AcomA and PcomA aneurysms using available studies.
Methods
This systematic review and meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The protocol for this meta-analysis was registered at https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=225149.
Search strategy
Relevant studies published before September 21, 2022 were selected by searching PubMed, Embase, and Web of Science databases. A search strategy was developed that combines both free text words and medical subject headings covering, “intracranial aneurysm,” “variation,” and “Circle of Willis.” The detailed search strategy was listed in Supplementary Tables 1–3. The citations of the included reviews and articles were manually searched to obtain additional relevant studies.
Study selection
Studies that satisfied the following criteria were included: (1) investigated the relationship of COW configuration and formation or rupture of AcomA and PcomA aneurysms; (2) were cross-sectional, case-control, or cohort studies; (3) reported odds ratios (ORs) with 95% confidence intervals (CIs) (or reported data that was sufficient to calculate ORs and 95% CIs); and (4) published in English.
The exclusion criteria were as follows: (1) were case reports, editorials, conference abstract, or letters; and (2) were model/computer-generated brain studies. If the study population overlapped in two or more papers by the same authors, only the study with the largest number of participants were included.
Data extraction and study quality evaluation
Two investigators (LF and H-JM) independently reviewed the study titles and abstracts, and studies that satisfied the inclusion criteria underwent full-text assessment. Data were extracted independently by the two authors according to the pre-designed forms. The extracted data of the selected study included: first author, year of publication, country or area, study design, total number of participants, age, sex, COW variation types and definitions, aneurysm size, imaging techniques, and outcome variables. A consensus was reached through discussion when there were any disagreements.
The Newcastle–Ottawa scale (NOS) was used to assess the quality of cohort and case-control studies (21). The version of the scale consists of three categories: selection, comparability, and outcome. A study can be given a score ranging from zero to nine stars (low-quality: 0–3, medium-quality: 4–6, high-quality: 7–9). The Agency for Healthcare Research and Quality (AHRQ) scale was used to assess the quality of cross-sectional studies (22). A study can be given a score ranging from zero to 11 points (low-quality: 0–3, medium-quality: 4–7, high-quality: 8–11).
Statistical analysis
The effect measures of interest were ORs and 95% CIs. The heterogeneity of the studies was assessed using the Cochran Q-test and I2 statistic (23). For the pooling of study results, a random-effects model was used when between-study heterogeneity was statistically significant (P < 0.05 or I2 > 50%), while a fixed-effects model was applied when the heterogeneity was not significant (P > 0.05 and I2 <50%). Subgroup analysis was performed based on the locations and types of study. Sensitivity analysis was performed by removing each study in turn and evaluating whether the pooled estimate was affected significantly (24). The Egger test was used to assess publication bias when the included studies were more than 10, and significant publication bias was defined as p < 0.1 (25). Statistical analysis was performed using Stata 12.0.
Results
Identified studies
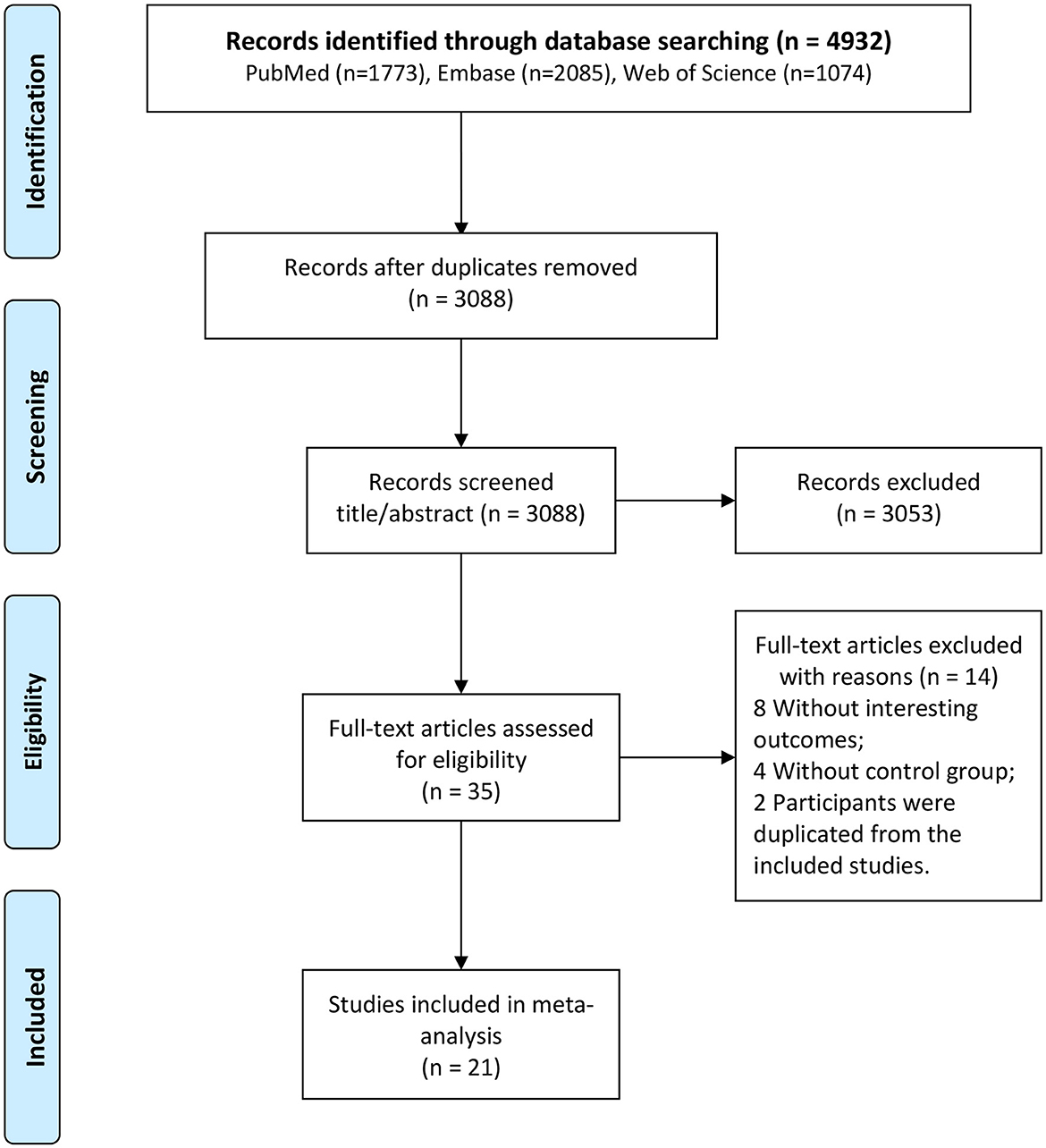
A total of 4,932 studies were identified after a literature search (1,773 from PubMed, 2,085 from Embase, and 1,074 from Web of Science) (Figure 1). Among these, 1,844 were replicated, 3,053 were excluded after reviewing the title and abstract, and 35 were selected for full-text assessment. Moreover, among these, 14 were excluded, and 21 studies fulfilled the eligibility criteria and were included in the analysis (10–20, 26–35).
Study characteristics and quality
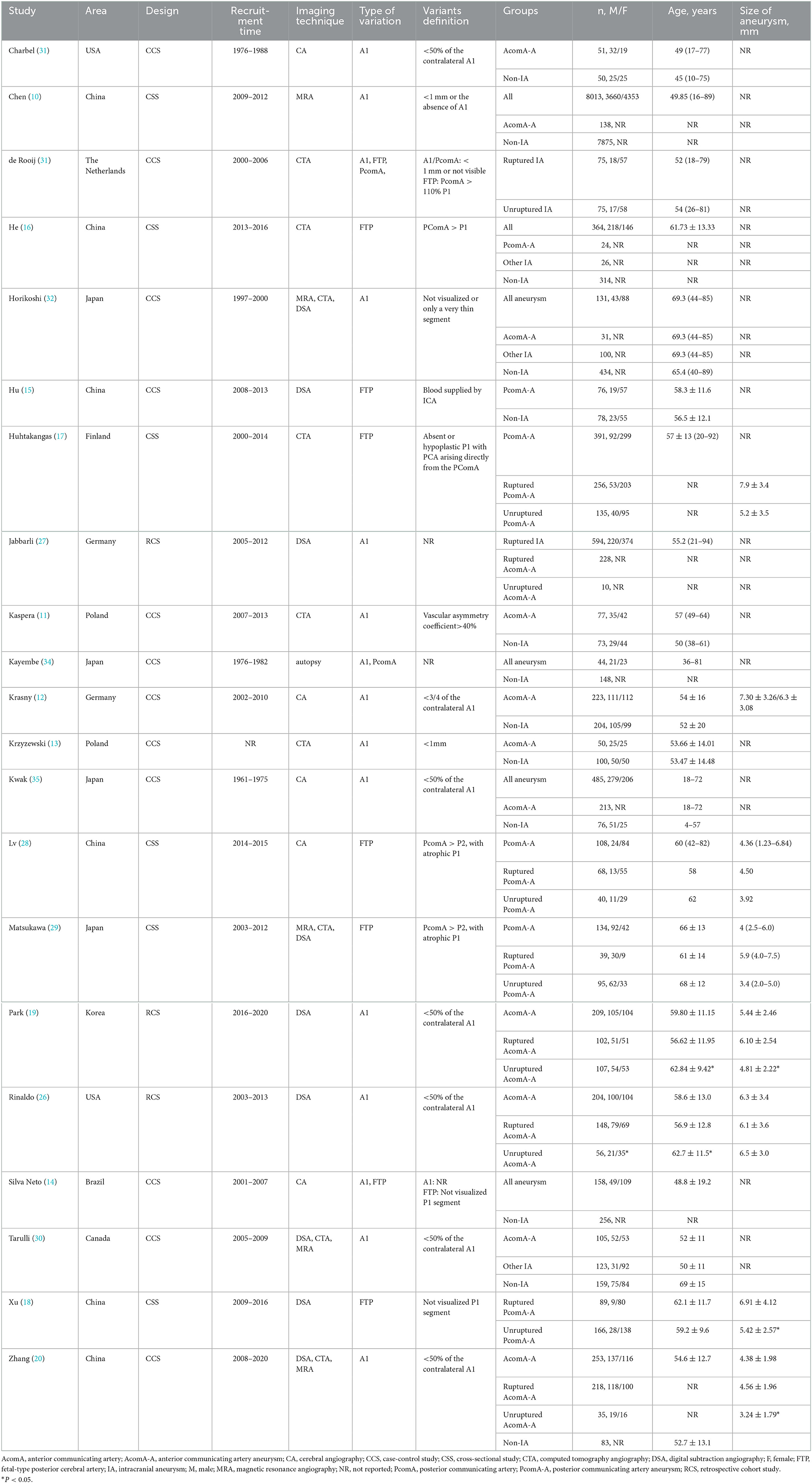
The 21 studies included in this systematic review and meta-analysis were published between 1980 and 2021 and conducted in China, Japan, Korea, Poland, United States of America, and other countries. A total of six cross-sectional studies, three retrospective cohort studies, and 12 case-control studies were included. A summary of the study characteristics is listed in Tables 1, 2.
Overall, the included cross-sectional studies that were assessed using the AHRQ scale received 6–9 scores, while the cohort and case-control studies that were assessed using NOS received 6–7 and 5–7 scores, respectively. Five studies were of high quality (15, 19, 20, 26, 29), while the others were of medium quality. A summary of the study quality assessment is provided in Supplementary Tables 4–6.
Hypoplastic/aplastic A1 and AcomA aneurysms
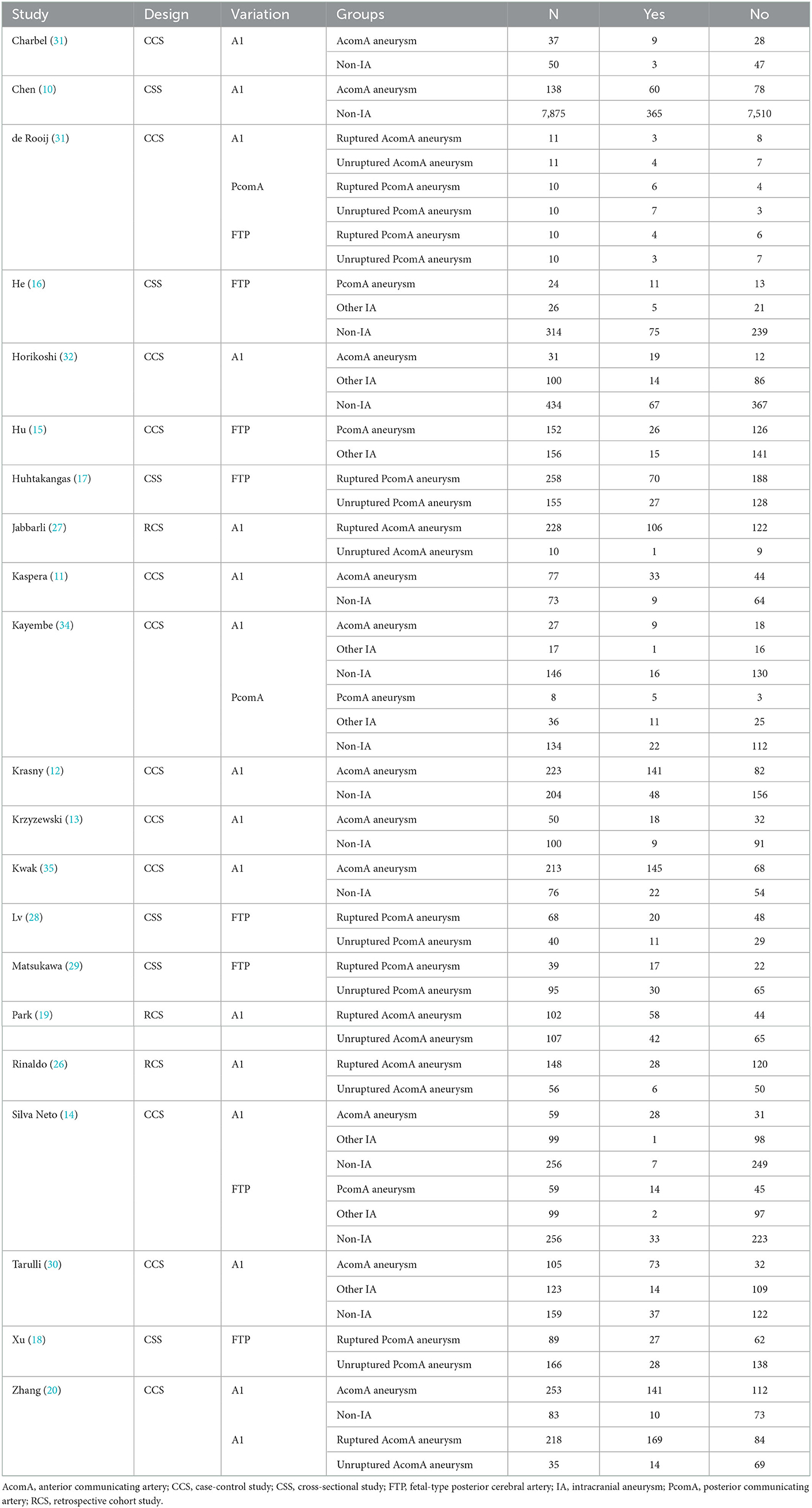
A total of 15 studies investigated the association between hypoplastic/aplastic A1 and the formation and rupture of AcomA aneurysms (Figures 2A, B). Moreover, 11 trials on the formation of AcomA aneurysms showed significant between-study heterogeneity (I2 = 68.7%, P < 0.001). Pooled analysis using random-effects model showed a prominent relationship between hypoplastic/aplastic A1 and the formation of AcomA aneurysms (OR (95% CI) = 7.97 (5.58, 11.39), P < 0.001). All studies showed a significant higher incidence of hypoplastic/aplastic A1 in patients with AcomA aneurysms than in healthy controls. In addition, pooled analysis of five studies showed a higher prevalence of hypoplastic/aplastic A1 in ruptured AcomA aneurysms than in unruptured ones, with no significant between-study heterogeneity (OR (95% CI) = 1.87 (1.29, 2.72), P < 0.001; I2 = 0.0%, P = 0.427).
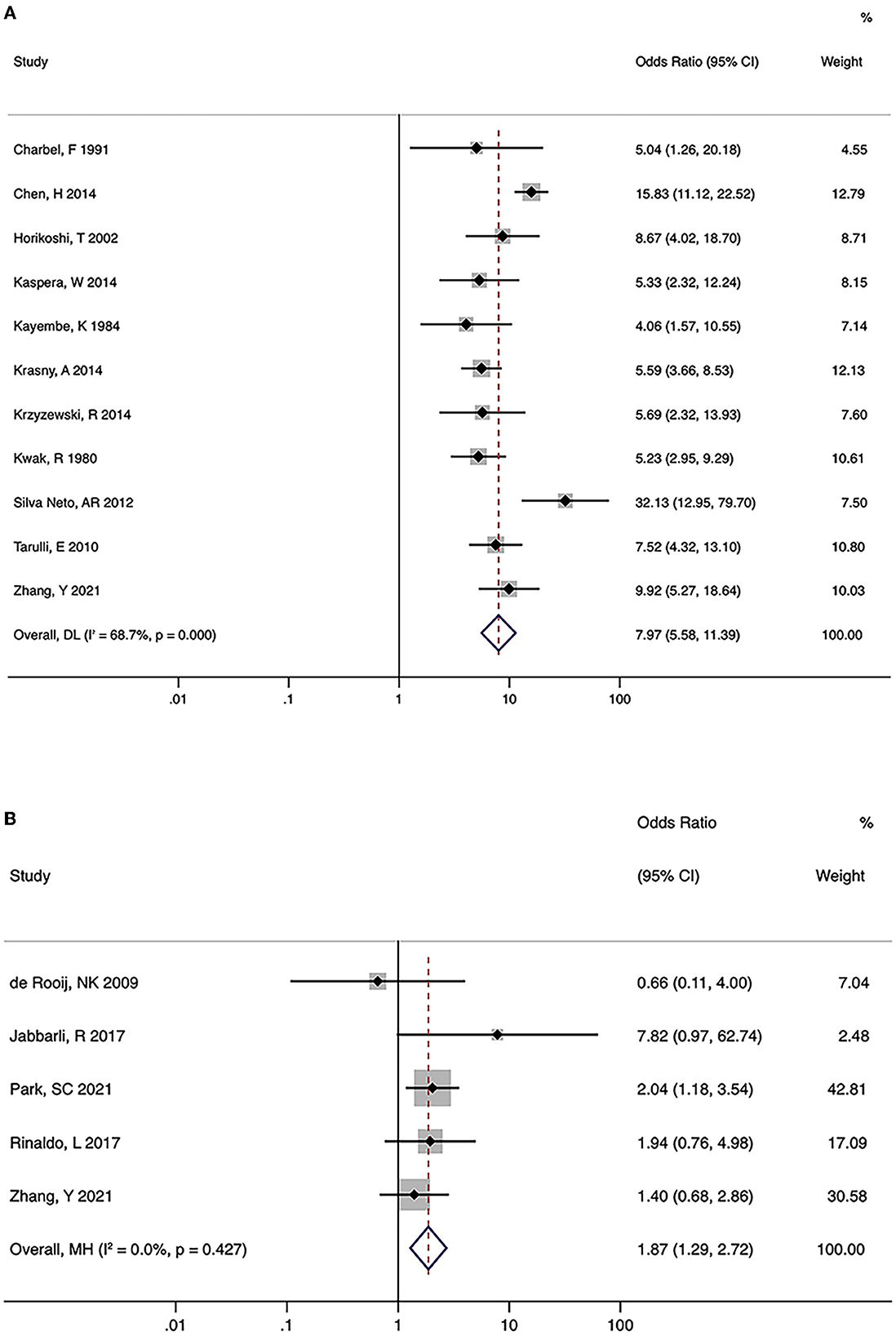
Figure 2. Summary odds ratios of hypoplastic/aplastic A1 and anterior communicating artery (AcomA) aneurysms. (A) AcomA aneurysms vs. healthy controls (B) ruptured vs. unruptured AcomA aneurysms.
Significant heterogeneity was seen in studies on the formation of AcomA aneurysms; hence, subgroup analysis was performed to explore potential sources (Table 3). After stratifying the studies by location (Asian or Western) and study design (case-control or cross-sectional study), the significant relationship between hypoplastic/aplastic A1 and the formation of AcomA aneurysms remained. After stratifying by study design, the heterogeneity of each group became non-significant (I2 <50%, P > 0.05), suggesting that the study design was a source of heterogeneity.
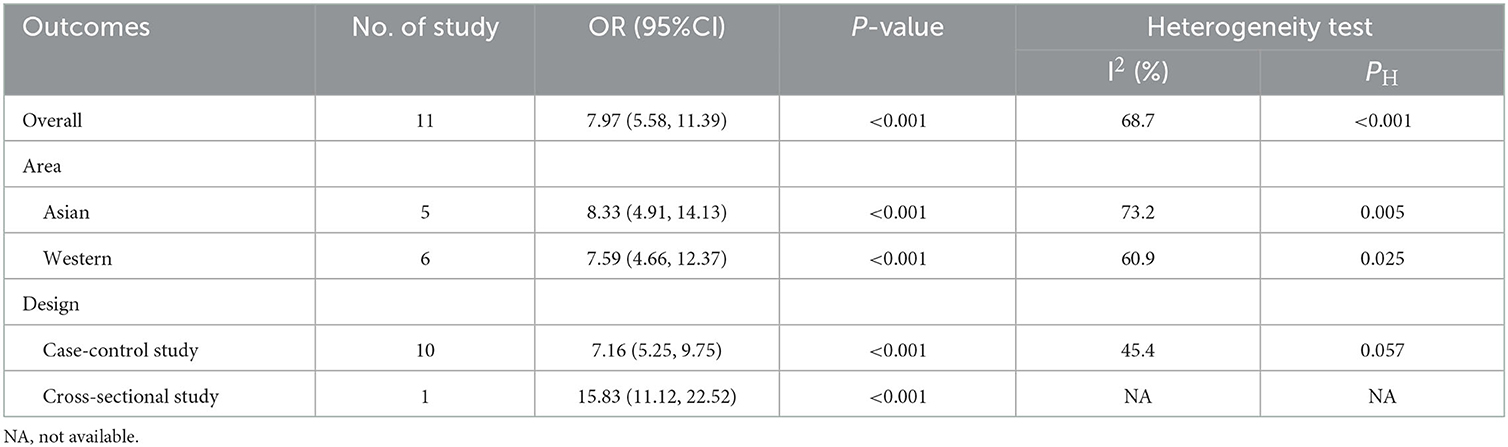
Table 3. Subgroup analyses of hypoplastic/aplastic A1 and anterior communicating artery (AcomA) aneurysm formation.
In the sensitivity analysis of both the formation and rupture of AcomA aneurysms, when a named study was removed, the pooled estimates remained significant (P < 0.05), suggesting that none of the studies had a large influence on the overall estimate.
No indication of publication bias for the studies on the formation of AcomA aneurysms was found using the Egger test (p = 0.373). However, due to the limited number of studies, the Egger test was not performed for studies on rupture.
FTP and PcomA aneurysms
There were three and five studies on the relationship between FTP and the formation and rupture of PcomA aneurysms, respectively. No significant heterogeneity was observed (formation: I2 = 0.0%, P = 0.832; rupture: I2 = 0.0%, P = 0.816). Pooled analysis using the fixed-effects model indicated a significant association between FTP and both the formation and rupture of PcomA aneurysms (formation: OR (95% CI) = 2.15 (1.41, 3.30), P < 0.001; rupture: OR (95% CI) = 1.72 (1.26, 2.36), P < 0.001) (Figures 3A, B). The sensitivity analysis of the two groups suggested stable pooled estimates after removing a named study in turn. However, due to the limited number of studies, Egger test was not performed.
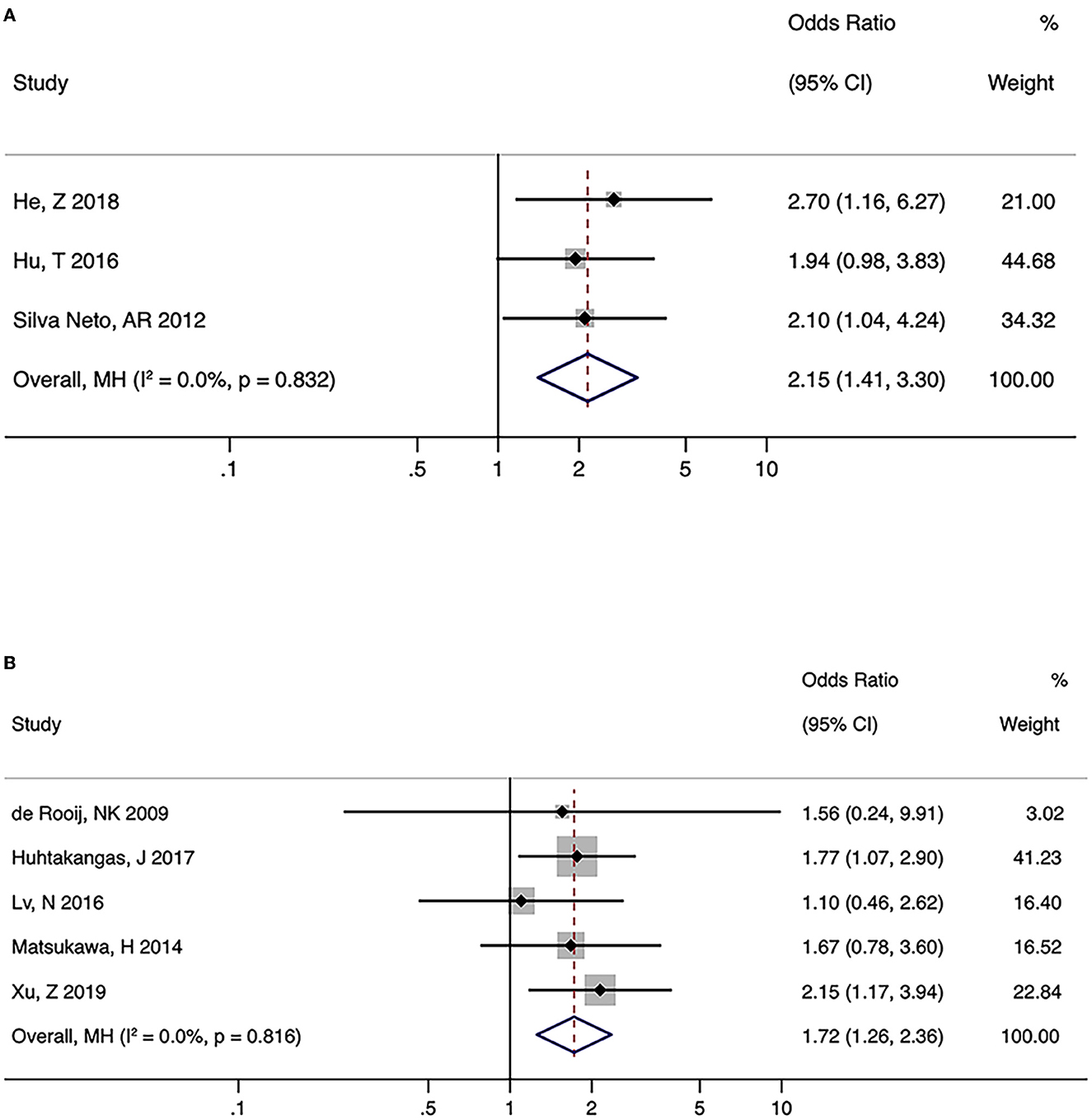
Figure 3. Summary odds ratios of fetal-type posterior cerebral artery (FTP) and posterior communicating artery (PcomA) aneurysms. (A) PcomA aneurysms vs. controls (B) ruptured vs. unruptured PcomA aneurysms.
Hypoplastic/aplastic PcomA and PcomA aneurysms
There were two studies on hypoplastic/aplastic PcomA, in which one focused on the formation of PcomA aneurysms and the other on rupture. The OR for hypoplastic/aplastic PcomA and PcomA aneurysm formation was 8.5 (95% CI = 1.9 to 38.1), and that for PcomA rupture was 0.6 (95% CI = 0.1 to 4.1).
Discussion
This systematic review and meta-analysis investigated the association between COW variations and the formation and rupture of AcomA and PcomA aneurysms. For hypoplastic/aplastic A1 and AcomA aneurysms, a strong association was identified with AcomA aneurysm formation (OR = 7.97), and a moderate association with aneurysm rupture (OR = 1.87). FTP is a risk factor for both the formation and rupture of PcomA aneurysms. However, evidence on hypoplastic/aplastic PcomA and PcomA aneurysms is lacking.
COW variations and aneurysm formation
COW variations play an important role in the formation of intracranial aneurysms, as the incidence of variations was significantly higher in patients with aneurysms than in controls (34). This association arises from hemodynamic changes, because the elevated wall shear stress caused by COW variations may be a predisposition to aneurysm formation (6).
Numerous imaging and cadaver studies described the coincidence of hypoplastic/aplastic A1 and AcomA aneurysms (10–14, 20, 30, 32–35). In this systematic review and meta-analysis, the prevalence of hypoplastic/aplastic A1 ranged from 24.3 to 69.5% in patients with AcomA aneurysms. The difference in prevalence was due to the limited sample size and different definitions of A1 segment hypoplasia and aplasia (26). With one dominant A1 perfusing the bilateral A2s (30), the increased blood flow across the AcomA elevated the hemodynamic stress dramatically at the artery wall, which may contribute to the formation of AcomA aneurysms (8).
The incidence of FTP among patients with PcomA aneurysms ranged from 17.1 to 45.8%. FTP is formed during embryogenesis. After the internal carotid arteries (ICA) give rise to each arterial segment of COW, the blood of the P2-segment of the posterior cerebral artery (PCA-P2) is equally supplied by the P1-segment of the posterior cerebral artery (PCA-P1) and PcomA. Subsequently, when the occipital lobe develops rapidly, either the PCA-P1 or PcomA enlarges to maintain its blood supply. Considering this, FTP refers to the configuration in which the PcomA outsizes the ipsilateral PCA-P1 (36). When the blood supply to the PCA is predominantly from the ICA-PcomA, blood flow increases in the ICA (37), leading to elevated wall shear stress at the ICA-PcomA junction, which may induce the formation of PcomA aneurysms (9).
COW variations and aneurysm rupture
The size and location of intracranial aneurysms were significantly correlated with the risk of rupture. Although the International Study of Unruptured Intracranial Aneurysms indicated that small aneurysms (<7 mm) located in the ICA system had a minimal risk of rupture (2), a pooled analysis of six cohort studies observed that AcomA and PcomA aneurysms were associated with a higher risk of rupture, even those that have sizes <7 mm (4). Therefore, reliable predictors for rupture risk are needed for managing patients with AcomA and PcomA aneurysms.
Given its potential effects on wall shear stress, COW configurations might also potentially increase the risk of aneurysm rupture (6). However, the number of studies is limited, and the conclusions were conflicting. One possible reason is that the sample sizes of most studies were limited to one or two hundred, leading to an insufficient number of patients with those COW variations. In this pooled analysis, we confirmed the association between hypoplastic/aplastic A1 and the rupture status of AcomA aneurysms, and FTP and the rupture of PcomA aneurysms.
Strengths and limitations
First, since the past studies were largely narrative, we chose systematic review and meta-analysis to provide a more comprehensive and unbiased synthesis of relevant studies, and also offered an explicit and exhaustive reporting of the methods used here. By stratifying the variations and site of aneurysms, the localization corresponds to where the hemodynamic stresses were prominently increased, which gives a better understanding of the role of COW configuration on hemodynamics in the development of aneurysms. Second, although there was significant heterogeneity in studies on hypoplastic/aplastic A1 and the formation of AcomA aneurysms, the pooled estimate showed a high correlation strength between the two. In addition, the sensitivity analysis suggested a stable overall estimate. Third, the Egger test performed showed no evidence of publication bias in the meta-analysis, indicating the high credibility of the pooled results.
Nevertheless, this study has several limitations. Only articles published in English were included. Furthermore, the number of studies on aneurysm rupture is limited and are based on small series, which makes it hard to perform subgroup analysis by stratifying the studies by location. Thus, further high-quality research with a large sample size is needed to validate the stability of the results across populations.
Implications for research and clinical practice
Because of the greater availability of non-invasive imaging techniques, including computed tomography angiography and magnetic resonance angiography, a great number of unruptured intracranial aneurysms are observed. According to our findings, AcomA aneurysms with hypoplastic/aplastic A1 and PcomA aneurysms with FTP require close monitoring and can be an indication for treatment in addition to other morphological features of aneurysms.
Since COW configurations can influence aneurysm morphology and endovascular access to aneurysms, the effect of COW variations on the efficacy or complication rate of aneurysm treatment may warrant further investigation (27, 38, 39).
Conclusions
In summary, COW variations and both the formation and rupture of AcomA and PcomA aneurysms were significantly associated. These findings could increase awareness for the need to improve screening among patients with relevant COW variations, and also help with intervention determination for patients with aneurysms. Further studies with high quality and large sample sizes are needed.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LF and H-JM contributed to data analysis and manuscript drafting. D-DZ, FH, and Y-CZ revised the data for the intellectual contents. FH and Y-CZ were responsible for study conception and interpretation. All authors contributed to the article and approved the submitted version.
Funding
The study was funded by the National Natural Science Foundation of China (grant number: 82271368), and the National High Level Hospital Clinical Research Funding (grant number: 2022-PUMCH-A-068).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1098950/full#supplementary-material
References
1. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13:393–404. doi: 10.1016/S1474-4422(14)70015-8
2. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
3. Morita A, Kirino T, Hashi K, Aoki N, Fukuhara S, Hashimoto N, et al. The natural course of unruptured cerebral aneurysms in a Japanese cohort. N Engl J Med. (2012) 366:2474–82. doi: 10.1056/NEJMoa1113260
4. Greving JP, Wermer MJ, Brown RD Jr, Morita A, Juvela S, Yonekura M, et al. Development of the phases score for prediction of risk of rupture of intracranial aneurysms: a pooled analysis of six prospective cohort studies. Lancet Neurol. (2014) 13:59–66. doi: 10.1016/S1474-4422(13)70263-1
5. Bijlenga P, Ebeling C, Jaegersberg M, Summers P, Rogers A, Waterworth A, et al. Risk of rupture of small anterior communicating artery aneurysms is similar to posterior circulation aneurysms. Stroke. (2013) 44:3018–26. doi: 10.1161/STROKEAHA.113.001667
6. Can A, Du R. Association of hemodynamic factors with intracranial aneurysm formation and rupture: systematic review and meta-analysis. Neurosurgery. (2016) 78:510–20. doi: 10.1227/NEU.0000000000001083
7. Hindenes LB, Håberg AK, Johnsen LH, Mathiesen EB, Robben D, Vangberg TR. Variations in the circle of willis in a large population sample using 3d tof angiography: the tromsø study. PLoS ONE. (2020) 15:e0241373. doi: 10.1371/journal.pone.0241373
8. Ujiie H, Liepsch DW, Goetz M, Yamaguchi R, Yonetani H, Takakura K. Hemodynamic study of the anterior communicating artery. Stroke. (1996) 27:2086–93. doi: 10.1161/01.STR.27.11.2086
9. Xu J, Yu Y, Wu X, Wu Y, Jiang C, Wang S, et al. Morphological and hemodynamic analysis of mirror posterior communicating artery aneurysms. PLoS ONE. (2013) 8:e55413. doi: 10.1371/journal.pone.0055413
10. Chen H, Li MH. A1 segment hypoplasia accompanied by acoma aneurysms assessed with magnetic resonance angiography. Surg Radiol Anat. (2014) 36:353–7. doi: 10.1007/s00276-013-1182-5
11. Kaspera W, Ladzinski P, Larysz P, Hebda A, Ptaszkiewicz K, Kopera M, et al. Morphological, hemodynamic, and clinical independent risk factors for anterior communicating artery aneurysms. Stroke. (2014) 45:2906–11. doi: 10.1161/STROKEAHA.114.006055
12. Krasny A, Nensa F, Sandalcioglu IE, Göricke SL, Wanke I, Gramsch C, et al. Association of aneurysms and variation of the A1 segment. J Neurointerv Surg. (2014) 6:178–83. doi: 10.1136/neurintsurg-2013-010669
13. Krzyzewski RM, Tomaszewska IM, Lorenc N, Kochana M, Goncerz G, Klimek-Piotrowska W, et al. Variations of the anterior communicating artery complex and occurrence of anterior communicating artery aneurysm: A2 segment consideration. Folia Med Cracov. (2014) 54:13–20.
14. Neto ÂRS, Câmara RLB, Valença MM. Carotid siphon geometry and variants of the circle of willis in the origin of carotid aneurysms. Arq Neuropsiquiatr. (2012) 70:917–21. doi: 10.1590/S0004-282X2012001200003
15. Hu T, Wang D. Association between anatomical variations of the posterior communicating artery and the presence of aneurysms. Neurol Res. (2016) 38:981–7. doi: 10.1080/01616412.2016.1238662
16. He Z, Wan Y. Is fetal-type posterior cerebral artery a risk factor for intracranial aneurysm as analyzed by multislice Ct angiography? Exp Ther Med. (2018) 15:838–46. doi: 10.3892/etm.2017.5504
17. Huhtakangas J, Lehecka M, Lehto H, Jahromi BR, Niemelä M, Kivisaari R. Cta analysis and assessment of morphological factors related to rupture in 413 posterior communicating artery aneurysms. Acta Neurochir. (2017) 159:1643–52. doi: 10.1007/s00701-017-3263-4
18. Xu Z, Kim BS, Lee KS, Choi JH, Shin YS. Morphological and clinical risk factors for the rupture of posterior communicating artery aneurysms: significance of fetal-type posterior cerebral artery. Neurol Sci. (2019) 40:2377–82. doi: 10.1007/s10072-019-03991-4
19. Park SC, Jung NY, Park ES, Kwon SC. Could A1 aplasia or hypoplasia affect the morphology and rupture risk of anterior communicating artery aneurysm? J Korean Neurosurg Soc. (2021) 65:531–8. doi: 10.3340/jkns.2021.0283
20. Zhang Y, Zhou G, Liu W, Gu W, Zhu Y, Meng L, et al. Analysis of risk factors for anterior communicating artery aneurysm rupture: a single-center study. World Neurosurg. (2021) 153:e59–e65. doi: 10.1016/j.wneu.2021.06.007
21. Stang A. Critical evaluation of the newcastle-ottawa scale for the assessment of the quality of non-randomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
22. Rostom A, Dubé C, Cranney A, Saloojee N, Sy R, Garritty C, et al. Appendix D. Quality Assessment Forms. Rockville(Md): Agency for Healthcare Research Quality (Us). (2004). (Evidence Reports/Technology Assessments, No. 104.). Available online at: http://www.ncbi.nlm.nih.gov/books/NBK35156 (accessed September 27, 2022).
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
24. Aurelio T. Assessing the influence of a single study in the meta-anyalysis estimate. Stata Tech Bull. (1999) 8:15–7.
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
26. Rinaldo L, McCutcheon BA, Murphy ME, Bydon M, Rabinstein AA, Lanzino G. Relationship of A1 segment hypoplasia to anterior communicating artery aneurysm morphology and risk factors for aneurysm formation. J Neurosurg. (2017) 127:89–95. doi: 10.3171/2016.7.JNS16736
27. Jabbarli R, Reinhard M, Roelz R, Kaier K, Weyerbrock A, Taschner C, et al. Clinical relevance of anterior cerebral artery asymmetry in aneurysmal subarachnoid hemorrhage. J Neurosurg. (2017) 127:1070–6. doi: 10.3171/2016.9.JNS161706
28. Lv N, Feng Z, Wang C, Cao W, Fang Y, Karmonik C, et al. Morphological risk factors for rupture of small (<7 mm) posterior communicating artery aneurysms. World Neurosurg. (2016) 87:311–5. doi: 10.1016/j.wneu.2015.12.055
29. Matsukawa H, Fujii M, Akaike G, Uemura A, Takahashi O, Niimi Y, et al. Morphological and clinical risk factors for posterior communicating artery aneurysm rupture. J Neurosurg. (2014) 120:104–10. doi: 10.3171/2013.9.JNS13921
30. Tarulli E, Fox AJ. Potent risk factor for aneurysm formation: termination aneurysms of the anterior communicating artery and detection of A1 vessel asymmetry by flow dilution. AJNR Am J Neuroradiol. (2010) 31:1186–91. doi: 10.3174/ajnr.A2065
31. ij NK, Velthuis BK. Algra A, Rinkel GJ. Configuration of the Circle of Willis, direction of flow, and shape of the aneurysm as risk factors for rupture of intracranial aneurysms. J Neurol. (2009) 256:45–50. doi: 10.1007/s00415-009-0028-x
32. Horikoshi T, Akiyama I, Yamagata Z, Sugita M, Nukui H. Magnetic resonance angiographic evidence of sex-linked variations in the circle of willis and the occurrence of cerebral aneurysms. J Neurosurg. (2002) 96:697–703. doi: 10.3171/jns.2002.96.4.0697
33. Charbel FT, Seyfried D, Mehta B, Dujovny M, Ausman JI. Dominant A1: angiographic and clinical correlations with anterior communicating artery aneurysms. Neurol Res. (1991) 13:253–6. doi: 10.1080/01616412.1991.11740001
34. Kayembe KN, Sasahara M, Hazama F. Cerebral aneurysms and variations in the Circle of Willis. Stroke. (1984) 15:846–50. doi: 10.1161/01.STR.15.5.846
35. Kwak R, Niizuma H, Suzuki J. Hemodynamics in the anterior part of the circle of willis in patients with intracranial aneurysms: a study of cerebral angiography. Tohoku J Exp Med. (1980) 132:69–73. doi: 10.1620/tjem.132.69
36. Menshawi K, Mohr JP, Gutierrez J, A. Functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke. (2015) 17:144–58. doi: 10.5853/jos.2015.17.2.144
37. Hendrikse J, van Raamt AF, van der Graaf Y, Mali WP, van der Grond J. Distribution of cerebral blood flow in the Circle of Willis. Radiology. (2005) 235:184–9. doi: 10.1148/radiol.2351031799
38. Tarulli E, Sneade M, Clarke A, Molyneux AJ, Fox AJ. Effects of Circle of Willis anatomic variations on angiographic and clinical outcomes of coiled anterior communicating artery aneurysms. AJNR Am J Neuroradiol. (2014) 35:1551–5. doi: 10.3174/ajnr.A3991
Keywords: Circle of Willis, intracranial aneurysm, variation, rupture, anterior communicating artery, posterior communicating artery
Citation: Feng L, Mao H-J, Zhang D-D, Zhu Y-C and Han F (2023) Anatomical variations in the Circle of Willis and the formation and rupture of intracranial aneurysms: A systematic review and meta-analysis. Front. Neurol. 13:1098950. doi: 10.3389/fneur.2022.1098950
Received: 15 November 2022; Accepted: 30 December 2022;
Published: 16 January 2023.
Edited by:
Li Ma, Beijing Tiantan Hospital, Capital Medical University, ChinaReviewed by:
S. Ottavio Tomasi, Paracelsus Medical University, AustriaCamillo Sherif, Karl Landsteiner University of Health Sciences, Austria
Copyright © 2023 Feng, Mao, Zhang, Zhu and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Han,  bG91cmFoYW5AMTYzLmNvbQ==
bG91cmFoYW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Lu Feng
Lu Feng He-Jiao Mao
He-Jiao Mao Ding-Ding Zhang
Ding-Ding Zhang Yi-Cheng Zhu
Yi-Cheng Zhu Fei Han
Fei Han