
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Neurol. , 24 January 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1090133
This article is part of the Research Topic Women in Multiple Sclerosis and other demyelinating disorders: a global perspective View all 8 articles
The immunoprotective role of pregnancy in multiple sclerosis (MS) has been known for decades. Conversely, there has been rich debate on the topic of breastfeeding and disease activity in MS. In clinical practice, women are often offered to restart their disease-modifying drug (DMD) soon after delivery to maintain their relapse risk protection. Limited available information about peri-partum DMD safety can discourage women to choose breastfeeding, despite the World Health Organization's recommendation to breastfeed children for the first 6 months of life exclusively. New evidence is emerging about the protective role of exclusive breastfeeding on relapse rate. Research studies shed light on the hormonal and immunological mechanisms driving the risk of relapses during pregnancy and postpartum. Finally, case reports, real-world data, and clinical trials are increasing our knowledge of the safety of DMDs for the fetus and infant. While some DMDs must be avoided, others may be considered in highly active pregnant or lactating women with MS. This mini-review conveys recent evidence regarding the protective role of exclusive breastfeeding in MS and offers clinicians practical considerations for a patient-tailored approach.
Since the PRISM study in 1998 (1), neurologists have reported that the likelihood of a woman experiencing a multiple sclerosis (MS) relapse was reduced during pregnancy, especially during the third trimester, and again increases in the first 3–6 months postpartum, compared with her pre-pregnancy risk status (1–3).
In this scenario, the effect of breastfeeding on postpartum relapses was unclear: some studies showed potential benefits (4, 5), and others did not (6, 7). Consequently, in clinical practice, women were often offered to restart their disease-modifying drug (DMD) soon after delivery to reduce the chances of having an MS relapse. Limited available evidence about DMD safety meant many women avoided breastfeeding, despite the World Health Organization's recommendation to exclusively breastfeed children for the first 6 months of life (8).
This mini-review discusses recent evidence about a protective role for breastfeeding and provides clinicians with a practical tool for a patient-driven approach in evaluating women with MS planning to breastfeed.
To understand the biological mechanisms behind the protective role of exclusive breastfeeding in MS, we consider the hormonal and immunological changes characterizing women's reproductive years, from menstruation to pregnancy and puerperium (Figure 1).
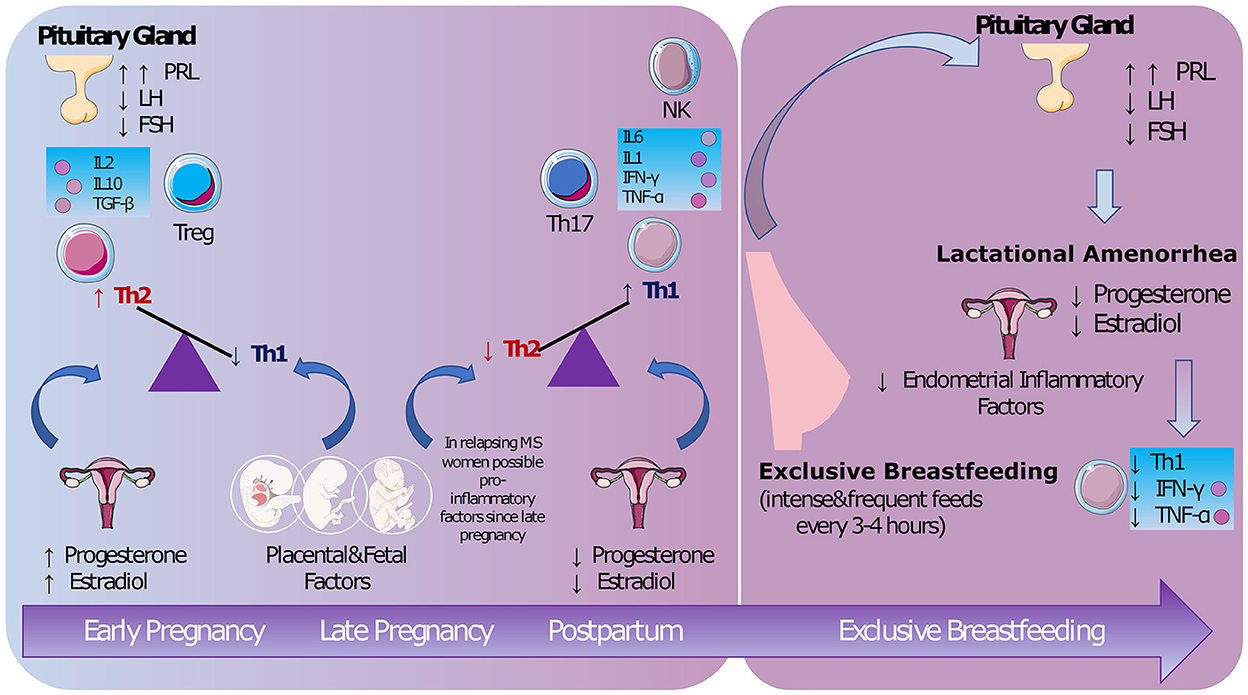
Figure 1. The biological mechanisms. During pregnancy both hormonal and fetal/placental factors maintain a non-inflammatory status. A pro-inflammatory response is restored after delivery [possibly from late pregnancy (5)]. Lactational amenorrhea promoted by exclusive breastfeeding seems to decrease lymphocyte Th1 activity (9). PRL, prolactin; LH, luteinizing hormone; FSH, follicle-stimulating hormone; IL, interleukin; TGF, transforming growth factor; Treg, regulatory T cells; Th, lymphocyte T-helper; NK, natural killer; IFN, interferon; TNF, tumor necrosis factor (Images from Servier Medical Art by Servier https://smart.servier.com/).
The protective role of pregnancy, which MS shares with other autoimmune conditions, has been known for decades (1).
During pregnancy, there is a shift from a lymphocyte T-helper (Th)-1 [interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin (IL)-2] to a Th-2 (IL-4, IL-10) immune response to protect the fetal-placental unit (10). High levels of estradiol and progesterone suppress lymphocyte Th-1 activity (11), which plays a crucial role in promoting MS inflammatory activity, while placental factors sustain a Th-2 response (10). The effects of estrogen on MS activity have been studied in experimental autoimmune encephalomyelitis (12), cuprizone mice (13), and in clinical trials using estriol (14, 15).
As drop in sex hormones after delivery is associated with increased annualized relapse rate (ARR) postpartum, researchers suggested starting protective treatments from the third trimester or immediately postpartum (16).
However, not all women with MS experience rebound activity after delivery. Other immunological factors may influence the changes in relapse risk in pregnancy and postpartum, such as changes in lymphocyte T-regulatory cells (Th-17) (17) and natural killer cells (18) that occur from late pregnancy. In these studies, however, researchers did not investigate if exclusive breastfeeding impacted the immunological changes.
Researchers started to observe reduced ARR in MS women who exclusively breastfeed compared with non-breastfeeding women (5). A small study showed that MS women having relapses postpartum had increasing levels of IFN-gamma from the third trimester, but this remained low in women who exclusively breastfed (9).
Exclusive and frequent feedings (every 3–4 h) maintain high prolactin and low luteinizing hormone (LH) levels, inducing ovarian suppression (lactational amenorrhea). Since menstrual cycles are involve inflammatory processes that probably serve to prepare the endometrium for embryonic implantation (19), their suppression is could be related to anti-inflammatory effects (20). Prolactin has a controversial role: on the one hand, this hormone has recognized inflammatory activity (21), on the other hand, prolactin may help oligodendrocyte precursors to repair damaged myelin (22).
Whether a predisposition emerges during pregnancy has yet to be defined, and studies that carefully evaluate the role of breastfeeding are still lacking. Furthermore, other factors such as parity, body mass index, and fetal sex may influence the postpartum ARR risk (23).
For instance, fetal sex may influence maternal immune activity during pregnancy. A study found that a male fetus may increase maternal levels of pro-inflammatory cytokines (e.g., IL-12p70, IL-21) (24). In fact, histological studies showed that placentae from male premature babies had greater markers of inflammation than female ones, suggesting a more robust maternal immune response to male fetuses (25). However, other studies could not confirm the association between fetal sex and increased maternal production of inflammatory proteins (26). A recent study by Ross et al. considered multiple factors (23), including fetal sex, body mass index and parity, showing different patterns of inflammatory markers in pregnant women. Their findings highlight the complexity of pregnancy and postpartum regarding immune system maternal adaptation, which is likely to be influenced by multiple factors.
The PRIMS study (1) was the first large prospective study on MS and pregnancy. The effects of exclusively breastfeeding on relapses were not a specified outcome. However, a subgroup analysis reported no associations between breastfeeding and relapse rate in the first 3 months postpartum.
Since then, there has been controversy about the protective role of breastfeeding on postpartum annualized relapse rate (ARR). Prospective and retrospective studies have followed, and, although authors agree that breastfeeding has no adverse effects, some have reported beneficial effects on ARR (27, 28), whilst others have not (16, 29). One of the main biases was that the early studies included patients who were breastfeeding in general, regardless of its exclusivity and duration. As discussed before, it is thought to be lactational amenorrhea, promoted by exclusive breastfeeding, that seems to suppress inflammatory activity.
In 2009, Langer-Gould et al. (5) were the first to differentiate between exclusive and non-exclusive breastfeeding, finding an ARR significantly higher in women who did not exclusively breastfeed, irrespective of their 2-year pre-pregnancy ARR or treatment history. This observation was in line with evidence for the protective role of lactational amenorrhea (Figure 1). A meta-analysis (30) found women with MS who breastfeed were almost half as likely to experience a postpartum relapse as women who did not. However, significant heterogeneity was present (63%), driven mainly by the variable duration of the postpartum follow-up.
Although two large observational studies nourished the debate (31, 32) on breastfeeding and MS, showing different results (4, 7), recent works have supported the benefits of breastfeeding (33, 34), as summarized by a recent meta-analysis (35). Krysko et al. (35), with an overall estimated heterogeneity of 48%, showed that those who breastfed had 37% lower odds of postpartum relapse than those who did not breastfeed or did not exclusively breastfeed postpartum.
DMDs target neuroinflammation and are approved for patients with relapsing-remitting MS (RRMS) and active secondary-progressive MS (SPMS) patients (36). Approximately 30% of mothers with MS may relapse within the first 3 months postpartum (1). Effective disease management during this challenging period is essential for the wellbeing of the mother and baby.
Nine classes of DMDs with different mechanisms of action and routes of administration are currently available in MS. For each class, there are limited data available, with some studies showing individual variability (37). However, most DMDs for RRMS are not advised during breastfeeding by manufactures, the Food and Drug Administration (FDA) and European Medicines Agency (EMA) (38, 39).
The drug's molecular weight is probably the most critical factor in determining transfer to breast milk, but oral absorption is also crucial for the child. Poorly absorbed drugs are not likely to enter the child's bloodstream and cause pharmacological effects (40).
Although the FDA and EMA advise caution, first-line modest efficacy injectable treatments for RRMS, such as glatiramer acetate (GA) and interferon beta (IFN-β), are considered safe to use in pregnancy and during lactation (41, 42).
Oral agents, including modest and high-efficacy treatments, must be avoided during pregnancy and breastfeeding (43, 44). Although there have been case studies in the literature showing a very low Relative Infant Dose (RID) in the breastmilk of lactating mothers for dimethyl fumarate (DMF), larger studies are required to establish its safety (45). Cladribine is a potential choice for MS patients who are planning to conceive because it allows conception 6 months after the last dose (46). The evidence suggests withholding breastfeeding for at least 48 h after a dose (47, 48), but manufacturers recommend a 7-day (Europe) or 10-day (US) abstinence period.
Monoclonal antibodies are highly effective DMDs. Due to their high molecular weight, these drugs do not pass into breast milk in significant amounts. Studies show minimal concentrations and, at least in the short term, no negative impact on breastfed infants. Although the evidence for relative safety is sparse in MS, it is widespread in non-neurological inflammatory diseases (49). Natalizumab, a humanized recombinant anti-α4-integrin antibody, is not recommended in the third trimester (50). In the postpartum, although it is detected in breast milk, its RID is <10%, the recommended safety threshold. As the accumulation of the drug in milk was identified in women receiving multiple infusions (51, 52), longer follow-up is needed to inform definitive recommendations for breastfeeding.
For anti-CD20 therapies, e.g., Ocrelizumab and Ofatumumab, only a few studies of humans and infants with small numbers have been performed, mainly due to the prolonged dose interval (50). Therefore, EMA and FDA recommend high caution for adverse effects in infants if its use is unavoidable in the lactating mother. Nevertheless, different guidelines (53, 54) suggest the resumption of anti-CD20 therapies, if needed, after the first weeks of breastfeeding. Vaccinations, which are contraindicated in breastfeeding women, but needed for anti-CD20 therapies, may be discussed with patients. The first prospective study, SOPRANINO (55), on breastfed infants of mothers on ocrelizumab may establish whether the drug is safe during breastfeeding.
Finally, there is no safety information on anti-CD52 therapy (Alemtuzumab). The EMA suggests stopping breastfeeding 4 months after the last infusion, while the FDA asks to balance the risk of the need of feeding to the infant and the clinical need for infusion to the mother.
High-dose intravenous or oral methylprednisolone is first-line treatment for acute, disabling MS relapses to hasten recovery. It is typically administered for 3 or 5 days (56).
For the mother, potential side effects of corticosteroid exposure on the baby may include suppression of growth, changes in behavior or appetite, acne, and sleep disorders (57). Particular attention should be paid to mothers suffering from postpartum depression.
A study on the concentrations of methylprednisolone in the breast milk of 16 patients with MS showed that transfer of corticosteroids into breast milk is very low (58). Methylprednisolone levels dropped significantly 4 h after intravenous infusion, and the RID was 0.71% of the weight-adjusted maternal dose, which was >10 times lower than the accepted theoretical RID of 10%. It also found that fully breastfed infants would receive a lower dose than their daily cortisol output. These findings are consistent with previous reports obtained in smaller sample sizes (58). If possible, mothers should wait 2–4 h after infusion to further limit the infant's exposure.
MRI with gadolinium to confirm a relapse is not usually necessary in clinical practice. However, if its administration is considered necessary (e.g., in a differential diagnosis with progressive multifocal leukoencephalopathy), most guidelines state that breastfeeding need not be disrupted after a nursing mother receives a gadolinium-containing contrast medium (59), as only small amounts of this molecule are detectable in the milk.
Prepartum choices influence decisions about DMDs in the postpartum period. DMD initiation/discontinuation must be carefully evaluated and agreed with the patient before family planning. Clinicians should pay particular attention to natalizumab and fingolimod, whose cessation can cause relapse exacerbation during pregnancy and postpartum.
At present, there are no studies directly comparing exclusive breastfeeding vs. DMDs in terms of efficacy on postpartum ARR.
Figure 2 offers a flowchart for possible treatment choices.
For women planning to conceive at the time of the MS diagnosis, reasonable treatment options include:
• Cladribine, which can be stopped 6 months before conception, offers exceptional control during pregnancy and can be resumed either after breastfeeding or when solids are introduced, allowing seven to 10 days between the last dose and breastfeeding (60) (mothers may want to consider pumping to maintain their milk supply);
• anti-CD20 (ocrelizumab or ofatumumab) because even with exposure around the time of conception, they clear from the maternal system by the time the placental transfer is established and can be resumed during lactation if needed (61);
• GA or IFN, as they can be continued during pregnancy and lactation. However, resuming these drugs in the early postpartum may not affect the risk of relapses (16).
For a patient with high disease activity, defined as frequent relapses with incomplete recovery. natalizumab can be offered, continued up to 28–34 weeks of gestation, and resumed soon after delivery to minimize relapse risk (50, 62).
If the patient is not on one of the DMDs mentioned above, a careful plan for treatment cessation should be in place before conception.
Dimethyl fumarate requires a two-month washout period, whilst Teriflunomide needs to be eliminated via chelation. Studies and clinical trials have not associated these two DMDs with rebound activity (43, 62), so it is safe to conclude that patients can resume them after breastfeeding unless pregnancy or postpartum relapses occur. According to the United States label (63), mothers can potentially resume dimethyl fumarate during lactation, but clinicians should monitor infants for potential side effects and weight gain.
Cessation of Sphingosine-1-Phosphate receptor modulators can be associated with rebound disease activity. Authors have also reported fatal outcomes and pregnancy discontinuation caused by severe relapses after fingolimod termination (64). Clinicians should inform patients of childbearing age of these risks. The washout period from fingolimod is at least 2 months, though the availability of ponesimod, which has a shorter half-life, may reduce this time range. Pregnant patients previously on S1P inhibitors can switch to natalizumab and continue for up to 34 weeks of gestation and resume it early after delivery, which, as discussed, can be compatible with breastfeeding. Otherwise, clinicians should carefully monitor the patients during pregnancy and postpartum introducing rapid-action drugs, such as anti-CD20 or natalizumab, if a relapse occurs.
The last infusion of Alemtuzumab should be administered at least 4 months before conception. Lymphocyte depletion should protect women from relapses during pregnancy, but there is no data on breastfeeding, so a second infusion should be postponed until after breastfeeding. Patients should be closely monitored during postpartum and switched to other monoclonal antibodies if there are signs of disease activity before the second infusion.
As discussed before, both FDA and EMA have now dropped the “Category C” classification for pregnancy and breastfeeding from the GA and IFN labels. Consequently, clinicians can consider switching MS patients from potentially teratogenic DMDs to these injectables. However, evidence on the safety of switching during pregnancy and postpartum is very limited. Most existing studies included patients who switched therapy either because of treatment failure (65) or for safety issues (66). Thus, ARR may have influenced the results in the former studies, and both stable and unstable patients were included in the latter studies.
Hence, in pregnant patients on teratogenic DMDs with low risk of rebound activity, clinicians may discuss with the patients the choice of remaining treatment-free during pregnancy and postpartum or using GA and INF as bridging treatment before re-starting the prepartum DMD.
For breastfeeding and MS, evidence only comes from observational studies. Over the years, increasing attention toward women-related issues in MS has stimulated the setup of well-designed prospective studies. However, many studies are still retrospective with the number of relapses based on patients' recall.
For many studies, an inclusion criterion for the breastfeeding group is to breastfeed for at least 2 months. Therefore, women experiencing a relapse before this time are automatically assigned to the non-breastfeeding cohort. However, this creates bias (i.e., the immortal person-time bias) by artificially decreasing the number of relapsing patients in the breastfeeding group. Only one study included this bias by defining the inclusion criterion for the breastfeeding group as the intent to breastfeed exclusively for at least 2 months and not actual breastfeeding (34).
It is challenging to quantify exclusive breastfeeding in the research setting. In retrospective studies, occasional integration with formula milk may not be correctly recalled. Furthermore, mothers may over report breastfeeding because of social or family pressure. In addition, although Hellwig et al. (34) suggest that benefits are limited to exclusive breastfeeding, no studies have focused so far on partial breastfeeding only. Midwives usually advise exclusively breastfeeding for at least 6 weeks to let the milk supplies stabilize. Afterward, women may decide to integrate with formula milk, occasionally, possibly maintaining ovarian suppression. However, studies usually either group together exclusive and non-exclusive breastfeeding or do not stratify the non-exclusive breastfeeding cohort according to the number of feeds missed in a day. Finally, no studies have investigated breastfeeding along with the hormonal levels to get a further inside on the biological mechanisms behind possible relapse prevention.
Krysko et al. possibly highlight that the benefits of exclusive breastfeeding are more significant in the presence of high ARR pre-pregnancy (35). However, this evidence is based on few studies, as many authors did not adjust their analysis for prepartum MS activity. In addition, MS treatment is the primary factor preventing women from breastfeeding, potentially biasing the observations.
Finally, it is not clear how long the benefits of exclusive breastfeeding may last over time. Most of the studies have only accounted for relapses in the first year postpartum. However, two studies extended the observation period and found prolonged beneficial effects on ARR (67, 68). Real-world evidence showed that breastfeeding did not substantially modify the risk of disability over time (69), but prospective studies with a longer observational time are still required.
The primary biological mechanism driving the protective role of exclusive breastfeeding is ovarian suppression. This suppression is usually maintained until the infant has been weaned consistently to solids (usually around 6–9 months postpartum). Therefore, it is safe to conclude that women with MS can exclusively breastfeed until solids are needed, and this could have a protective effect for at least 1 year (35). More evidence is required for longer durations. Although it has been highlighted (35) that higher prepartum ARRs might be even related to lower postpartum ARRs in exclusively breastfeeding women, further studies must confirm this. Therefore, neurologists should adopt a careful approach for women with high prepartum ARR. Finally, MS women planning pregnancy should be offered multi-disciplinary consultations to decide on their treatment journey. Particular attention should be paid to DMDs that have a relatively high risk of rebound. Then, during pregnancy and breastfeeding, MS women should be carefully monitored for relapses and DMDs should be reinstituted if indicated.
SC has done the figures included in the manuscript. All authors have contributed in the literature review and in drafting the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by Rosetrees and Bermuda Trust (PGL21/10079) and Medical Research Council (MR/S026088/1).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in multiple sclerosis group. N Engl J Med. (1998) 339:285–91. doi: 10.1056/NEJM199807303390501
2. Salemi G, Callari G, Gammino M, Battaglieri F, Cammarata E, Cuccia G, et al. The relapse rate of multiple sclerosis changes during pregnancy: a cohort study. Acta Neurol Scand. (2004) 110:23–6. doi: 10.1111/j.1600-0404.2004.00270.x
3. Vukusic S, Hutchinson M, Hours M, Moreau T, Cortinovis-Tourniaire P, Adeleine P, et al. Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. (2004) 127:1353–60. doi: 10.1093/brain/awh152
4. Hellwig K, Haghikia A, Rockhoff M, Gold R. Multiple sclerosis and pregnancy: experience from a nationwide database in Germany. Ther Adv Neurol Disord. (2012) 5:247–53. doi: 10.1177/1756285612453192
5. Langer-Gould A, Huang SM, Gupta R, Leimpeter AD, Greenwood E, Albers KB, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. (2009) 66:958–63. doi: 10.1001/archneurol.2009.132
6. Airas L, Jalkanen A, Alanen A, Pirttilä T, Marttila RJ. Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology. (2010) 75:474–6. doi: 10.1212/WNL.0b013e3181eb5860
7. Portaccio E, Ghezzi A, Hakiki B, Martinelli V, Moiola L, Patti F, et al. Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology. (2011) 77:145–50. doi: 10.1212/WNL.0b013e318224afc9
8. WHO Breastfeeding. Available online at: https://www.who.int/health-topics/breastfeeding#tab=tab_1 (accessed October 27, 2022).
9. Langer-Gould A, Gupta R, Huang S, et al. Interferon-gamma-producing T cells, pregnancy, and postpartum relapses of multiple sclerosis. Arch Neurol. (2010) 67:51–7. doi: 10.1001/archneurol.2009.304
10. Wegmann TG, Lin H, Guilbert L, Mosmann TR. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today. (1993) 14:353–6. doi: 10.1016/0167-5699(93)90235-D
11. Koetzier SC, Neuteboom RF, Wierenga-Wolf AF, Melief MJ, de Mol CL, van Rijswijk A, et al. Effector T helper cells are selectively controlled during pregnancy and related to a postpartum relapse in multiple sclerosis. Front Immunol. (2021) 12:642038. doi: 10.3389/fimmu.2021.642038
12. Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. (2006) 129:1953–71. doi: 10.1093/brain/awl075
13. Acs P, Kipp M, Norkute A, Johann S, Clarner T, Braun A, et al. 17beta-estradiol and progesterone prevent cuprizone provoked demyelination of corpus callosum in male mice. Glia. (2009) 57:807–14. doi: 10.1002/glia.20806
14. Kim S, Liva SM, Dalal MA, Verity MA, Voskuhl RR. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. (1999) 52:1230–8. doi: 10.1212/WNL.52.6.1230
15. Sicotte NL, Liva SM, Klutch R, Pfeiffer P, Bouvier S, Odesa S, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. (2002) 52:421–8. doi: 10.1002/ana.10301
16. Achiron A, Kishner I, Dolev M, Stern Y, Dulitzky M, Schiff E, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. (2004) 251:1133–7. doi: 10.1007/s00415-004-0495-z
17. Neuteboom RF, Verbraak E, Wierenga-Wolf AF, et al. Pregnancy-induced fluctuations in functional T-cell subsets in multiple sclerosis patients. Mult Scler. (2010) 16:1073–8. doi: 10.1177/1352458510373939
18. Airas L, Saraste M, Rinta S, Elovaara I, Huang Y-H, Wiendl H, et al. Immunoregulatory factors in multiple sclerosis patients during and after pregnancy: relevance of natural killer cells. Clin Exp Immunol. (2008) 151:235. doi: 10.1111/j.1365-2249.2007.03555.x
19. Berbic M, Fraser IS. Immunology of normal and abnormal menstruation. Womens Health. (2013) 9:387–95. doi: 10.2217/WHE.13.32
20. Langer-Gould A, Beaber BE. Effects of pregnancy and breastfeeding on the multiple sclerosis disease course. Clin Immunol. (2013) 149:244–50. doi: 10.1016/j.clim.2013.01.008
21. Desai MK, Brinton RD. Autoimmune disease in women: endocrine transition and risk across the lifespan. Front Endocrinol. (2019) 10:265. doi: 10.3389/fendo.2019.00265
22. Gregg C, Shikar V, Larsen P, Mak G, Chojnacki A, Yong VW, et al. White matter plasticity and enhanced remyelination in the maternal CNS. J Neurosci. (2007) 27:1812–23. doi: 10.1523/JNEUROSCI.4441-06.2007
23. Ross KM, Schetter CD, Carroll JE, Mancuso RA, Breen EC, Okun ML, et al. Inflammatory and immune marker trajectories from pregnancy to one-year post-birth. Cytokine. (2022) 149:155758. doi: 10.1016/j.cyto.2021.155758
24. Enninga EAL, Nevala WK, Creedon DJ, Markovic SN, Holtan SG. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am J Reprod Immunol. (2015) 73:251–62. doi: 10.1111/aji.12303
25. Ghidini A, Salafia CM. Gender differences of placental dysfunction in severe prematurity. BJOG. (2005) 112:140–4. doi: 10.1111/j.1471-0528.2004.00308.x
26. Mitchell AM, Palettas M, Christian LM. Fetal sex is associated with maternal stimulated cytokine production, but not serum cytokine levels, in human pregnancy. Brain Behav Immun. (2017) 60:32–7. doi: 10.1016/j.bbi.2016.06.015
27. Gulick EE, Halper J. Influence of infant feeding method on postpartum relapse of mothers with MS. Int J MS Care. (2002) 4:183–91. doi: 10.7224/1537-2073-4.4.183
28. Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting. Mult Scler. (2007) 13:900–8. doi: 10.1177/1352458506075654
29. De las Heras V, De Andrés C, Téllez N, Tintoré M. Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult Scler. (2007) 13:981–4. doi: 10.1177/1352458507077896
30. Pakpoor J, Disanto G, Lacey MV, et al. Breastfeeding and multiple sclerosis relapses: a meta-analysis. J Neurol. (2012) 259:2246–8. doi: 10.1007/s00415-012-6553-z
31. Hellwig K. We need to conduct clinical trials of disease-modifying therapy in pregnancy to optimize care of women with MS - No. Mult Scler. (2019) 25:189–90. doi: 10.1177/1352458518795398
32. Alvarez E, Mowry EM. We need to conduct clinical trials of disease-modifying therapy in pregnancy to optimize care of women with MS - yes. Mult Scler. (2019) 25:187–8. doi: 10.1177/1352458518794061
33. Langer-Gould A, Smith JB, Albers KB, Xiang AH, Wu J, Kerezsi EH, et al. Pregnancy-related relapses and breastfeeding in a contemporary multiple sclerosis cohort. Neurology. (2020) 94:E1939–49. doi: 10.1212/WNL.0000000000009374
34. Hellwig K, Rockhoff M, Herbstritt S, Borisow N, Haghikia A, Elias-Hamp B, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol. (2015) 72:1132–8. doi: 10.1001/jamaneurol.2015.1806
35. Krysko KM, Rutatangwa A, Graves J, Lazar A, Waubant E. Association between breastfeeding and postpartum multiple sclerosis relapses: a systematic review and meta-analysis. JAMA Neurol. (2020) 77:327–38. doi: 10.1001/jamaneurol.2019.4173
36. Cree BAC, Mares J, Hartung HP. Current therapeutic landscape in multiple sclerosis: an evolving treatment paradigm. Curr Opin Neurol. (2019) 32:365–77. doi: 10.1097/WCO.0000000000000700
37. Almas S, Vance J, Baker T, Hale T. Management of multiple sclerosis in the breastfeeding mother. Mult Scler Int. (2016) 2016:6527458. doi: 10.1155/2016/6527458
38. Capone F, Albanese A, Quadri G, Di Lazzaro V, Falato E, Cortese A, et al. Disease-modifying drugs and breastfeeding in multiple sclerosis: a narrative literature review. Front Neurol. (2022) 13:851413. doi: 10.3389/fneur.2022.851413
39. Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of women with multiple sclerosis planning pregnancy. Curr Treat Options Neurol. (2021) 23:11. doi: 10.1007/s11940-021-00666-4
40. Rowe H, Baker T, Hale TW. Maternal medication, drug use, and breastfeeding. Pediatr Clin North Am. (2013) 60:275–94. doi: 10.1016/j.pcl.2012.10.009
41. Ciplea AI, Langer-Gould A, Stahl A, Thiel S, Queisser-Wahrendorf A, Gold R, et al. Safety of potential breast milk exposure to IFN-β or glatiramer acetate: one-year infant outcomes. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e757. doi: 10.1212/NXI.0000000000000757
42. Ciplea AI, Kurzeja A, Thiel S, Haben S, Alexander J, Adamus E, et al. Eighteen-month safety analysis of offspring breastfed by mothers receiving glatiramer acetate therapy for relapsing multiple sclerosis – COBRA study. Mult Scler J. (2022) 28:1641–50. doi: 10.1177/13524585221083982
43. Confavreux C, O'Connor P, Comi G, Freedman MS, Miller AE, Olsson TP, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. (2014) 13:247–56. doi: 10.1016/S1474-4422(13)70308-9
44. Datta P, Ciplea AI, Rewers-Felkins K, Baker T, Gold R, Hale TW, et al. Cladribine transfer into human milk: a case report. Mult Scler. (2021) 27:799–801. doi: 10.1177/1352458520912173
45. Ciplea AI, Datta P, Rewers-Felkins K, Baker T, Gold R, Hale TW, et al. Dimethyl fumarate transfer into human milk. Ther Adv Neurol Disord. (2020) 13:1756286420968414. doi: 10.1177/1756286420968414
46. Giovannoni G, Galazka A, Schick R, Leist T, Comi G, Montalban X, et al. Pregnancy outcomes during the clinical development program of cladribine in multiple sclerosis: an integrated analysis of safety. Drug Saf. (2020) 43:635–43. doi: 10.1007/s40264-020-00948-x
47. Canibaño B, Deleu D, Mesraoua B, Melikyan G, Ibrahim F, Hanssens Y. Pregnancy-related issues in women with multiple sclerosis: an evidence-based review with practical recommendations. J drug Assess. (2020) 9:20–36. doi: 10.1080/21556660.2020.1721507
48. Dost-Kovalsky K, Thiel S, Ciplea AI, Gold R, Hellwig K. Cladribine and pregnancy in women with multiple sclerosis: the first cohort study. Mult Scler. (2022). doi: 10.1177/13524585221131486 [Epub ahead of print].
49. Matro R, Martin CF, Wolf D, Shah SA, Mahadevan U. Exposure concentrations of infants breastfed by women receiving biologic therapies for inflammatory bowel diseases and effects of breastfeeding on infections and development. Gastroenterology. (2018) 155:696–704. doi: 10.1053/j.gastro.2018.05.040
50. Ciplea AI, Langer-Gould A, de Vries A, Schaap T, Thiel S, Ringelstein M, et al. Monoclonal antibody treatment during pregnancy and/or lactation in women with MS or neuromyelitis optica spectrum disorder. Neurol Neuroimmunol neuroinflamm. (2020) 7:e723. doi: 10.1212/NXI.0000000000000723
51. Proschmann U, Thomas K, Thiel S, Hellwig K, Ziemssen T. Natalizumab during pregnancy and lactation. Mult Scler. (2018) 24:1627–34. doi: 10.1177/1352458517728813
52. Baker TE, Cooper SD, Kessler L, Hale TW. Transfer of natalizumab into breast milk in a mother with multiple sclerosis. J Hum Lact. (2015) 31:233–6. doi: 10.1177/0890334414566237
53. Dobson R, Rog D, Ovadia C, Murray K, Hughes S, Ford HL, et al. Anti-CD20 therapies in pregnancy and breast feeding: a review and ABN guidelines. Pract Neurol. (2022). doi: 10.1136/pn-2022-003426
54. Vukusic S, Carra-Dalliere C, Ciron J, Maillart E, Michel L, Leray E, et al. Pregnancy and multiple sclerosis: 2022 recommendations from the French multiple sclerosis society. Mult Scler. (2022). doi: 10.1177/13524585221129472
55. Bove R, Hellwig K, Pasquarelli N, Borriello F, Dobson R, Oreja-Guevara C, et al. Ocrelizumab during pregnancy and lactation: Rationale and design of the MINORE and SOPRANINO studies in women with MS and their infants. Mult Scler Relat Disord. (2022) 64:1–9. doi: 10.1016/j.msard.2022.103963
56. Sellebjerg F, Barnes D, Filippini G, Midgard R, Montalban X, Rieckmann P, et al. EFNS guideline on treatment of multiple sclerosis relapses: report of an EFNS task force on treatment of multiple sclerosis relapses. Eur J Neurol. (2005) 12:939–46. doi: 10.1111/j.1468-1331.2005.01352.x
57. Greenberger PA, Odeh YK, Frederiksen MC. Pharmacokinetics of prednisolone transfer to breast milk. Clin Pharmacol Ther. (1993) 53:324–8. doi: 10.1038/clpt.1993.28
58. Karahan SZ, Boz C, Terzi M, Aktoz G, Sen S, Ozbudun B, et al. Methylprednisolone concentrations in breast milk and serum of patients with multiple sclerosis treated with IV pulse methylprednisolone. Clin Neurol Neurosurg. (2020) 197:106118. doi: 10.1016/j.clineuro.2020.106118
59. Committee Opinion No. 723: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol. (2017) 130:e210–6. doi: 10.1097/AOG.0000000000002355
60. Giovannoni G, Sorensen PS, Cook S, Rammohan KW, Rieckmann P, Comi G, et al. Efficacy of cladribine tablets in high disease activity subgroups of patients with relapsing multiple sclerosis: a post hoc analysis of the CLARITY study. Mult Scler J. (2018) 25:819–27. doi: 10.1177/1352458518771875
61. Dobson R, Ramagopalan S, Giovannoni G. The effect of gender in clinically isolated syndrome (CIS): a meta-analysis. Mult Scler J. (2012) 18:600–4. doi: 10.1177/1352458511426740
62. Yeh WZ, Widyastuti PA, Van der Walt A, Stankovich J, Havrdova E, Horakova D, et al. Natalizumab, fingolimod, and dimethyl fumarate use and pregnancy-related relapse and disability in women with multiple sclerosis. Neurology. (2021) 96:e2989. doi: 10.1212/WNL.0000000000012084
63. U.S. Food and Drug Administration. Tecfidera® (dimethyl fumarate). Full prescribing information: Biogen Inc., Cambridge, MA. Available online at: https://www.tecfidera.com/content/dam/commercial/tecfidera/pat/en_us/pdf/full-prescribing-info.pdf (accessed November 28, 2022).
64. Novi G, Ghezzi A, Pizzorno M, Lapucci C, Bandini F, Annovazzi P, et al. Dramatic rebounds of MS during pregnancy following fingolimod withdrawal. Neurol Neuroimmunol Neuroinflamm. (2017) 4:e377. doi: 10.1212/NXI.0000000000000377
65. Río J, Tintoré M, Sastre-Garriga J, Nos C, Castilló J, Tur C, et al. Change in the clinical activity of multiple sclerosis after treatment switch for suboptimal response. Eur J Neurol. (2012) 19:899–904. doi: 10.1111/j.1468-1331.2011.03648.x
66. Villaverde-González R, Gil JG, Sempere AP, Pascual JM, Marín JM, Gadea MC, et al. Observational study of switching from natalizumab to immunomodulatory drugs. Eur Neurol. (2017) 77:130–6. doi: 10.1159/000453333
67. Ghiasian M, Nouri M, Moghadasi AN, Ghaffari M. Effect of pregnancy and exclusive breastfeeding on multiple sclerosis relapse rate and degree of disability within two years after delivery. Clin Neurol Neurosurg. (2020) 194:105829. doi: 10.1016/j.clineuro.2020.105829
Keywords: multiple sclerosis, breastfeeding, pregnancy, immune system, drug therapy
Citation: Collorone S, Kodali S and Toosy AT (2023) The protective role of breastfeeding in multiple sclerosis: Latest evidence and practical considerations. Front. Neurol. 13:1090133. doi: 10.3389/fneur.2022.1090133
Received: 04 November 2022; Accepted: 23 December 2022;
Published: 24 January 2023.
Edited by:
Ethel Ciampi, Pontificia Universidad Católica de Chile, ChileReviewed by:
Klaus Berek, Innsbruck Medical University, AustriaCopyright © 2023 Collorone, Kodali and Toosy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Collorone,  cy5jb2xsb3JvbmVAdWNsLmFjLnVr
cy5jb2xsb3JvbmVAdWNsLmFjLnVr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.