
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Neurol., 09 January 2023
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1087220
 Mao Mukai1
Mao Mukai1 Ai Hamano1
Ai Hamano1 Ikuko Mizuta1
Ikuko Mizuta1 Isao Yokota2
Isao Yokota2 Akiko Watanabe-Hosomi1
Akiko Watanabe-Hosomi1 Hiraku Matsuura1
Hiraku Matsuura1 Takashi Koizumi1,3
Takashi Koizumi1,3 Jun Matsuura1
Jun Matsuura1 Tomoyuki Ohara1
Tomoyuki Ohara1 Shigenori Matsushima4
Shigenori Matsushima4 Satoshi Teramukai5
Satoshi Teramukai5 Kei Yamada4
Kei Yamada4 Toshiki Mizuno1*
Toshiki Mizuno1*Background: Impaired cerebrovasoreactivity is thought to play an important role in the pathophysiology of cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL). We aimed to clarify the association between cerebrovascular reactivity and stroke in patients with CADASIL.
Methods: We retrospectively recruited 14 patients with CADASIL, eight of whom had symptomatic stroke. They underwent quantitative single-photon emission computed tomography using an autoradiographic method at rest and after acetazolamide (ACZ) administration. Regional cerebral blood flow (rCBF) in the cerebral cortex, lenticular nucleus, thalamus, and cerebellum was measured. We compared the rCBF parameters between patients with and without stroke.
Results: The baseline characteristics and magnetic resonance imaging findings were similar between the two groups, except for a higher frequency of pyramidal tract sign (75% vs. 0%) and a larger number of old lacunes (15.4 ± 8.8 vs. 2.2 ± 1.8) in the patients with stroke. Of the rCBF parameters measured, significantly lower flow (mL/100 g/min) was observed in ACZ-rCBF in the thalamus (35.6 ± 9.4 vs. 51.1 ± 7.6, p = 0.01) and ΔrCBF in the thalamus (10.6 ± 3.7 vs. 21.0 ± 7.9, p = 0.02) in the patients with stroke.
Conclusion: Cerebrovasoreactivity in the thalamus was significantly associated with stroke in patients with CADASIL.
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), caused by NOTCH3 mutations, is one of the most common hereditary small vessel diseases (1). CADASIL is clinically characterized by migraine with aura, cerebral ischemic events, apathy, mood disturbance, and cognitive decline, progressing to dementia. Typical neuroimaging features include white matter lesions (WML) and subcortical infarcts (2). Cerebral ischemic events are the most frequent symptoms in patients with CADASIL, occurring in 60–85% of cases (2). Most patients experience recurrent stroke episodes, leading to irreversible dysfunctions such as gait disturbance, pseudobulbar palsy, and vascular dementia.
CADASIL arteriopathy is characterized by the loss of vascular smooth muscle cells and the thickening of the vascular wall, which are thought to impair vascular function (2, 3). Impaired cerebrovasoreactivity is thought to play an important role in the pathophysiology of CADASIL based on animal model studies (3) and comparisons between patients and controls (4, 5). Cerebrovasoreactivity is a hemodynamic biomarker that can be monitored using several instruments. Previous studies have examined cerebrovasoreactivity in patients with CADASIL using single-photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance imaging (MRI), or Doppler sonography (4, 6–11). Pfefferkorn et al. reported that cerebrovasoreactivity in response to CO2 was significantly reduced in patients with CADASIL compared to controls (7). Some studies have analyzed MRI parameters and cerebrovascular reactivity in relation to disease progression. Cerebral hypoperfusion and impaired cerebrovasoreactivity were associated with cognitive dysfunction in patients with CADASIL (5, 12). Liem et al. reported that lower cerebrovasoreactivity at baseline was associated with a larger increase in white matter hyperintensity (10). However, to our knowledge, cerebrovasoreactivity in relation to the occurrence of symptomatic stroke in patients has not yet been reported. To address this issue, we compared regional CBF (rCBF) at rest and after the administration of acetazolamide (ACZ) between CADASIL patients with and without stroke.
Fourteen patients with CADASIL who underwent ACZ challenge tests between January 2005 and December 2015 were recruited for this study. If a patient underwent multiple ACZ challenge tests, the last test was used in the analysis. Clinical information, including neurological findings, medical treatment, vascular risk factors, MRI findings, and ischemic events, was collected. Symptomatic stroke was defined as having both neurological signs and imaging findings indicative of stroke. Based on the records of symptomatic stroke before the SPECT study, the patients were divided into two groups: those with stroke (n = 8) and those without stroke (n = 6). Eight patients with stroke were further divided into two groups according to critical events, including symptomatic stroke during the 2 years after the SPECT study: those with critical events (n = 4, three patients with recurrence of symptomatic stroke and one patient deceased suddenly) and those without critical events (n = 4). The patients were suspected as CADASIL according to history of stroke, neurological symptoms, MRI findings, and family history. All the patients were definitively diagnosed with CADASIL based on the presence of typical cysteine-related mutations in the EGF-like repeat domain of NOTCH3.
Measurements of rCBF after ACZ administration (ACZ-rCBF) and at rest (REST-rCBF) were performed using quantitative SPECT with the ARG method (13). Acetazolamide (1 g) was administered intravenously, starting 10 min before the intravenous injection of 185 MBq 123I-IMP (Nihon Medi-physics, Hyogo, Japan). After 10 min, one-point arterial blood sampling was performed from the brachial artery to assess whole-blood radioactivity concentration. The SPECT study was started 22 min after 123I-IMP injection and was conducted for 16 min using a triple-head gamma camera system (IRIX; Philips Healthcare, Cleveland, Ohio, USA) equipped with low-energy, parallel collimators. SPECT images with a 128 × 128 matrix were reconstructed using ordered-subset expectation maximization reconstruction with four iterations and 12 subsets. Attenuation correction was performed using Chang's method, and scatter was corrected using the triple-energy window method. To quantify rCBF, 123I-IMP SPECT images were analyzed using a three-dimensional stereotactic region of interest (ROI) template 3DSRT (FUJIFILM RI Pharma, Tokyo, Japan) (14).
3DSRT is composed of 318 ROIs in 12 segments (1: callosomarginal, 2: precentral, 3: central, 4: parietal, 5: angular, 6: temporal, 7: posterior, 8: pericallosal, 9: lenticular nucleus, 10: thalamus, 11: hippocampus, and 12: cerebellum) on each side. We measured the rCBF values (mL/100 g/min) of the 636 ROIs of patients with CADASIL and calculated the area-weighted average of the four main regions: cortex, lenticular nucleus, thalamus, and cerebellum. The cerebral cortex consists of the callosomarginal, precentral, central, parietal, angular, temporal, posterior, pericallosal, and hippocampal regions. REST-rCBF was measured 19.6 ± 12.9 (5–39) days before the ACZ-rCBF test.
The increase in rCBF (ΔrCBF) and regional cerebrovasoreactivity (rCVR) were calculated as follows:
MRI was performed using a 1.5T MR instrument (Gyroscan Intera Nova; Philips Medical Systems, Best, Netherlands). The severity of WML on fluid-attenuated inversion recovery images was classified into four grades according to a previous report (15). Specifically, T2-WI images were used to evaluate white matter lesions on four grades: A (no lesion), B (punctiform or slight periventricular hyperintensities, or both), C (nodular or moderate periventricular hyperintensities, or both) and D (confluent lesions or severe periventricular hyperintensities, or both) (15). White matter hyperintensity (WMH) and total brain parenchyma volumes were calculated using a 3D slicer (http://www.slicer.org). Old lacunes were identified as hypointense lesions on T1-weighted images with a signal identical to that of cerebrospinal fluid, sharp delineation, and a diameter >2 mm.
The demographics, CBF parameters, and MRI parameters were compared between patients with and without symptomatic stroke, which were diagnosed based on neurological signs and MR findings. Fisher's exact test or Wilcoxon rank sum test was used. We further analyzed association between representative rCBF parameter and occurrence of critical events (symptomatic stroke or death) during two years after the SPECT examination in the patients with stroke. Univariate logistic regression models were used to calculate non-adjusted odds ratios with 95% confidence intervals (CIs) Receiver operating characteristic analysis/area under the curve (AUC) was used to assess relevance of the parameter. All the statistical methods were performed using JMP 14.2.0 (SAS Institute Japan Ltd., Japan). Statistical significance was set at P < 0.05.
Fourteen patients (nine males and five females; mean age: 56.3 ± 10.2 years) were included in this study. In the patients with symptomatic stroke, the intervals between past symptomatic stroke to SPECT examination were 1.6 ± 1.6 (range: 0.04–4.4) years. The background and clinical characteristics at the SPECT examinations are shown in Table 1. No significant difference was observed in the background variables between patients with symptomatic stroke and those without symptomatic stroke, except for the frequency of pyramidal tract signs. Regarding the MRI findings, the number of old lacunes was significantly higher in those with stroke than in those without stroke (p = 0.007). White matter hyperintensities in temporal tip and external capsule were identified in all the patients. The frequency of grade D and % volume of WMLs was higher in those with stroke than in those without stroke; however, the differences were not significant (Table 1).
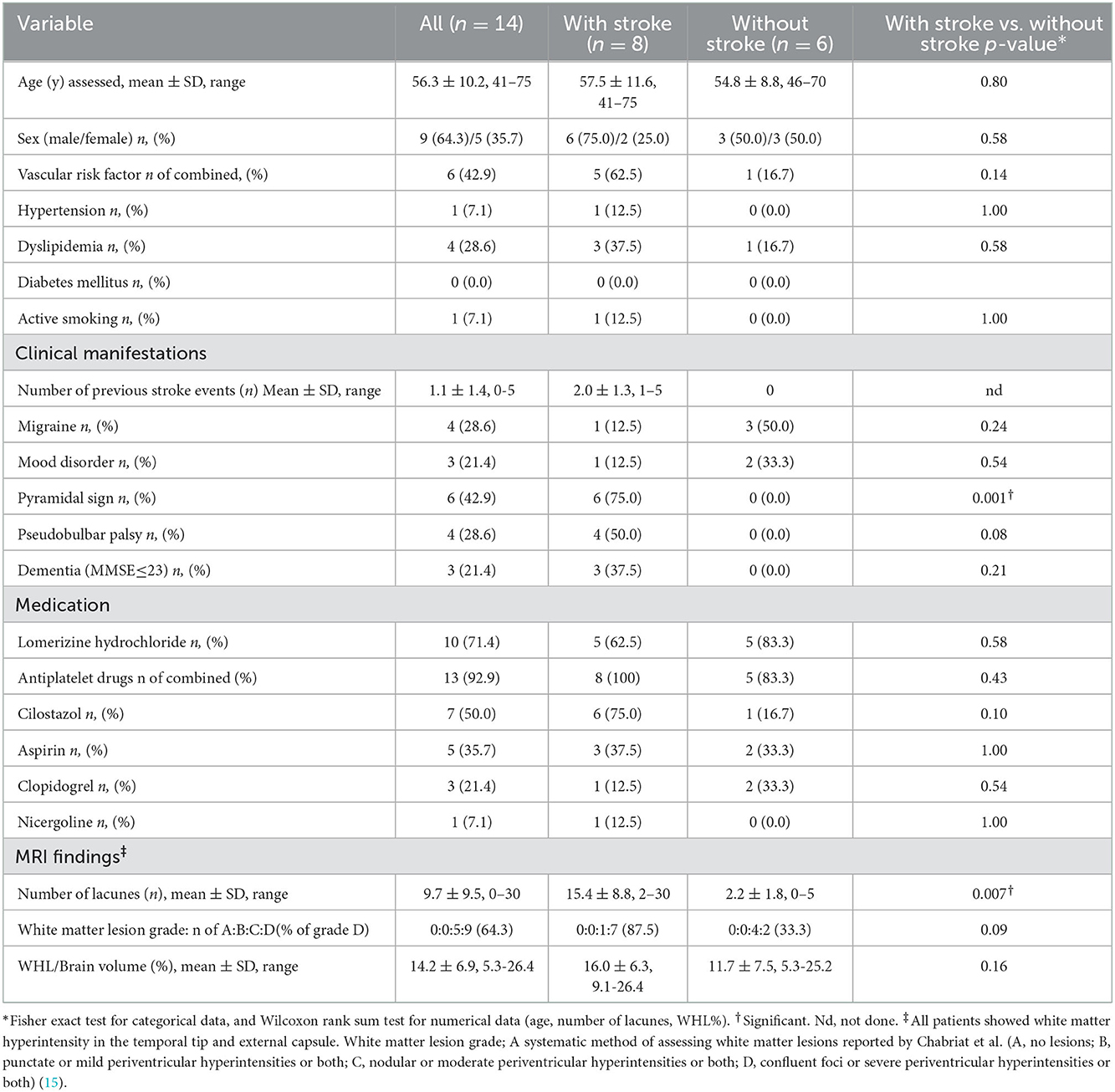
Table 1. Comparison of the baseline characteristics and MRI findings between patients with and without stroke.
SPECT images of representative patients, a good responder to ACZ without symptomatic stroke (Figure 1, upper panel), and a poor responder to ACZ with symptomatic stroke (Figure 1, lower panel) are shown. Among the 16 CBF parameters, a significant difference was observed between those with and without stroke in the thalamus. ACZ-rCBF in the thalamus was significantly lower in those with stroke, at 35.6 ± 9.4, than without stroke, at 51.1 ± 7.6 (p = 0.007). ΔrCBF in the thalamus was significantly lower in those with stroke, at 10.6 ± 3.7, than in those without stroke, at 21.0 ± 7.9 (p = 0.02), but the rCVR (%) was not significantly different between the two groups (Table 2).
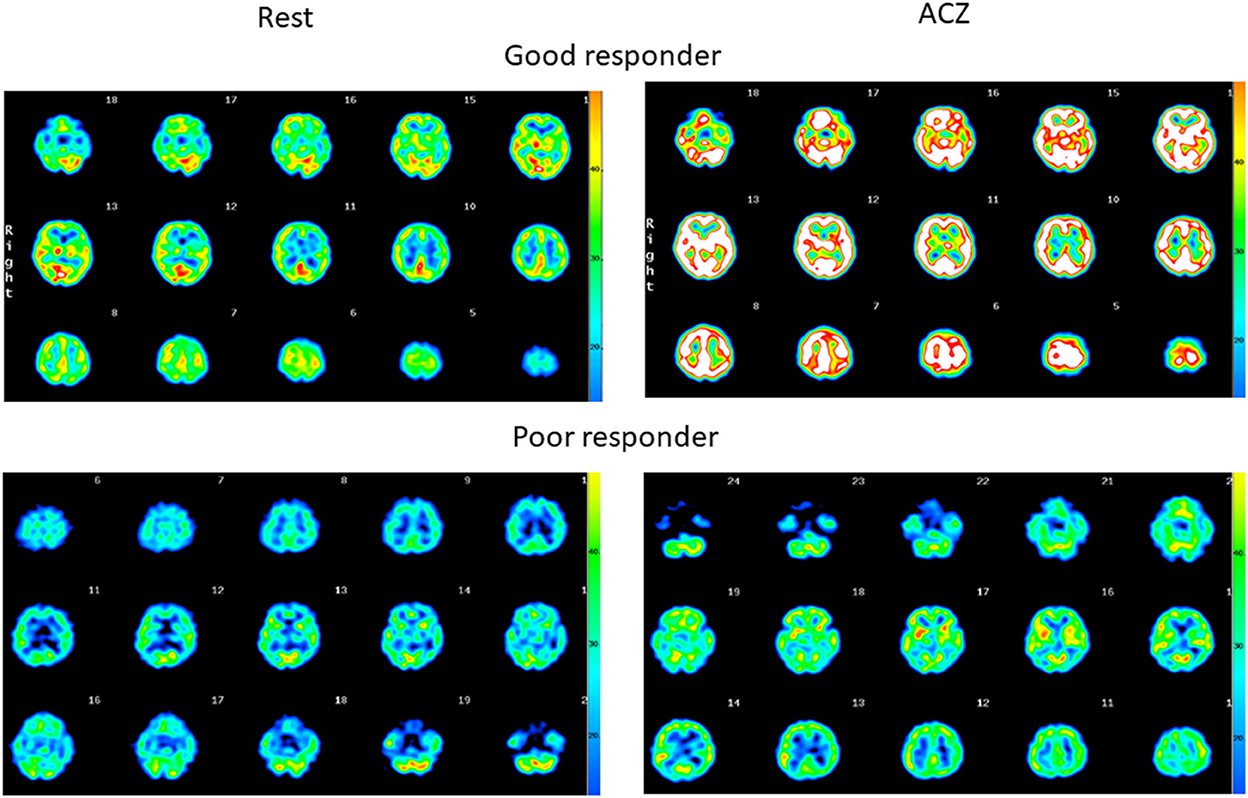
Figure 1. Quantitative SPECT images at rest and after ACZ injection. (Upper) Diffuse increase in the rCBF, indicating the maintenance in cerebrovasoreactivity in the patient (64-year-old female without stroke). (Lower) Only a slight increase in the rCBF, indicating little residual cerebrovasoreactivity in the patient (42-year-old male with stroke).
As described above, we found that ACZ-rCBF in the thalamus was significantly associated with past incidents of symptomatic stroke in patients with CADASIL. We further examined the relationship between ACZ-rCBF in the thalamus and the prognosis of patients with stroke. We performed a logistic regression analysis of ACZ-rCBF in the thalamus and MRI findings in association with critical events (stroke or death) during the 2 years following SPECT examination. Receiver operating characteristic analysis showed significance for ACZ-rCBF (odds ratio = 0.44, 95% CI = 0.02–0.92), but not for the number of lacunes or WHL/brain volume (Table 3).
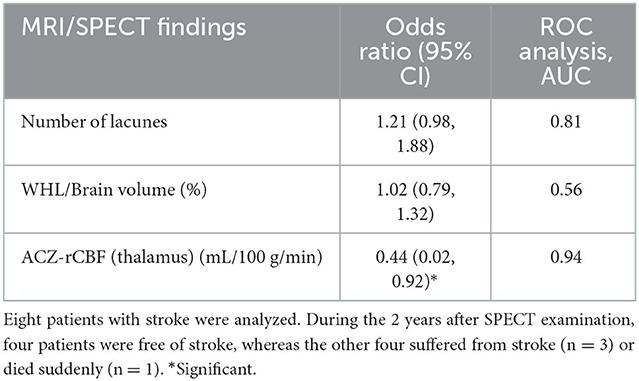
Table 3. Univariate logistic regression analysis of MRI/SPECT findings in association with the recurrence of stroke or critical event during 2 years.
In this study, we found that cerebrovasoreactivity in the thalamus was significantly lower in CADASIL patients with symptomatic stroke than in those without symptomatic stroke. A similar association was not observed in regions other than the thalamus.
Some imaging studies in patients with CADASIL have shown significant findings in the thalamus. Two diffusion tensor imaging (DTI) studies have identified significant microstructural changes in the thalamus and putamen of patients with CADASIL compared to controls (16, 17). One DTI study showed a negative correlation with the mini-mental state examination score in patients (16), and the other showed a correlation with executive function tests in patients without dementia (17). An 18F-FDG PET study reported that the regional cerebral metabolic rate of glucose (rCMRglc) was lower in the thalamus and caudate than in the putamen and cortical lobes in patients with CADASIL (18).
The significant cerebrovasoreactivity parameters in the thalamus were the ACZ-rCBF and ΔrCBF. Rest-rCBF and rCVR in the thalamus were not significantly different. This suggests that the maximal dilatation capacity, rather than basal flow, of cerebral small arteries may influence the occurrence of stroke.
As for location of previous symptomatic stroke, only one out of 16 cumulative prior strokes occurred in the thalamus (Supplementary Table 1), suggesting that the reduced vasoreactivity of the thalamus in eight patients with symptomatic stroke was not due to the site of previous stroke in the thalamus.
Regarding location of lacunes and microbleeds between the two groups, the number of lacunes and also microbleeds in thalamus and that in lenticular nucleus were larger in patients with stroke than in those without stroke (Supplementary Table 2). On the other hand, vasoreactivity in thalamus was decreased in patients with stroke compared with those without stroke, whereas vasoreactivity in lenticular nucleus was similar between with and without stroke (Table 2). Taken together, we think that the decreased vasoreactivity in the thalamus may reflect the disease process of small vessel in the CADASIL, and not be due solely to increased lacunes and microbleeds in the thalamus.
A previous report showed total cerebrovasoreactivity at baseline and an increase in WMH at the seven-year follow-up (10), suggesting that decreased cerebrovasoreactivity may be a potential predictor of the clinical progression of CADASIL. Based on this hypothesis, we further analyzed the recurrence of symptomatic stroke during the 2 years following SPECT in relation to cerebrovasoreactivity in the thalamus. Logistic regression analysis revealed that critical events (stroke or death) during the 2 years following SPECT examination were significantly related to ACZ-rCBF in the thalamus but not to WMH volume or the number of lacunar infarctions (Table 3). Our results suggest that ACZ-rCBF in the thalamus may be a new biomarker of CADASIL progression.
Diameter of perforating arteries supplying central gray matter lesions are similar, but the length of the thalamoperforating artery is shorter than the lenticulostriate artery or anterior choroidal artery from the anatomical construction. Anatomical construction of cerebral vessel also suggested that the thalamoperforating artery may be more easily influenced by changes of blood pressure directly more than the lenticulostriate artery or anterior choroidal artery. These factors might contribute that the thalamoperforating artery is the most sensitivity artery against ACZ to evaluate the pathological process of small vessel in CADASIL.
It is uncertain whether vasoreactivity in thalamus decreases at early stage of the disease. Comparison between patients at early stage and controls may be informative, but SPECT data of controls were not available due to the radiation exposure. Previous study compared q-space imaging values between patients with preclinical CADASIL and healthy controls and detected early neuronal change in frontal lobe and central gray matter in preclinical CADASIL (19). From the previous finding, we speculate that vasoreactivity in central gray matter, which includes thalamus, might occur from early stage of the disease.
A limitation of this study was the small number of patients, mainly because of the rare prevalence of CADASIL. In addition, we needed to identify patients who underwent ACZ-challenged SPECT using the ARG method for quantitative analysis. Another limitation was that this was a retrospective study. Further large-scale prospective studies are required to confirm our findings.
In conclusion, we found that cerebrovasoreactivity in the thalamus after ACZ administration was significantly associated with stroke in patients with CADASIL. This study indicates that quantitative cerebrovasoreactivity using ACZ could be a useful marker for monitoring the disease course in patients with CADASIL.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Kyoto Prefectural University of Medicine. The patients/participants provided their written informed consent to participate in this study.
TM, MM, SM, and KY conceived and designed the study. MM, AH, AW-H, TK, JM, SM, HM, and TM acquired the data. MM, AH, IM, IY, AW-H, TK, JM, SM, ST, KY, and TM analyzed and interpreted the data. MM, IM, TM, and TO drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the Japan Agency for Medical Research and Development (AMED, 17ek0109130s0703), by JSPS KAKENHI Grant Number JP18K07533, and by a Grant-in-Aid for Research on Intractable Disease from the Japanese Ministry of Health, Labor, and Welfare, Japan (H28-Nanchitou(Nan)-Ippan-029, H30-Nanchitou(Nan)-Ippan-006, and 21FC0201).
We acknowledge all the patients who participated in this study. We would like to thank Dr. Yasuhiro Fujiwara for their helpful comments on rCBF analysis and the radiological technologists who helped collect the raw rCBF data. We also would like to thank Dr. Daiki Fukunaga for his suggestive discussion. We would like to thank Editage (www.editage.com) for English language editing.
IY reports grants from KAKENHI, AMED, and Health, Labor and Welfare Policy Research Grants, a research fund from Nihon Medi-Physics, and speaker fees from Chugai Pharmaceutical Co, AstraZeneca plt, Japan Tobacco Pharmaceutical Division, and Nippon Shinyaku Co., outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1087220/full#supplementary-material
CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; rCBF, regional cerebral blood flow; SPECT, single-photon emission computed tomography; ACZ-rCBF, regional cerebral blood flow after acetazolamide; REST-rCBF, regional cerebral blood flow at rest; PET, positron emission tomography; MRI, magnetic resonance imaging; ACZ, acetazolamide; ROI, region of interest; ΔrCBF, increase in rCBF; rCVR, regional cerebral vasoreactivity; WML, white matter lesions; OR, odds ratio; CI, confidence interval; rCMRglc, regional cerebral metabolic rate of glucose; GOM, granular osmiophilic material.
1. Chabriat H, Joutel A, Tournier-Lasserve E, Bousser MG, CADASIL. yesterday, today, tomorrow. Eur J Neurol. (2020) 27:1588–95. doi: 10.1111/ene.14293
2. Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. (2009) 8:643–53. doi: 10.1016/S1474-4422(09)70127-9
3. Joutel A. Pathogenesis of CADASIL: transgenic and knock-out mice to probe function and dysfunction of the mutated gene, Notch3, in the cerebrovasculature. Bioessays. (2011) 33:73–80. doi: 10.1002/bies.201000093
4. Chabriat H, Pappata S, Ostergaard L, Clark CA, Pachot-Clouard M, Vahedi K, et al. Cerebral hemodynamics in CADASIL before and after acetazolamide challenge assessed with MRI bolus tracking. Stroke. (2000) 31:1904–12. doi: 10.1161/01.STR.31.8.1904
5. Moreton FC, Cullen B, Delles C, Santosh C, Gonzalez RL, Dani K, et al. Vasoreactivity in CADASIL: Comparison to structural MRI and neuropsychology. J Cereb Blood Flow Metab. (2018) 38:1085–95. doi: 10.1177/0271678X17710375
6. Mellies JK, Baumer T, Muller JA, Tournier-Lasserve E, Chabriat H, Knobloch O, et al. SPECT study of a German CADASIL family: a phenotype with migraine and progressive dementia only. Neurology. (1998) 50:1715–21. doi: 10.1212/WNL.50.6.1715
7. Pfefferkorn T, von Stuckrad-Barre S, Herzog J, Gasser T, Hamann GF, Dichgans M. Reduced cerebrovascular CO(2) reactivity in CADASIL: a transcranial Doppler sonography study. Stroke. (2001) 32:17–21. doi: 10.1161/01.STR.32.1.17
8. van den Boom R, Lesnik Oberstein SA, Spilt A, Behloul F, Ferrari MD, Haan J, et al. Cerebral hemodynamics and white matter hyperintensities in CADASIL. J Cereb Blood Flow Metab. (2003) 23:599–604. doi: 10.1097/01.WCB.0000062341.61367.D3
9. Tuominen S, Miao Q, Kurki T, Tuisku S, Poyhonen M, Kalimo H, et al. Positron emission tomography examination of cerebral blood flow and glucose metabolism in young CADASIL patients. Stroke. (2004) 35:1063–7. doi: 10.1161/01.STR.0000124124.69842.2d
10. Liem MK, Lesnik Oberstein SA, Haan J, Boom R, Ferrari MD, Buchem MA, et al. Cerebrovascular reactivity is a main determinant of white matter hyperintensity progression in CADASIL. AJNR Am J Neuroradiol. (2009) 30:1244–7. doi: 10.3174/ajnr.A1533
11. Fujiwara Y, Mizuno T, Okuyama C, Nagakane Y, Watanabe-Hosomi A, Kondo M, et al. Simultaneous impairment of intracranial and peripheral artery vasoreactivity in CADASIL patients. Cerebrovasc Dis. (2012) 33:128–34. doi: 10.1159/000334185
12. Yin X, Zhou Y, Yan S, Lou M. Effects of cerebral blood flow and white matter integrity on cognition in CADASIL patients. Front Psychiatry. (2018) 9:741. doi: 10.3389/fpsyt.2018.00741
13. Iida H, Itoh H, Nakazawa M, Hatazawa J, Nishimura H, Onishi Y, et al. Quantitative mapping of regional cerebral blood flow using iodine-123-IMP and SPECT. J Nuclear Med. (1994) 35:2019–30.
14. Takeuchi R, Yonekura Y, Matsuda H, Konishi J. Usefulness of a three-dimensional stereotaxic ROI template on anatomically standardised 99mTc-ECD SPET. Eur J Nucl Med Mol Imaging. (2002) 29:331–41. doi: 10.1007/s00259-001-0715-z
15. Chabriat H, Levy C, Taillia H, Iba-Zizen MT, Vahedi K, Joutel A, et al. Patterns of MRI lesions in CADASIL. Neurology. (1998) 51:452–7. doi: 10.1212/WNL.51.2.452
16. Molko N, Pappata S, Mangin JF, Poupon C, Vahedi K, Jobert A, et al. Diffusion tensor imaging study of subcortical gray matter in cadasil. Stroke. (2001) 32:2049–54. doi: 10.1161/hs0901.094255
17. O'Sullivan M, Singhal S, Charlton R, Markus HS. Diffusion tensor imaging of thalamus correlates with cognition in CADASIL without dementia. Neurology. (2004) 62:702–7. doi: 10.1212/01.WNL.0000113760.72706.D2
18. Tatsch K, Koch W, Linke R, Poepperl G, Peters N, Holtmannspoetter M, et al. Cortical hypometabolism and crossed cerebellar diaschisis suggest subcortically induced disconnection in CADASIL: an 18F-FDG PET study. J Nucl Med. (2003) 44:862–9.
Keywords: cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, stroke, cerebrovasoreactivity, single-photon emission computed tomography, acetazolamide
Citation: Mukai M, Hamano A, Mizuta I, Yokota I, Watanabe-Hosomi A, Matsuura H, Koizumi T, Matsuura J, Ohara T, Matsushima S, Teramukai S, Yamada K and Mizuno T (2023) Association between cerebrovasoreactivity and stroke in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Front. Neurol. 13:1087220. doi: 10.3389/fneur.2022.1087220
Received: 02 November 2022; Accepted: 16 December 2022;
Published: 09 January 2023.
Edited by:
Patricia Martínez Sánchez, Torrecárdenas University Hospital, SpainReviewed by:
Chih-Hao Chen, National Taiwan University Hospital, TaiwanCopyright © 2023 Mukai, Hamano, Mizuta, Yokota, Watanabe-Hosomi, Matsuura, Koizumi, Matsuura, Ohara, Matsushima, Teramukai, Yamada and Mizuno. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Toshiki Mizuno,  bWl6dW5vQGtvdG8ua3B1LW0uYWMuanA=
bWl6dW5vQGtvdG8ua3B1LW0uYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.