- In-Patient Ultrasound Department, Second Affiliated Hospital of Harbin Medical University, Harbin, China
Introduction: Carpal tunnel syndrome (CTS) is a common compression neuropathy of the median nerve in the wrist. Early diagnosis of CTS is essential for selecting treatment options and assessing prognosis. The current diagnosis of CTS is based on the patient's clinical symptoms, signs, and an electromyography (EMG) test. However, they have some limitations. Recently, ultrasound has been adopted as an adjunct diagnostic tool for electromyography (EMG). Ultrasound is a non-invasive and cost-effective technique. It provides a dynamic display of morphological changes in the median nerve and an assessment of CTS etiology such as tenosynovitis, mass compression, and tendon disease. This study aimed to investigate the value of conventional ultrasound and real-time shear wave elastography (SWE) in evaluation of median neuropathy in patients with carpal tunnel syndrome (CTS) before and after surgery.
Methods: First, the Boston Carpal Tunnel Questionnaire (BCTQ) was administered to patients with CTS. All subjects were measured at three levels: the distal 1/3 of the forearm, the carpal tunnel inlet, and the distal carpal tunnel using conventional ultrasound and SWE. Median nerve parameters were examined in patients with CTS 1 week after surgery.
Results: The cross-sectional area (CSA) and stiffness of the median nerve at the carpal tunnel inlet and distal carpal tunnel were significantly higher in patients with CTS than in healthy controls (p < 0.001). The CSA and stiffness of the median nerve at the carpal tunnel inlet were statistically significantly significantly between pre- and postoperative patients with CTS (p < 0.001). The CSA and stiffness of the nerve in patients with CTS had a positive correlation with electrophysiology severity.
Conclusions and discussion: Conventional ultrasound and elastography are valuable in the diagnosis of CTS and are useful in the clinical assessment of patient's nerve recovery after operation.
1. Introduction
Carpal tunnel syndrome (CTS) is a common compression neuropathy of the median nerve in the wrist. It accounts for 90% of all entrapment neuropathies, with a prevalence rate of approximately 3.8% in the population (1). Nerve damage can be reversed with early treatment and diagnosis to prevent permanent damage. Early diagnosis of CTS is essential for selecting treatment options and assessing prognosis (2, 3). The current diagnosis of CTS is based on the patient's clinical symptoms, signs, and an electromyography (EMG) test. However, symptoms and signs in patients with mild disease are unclear and lack objective judgment criteria (4). Electromyography is the gold standard for CTS diagnosis and yields 98% specificity and 75% sensitivity. This examination has some limitations, including being time-consuming, invasive, and has a 10–25% false-negative rate. In addition, it cannot directly assess the anatomical relationship between the median nerve and surrounding tissues (4–6). Certain imaging examinations are significant in CTS diagnosis. According to Park et al. (7), magnetic resonance imaging (MRI) can be a diagnostic tool for median neuropathy. This technology can measure the median nerve cross-sectional area (CSA), despite the procedure being expensive and time-consuming. Also, computed tomography (CT) can diagnose CTS by showing changes in the density of the compressed median nerve and occupying lesions (8, 9).
Recently, ultrasound has been adopted as an adjunct diagnostic tool for electromyography (EMG). Ultrasound is a non-invasive and cost-effective technique. It provides a dynamic display of morphological changes in the median nerve and an assessment of CTS etiology such as tenosynovitis, mass compression, and tendon disease (10–12). Previous studies have focused on the CSA of the median nerve at the carpal tunnel entrance in CTS patients with sensitivity and specificity comparable to electromyography examinations (13–16). On the contrary, shear-wave elastography (SWE) is a new technique for the quantitative measurement of tissue stiffness (17). It is a complement to conventional ultrasound in the assessment of neuropathy (18–21). Studies have reported the significance of elastography in the assessment of CTS (22–25). However, to the extent of our knowledge, there are few reports on the evaluation of pre- and post-operative changes in nerve stiffness in patients with CTS. Therefore, the purpose of this study was to investigate the value of conventional ultrasound and SWE on the median nerve in patients with CTS before and after surgery.
2. Materials and methods
The study was approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. All examinations were conducted after written informed consent was obtained from all subjects.
2.1. Patients
In total, 50 wrists of 25 healthy volunteers and 54 wrists of 30 patients who underwent carpal tunnel release surgery were studied from October 2020 to June 2021. Inclusion criteria were as follows: patients who reported abnormal sensation or pain in their extremities and had a positive electromyography examination (26). Exclusion criteria were as follows: age < 18 years, history of previous hand surgery, and other conditions affecting the measurement of the median nerve. The scale used to measure the severity of CTS was as follows: negative (grade 0), mild (grade 1), moderate (grade 2), severe (grade 3), and extremely severe (grade 4) according to the results of electromyography. Questionnaires were administered to patients with CTS, and basic information was recorded for all participants.
2.2. Questionnaires
The Boston Carpal Tunnel Questionnaire (BCTQ) consists of two self-rating scales: the Symptom Severity Scale (SSS) and the Functional Status Scale (FSS) (27). High scores indicated more severe disease conditions. It was used to assess all patients with CTS.
2.3. Ultrasound examination
All examinations were performed using a 4C15 MHz linear array transducer (Aixplorer; Supersonic Imagine, Aix en Provence, France). All patients were placed in a seated position, facing the examiner, with the forearm fully exposed, the elbow bent at 90°, and the hand kept in a resting position. The median nerve CSA and nerve flattening rate (NFR) were measured at three sites: the distal 1/3 of the forearm, the carpal tunnel inlet (at the level of the doughy bone), and the distal carpal tunnel (at the level of the hook bone). NFR is the ratio of the transverse diameter of the nerve to the anterior-posterior diameter when the median nerve is compressed by entrapment. It is an ultrasound parameter to determine if the nerve is compressed. The mean value was calculated by taking the average of the replicate measurements. The transducer was rotated 90° to obtain a longitudinal image, and the image was frozen after obtaining the ideal image. SWE was activated at the same three loci as the 2D ultrasound. Three regions of interest (ROI) with a diameter of 1 mm were selected to calculate the average value. Each locus was measured in triplicate. During the measurement, a sufficient coupling agent was applied, and the probe was placed gently on the skin to avoid pressure. A week after the surgery, the conventional ultrasound and SWE were repeated on the same patients. The above operations and image analysis were performed by a qualified ultrasound physician with over 10 years of research in musculoskeletal aspects.
2.4. Statistical analyses
Data were analyzed using the SPSS version 23.0 (SPSS Inc., Chicago, USA) and MedCalc 20.0 software (Ostend, West-Vlaanderen, Belgium). Continuous variables were expressed as mean ± standard deviation (SD). The Kolmogorov-Smirnov test was used to analyze the normal distribution. Mann–Whitney U test was used to analyze the non-normally distributed measures. The correlation was expressed by Spearman's correlation coefficient. Receiver operating characteristic (ROC) curves for CSA and elasticity were obtained to calculate sensitivity, specificity, accuracy, the area under the curve (AUC), and optimal cutoff values. Z-test was used for AUC. Statistical significance was considered at p < 0.05.
3. Results
3.1. Study demographics
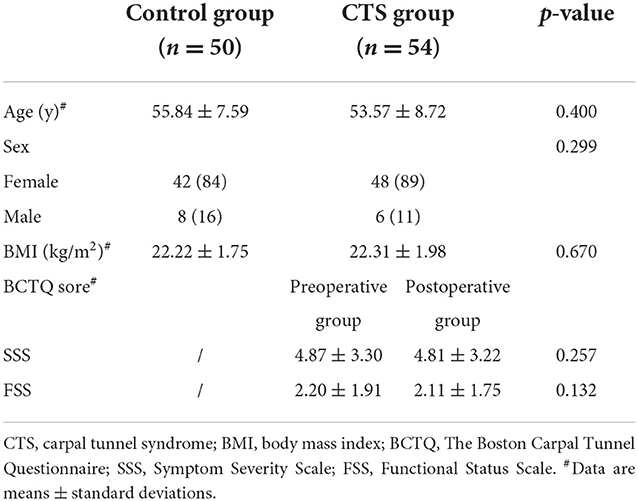
The demographic data for the patients are summarized in Table 1. There was no statistical difference in gender, age, and body mass index (BMI) between patients with CTS and healthy controls at p > 0.05. BCTQ scores (SSS and FSS) between pre- and postoperative groups with CTS did not show any significant statistical difference (p > 0.05). In total, six wrists (11.1%) reported negative electromyography results. Grading CTS by severity showed mild in 15 (27.8%), moderate in 18 (33.3%), severe in 13 (24.1%), and extremely severe in 2 (3.7%) cases with a positive neurophysiological result.
3.2. Conventional ultrasound and SWE parameters in healthy controls and preoperative groups
The distal 1/3 of the forearm in the preoperative group was not statistically different at p > 0.05 in CSA, flattening rate, and Emean of the median nerve compared to the healthy control group. However, the CSA, flattening rate, and Emean of the median nerve at the carpal tunnel inlet and the distal carpal tunnel in the preoperative group were significantly higher than in healthy controls at p < 0.001 (Table 2).
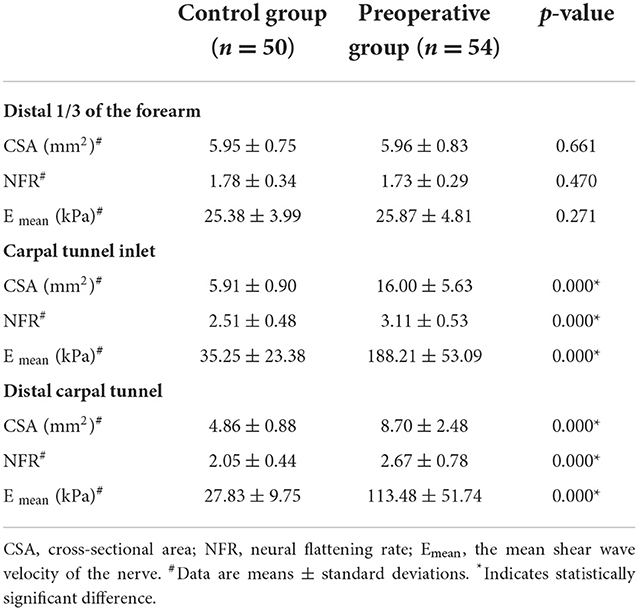
Table 2. Conventional ultrasound and shear wave elastography parameters of the control group and the preoperative group.
3.3. Conventional ultrasound and SWE parameters between pre- and postoperative CTS groups
There was no significant difference in CSA, flattening rate, and Emean of the median nerve at the distal 1/3 of the forearm (p > 0.05). The CSA, flattening rate, and Emean of the median nerve at the carpal tunnel inlet and distal carpal tunnel were significantly lower in the postoperative group than in the preoperative group (p < 0.05) (Table 3).
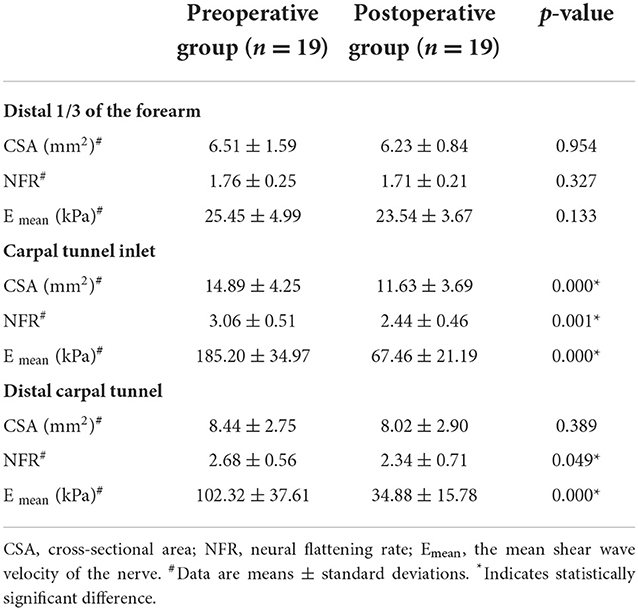
Table 3. Conventional ultrasound and shear wave elastography parameters of the preoperative group and the postoperative group.
3.4. Conventional ultrasound and SWE parameters of healthy controls and postoperative CTS groups
The postoperative group had a significantly lower CSA and Emean of the median nerve at the carpal tunnel inlet and the distal carpal tunnel than the healthy controls (p < 0.05) (Table 4).
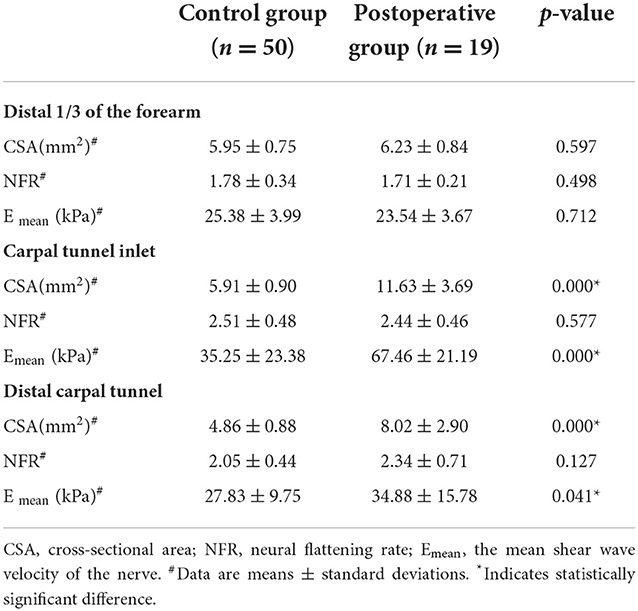
Table 4. Conventional ultrasound and shear wave elastography parameters of the control group and the postoperative group.
3.5. Diagnostic performance of conventional ultrasound and SWE parameters
The CSA and Emean at the carpal tunnel inlet and the distal 1/3 of the forearm showed good diagnostic performance (0.999, 0.993, 0.999, 0.992, p < 0.001). In this study, the corresponding cutoff value of CSA (8.2 mm2) was used to diagnose CTS, with a sensitivity of 96.2% and a specificity of 100% at the carpal tunnel inlet. We chose the optimal cutoff value of Emean (102.9 Kpa) to diagnose CTS, with a sensitivity of 96.3% and a specificity of 98%. The difference in AUC between CSA and Emean for CTS diagnosis was not statistically significant (Z = 1.15, p = 0.25). The CSA cutoff value (1.68 mm2) of the carpal tunnel inlet and the distal 1/3 of the forearm produced sensitivity and specificity of 86.6 and 100%, respectively. However, the cutoff value (3.72 mm2) of the Emean ratio of the carpal tunnel inlet and the distal 1/3 of the forearm produced a sensitivity of 96% and a specificity of 96% (Table 5).
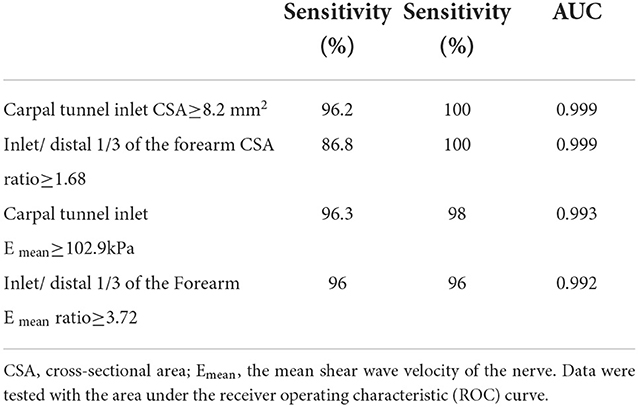
Table 5. AUC, sensitivity, specificity, and optimal cut-off values of nerve parameters for the diagnosis of CTS.
3.6. Correlation of electrophysiology severity with CSA and Emean
Electrophysiological severity had a positive correlation with CSA (r2 = 0.93, p < 0.001), whereas electrophysiological severity had a moderate correlation with Emean (r2 = 0.60, p < 0.001).
4. Discussion
Studies suggest high pressure in the carpal tunnel to be the cause of CTS (28). The increased pressure affects the microcirculation in the median nerve, leading to edema and demyelinating lesions. Hence, there is a thickening of the nerve bundle and vessel walls (29, 30). Nerve entrapment injury causes intraneural ischemia, which leads to abnormal vascular endothelial permeability and manifests as intra-nerve edema and increased CSA measurement (29, 31). High-frequency ultrasound can detect changes in the median nerve, such as hypoechoic nerve, blurred nerve bundle, and increased cross-sectional area.
Byra et al. (32) reported variability in the echogenicity and shape of the median nerve in CTS and healthy patients. Also, the CSA of patients with CTS was significantly higher than healthy individuals (33). Notably, these findings were consistent with our study.
Furthermore, we found that the lack of differences between CSA at the distal carpal tunnel pre- and postoperatively could be attributed to delayed decompression.
We also found that the injury to the nerve did not involve the distal 1/3 of the forearm, and because of this, we could use the CSA at the distal 1/3 of the forearm as a reference value in this experiment to obtain a truncated value with less individual differentiation compared with the CSA at other locations.
Compression of microcirculation in the median nerve leads to focal demyelination and axonal degeneration with a fibrotic response (14, 30). Wang et al. (34) documented a relationship between carpal tunnel pressure and shear wave velocity. In our study, the cutoff values of the median nerve at the carpal tunnel inlet were higher than in other studies. We concede that participants had moderate and severe CTS with a higher degree of neuropathy, thus leading to a higher Emean.
In our study, we found that the variance of Emean at the carpal tunnel inlet and outlet in all populations is very high compared to 1/3 of the distal forearm (Figure 1). We hypothesized that there are more bones in the carpal tunnel inlet and outlet, which cause the “bone-proximity” hardening artifacts. These artifacts occur when the nerve travels near a bone plane that prevents the shear wave from homogeneously propagating at depth, thus causing local stress inhomogeneity. As Bortolotto reported, the stiffness of the median nerve progressively increases in its distal portions, where the nerve approaches the bone surface (35).
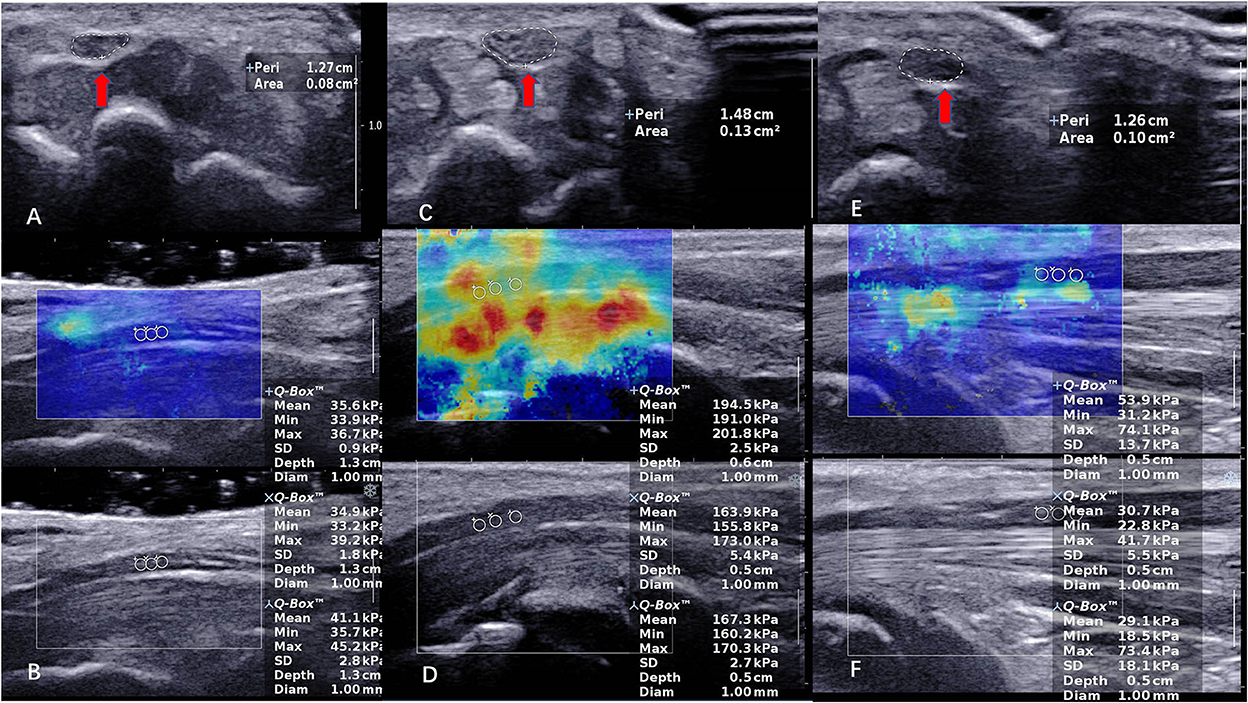
Figure 1. The CSA and SWE of the median nerve (MN) in a 40-year-old female control subject at carpal tunnel inlet. (A,B) (CSA = 0.08 cm2, Emean = 37.2 kPa). The CSA and SWE of the median nerve (MN) in a 51-year-old female patient with severe CTS at carpal tunnel inlet in preoperative. (C,D) (CSA = 0.13 cm2, Emean = 175.3 kPa). The CSA and SWE of the median nerve (MN) in a 51-year-old female patient with severe CTS at carpal tunnel inlet in postoperative. (E,F) (CSA = 0.10 cm2, Emean = 37.9 kPa). The red arrow indicates the CSA of the nerve.
The correlation of electrophysiology severity with CSA and Emean is consistent with the results of Choi et al. (36) and Drakopoulos et al. (37). Therefore, we suggest that it is feasible to determine the severity of the disease in patients with CTS based on conventional ultrasound and SWE. Conversely, another study showed that nerve stiffness was a better predictor of disease severity than CSA, due to substantial axonal loss in extremely severe cases, which produces less swelling of the compressed nerve than in moderately severe cases (38).
There were two cases of extreme severity in our study that had no significant difference in assessing CTS severity. The quantitative evaluation of the median nerve injury in patients by conventional ultrasound and SWE had high diagnostic values (Table 5). Although there were no significant differences between the two techniques, the application of the SWE technique did not improve the diagnostic efficacy of CTS. Kantarci et al. (28) and Arslanet et al. (39) documented similar findings.
Currently, the best treatment for CTS is carpal tunnel release. Approximately, 70– 90% of patients undergo carpal tunnel dissection to release the median nerve and achieve satisfactory results (40, 41). The questionnaires can be used as a method to assess postoperative recovery (42). Compared to the BCTQ score, ultrasound better reflects the recovery of neuropathy before the symptoms improve.
The reduction in CSA and elasticity is due to the successful severance of the transverse carpal ligament, which releases nerve compression and reduces nerve edema. In our study, the differences in CSA and Emean between postoperative patients with CTS and volunteers were statistically significant (Table 4). It can be attributed to the presence of severe permanent structural changes in the median nerve in some patients, as reported by Inui et al. (40). In addition, neurological symptoms were not completely relieved because of the short recovery time.
The differences in CSA of the median nerve measured at the carpal tunnel and proximal levels provided higher diagnostic efficacy than a single CSA measure (43). Scholars have suggested the use of different elasticity ratios to reduce individual variability and stiffness (13, 33, 44) due to the variability of CSA and elasticity in patients of different ages, weights, and genders.
In this experiment, we used CSA and Emean of the nerve at the distal 1/3 of the forearm as internal control parameters. We analyzed the diagnostic performance of the CSA ratio and Emean of the nerve at the carpal tunnel inlet and the distal 1/3 of the forearm. The ratio had a high diagnostic efficacy; this strategy increased the assessment efficiency and sensitivity of median nerve injury (45) but was not optimized by applying CSA or Emean alone due to the low number of patients.
This study has some limitations: (a) A single-center study with a small sample size, (b) a preliminary study of postoperative nerve recovery in the short term; in future studies, we will refine the records of longer follow-ups. (c) We detected microvascular changes within the median nerve in some moderate to severe patients, which we believe is also a sign of pathological changes. However, we did not conduct further analysis. (d) The pressure applied to the surface with the probe cannot be controlled completely at the carpal tunnel inlet and outlet. Therefore, the recommendations for future research are to (a) increase the number of patients with mild and extremely severe CTS, (b) increase the patient follow-up information to assess long-term nerve recovery and explore the potential correlations between SWE parameters and clinical score in a later stage after surgery, (c) examine the microvascular anatomy of the median nerve in all patients in future studies, and (d) add an ultrasound gel pad to reduce excessive pressure when measuring the elasticity of the superficial nerve.
5. Conclusion
Conventional ultrasound and shear wave elastography have a strong correlation with clinical symptom scores and electromyography severity grading. This allowed the assessment of changes in postoperative nerve stiffness, which assisted in the clinical judgment of postoperative nerve recovery in patients with CTS.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conception and design of the study: X-LW. Ultrasound data acquisition: HW. Analysis of data: W-LX, Y-CW, and W-YZ. Drafting the manuscript: HW and H-JZ. Revising and final approval of the version to be published: X-LW. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the scientific research project of the Heilongjiang Provincial Health Commission (Grant No. 202109020156).
Acknowledgments
We sincerely thank all participants in the study for their cooperation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Yao Y, Grandy E, Jenkins L, Hou J, Evans PJ, Seitz Jr WH, et al. Changes of median nerve conduction, cross–sectional area and mobility by radioulnar wrist compression intervention in patients with carpal tunnel syndrome. J Ortho Translat. (2019) 18:13–9. doi: 10.1016/j.jot.2019.01.002
2. Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, et al. Webb Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol. (2016) 15:1273–84. doi: 10.1016/S1474-4422(16)30231-9
3. Wipperman J, Goerl K. Carpal tunnel syndrome: diagnosis and management. Am Fam Phys. (2016) 94:993–999. doi: 10.1016/j.wneu.2022.11.115
4. Chang YW, Hsieh TC, Tzeng I, Chiu V, Huang PJ, Horng YS. Ratio and difference of the cross–sectional area of median nerve to ulnar nerve in diagnosing carpal tunnel syndrome: a case control study. BMC Med Imag. (2019) 19:52. doi: 10.1186/s12880-019-0351-3
5. Cartwright MS, Hobson-Webb LD, Boon AJ, Alter KE, Hunt CH, Flores VH, et al. Evidence–based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Mus Nerve. (2012) 46:287–93. doi: 10.1002/mus.23389
6. T Wee TC, Simon NG. Ultrasound elastography for the evaluation of peripheral nerves: A systematic review. Mus Nerve. (2019) 60:501–512. doi: 10.1002/mus.26624
7. Park JS, Won HC, Oh JY, Kim DH, Hwang SC, Yoo JI. Value of cross–sectional area of median nerve by MRI in carpal tunnel syndrome. As J Surg. (2020) 43:654–9. doi: 10.1016/j.asjsur.2019.08.001
8. Deniz EO, Sarikaya S, Kurt U, Erkorkmaz H, Ulusoy, Arslan S. Comparison of the diagnostic utility of electromyography, ultrasonography, computed tomography, and magnetic resonance imaging in idiopathic carpal tunnel syndrome determined by clinical findings. Neurosurgery. (2012) 70:610–6. doi: 10.1227/NEU.0b013e318233868f
9. Lee S, Cho HR, Yoo JS, Kim YU. The prognostic value of median nerve thickness in diagnosing carpal tunnel syndrome using magnetic resonance imaging: a pilot study. Kor J Pain. (2020) 33:54–9. doi: 10.3344/kjp.2020.33.1.54
10. Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic performance of two–dimensional shear wave elastography for evaluating tibial nerve stiffness in patients with diabetic peripheral neuropathy. Eur Radiol. (2019) 29:2167–74. doi: 10.1007/s00330-018-5858-4
11. Faeghi F, Ardakani AA, Acharya UR, Mirza-Aghazadeh-Attari M, Abolghasemi J, Ejtehadifar S, et al. Accurate automated diagnosis of carpal tunnel syndrome using radiomics features with ultrasound images: a comparison with radiologists' assessment. Eur J Radiol. (2021) 136:109518. doi: 10.1016/j.ejrad.2020.109518
12. Gonzalez-Suarez CB, Fidel BC, Cabrera JT, Dela Cruz FC, Gesmundo MV, et al. Diagnostic accuracy of ultrasound parameters in carpal tunnel syndrome: additional criteria for diagnosis. J Am Inst Ultrasound Med. (2019) 38:3043–52. doi: 10.1002/jum.15012
13. Moran L, Royuela A, de Vargas AP, Lopez A, Cepeda Y, Martinelli G. Carpal tunnel syndrome: diagnostic usefulness of ultrasound measurement of the median nerve area and quantitative elastographic measurement of the median nerve stiffness. J Am Inst Ultrasound Med. (2020) 39:331–9. doi: 10.1002/jum.15111
14. Miyamoto H, Halpern EJ, Kastlunger M, Gabl M, Arora R, Bellmann-Weiler R, et al. Carpal tunnel syndrome: diagnosis by means of median nerve elasticity—-improved diagnostic accuracy of US with sonoelastography. Radiology. (2014) 270:481–6. doi: 10.1148/radiol.13122901
15. Smerilli G, Di Matteo A, Cipolletta E, Carloni S, Incorvaia A, Di Carlo M, et al. Ultrasound assessment of carpal tunnel in rheumatoid arthritis and idiopathic carpal tunnel syndrome. Clin Rheumatol. (2021) 40:1085–92. doi: 10.1007/s10067-020-05293-z
16. Fowler JR, Munsch M, Tosti R, Hagberg WC, Imbriglia JE. Comparison of ultrasound and electrodiagnostic testing for diagnosis of carpal tunnel syndrome: study using a validated clinical tool as the reference standard. J Bone Joint Surg. (2014) 96:e148. doi: 10.2106/JBJS.M.01250
17. Schillizzi G, Alviti F, D'Ercole C, Elia D, Agostini F, Mangone M, et al. Evaluation of plantar fasciopathy shear wave elastography: a comparison between patients and healthy subjects. J Ultrasound. (2021) 24:417–22. doi: 10.1007/s40477-020-00474-7
18. Harmon B, Wells M, Park D, Gao J. Ultrasound elastography in neuromuscular and movement disorders. Clin Imag. (2019) 53:35–42. doi: 10.1016/j.clinimag.2018.10.008
19. Liao YY, Lee WN, Lee MR, Chen WS, Chiou HJ, Kuo TT, Yeh CK. Carpal tunnel syndrome: US strain imaging for diagnosis. Radiology. (2015) 275:205–14. doi: 10.1148/radiol.14140017
20. Paluch Ł Noszczyk B, Nitek Z, Walecki J, Osiak K, Pietruski P, Shear–wave elastography: a new potential method to diagnose ulnar neuropathy at the elbow. Eur Radiol. (2018) 28:4932–9. doi: 10.1007/s00330-018-5517-9
21. Zhu B, Yan F, He Y, Wang L, Xiang X, Tang Y, et al. Evaluation of the healthy median nerve elasticity: feasibility and reliability of shear wave elastography. Medicine. (2018) 97:e12956. doi: 10.1097/MD.0000000000012956
22. Zhang C, Li M, Jiang J, Zhou Q, Xiang L, Huang Y, et al. Diagnostic value of virtual touch tissue imaging quantification for evaluating median nerve stiffness in carpal tunnel syndrome. J Am Inst Ultrasound Med. (2017) 36:1783–91. doi: 10.1002/jum.14213
23. Lai ZH, Yang SP, Shen HL, Luo Y, Cai XH, Jiang WT, et al. Combination of high–frequency ultrasound and virtual touch tissue imaging and quantification improve the diagnostic efficiency for mild carpal tunnel syndrome. BMC Musculosk Disord. (2021) 22:112. doi: 10.1186/s12891-021-03982-7
24. Cingoz M, Kandemirli SG, Alis DC, Samanci C, Kandemirli GC, Adatepe NU. Evaluation of median nerve by shear wave elastography and diffusion tensor imaging in carpal tunnel syndrome. Eur J Radiol. (2018) 101:59–64. doi: 10.1016/j.ejrad.2018.02.005
25. Lin CP, Chen J, Chang KV, Wu WT, Özçakar L. Utility of ultrasound elastography in evaluation of carpal tunnel syndrome: a systematic review and meta–analysis. Ultrasound Med Biol. (2019) 45:2855–65. doi: 10.1016/j.ultrasmedbio.2019.07.409
26. Shen YP, Li TY, Chou YC, Ho TY, Ke MJ, Chen LC, et al. Comparison of perineural platelet–rich plasma and dextrose injections for moderate carpal tunnel syndrome: a prospective randomized, single–blind, head–to–head comparative trial. J Tissue Eng Regen Med. (2019) 13:2009–2017. doi: 10.1002/term.2950
27. Fischer J, Thompson NW, Harrison JW. A self–administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg. (1993) 75:1585–92. doi: 10.2106/00004623-199311000-00002
28. Kantarci F, Ustabasioglu FE, Delil S, Olgun DC, Korkmazer B, Dikici AS, et al. Median nerve stiffness measurement by shear wave elastography: a potential sonographic method in the diagnosis of carpal tunnel syndrome. Eur Radiol. (2014) 24:434–40. doi: 10.1007/s00330-013-3023-7
29. Yayama T, Kobayashi S, Nakanishi Y, Uchida K, Kokubo Y, Miyazaki T, et al. Effects of graded mechanical compression of rabbit sciatic nerve on nerve blood flow and electrophysiological properties. J Clin Neurosci. (2010) 17:501–5. doi: 10.1016/j.jocn.2009.07.110
30. Pindrik J, Belzberg AJ. Peripheral nerve surgery: primer for the imagers. Neuroimag Clin North Am. (2014) 24:193–210. doi: 10.1016/j.nic.2013.03.034
31. Xu C, Zhou Y, He Z, Liu W, Zou M, Sun Y, et al. Difference and ratio of the cross–sectional area of median nerve at the carpal tunnel and the pronator quadratus muscle in diagnosing carpal tunnel syndrome: a cross–sectional study. Annals Translat Med. (2022) 10:340. doi: 10.21037/atm-22-1128
32. Byra M, Hentzen E, Du J, Andre M, Chang EY, Shah S. Assessing the performance of morphologic and echogenic features in median nerve ultrasound for carpal tunnel syndrome diagnosis. J Ultrasound Med. (2020) 39:1165–74. doi: 10.1002/jum.15201
33. Paluch Ł, Pietruski P, Walecki J, Noszczyk BH. Wrist to forearm ratio as a median nerve shear wave elastography test in carpal tunnel syndrome diagnosis. J Plastic Reconst Aesth Surg JPRAS. (2018) 71:1146–52. doi: 10.1016/j.bjps.2018.03.022
34. Wang Y, Qiang B, Zhang X, Greenleaf JF, An KN, Amadio PC, et al. A non–invasive technique for estimating carpal tunnel pressure by measuring shear wave speed in tendon: a feasibility study. J Biomech. (2012) 45:2927–30. doi: 10.1016/j.jbiomech.2012.09.002
35. Bhatia KS, Cho C, Tong CS, Lee YY, Yuen EH, Ahuja AT. Shear wave elastography of focal salivary gland lesions: preliminary experience in a routine head and neck US clinic. Eur Radiol. (2012) 22:957–65. doi: 10.1007/s00330-011-2364-3
36. Choi J, Kim SU, Kim DG, Park KS. Characteristics of forearm mixed nerve conduction study in carpal tunnel syndrome: comparison with ultrasound assessments. J Neurosurg Soc Au. (2020) 73:155–8. doi: 10.1016/j.jocn.2019.12.053
37. Drakopoulos D, Mitsiokapa E, Karamanis E, Kontogeorgakos V, Mavrogenis AF. ultrasonography provides a diagnosis similar to that of nerve conduction studies for carpal tunnel. Synd Orthoped. (2019) 42:e460–4. doi: 10.3928/01477447-20190604-02
38. Shen. Neuromuscular ultrasound in the assessment of polyneuropathies and motor neuron disease. J Clin Neurophysiol. (2016) 33:86–93. doi: 10.1097/WNP.0000000000000241
39. Arslan H, Yavuz A, Ilgen F, Aycan A, Ozgokce M, Akdeniz H, et al. The efficiency of acoustic radiation force impulse (ARFI) elastography in the diagnosis and staging of carpal tunnel syndrome. J Med Ultrasonics. (2001) 45:453–9. doi: 10.1007/s10396-017-0857-7
40. Inui A, Nishimoto H, Mifune Y, Kokubu T, Sakata R, Kurosaka M. Ultrasound measurement of median nerve cross–sectional area at the inlet and outlet of carpal tunnel after carpal tunnel release compared to electrodiagnostic findings. Arc Ortho Trauma Surg. (2016) 136:1325–30. doi: 10.1007/s00402-016-2514-9
41. Peters BR, Martin AM, Memauri BF, Bock HW, Turner RB, Murray KA, et al. Morphologic analysis of the carpal tunnel and median nerve following open and endoscopic carpal tunnel. Rel Hand. (2021) 16:310–5. doi: 10.1177/1558944719861711
42. Schrier VJ, Evers S, Geske JR, Kremers WK, Villarraga HR, Kakar S, et al. Median nerve transverse mobility and outcome after carpal tunnel release. Ultra Med Biol. (2019) 45:2887–97. doi: 10.1016/j.ultrasmedbio.2019.06.422
43. Klauser AS, Halpern EJ, De Zordo T, Feuchtner GM, Arora R, Gruber J, et al. Carpal tunnel syndrome assessment with US: value of additional cross–sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. (2009) 250:171–7. doi: 10.1148/radiol.2501080397
44. Bedewi MA, Coraci D, Ruggeri F, Giovannini S, Gentile L, Padua L. Shear wave elastography of median nerve at wrist and forearm Heterogeneity of normative values. J Plast Reconst Aesth Surgery JPRAS. (2019) 72:137–71. doi: 10.1016/j.bjps.2018.09.015
Keywords: carpal tunnel syndrome, acoustic radiation force impulse, shear wave elastography, Boston Carpal Tunnel Questionnaire, cross-sectional area
Citation: Wu H, Zhao H-J, Xue W-L, Wang Y-C, Zhang W-Y and Wang X-L (2022) Ultrasound and elastography role in pre- and post-operative evaluation of median neuropathy in patients with carpal tunnel syndrome. Front. Neurol. 13:1079737. doi: 10.3389/fneur.2022.1079737
Received: 25 October 2022; Accepted: 23 November 2022;
Published: 16 December 2022.
Edited by:
Hui Lu, Zhejiang University, ChinaReviewed by:
Francesco Agostini, Sapienza University of Rome, ItalyLuca Leonardi, Sapienza University of Rome, Italy
Copyright © 2022 Wu, Zhao, Xue, Wang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Lei Wang, d3hsZ2hiQDEyNi5jb20=
†These authors have contributed equally to this work
 Han Wu
Han Wu Hong-Juan Zhao†
Hong-Juan Zhao†