
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 December 2022
Sec. Pediatric Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1073703
 Seh Hyun Kim1
Seh Hyun Kim1 Seung Han Shin1*
Seung Han Shin1* Hyo Ju Yang1
Hyo Ju Yang1 Seul Gi Park1
Seul Gi Park1 Soo Yeon Lim1
Soo Yeon Lim1 Young Hun Choi2
Young Hun Choi2 Ee-Kyung Kim1
Ee-Kyung Kim1 Han-Suk Kim1
Han-Suk Kim1Background: Cerebellar hemorrhage (CBH) is a major form of cerebellar injury in preterm infants. We aimed to investigate the risk factors and neurodevelopmental outcomes of isolated CBH and performed volumetric analysis at term-equivalent age.
Methods: This single-centered nested case-control study included 26 preterm infants with isolated CBH and 52 infants without isolated CBH and any significant supratentorial injury.
Results: Isolated CBH was associated with PCO2 fluctuation within 72 h after birth (adjusted odds ratio 1.007, 95% confidence interval 1.000–1.014). The composite score in the motor domain of the Bayley Scales of Infant and Toddler Development at 24 month of corrected age was lower in the punctate isolated CBH group than that in the control group (85.3 vs. 94.5, P = 0.023). Preterm infants with isolated CBH had smaller cerebellum and pons at term-equivalent age compared to the control group. Isolated CBH with adverse neurodevelopment had a smaller ventral diencephalon and midbrain compared to isolated CBH without adverse neurodevelopmental outcomes.
Conclusions: In preterm infants, isolated CBH with punctate lesions were associated with abnormal motor development at 24 months of corrected age. Isolated CBH accompanied by a smaller ventral diencephalon and midbrain at term equivalent had adverse neurodevelopmental outcomes.
Structural abnormalities of the cerebellum in premature infants can be characterized as destructive lesions with underdevelopment of the infratentorial structures. Major forms of destructive cerebellar disease include hemorrhage and infarction (1). Advances in neuroimaging techniques, such as ultrasound by mastoid fontanel view and magnetic resonance imaging (MRI) are used for diagnosing cerebellar hemorrhage (CBH), which is not an uncommon complication of preterm birth (1, 2).
There have been controversies over the neurodevelopmental outcomes of CBH based on the location and size of the lesions, such as punctate lesions in the cerebellum of preterm infants. In a retrospective study, punctate CBH was not associated with neurodevelopmental outcomes at 24 months of age (3). On the other hand, a multicenter study reported that 40% of infants with punctate CBH had abnormal developmental outcomes (4). The location of CBH is also an important determinant for neurodevelopmental outcomes as involvement of the cerebellar vermis showed global developmental delay (5, 6). As CBH often occurrs concomitant with supratentorial injury of the brain such as intraventricular hemorrhage (IVH) and periventricular leukomalacia (PVL), effect of CBH on neurodevelopmental outcomes could be biased by supratentorial lesions (7). Although there have been other studies regarding outcomes of CBH, most studies were performed with patients of CBH plus supratentorial injury (5, 8–10). Therefore, studies investigating neurodevelopmental outcomes of CBH without significant supratentorial injury are needed for better understanding of CBH in preterm infants.
Neurodevelopmental outcomes of preterm infants are not only mediated by acute destructive events of the brain but also by adverse development in regions close to and distant from the original injury (9, 11). Previous studies with volumetric analysis showed associations of reduced volume of the cerebellum and that of the total brain with poor neurological outcome at 24 months of corrected age in preterm infants (12, 13). Growth of the cerebellum is influenced by both direct damage to the cerebellum and by the growth of the contralateral cerebral hemisphere (14, 15).
In this study, we aimed to explore factors associated with isolated CBH and their neurodevelopmental outcomes and to conduct a volumetric analysis of brain structures, including the cerebellum, other infratentorial structures, and supratentorial structures of the brain, to assess the impact of isolated CBH on the volume of the cerebellum and other brain regions.
This was a nested case-control study of preterm infants who were admitted to the neonatal intensive care unit (NICU) of Seoul National University Children's Hospital and ha0d brain MRI examination between January 2010 and December 2019. Infants with major congenital anomalies and significant supratentorial lesions such as severe IVH, PVL, and infarction were excluded from the study population. Isolated CBH was defined based on MRI at term equivalent age, when supratentorial lesions were not present, except for low-grade (I-II) IVH (16). Infants with isolated CBH were compared with double the number of preterm infants with gestational age (GA), birth weight, and sex-matched controls without CBH and significant supratentorial lesions to assess the risk factors and neurodevelopmental outcomes of isolated CBH. Medical records of perinatal factors, including Apgar score, preterm premature rupture of the membrane, placental abruption, histologic chorioamnionitis, chest compression in the delivery room, body temperature at admission, intubation within 3 days after birth, inotrope use within 3 days after birth, and blood gas analysis within 3 days after birth were collected. Degree of PCO2 fluctuation was calculated by subtracting the trough PCO2 level from the peak PCO2 level from results of all blood gas analysis during first 72 h life. In preterm infants, brain sonography was first conducted within 3 days after birth and subsequently performed weekly or bi-weekly according to the baby's conditions and previous sonography results during the first month of life. During the study period, there were 3,294 preterm infants admitted to Seoul National University Children's Hospital NICU between 2010 and 2019. Among them, 59 (1.8%) infants were diagnosed with CBH, with 26 (0.8%) being diagnosed with isolated CBH (Figure 1).
To evaluate the neurodevelopmental outcomes, Bayley Scales of Infant and Toddler Development 3rd Edition (BSID-III) results were gathered for each group at a corrected age (CA) of 18–24 months. On BSID-III, scores of <85 (< -1 SD) in both cognitive and language domains, or a motor score of < 85, were defined as developmental delay (17). Infants who had delay in BSID-III, hearing impairment, blindness or cerebral palsy (CP) at a CA of 18–24 months were defined as having combined neurodevelopmental impairment (NDI) (18). Hearing impairment was defined as the need for unilateral or bilateral hearing aids. Neurodevelopmental outcomes were compared between the no CBH and CBH with punctate lesion groups.
The conventional neonatal brain MRI protocol includes sagittal 3d gradient echo sequence, T2- and T1-weighted axial turbo spin echo sequences, susceptibility weighted images and diffusion weighted images. T2- and T1-weighted axial turbo spin echo images and diffusion weighted images were obtained at a slice thickness of 4 mm without a gap. The sagittal 3d gradient echo sequence was obtained at a 1 mm-iso voxel and then presented as axial, coronal and sagittal reformatted images of 1 mm-thickness. Brain MRI at term-equivalent age (TEA) was routinely conducted in all preterm infants born at <29 weeks of gestation or birth weight < 1,000 g, as well as in those with parenchymal brain injury on sonography.
CBH were categorized by an experienced pediatric radiologist into four types: punctate (lesion ≤ 4 mm) CBH, limited CBH (lesion > 4 mm, involving < 1/3 of the cerebellar hemisphere), vermian hemorrhage, and massive CBH (lesion involving ≥ 1/3 of the cerebellar hemisphere), which were modified from a study by Gerda Meijler (19). Isolated CBH was defined as CBH without significant supratentorial parenchymal lesions or hydrocephalus. In present study, among 26 isolated CBH, there were 18 (69.2%) with punctate CBH (Figure 2), 7 (27.0%) with limited CBH, and 1 (3.8%) with vermian hemorrhage. No infants had massive CBH in the study population of isolated CBH.
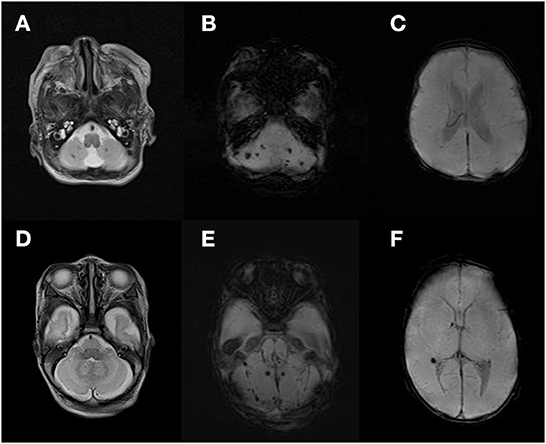
Figure 2. Image of isolated punctate cerebellar hemorrhage. T2 weighted image of punctate cerebellar lesion (A,D). Susceptibility weighted imaging of punctate cerebellar hemorrhage (B,E) and supratentorial area (C,F).
For volumetric analysis of brain structures, the Infant Freesurfer software (https://surfer.nmr.mgh.harvard.edu/fswiki/infantFS) was used by one individual. Infant Freesurfer is a pipeline of automated segmentation and surface extraction based on Freesurfer for T1-weighted neuroimaging data for infants (20). Infant Freesurfer can calculate brain segment volumes including cerebral white matter, cerebral cortex, both cerebellar hemisphere, vermis, midbrain, pons, medulla, thalamus, hippocampus, amygdala, accumbens, and ventral diencephalon. After automatic segmentation by Infant Freesurfer, visual rechecking of each brain structure was performed. Volumetric analysis was conducted between the isolated CBH group and the control group, and then among the isolated CBH groups according to neurodevelopmental outcomes.
Statistical analysis was executed using the R statistical software v 4.1.1. Data was expressed as number (%) or mean ± standard deviation. Wilcoxon rank-sum tests were conducted for continuous data and chi-square for categorical data. Statistical significance was set at P < 0.05. Univariate logistic regression analysis of GA and factors which were different between two groups was used for isolated CBH, and factors with a P < 0.05 in univariate analysis were inserted in the multivariate analysis.
The GA at birth (26.0 ± 2.0 vs. 26.2 ± 1.6 weeks) and birthweights (872.5 ± 587.2 vs. 867.5 ± 212.1 g) were comparable between the isolated CBH group and matched-control group (Table 1). The range of GA of both groups were 23.4–32.4 weeks. The Apgar score at 5 min was lower in the isolated CBH group than in the control group (4.5 ± 2.1 vs. 5.6 ± 2.0, P = 0.034). Chest compression in the delivery room, admission temperature, and intubation within 3 days after birth were comparable between the two groups. In the blood gas analysis performed within 3 days after birth, trough PCO2 levels were lower in the isolated CBH group (27.4 ± 6.5 vs. 30.8 ± 6.2 mmHg, P = 0.042) and PCO2 fluctuation levels (peak – trough) were higher in the isolated CBH group (37.9 ± 15.4 vs. 28.7 ± 14.7 mmHg, P = 0.019) (Table 2).
Univariate regression analysis showed that 5 min Apgar score and PCO2 fluctuation level within 3 days after birth were associated with isolated CBH (Table 3). Multivariate logistic regression showed that the PCO2 fluctuation level was significantly associated with isolated CBH [adjusted odds ratio (OR) 1.007, 95% confidence interval (CI) 1.000–1.014, P = 0.044), while the association with 5 min Apgar score became insignificant (adjusted OR 0.961, 95% CI 0.914–1.011, P = 0.126).
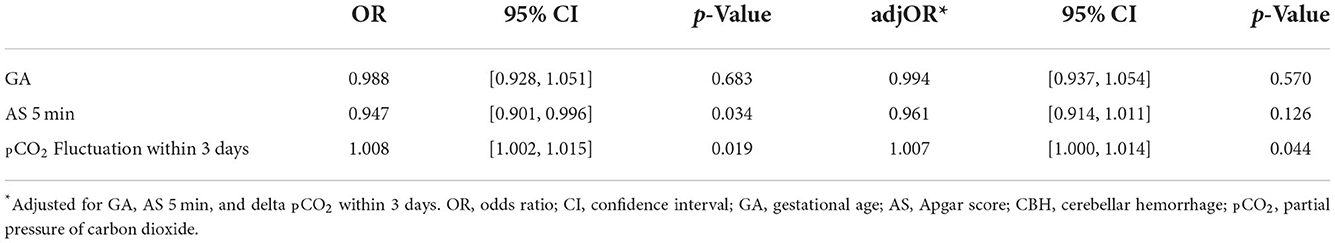
Table 3. Univariate and multivariate logistic regression analyses for factors related to isolated CBH.
In terms of neurodevelopmental outcome, there were no cases of CP, hearing loss and blindness in the study population and overall NDI was comparable between the isolated punctate CBH and matched-control groups. The motor domain in the BSID showed lower scores in the isolated punctate CBH group (85.3 ± 10.3 vs. 94.5 ± 11.5, P = 0.023) (Table 4).
In the volumetric analysis of the brain structures, the isolated CBH group showed smaller cerebellum (11.7 ± 3.0 vs. 13.5 ± 3.0 ml, P = 0.007) and pons (1.5 ± 0.4 vs. 1.8 ± 0.3 ml, P = 0.001) volume compared to the control group. The estimated total intracranial and vermian volumes were comparable between the two groups (Table 5). In the subgroup analysis of isolated CBH, those with NDI had smaller ventral diencephalon (1.5 ± 0.2 vs. 1.7 ± 0.2 ml, P = 0.028) and midbrain (1.3 ± 0.2 vs. 1.4 ± 0.1 ml, P = 0.032) than those without NDI at CA 18–24 months (Table 6).
The current study showed that preterm infants with isolated CBH had more fluctuations in PCO2 levels within 72 h after birth than those without CBH. Isolated CBH with punctate hemorrhage is associated with abnormal motor development in preterm infants. Among preterm infants with isolated CBH, the ventral diencephalon and midbrain were smaller in those with NDI than in those without NDI. The incidence of CBH and isolated CBH in the present study was 1.8 and 0.8%, respectively, which were consistent with previous studies that reported incidence ranges of CBH in preterm infants from 1.5 to 19%, and the incidence of isolated CBH from 0.9 to 5.2% depending on the imaging modality (2, 10, 21).
CBH is known to be more prevalent in smaller and more immature preterm infants, and several factors have been reported as risk factors such as preeclampsia, sepsis, prolonged mechanical ventilation, patent ductus arteriosus, lower Apgar scores, acidosis, and hypotension (8, 22–25). In the present study, we investigated the risk of isolated CBH, wherein fluctuation in PCO2 within 72 h after birth was the only factor associated with isolated CBH. Impaired autoregulation of the cerebral circulation in preterm infants is associated with CBH (4, 26). As hypercapnia alters cerebral hemodynamics by increasing blood flow and on the contrary, low PCO2 has vasoconstrictive effects (27, 28). These effects of PCO2 that alter cerebral hemodynamics might explain the association of isolated CBH with higher PCO2 fluctuation in the present study. Thus, careful ventilator strategy during the first 3 days after birth to avoid over- or under-ventilation might be helpful to avoid CBH.
The cerebellum undergoes exponential growth such that the volume expands 5-fold from 24 weeks to 40 weeks of gestation, and this growth rate is faster than any other structures of the human brain (1). Due to this exponential growth, the cerebellum of a premature infant is vulnerable to multiple insults, and large CBH are associated with poorer neurodevelopmental outcomes and cerebellar injury is associated with various degree of motor delay (1, 25). However, there is a limited understanding of the effect of CBH on neurodevelopment in preterm infants because CBH is often accompanied by supratentorial injuries of brain in this population. A systematic review by Hortensius et al. contributed to expanding the understanding of this problem to some extent and reported that isolated CBH in preterm infants was associated with neurodevelopmental delay in the cognitive, language, behavioral, and motor domains, with the highest incidence of vermian involvement and CBH with large hemorrhages (10).
Punctate hemorrhage accounts for 39–67% of isolated CBH in preterm infants (4, 29). Size of hemorrhage in CBH is one of the important determents for prognosis, as large CBH had higher incidence of motor impairment (10). These small punctate CBH are usually not found on head ultrasounds but are often diagnosed on TEA brain MRI (4). And punctate CBH are known to have more favorable neurodevelopmental outcomes (3, 4). In a retrospective study, CBH with punctate lesions was not associated with adverse neurodevelopment (3). However, the study included preterm infants with supratentorial lesions, which might mask the effect of isolated CBH. On the contrary, in a systematic review of isolated CBH, 13% of the patients with punctate CBH had significant neurodevelopmental impairment (10). The present study showed that punctate isolated CBH was associated with poorer motor function, showing NDI in 33% and BSID-III motor scores below 85 in 55% among isolated CBH with punctate lesion. However, there was no difference in the incidence of CP between the isolated CBH group and the control group. Distinguishing punctate cerebellar hemorrhage from the simple deposition of circulating blood in the cerebrospinal fluid is not simple. Disproportional deposition of hemosiderin in the posterior fossa, accompanied parenchymal hemosiderin deposits in the susceptibility weighted imaging, and parenchymal change such as atrophy might be helpful to distinguish the condition.
Decreased cerebellar volume is associated with poor neurodevelopmental outcomes, which could be mediated by effects on adverse development in regions close to and remote from the original injury (9, 11). The present study demonstrated that the isolated CBH group had smaller cerebellar and pons volumes than the control group. In a previous study, cerebellar atrophy developed after hemorrhages or infarcts with a prevalence of 20–37% in infants with CBH (2, 8). In addition, smaller pons was associated with CBH in premature infants in a case series by Parodi et al., which showed that unilateral CBH and crossed pontine hemiatrophy were related (30). In a MRI study that compared volumetric analysis of in-utero and ex-utero brain of term and preterm infants, volume of cerebellar hemisphere and pons at TEA were smaller in preterm infant than term infants (31).
Among preterm infants with isolated CBH, the ventral diencephalon and midbrain were smaller in the isolated CBH with NDI group than in the isolated CBH without NDI group. The ventral diencephalon is a combination of several structures, including the basal forebrain, ventral tegmentum, and hypothalamus. The midbrain connects the cerebellum, pons with the forebrain and the ventral midbrain, which contains the pyramidal and corticopontine tracts, which are related to motor functions (32). Also, the ventral tegmentum is known to play an important role in dopamine-related functions, including motor functioning (33). As shown in the present study, if isolated CBH is accompanied by a reduced volume of specific brain structures, including the ventral diencephalon and midbrain, the probability of developmental impairment might increase. This was also well-demonstrated in the study by Limperopoulos et al., which reported that neurodevelopmental impairment in preterm infants with cerebellar injury was accompanied by remoted growth restriction of cerebral cortical growth (34).
The current study had several limitations. This was a retrospective study, and brain MRI was performed in the selected high-risk population of preterm infants. In addition, only two-thirds of the infants were evaluated by Bayley-III at 18–24 months of CA, although data regarding CP, hearing loss and blindness were well-collected. Those who was not tested by Bayley-III among the study population were those whose parents thought that their child had normal development during the follow-up period. In addition, there is a possibility that some punctate CBH lesions may not be visible on TEA brain MRI in the study population and this may influence the results. However, the risk factors, neurodevelopmental outcomes, and brain structure volumetric analysis of isolated CBH were thoroughly analyzed. Volumetric MRI analysis of isolated CBH with or without NDI was also performed. This retrospective case-control study suggests that isolated CBH is associated with higher fluctuations in PCO2 levels within 72 h after birth. Additionally, when isolated CBH was combined with a reduced volume of the ventral diencephalon and midbrain, poorer neurodevelopmental outcomes were observed. Further studies with larger populations are required to clarify the neurodevelopmental outcomes of isolated CBH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Seoul National University Hospital Institutional Review Board. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
SK interpreted the collected MRI data, performed statistical analysis, and wrote the initial draft of the manuscript. HY, SP, and SL interpreted and collected clinical data. SS and YC developed the study idea, performed statistical analysis, and critically reviewed the manuscript. E-KK and H-SK critically reviewed the manuscript. All authors gave substantial contributions to the work, drafted, revised, reviewed, approved of the final manuscript, and agreed to be accountable for all aspects of the work.
This research was supported and funded by Seoul National University Hospital Kun-hee Lee Child Cancer and Rare Disease Project, Republic of Korea (Grant Number: 22C-014-0100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Volpe JJ. Cerebellum of the premature infant: rapidly developing, vulnerable, clinically important. J Child Neurol. (2009) 24:1085–104. doi: 10.1177/0883073809338067
2. Steggerda SJ, Leijser LM, Wiggers-de Bruine FT, van der Grond J, Walther FJ, van Wezel-Meijler G. Cerebellar injury in preterm infants: incidence and findings on US and MR images. Radiology. (2009) 252:190–9. doi: 10.1148/radiol.2521081525
3. Steggerda SJ, De Bruine FT, van den Berg-Huysmans AA, Rijken M, Leijser LM, Walther FJ, et al. Small cerebellar hemorrhage in preterm infants: perinatal and postnatal factors and outcome. Cerebellum. (2013) 12:794–801. doi: 10.1007/s12311-013-0487-6
4. Boswinkel V, Steggerda SJ, Fumagalli M, Parodi A, Ramenghi LA, Groenendaal F, et al. The chopin study: a multicenter study on cerebellar hemorrhage and outcome in preterm infants. Cerebellum. (2019) 18:989–98. doi: 10.1007/s12311-019-01053-1
5. Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long-term cognitive, learning, and behavioral disability in survivors? Pediatrics. (2007) 120:584–93. doi: 10.1542/peds.2007-1041
6. Zayek MM, Benjamin JT, Maertens P, Trimm RF, Lal CV, Eyal FG. Cerebellar hemorrhage: a major morbidity in extremely preterm infants. J Perinatol. (2012) 32:699–704. doi: 10.1038/jp.2011.185
7. Fumagalli M, Bassi L, Sirgiovanni I, Mosca F, Sannia A, Ramenghi LA. From germinal matrix to cerebellar haemorrhage. J Matern Fetal Neonatal Med. (2015) 28(Suppl 1):2280–5. doi: 10.3109/14767058.2013.796168
8. Limperopoulos C, Benson CB, Bassan H, Disalvo DN, Kinnamon DD, Moore M, et al. Cerebellar hemorrhage in the preterm infant: ultrasonographic findings and risk factors. Pediatrics. (2005) 116:717–24. doi: 10.1542/peds.2005-0556
9. Limperopoulos C, Chilingaryan G, Guizard N, Robertson RL, Du Plessis AJ. Cerebellar injury in the premature infant is associated with impaired growth of specific cerebral regions. Pediatr Res. (2010) 68:145–50. doi: 10.1203/PDR.0b013e3181e1d032
10. Hortensius LM, Dijkshoorn ABC, Ecury-Goossen GM, Steggerda SJ, Hoebeek FE, Benders M, et al. Neurodevelopmental consequences of preterm isolated cerebellar hemorrhage: a systematic review. Pediatrics. (2018) 142:e20180609. doi: 10.1542/peds.2018-0609
11. Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. (2009) 8:110–24. doi: 10.1016/S1474-4422(08)70294-1
12. Lind A, Parkkola R, Lehtonen L, Munck P, Maunu J, Lapinleimu H, et al. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr Radiol. (2011) 41:953–61. doi: 10.1007/s00247-011-2071-x
13. Zwicker JG, Miller SP, Grunau RE, Chau V, Brant R, Studholme C, et al. Smaller cerebellar growth and poorer neurodevelopmental outcomes in very preterm infants exposed to neonatal morphine. J Pediatr. (2016) 172:81–7.e2. doi: 10.1016/j.jpeds.2015.12.024
14. Limperopoulos C, Soul JS, Haidar H, Huppi PS, Bassan H, Warfield SK, et al. Impaired trophic interactions between the cerebellum and the cerebrum among preterm infants. Pediatrics. (2005) 116:844–50. doi: 10.1542/peds.2004-2282
15. Limperopoulos C, Soul JS, Gauvreau K, Huppi PS, Warfield SK, Bassan H, et al. Late gestation cerebellar growth is rapid and impeded by premature birth. Pediatrics. (2005) 115:688–95. doi: 10.1542/peds.2004-1169
16. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
17. Johnson S, Moore T, Marlow N. Using the Bayley-III to assess neurodevelopmental delay: which cut-off should be used? Pediatr Res. (2014) 75:670–4. doi: 10.1038/pr.2014.10
18. Kilbride HW, Aylward GP, Doyle LW, Singer LT, Lantos J. Prognostic neurodevelopmental testing of preterm infants: do we need to change the paradigm? J Perinatol. (2017) 37:475–9. doi: 10.1038/jp.2017.12
20. Zollei L, Iglesias JE, Ou Y, Grant PE, Fischl B. Infant freesurfer: an automated segmentation and surface extraction pipeline for T1-weighted neuroimaging data of infants 0-2 years. Neuroimage. (2020) 218:116946. doi: 10.1016/j.neuroimage.2020.116946
21. Hou D, Shetty U, Phillips M, Gray PH. Cerebellar Haemorrhage in the Extremely Preterm Infant. J Paediatr Child Health. (2012) 48:350–5. doi: 10.1111/j.1440-1754.2011.02232.x
22. Villamor-Martinez E, Fumagalli M, Alomar YI, Passera S, Cavallaro G, Mosca F, et al. Cerebellar hemorrhage in preterm infants: a meta-analysis on risk factors and neurodevelopmental outcome. Front Physiol. (2019) 10:800. doi: 10.3389/fphys.2019.00800
23. Neubauer V, Djurdjevic T, Griesmaier E, Biermayr M, Gizewski ER, Kiechl-Kohlendorfer U. Routine magnetic resonance imaging at term-equivalent age detects brain injury in 25% of a contemporary cohort of very preterm infants. PLoS ONE. (2017) 12:e0169442. doi: 10.1371/journal.pone.0169442
24. Sehgal A, El-Naggar W, Glanc P, Asztalos E. Risk factors and ultrasonographic profile of posterior fossa haemorrhages in preterm infants. J Paediatr Child Health. (2009) 45:215–8. doi: 10.1111/j.1440-1754.2008.01456.x
25. Spoto G, Amore G, Vetri L, Quatrosi G, Cafeo A, Gitto E, et al. Cerebellum and prematurity: a complex interplay between disruptive and dysmaturational events. Front Syst Neurosci. (2021) 15:655164. doi: 10.3389/fnsys.2021.655164
26. Tam EWY. Cerebellar injury in preterm infants. Handb Clin Neurol. (2018) 155:49–59. doi: 10.1016/B978-0-444-64189-2.00003-2
27. Travers CP, Carlo WA. Carbon dioxide and brain injury in preterm infants. J Perinatol. (2021) 41:183–4. doi: 10.1038/s41372-020-00842-5
28. Hoffman SB, Lakhani A, Viscardi RM. The association between carbon dioxide, cerebral blood flow, and autoregulation in the premature infant. J Perinatol. (2021) 41:324–9. doi: 10.1038/s41372-020-00835-4
29. Garfinkle J, Guo T, Synnes A, Chau V, Branson HM, Ufkes S, et al. Location and size of preterm cerebellar hemorrhage and childhood development. Ann Neurol. (2020) 88:1095–108. doi: 10.1002/ana.25899
30. Parodi A, Ramenghi LA, Malova M, Tortora D, Severino M, Morana G, et al. Crossed pontine hemiatrophy associated with unilateral cerebellar hemorrhage in premature infants. Neuropediatrics. (2016) 47:404–7. doi: 10.1055/s-0036-1587595
31. Wu Y, Stoodley C, Brossard-Racine M, Kapse K, Vezina G, Murnick J, et al. Altered local cerebellar and brainstem development in preterm infants. Neuroimage. (2020) 213:116702. doi: 10.1016/j.neuroimage.2020.116702
32. Sciacca S, Lynch J, Davagnanam I, Barker R. Midbrain, pons, and medulla: anatomy and syndromes. Radiographics. (2019) 39:1110–25. doi: 10.1148/rg.2019180126
33. Trutti AC, Mulder MJ, Hommel B, Forstmann BU. Functional neuroanatomical review of the ventral tegmental area. Neuroimage. (2019) 191:258–68. doi: 10.1016/j.neuroimage.2019.01.062
Keywords: preterm infant, cerebellar hemorrhage, brain injury, neurodevelopmental outcome, magnetic resonance imaging
Citation: Kim SH, Shin SH, Yang HJ, Park SG, Lim SY, Choi YH, Kim E-K and Kim H-S (2022) Neurodevelopmental outcomes and volumetric analysis of brain in preterm infants with isolated cerebellar hemorrhage. Front. Neurol. 13:1073703. doi: 10.3389/fneur.2022.1073703
Received: 18 October 2022; Accepted: 14 November 2022;
Published: 01 December 2022.
Edited by:
Carlotta Spagnoli, Santa Maria Nuova Hospital, ItalyReviewed by:
Manuel Hinojosa Rodríguez, National Autonomous University of Mexico, MexicoCopyright © 2022 Kim, Shin, Yang, Park, Lim, Choi, Kim and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Seung Han Shin, cmV2aXZhbDQyMUBzbnUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.