
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 11 January 2023
Sec. Dementia and Neurodegenerative Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1065335
This article is part of the Research TopicAdvances in understanding the pathogenetic mechanisms of neurodevelopmental disorders and neurodegenerative disease - The environment as a putative risk factorView all 12 articles
Background: Alzheimer's disease (AD) is a neurological disorder of unknown cause, resulting in the death of brain cells. Identifying some of the modifiable risk factors for AD could be crucial for primary prevention and could lead to a reduction in the incidence of AD.
Objective: This study aimed to perform a meta-meta-analysis of studies in order to assess the effect of blood pressure (BP) on the diagnosis of AD.
Method: The search was restricted to meta-analyses assessing high systolic BP (SBP) and diastolic BP (DBP) and AD. We applied the PRISMA guidelines.
Results: A total of 214 studies were identified from major databases. Finally, five meta-analyses (52 studies) were analyzed in this review. Results confirm that high SBP is associated with AD. The exploration of parameters (sex, age, study design, region, and BP measurements) shows that only region significantly moderates the relationship between BP and AD. Asian people are those whose SBP levels >140 mmHg are associated with AD. BP is associated with AD in both people aged ≤65 years and those aged ≥65 years and in cross-sectional and longitudinal studies. In the case of DBP, only women are at a higher risk of AD, particularly when its levels are >90.
Conclusion: SBP is associated with both cerebrovascular disease and AD. Therefore, future studies should use other uncontrolled factors, such as cardiovascular diseases, diabetes, and stroke, to explain the relationship between SBP and AD.
There are 55 million people affected by dementia worldwide (1). Alzheimer's disease (AD) is the most common cause of dementia, accounting for up to 75% of all dementia cases (2). The prevalence of AD increases every year in individuals between the ages of 65 and 85 years (3), and by the year 2050, the worldwide prevalence of AD will grow four-folds, to 106.8 million (range 47.2–221.2) (4). While between the ages of 65 and 74 years, about 10% of people have AD, and in those over 85 years old, the risk increases by 50% (3). According to estimates by the World Health Organization (WHO), the projected global prevalence of AD by 2050 will increase by 110% from 2010 (5).
Alzheimer's disease is a neurological disorder of unknown cause, resulting in the death of brain cells (3). AD is the most common cause of cognitive impairment (6). AD is characterized by hallmark pathological changes such as extracellular Aβ plaques and intracellular neurofibrillary pathology, which selectively affect specific subclasses of neurons and brain circuits. While dementia is a general term, Alzheimer's disease is a specific brain disease. It is marked by symptoms of dementia that gradually get worse over time (7). Dementia is a rather broad syndrome of global cognitive decline. However, AD first affects the part of the brain associated with specific cognitive functions, such as language (aphasia), motor skills (apraxia), and perception (agnosia) (8, 9). Moreover, in AD, early symptoms often include changes in memory, thinking, and reasoning skills (10).
Some of the first symptoms that occur with AD (neuropsychiatric) are a direct cause of early institutionalization (11). In AD, there is an identity loss (12) and worsening in the physical and social areas (11), along with the progressive deterioration of basic cognitive (episodic memory, linguistic, and spatial orienting) and executive functions (inhibitory abilities and the visuospatial functions) (13). Emotional and mental health problems (e.g., delusions and hallucinations, abnormal behaviors, or physical violence and hitting) are common, cause distress to caregivers, and may be amenable to treatment (14, 15). All these symptoms affect the quality of life and activities of daily living in individuals diagnosed with this disease (15).
The most important non-modifiable risk factor for developing AD is age. Many cardiovascular risk factors increase with age, such as high blood pressure (BP), which, in turn, could affect the mechanisms that lead to impairment in the brain (16).
According to Ballard et al. (17), the development of dementia is associated with not only genetic factors but also acquired factors (i.e., hypertension) that could predict a higher risk of AD. In this study, we particularly focused on analyzing high BP as a risk factor for the development of AD (18, 19). The overall prevalence of high BP in adults is 25%, with more than 50% of those individuals over 60 years (20). Vascular risk factors like BP could change the anatomy of the human body by modifying vascular walls or causing ischemia and cerebral hypoxia, which may consequently lead to the development of AD (21). Furthermore, BP could generate dysfunction in the blood–brain barrier, which has been associated with the genesis of AD (22). Studies on the relationship between BP and AD have yielded inconsistent results, showing an association between AD and high BP, or no significant association between these variables (23–25). For example, Mielke found that systolic hypertension was associated with an increased risk of AD. However, the authors did not find an association between diastolic hypertension and AD (22).
Findings also established that the association between AD and hypertension was determined by age of onset (early-onset AD ≤ 65 years and late-onset AD ≥ 65 years). In fact, AD has been classified as presenile or early onset (≤ 65 years) and as senile or late onset (≥65 years) that tend to be sporadic and slow moving (26). However, it is still not clear in the current literature whether age moderates the relationship between BP and AD. Indeed, some researchers have indicated that elevated BP occurring in either middle age or late life may be involved in the development of AD (23, 27, 28). Also, one study concluded that high systolic BP (SBP) and diastolic BP (DBP) were related to worse cognitive function for persons aged 65–74 years. However, in older age (≥75), higher SBP and DBP were related to adequate cognitive function (29).
Other studies have studied the relationship between hypertension and gender. Gillis and Sullivan (30) concluded that women are more likely to be prehypertensive than men. Furthermore, Anstey et al. (31) concluded that hypertension in middle-aged women was associated with greater cognitive impairment and AD. However, recent studies have shown that the prevalence of hypertension is higher in men before the sixth decade of life, although it increases in women after menopause (32).
Related to regions due to the high incidence of hypertension in developed countries, studies are aimed at prevention strategies (33, 34). In addition, the earlier onset and more aggressive development of AD in the young population have been identified as risk factors for hypertension in these countries (35).
The literature refers to various degrees of hypertension. This study was based on the cutoff points established by the International Society of Hypertension (ISH) (36). On the one hand, the ISH establishes the following measures for SBP: elevated (130–139 mmHg), grade 1 (140–159 mmHg), and grade 2 (160–179 mmHg). On the other hand, there are also three cutoff measurements for DBP: elevated (85–89 mmHg), grade 1 (90–99 mmHg), and grade 2 (100–109 mmHg) (36, 37). Mielke et al. (38) concluded that SBP measurements greater than 160 mmHg were associated with greater cognitive impairment in the elderly, which may lead to AD. Similarly, according to Launer et al. (23), elevated midlife SBP > 160 mmHg and DBP ≥ 90 mmHg were particularly associated with an increased risk of AD.
Furthermore, longitudinal (39, 40) and cross-sectional (41, 42) studies have been used to identify risk factors and elucidate some characteristics of AD. To this end, we aggregated data from longitudinal and cross-sectional studies and used meta-analytic equation modeling to test for causal relationships. One major advantage of meta-analytic equations is that it allows an integration of the given data from all studies into one model and specify models that have not been tested in the primary studies (43).
Based on the results and evidence of other articles and meta-analyses, we aimed to perform a meta-meta-analysis of longitudinal and cross-sectional studies to test the association between BP (high SBP and high DBP) and the risk of AD. We also aimed to pool findings separately from cross-sectional and longitudinal studies and assess the effect of BP on the risk of subsequent diagnosis of AD.
The search was restricted to meta-analyses assessing high SBP and DBP and AD. We applied the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (44). The literature searches were carried out in five electronic databases, including ISI Web of Science, Scopus, PubMed, Elsevier Science Direct, and Google Scholar. No publication date was set. The list of keywords was generated through a system of successive approximations: “blood pressure” and “Alzheimer's disease” and “meta-analysis.” A Google Scholar search was also performed but was limited to the title. The literature search was carried out in English and Spanish.
The procedures applied to carry out this meta-meta-analysis were as follows: (1) search and selection of meta-analyses assessing high SBP and DBP and AD and (2) selection of primary studies contained in the meta-analyses and the deletion of duplicates.
Meta-analyses and primary studies that met each of the following criteria were selected: (1) meta-analysis and primary studies that measured the relationship between hypertension (high SBP and DBP) and the risk of AD; (2) meta-analysis and primary studies reported data that allowed the estimation of a pooled effect size; (3) meta-analysis and primary studies that diagnosed AD through clinical examination, using defined diagnostic criteria, DSMV (9) and NINCDS-ADRDA (45); (4) meta-analysis and primary studies that reported the sample size; and (5) meta-analysis and primary studies written in English or Spanish.
To avoid bias in eligible studies, all abstracts were independently reviewed by two investigators (O.S. and A.P.). After excluding all irrelevant abstracts, the remaining articles were analyzed, and data precision was examined in detail. In meta-analysis where relevant data were lacking (k = 1), the authors were contacted to request additional data to be subsequently added to the meta-analysis. Then, duplicate reports were excluded to pool the primary studies. After all meta-analyses and primary studies were selected, a third researcher independently extracted the highlighted data (S.U.). Information on all data collected from the primary studies included in the meta-analysis is presented in the Supplementary Table 1.
The qualities of the meta-analyses were independently coded by two co-authors using the 11-item Assessment of Multiple Systematic Reviews (AMSTAR) tool (46), which has been shown to have a good inter-rater agreement, reliability, and content validity (46, 47). Total scores for the meta-analyses were calculated as the sum of the 11 items on a binary scale. Quality classifications were set as low quality (0–4), moderate quality (5–8), and high quality (9–11).
Initially, we reported the associations between hypertension and AD for each primary study included in the previous meta-analysis (see Supplementary material).
Then, for this review of meta-analyses, first, we calculated the cumulative incidence ratio [or log risk ratio (LnRR)] of AD for both SBP and DBP for each primary study. Second, we identified separate effect sizes for SBP and DBP measurements and their relationships with the risk of AD. Third, study outcomes were grouped according to the definition of BP (SBP or DBP) and the measurement of hypertension established by the ISH: (1) SBP: elevated (130–139 mmHg), grade 1 (140–159 mmHg), and grade 2 (160–179 mmHg), and (2) DBP: elevated (85–89 mmHg), grade 1 (90–99 mmHg), and grade 2 (100–109 mmHg) (36, 37). Heterogeneity between study samples was assessed using Cochran's Q statistic (48). The I2 statistic was calculated to express the fraction of variation between studies that was due to heterogeneity. The I2 statistic explains the percentage of variance in the observed effects due to variance in the true effects. An I2 value < 25% was considered low heterogeneity, between 25 and 50% was considered moderate heterogeneity, and >50% was considered high heterogeneity (48). Statistical significance was set at p ≤ 0.05. Data were analyzed using Comprehensive Meta-Analysis version 3.1 (Biostat Inc, NJ, USA) (49). Additionally, to test for the possibility of publication bias, we computed the Egger regression test. Results revealed no evidence for a publication bias (50).
For each primary study included in the meta-analysis, we calculated the following (see Table 1): (a) k or number of studies, (b) effect size, (c) 95% confidence interval (95%CI) of the effect, and (d) p (two-tailed significance) (55). We used a random-effect model for the calculation of pooled effect estimates. Then, to assess the heterogeneity of our results, subgroup analyses were performed to examine the differential effects of type of BP: (1) SBP, (2) DBP, and (3) BP (total) on the risk of AD. We did not assume a common among-study variance component across subgroups. High-resolution forest plots were also developed separately with random effects.
Additionally, moderating variables were selected based on substantive considerations and the availability of data across studies included in the meta-analysis. We anticipated interstudy heterogeneity as there was some variation between studies according to the study design (longitudinal k effect size = 29 vs. cross-sectional k effect size = 46) and the measures of SBP (>140 mmHg k effect size = 52 and >160 mmHg k effect size = 8) and DBP (>85 mmHg k effect size = 2 and >90 mmHg k effect size = 9). Finally, we also considered whether age at exposure assessment (early age of onset ≤ 65 k effect size = 39 vs. late age of onset or ≥65 k effect size = 36) could account for heterogeneity in associations. When possible, we used separate summary measures for early- and late-life measures of BP. Otherwise, BP in early life or late life was defined according to the mean of age. Moreover, we also analyzed the sex (male or female) in the different BP measurements. In the same line, we also analyzed the continent where the sample was recruited (Europe, Asia, and North America) in the different BP measurements.
A total of 214 studies were identified from major databases: 61 in ISI Web of Science, 55 in Scopus, 17 in PubMed, 79 in Elsevier Science Direct, and 2 in Google Scholar. In total, 189 articles were excluded from this review for various reasons: (a) k = 89 were duplicates and (b) k = 100, in which no information was provided on the relationship between BP and AD.
A total of 25 meta-analyses were eligible for inclusion in this review of meta-analyses. Of these meta-analyses, 20 were excluded: (a) k = 14 studies were duplicated data; (b) k = 2 were systematic reviews about other issues; (c) k = 2 aimed to study the effect of antihypertensives on AD; and (d) k = 2 aimed to study genetic factors (Figure 1).
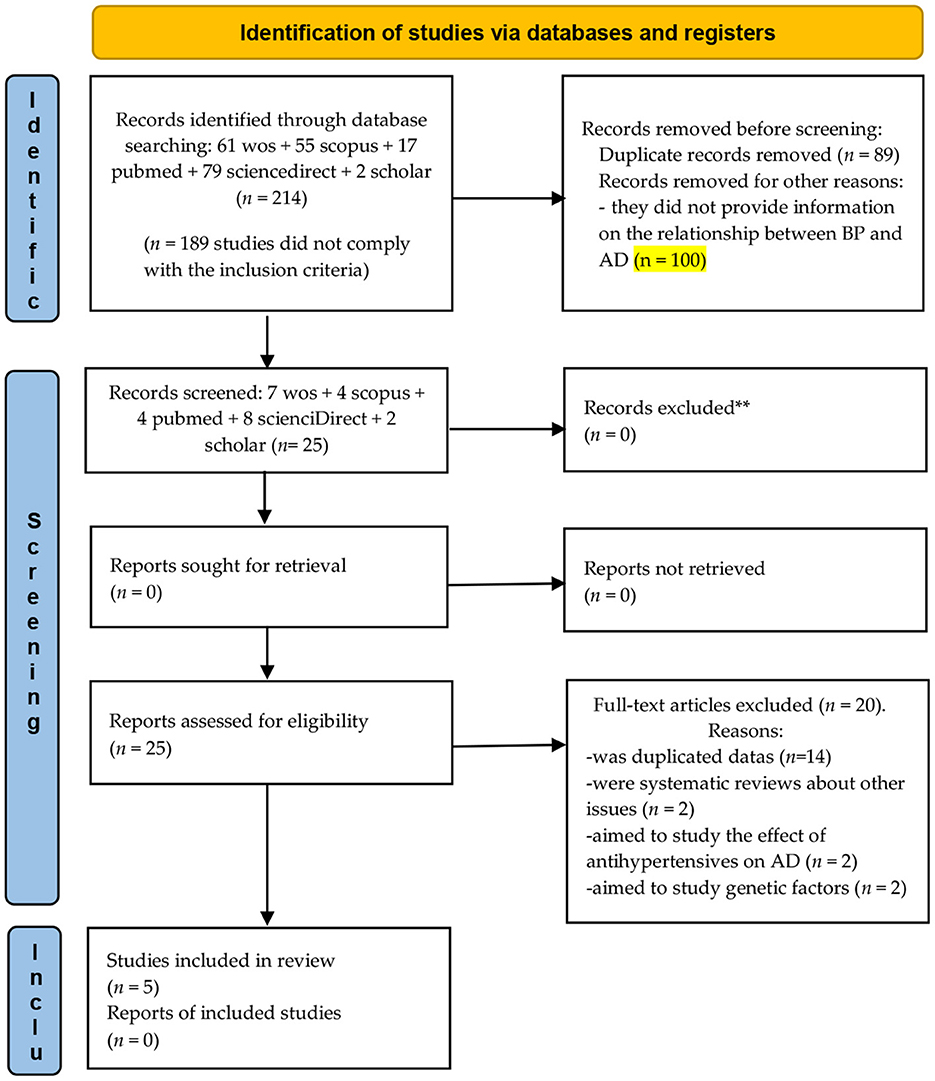
Figure 1. Flowchart depicting the selection of articles for our meta-analysis. From Page et al. (56).
Table 1 summarizes key features of the included primary diagnosis, design, number of primary studies, regions of origin of the study, sample size, gender, mean age, results, effect sizes of the relationships between BP and AD, and AMSTAR scores. Although the meta-meta-analyses were based on the criteria established by ISH, the studies only showed values for the following cutoff points: SBP (>140 mmHg and >160 mmHg) and DBP (>85 mmHg and >90 mmHg). Eggers' test was not significant: the intercept (B0) is 0.47, Se = 0.28, 95%CI (−0.09, 1.04), with t = 1.65, df = 73, indicating no publication bias.
A total of 75 effect sizes were extracted from a total of five meta-analyses that included k = 52 primary studies. Also, 60 effect sizes provided information about high SBP and risk of AD (80%); k = 11 about high DBP (14.7%); and k = 4 about the combined effect (5.3%) (Supplementary Table 1).
For the pooling LnRR analysis, we analyzed primary studies. The total effect size was LnRR = 0.07, Se = 0.02 (0.031, 0.125), Z = 3.27, p = 0.001, and heterogeneity was high (Qb = 415.56, df = 74, p = 0.0000; I2 = 82.19). These findings suggest that heterogeneity of effect may be present in some analyses.
Four meta-analyses examined the relationship between high SBP and AD. The meta-analyses carried out by Lennon et al. (22) (k = 11 effect sizes; N = 7,666; n = 1,520 participants with AD and high SBP; nHC = 6,146 HC participants), Xu et al. (51) (k = 40 effect sizes; N = 1,443,213; n = 17,113 participants with AD and high SBP; n = 1,426,100 HC participants), Meng et al. (52) (k = 1 effect size; N = 786; n = 79 participants with AD and high SBP; n = 707 HC participants), and Wang et al. (54) (k = 8 effect sizes; N = 5,885; n = 385 participants with AD and high SBP; n = 5,500 HC participants) compared HC and AD subjects with high SBP. Only two of them (22, 52) found significant associations between high SBP and the risk of AD (Figures 2–4).
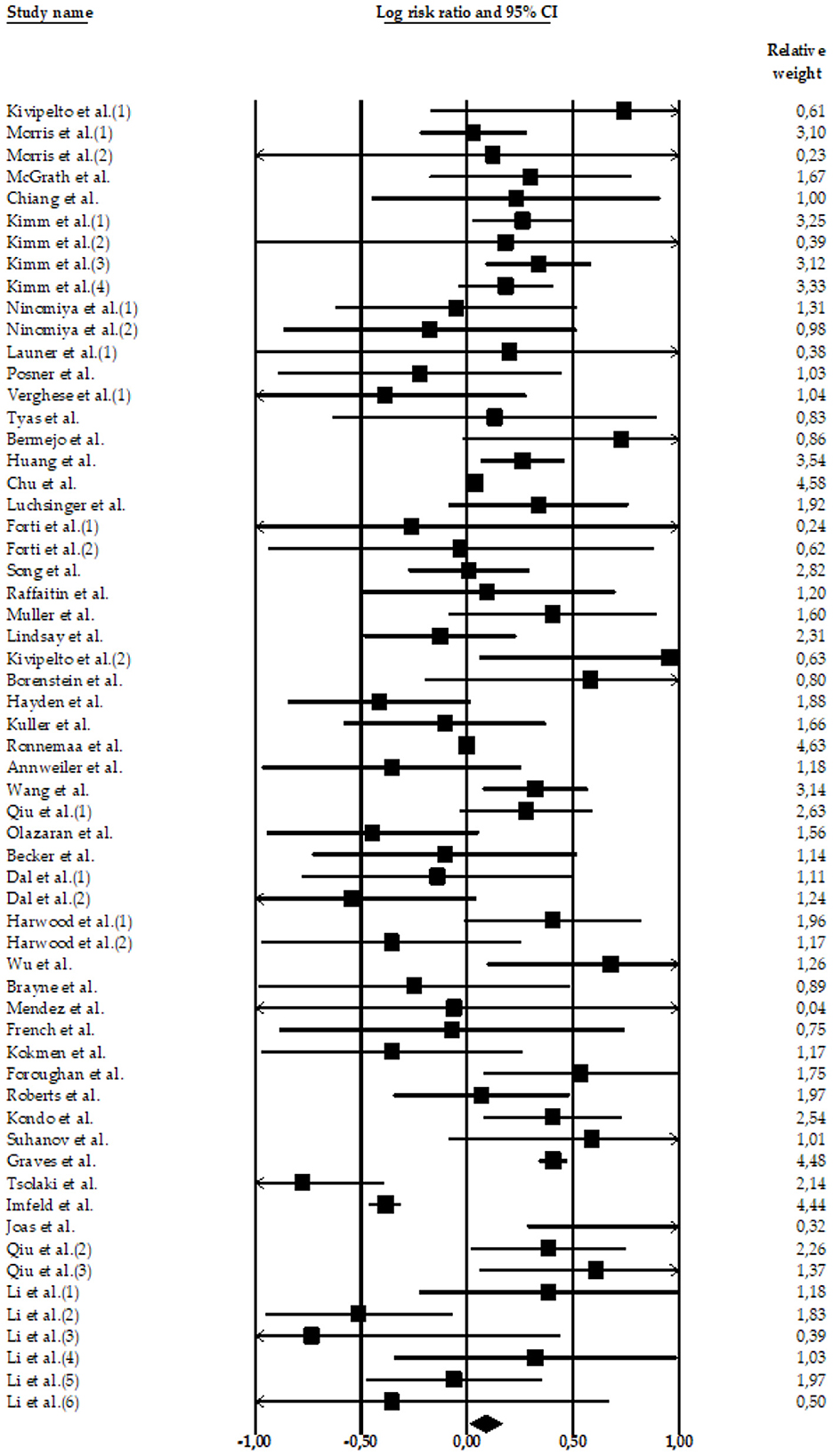
Figure 2. Forest plot of the meta-analysis of incidence rates of AD in participants with high SBP. Individual and pooled estimates of the association between measures of hypertension and AD. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
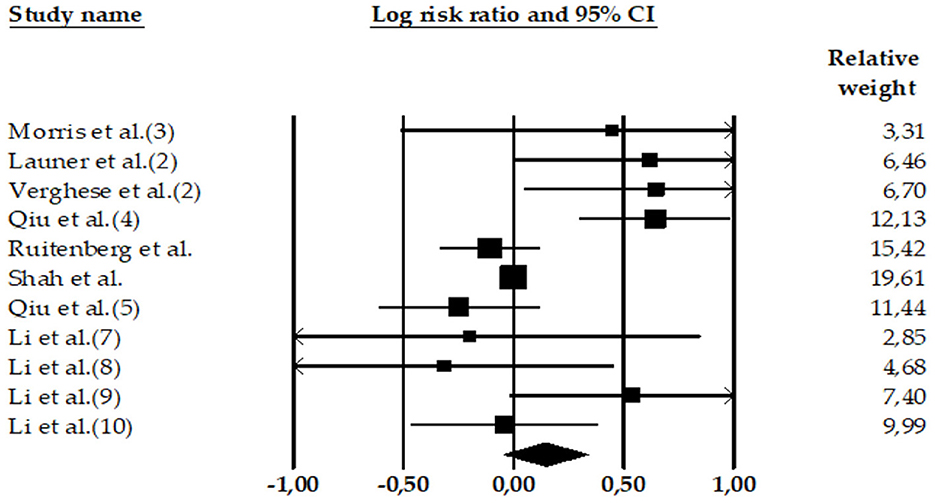
Figure 3. Forest plot of the meta-analysis of incidence rates of AD in participants with high DBP. Individual and pooled effect estimates of the association between DBP hypertension and AD. The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
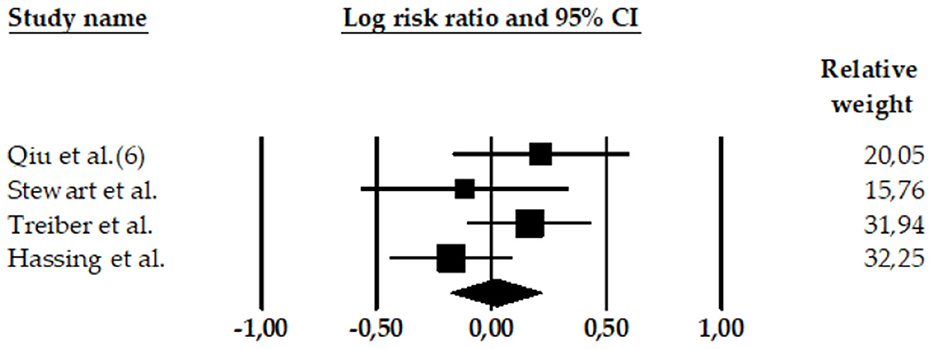
Figure 4. Forest plot of the meta-analysis of rates of AD in participants with high BP (high SBP and high DBP). The size of the box representing the point estimate for each study in the forest plot is proportional to the contributing weight of that study estimate to the summary estimate.
The total random effect of the high SBP value was k = 60 effect sizes; N = 1,457,550 participants; nAD = 19,097 participants; nHC = 1,438,453 (LnRR = 0.09, 95%CI = 0.013–0.166, Z = 2.28, p = 0.022) (see Table 2). The heterogeneity was high: Q-value= 380.08, df = 59, and I2 = 84.
Three meta-analyses showed the relationship between DBP and AD: Lennon et al. (22) (k = 1 effect size; N = 378; n = 78 with AD and high DBP; n = 300 HC participants), Xu et al. (51) (k = 5 effect sizes; N = 12,225; n = 497 with AD and high DBP; n = 11,728 HC participants), and Wang et al. (54) (k = 5 effect sizes; N = 7,745; n = 306 with AD and high DBP; n = 7,439 HC participants). None of the three meta-analyses show significant associations between high DBP and AD.
Consistently, our results (k = 11 effect sizes; N = 20,348; nAD = 881; HC = 19,467) did not find an association between high DBP and the risk of AD (LnRR = 0.15, 95% CI = −0.045 to 0.338, Z = 1.50, p = 0.133) (see Table 3). The heterogeneity was high: Q-value = 29.99, df = 10, and I2 = 66.65.
A meta-analysis reported a combined effect size for high SBP and high DBP (97). This study (k = 4 effect sizes; N = 7,494; n = 211 with AD and high SBP/DBP; n = 7,283 HC participants) found a non-significant association between high SBP and high DBP and AD (LnRR = 0.02, 95% CI = −0.179 to 0.222, Z = 0.21, p = 0.835) (see Table 4). The heterogeneity was medium: Q-value = 4.52, df = 3, and I2 = 33.69.
Results of the subgroup analysis on the primary outcomes are presented in Table 5. Study outcomes were grouped by definition of hypertension and measures of BP (e.g., SBP, DBP, or total BP). Notably, 60 effect sizes examined SBP at both grades (22): 52 effect sizes examined only grade 1 (>140 mmHg) (51, 54) and 8 effect sizes examined only grade 2 (>160 mmHg) (53). Eleven effect sizes examined DBP at both grades: 2 effect sizes examined DBP using a cutoff point of >85 mmHg (51, 54) and 9 effect sizes >90 mmHg. Four effect sizes combined both types of hypertension (53). Moderator analyses were performed comparing effect sizes according to sex (men and women), age (≤ 65 and ≥66), study design (cross-sectional or C and longitudinal or L), and regions (Europe, Asia, and North America).
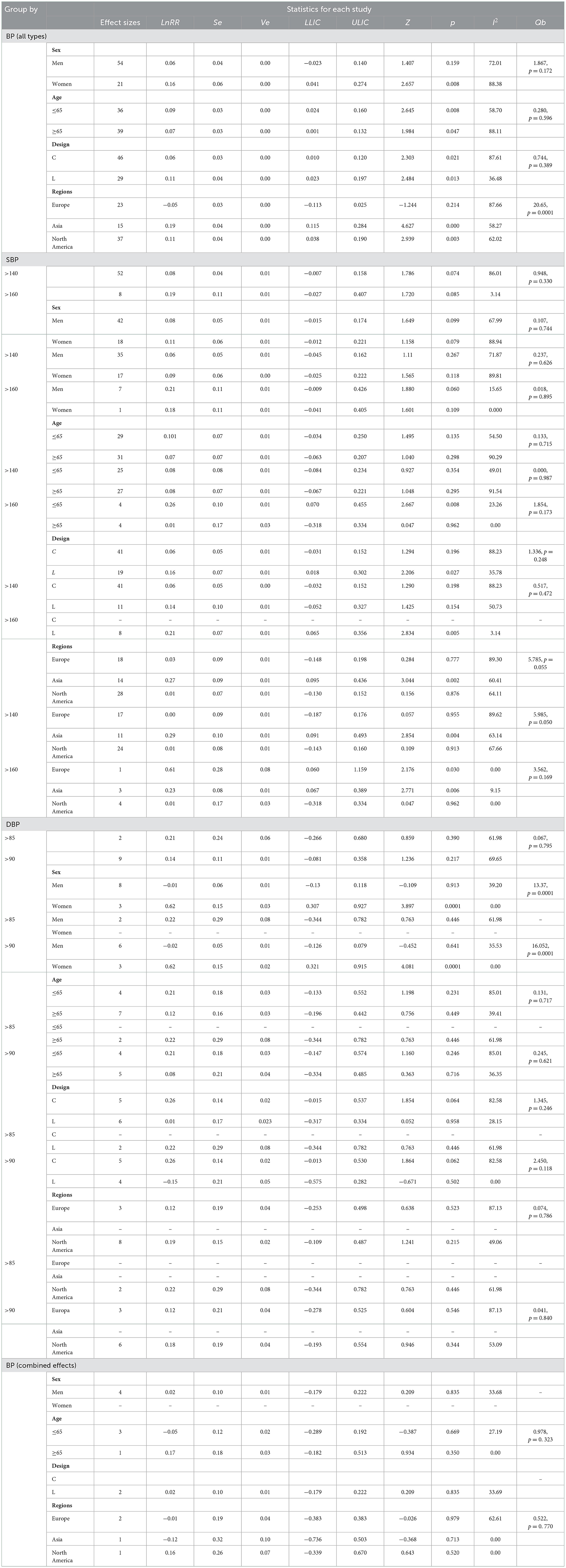
Table 5. Effects of sex, age, design, and regions in different types of SBP (>140 and >160 mmHg) and DBP (>85 and >90 mmHg).
The results of pooling studies that reported RRs for a total score of BP showed that sex, age, and design did not moderate the relationship between hypertension and AD risk (Qb: p ≤ 0.50). These results indicate that the risk of AD in participants with hypertension did not change significantly according to sex, age, and study design groups. However, it can be observed that there are significant relationships between different categories of the variables such as sex, age, study design, and AD (Z: p ≤ 0.50). Findings revealed a significant relationship only between being women and a greater risk of AD (p = 0.008). Age was also associated with increased risk of AD in early (p = 0.008) and late (p = 0.047) age of onset, and this association was also significant in cross-sectional (p = 0.021) and longitudinal (p = 0.013) studies. Regions moderated the association between BP and AD. The risk of AD was greater in studies that used samples from Asia and North America than those performed in Europe.
Results did not find significant differences in the risk of AD according to the measures of SBP (>140 and >160 mmHg) and DBP (>85 and >90 mmHg). Similarly, sex, age, design, and region did not moderate the relationship between SBP and DBP and the risk of AD, except sex in the case of DBP. Results found that women showed a stronger risk of developing AD than men. It is also observed that only in longitudinal studies and Asia regions, significant associations were found between SBP and AD.
According to measures of SBP (>140 and >160 mmHg), results indicated that SBP had no significant differences in effect sizes on the risk of AD at different sexes, ages, and designs. However, for SBP > 140 mmHg, there was evidence of heterogeneity between regions in RRs of AD. Asian countries showed stronger effect sizes between SBP and risk of AD than European and North American countries. Also, results found that elevated SBP (>160 mmHg) was significantly associated with AD risk in the young elderly (≤ 65), longitudinal studies, and in Europa and Asia.
For DBP (>85 and >90 mmHg), there was evidence of heterogeneity between the sexes. Women with elevated DBP (>90 mmHg) showed a greater risk of AD than men. Furthermore, there were no significant differences in AD risk according to age, design, and region.
Finally, age and region did not moderate the relationship between the combined effects of BP and the risk of AD.
This study analyzes the association between high BP and the risk of AD. This is the first study to evaluate this relationship by identifying previous meta-analyses and analyzing primary studies worldwide. The present study summarized the information on meta-analyses of hypertension (DBP and SBP) and AD and expanded the findings from individual studies. In this study, 52 primary studies and 75 effect sizes were extracted. Furthermore, we included some moderator variables between high DBP and high SBP and AD, such as sex, age, study design, regions, and measures of SBP and DBP.
Overall, results suggest that hypertension is associated with an increased risk of AD (RR = 1.08, 95%CI: 1.032, 1.13, Z = 3.273, p = 0.001). It indicates that the risk of AD increases by 8% for patients with SBP.
In this study, 46 primary studies and 60 effect sizes extracted from four meta-analyses (22, 51–53) confirm the relationship between high SBP and AD (RR = 1.09, 95%CI: 1.013, 1.181, Z = 2.285, p = 0.022). These results indicate that participants with high SBP increase the rate risk of AD by 9% and support findings of previous studies, suggesting that there were consistent demonstrations of a relationship between SBP and the risk of developing AD. In this vein, research demonstrated that high SBP could increase the risk of AD since it could cause neurobiological alterations (deposits of beta-amyloid protein), which lead to lesions in the brain, such as cerebral atrophy, senile plaques, and neurofibrillary tangles, which could be explanatory factors of the development of AD (103, 104). Other studies also suggest that high SBP could cause brain vascular injury, leading to increased flow of blood, cerebral patency, and cerebral amyloid angiopathy which were also associated with a higher risk of AD (105–107). However, our analysis cannot underlie the pathophysiology of AD and could only define SBP as a risk factor.
The relationship between high DBP and AD was studied through k = 8 primary studies and eleven effect sizes (three meta-analyses) (22, 51, 54). Findings did not find a significant association between high DBP and the risk of AD. Nevertheless, according to previous studies, these results could be explained by confounding due to associations between BP and advanced disease or other unknown modifiable risk factors (108–110). For instance, secondary diseases, such as obesity, cardiovascular diseases, silent infarcts, and vascular risk factors (111) or type 2 diabetes (103, 108, 109), could be closely related to the development of AD. Hence, in these cases, it is not clear if hypertension is directly related to the risk of AD or whether AD is indirectly motivated by a secondary disease (110). Finally, there was a small number of studies analyzing DBP and AD in comparison with SBP, and in consequence, it is possible that we did not have sufficient statistical power to obtain a significant pooled estimate of the association between DBP and AD.
Related to the combined BP hypertension, only a meta-analysis (53) with four independent studies and effect sizes compared the incidence of AD between subjects with and without hypertension. These studies found that high BP is not associated with an increased risk of AD. This result is contradictory to the general view on the association between risk for AD and hypertension. For example, Guan et al. (53) highlighted that AD and hypertension are independent diseases with some common etiopathogenesis, which is a risk factor in AD.
To explore the influence of other research parameters in the relationship between high SBP and high DBP with AD, we analyzed different moderators: sex, age, study design, and region. This study does not find differences in the risk of AD according to the type of measure of SBP (>140 and >160 mmHg) and DBP (>85 and >90 mmHg). Total scores reveal significant differences between men (RR = 0.99, 95%CI: 0.887, 1.125, Z = −0.109, p = 0.913) and women (RR = 1.85, 95% CI: 1.359, 2.527, Z = 3.897, p = 0.001) (rate risk of AD increases by 85%) in the relationship of high DBP and AD, but not between SBP and AD. Specifically, the data suggest that women with high DBP (>90 mmHg) had an increased risk of AD compared with men (RR = 1.86, 95%CI: 1.379, 2.498, Z = 16.05, p = 0.001), which increase the rate risk of AD by 86%. These results have been shown in previous studies that worked with different samples (women and men), where AD was also associated with high DBP mainly in women (107, 108). For instance, Benetos et al. (112) found that DBP in women is associated with a higher cardiac output, pulse pressure, and heart rate (HR) factors that are related to a higher risk of AD (63.8%).
Total scores of BP show that age is associated with increased risk of AD in the early and late age of onset (RR = 1.10, 95%CI: 1.024, 1.174, Z = 2.645, p = 0.008; RR = 1.07, 95%CI: 1.001, 1.141, Z = 0.047, p = 0.047), with the rate risk of AD increases by 10% and 7%. However, the age of onset (early onset ≤ 65 years and late onset ≥65 years) does not moderate the relationship between high SBP/DBP and AD, showing similar effect sizes for both categories. Related to the measure of BP, this study found that elevated SBP > 160 mmHg was associated with the risk of AD in the young elderly (≤ 65 years), but not in those ≥65 years of age. In this vein, several studies have found that hypertension has different impacts on cognitive function at different ages (19, 22, 110). Current literature indicates that hypertension is a risk factor for cognitive decline in midlife and young old age but may be protective against cognitive decline in late life (22). For example, some authors concluded that high BP at the early age of onset impacted cognitive functions and increased the risk of developing AD in older age (19, 113). Iadecola et al. (114) also found that hypertension in early onset is associated with a higher risk of AD. Therefore, changes in BP may be due to hemodynamic regulation being altered by neurodegenerative processes in the years preceding disease onset (22).
The only variable that moderates the relationship between BP and AD is the region. We observe a higher risk of AD in Asia with SBP >140 mmHg (RR = 1.34, 95%CI: 1.096, 1.637, Z = 2.854, p = 0.004) compared with European (RR = 0.99, 95%CI: 0.829, 1.193, Z = −0.057, p = 0.955) and North America (RR = 1.01, 95%CI: 0.866, 1.174, Z = 0.109, p = 0.913). Therefore, the rate risk of AD in Asia increases by 34%. These results are related to the findings of some studies. During the past four decades, the highest BP measurements worldwide have shifted from high-income countries to low-income countries, such as South Asia and Africa (115), which could explain our results (116, 117). On the one hand, several authors suggest that recent lifestyle changes in Asia countries, such as diet, changing demographics, urbanization, environmental interactions, and other factors, may help explain this relationship (117). On the other hand, one study with data from 90 countries showed that the percentage of people with hypertension receiving treatment increased in both high-income and low- and middle-income countries, but the gap between them widened (118). Moreover, our results also show that the risk of AD related to SBP > 160 mmHg in Europe (RR = 0.61, 95%CI: 0.060, 1.159, Z = 2.176, p = 0.030) and Asia (RR = 0.23, 95%CI: 0.067, 0.389, Z = 2.771, p = 0.006) is significant. However, North America (RR = 0.01, 95%CI: −0.318, 0.334, Z = 0.047, p = 0.962) did not find a significant relationship. Despite these results, the strength of the association between SBP (>160 mmHg) and AD risk is similar in the three regions.
Finally, results do not find differences in the effect size of the association between high SBP and DBP and the risk of AD according to the type of design (cross-sectional and longitudinal). Our results found an association between BP and the risk of AD in both types of studies. However, findings confirm that the relationship between higher SBP and AD is only significant in longitudinal studies and with SBP > 160 mmHg (RR = 1.23, 95%CI: 1.067, 1.428, Z = 2.834, p = 0.005), so the rate risk of AD increases by 23%, while high DBP (>85 and >90 mmHg) is not related to increased AD risk. In this vein, previous work found differences according to the type of design that may result in part from the use of different definitions of hypertension and non-uniform measures of high or low BP. In this study, we use standardized criteria to define BP (SBP > 140/160 mmHg and DBP > 85/90 mmHg) and AD (clinical criteria) which could explain that there are no differences according to the study design. After controlling for this confounding factor, the effect size of longitudinal studies is higher in all the BP and SBP measures, although the differences do not reach significance. Longitudinal studies provide an opportunity to assess the temporal relationship between BP and AD and the length of follow-up remains relevant since hypertension could render individuals more vulnerable to comorbid conditions, such as cerebrovascular disease, that confer greater risk for AD during long periods of follow-up.
However, there are some limitations to our study. The key limitation is that only a small number of studies examined the association between DBP, both types of BP combined, and AD compromising the generalizability of the results. Furthermore, it is likely that due to the procedure used in this meta-meta-analysis, some primary studies were not included. Another challenge was that studies reported outcomes using different metrics (OR, HR, and RR). Likewise, not all the cutoff points established by ISH could be analyzed since the stages of SBP ≥ 130–139 and DBP ≥ 100 could not be defined due to the lack of primary studies. Other confounders may also influence the study's findings. For example, results were not adjusted for other risk variables including cardiovascular disease, stroke, alcohol consumption, smoking, kidney disease, and many others. Also, two studies did not report the mean age of the sample, and they were not included in the moderator analysis. Moreover, the relationship between hypertension and AD could not be thought of as binary but rather as a dynamic one, changing with life stage and disease state. Hence, a single measurement of BP may not accurately reflect the participant's average BP measurements. Additionally, data on the age at the onset of hypertension and years of living with the condition may be important in clarifying temporal relationships between hypertension and AD. Also, we did not examine the potentially modifying impact of antihypertensive therapy on the relationship between hypertension and AD. In addition, another limitation is the absence of studies from South America and Australia. Finally, we did not include educational level as a moderator variable since the external validity of some of the results has been questioned. The primary studies contained in this meta-analysis used very different forms of measurement. For instance, some studies analyzed education using individual (i.e., no formal education, mandatory education, secondary studies, university studies) (79, 88) or community-based samples (i.e., family education level, region, or country) (80, 88), quantitative (linear relation between the number of years of education and the risk of dementia) (81, 83) or qualitative measures (a threshold effect at a given level of education) (86), and composite measures (i.e., socioeconomic status, SES defines education plus income) (67, 119) that show different results. Therefore, we should interpret our results cautiously.
Several strengths of our review of a meta-analysis should be emphasized. First, most prior studies were drawn from general community samples or non-AD-specific studies (vascular dementia, cortical dementia, or dementia in general), whereas the current study relied on AD. Second, we add to the current literature by analyzing 52 primary studies extracted from the previous meta-analysis increasing the statistical power of our results. Third, we analyzed the impact of different moderators (sex, age, study design, region, and measures of SBP/DBP) to explore the influence of other research parameters in the relationship between high SBP and DBP and AD. Finally, we want to focus on effect sizes since the statistical significance should never be interpreted as evidence that an effect had clinical importance. It is important to note that the effect sizes were “relatively small” and the variation is great within the same meta-analysis. Therefore, the clinical significance and practical importance of these results should be considered in relation to the patient's status, goals, and clinician experience.
As a practical implication, this study suggests that high SBP could be a risk factor for AD. There is limited evidence that single cardiovascular risk factors affect AD risk, but the strength of the association is influenced greatly by changing the parameters of the risk factors and in particular by identifying interactions between the factors. Future research should confirm this and determine whether stabilizing BP might be a target to slow or decline the development of AD.
This study analyzes the association between SBP/DBP/combined BP and the risk of developing AD. A total of five meta-analyses and 52 primary studies were analyzed in this review of meta-analysis. Our study found that SBP is associated with an increased risk of AD by 11%, although no association was found for DBP. Measures of SBP >140, SBP >160, DBP >85, and DBP >90 do not moderate the relationship between SBP and DBP and AD. Moderator analysis (sex, age, study design, region, and measures of SBP/DBP) shows a significant association between high DBP (>90) and AD in women. The age of onset (early-onset AD ≤ 65 years and late-onset AD or senile AD ≥65 years) did not moderate the relationship between SBP and DBP and AD. Finally, regarding the type of study, there were no differences in the association between BP and AD between longitudinal and cross-sectional studies. However, Asian countries showed stronger effect sizes between SBP > 140 and risk of AD than European and North American countries. Future work should use other uncontrolled factors (e.g., cardiovascular diseases, diabetes, and stroke) to explain the relationship between high BP and AD.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
OS-V and AP-M conceived and designed the analysis, collected the data, contributed data or analysis tools, performed analysis, and wrote the paper. JP-B wrote the paper. SU-L contributed data or analysis tools, performed analysis, and wrote the paper. All authors take full responsibility for the data, the analyses and interpretation, and the conduct of the research, full access to all of the data and the right to publish any and all data. All authors contributed to the article and approved the submitted version.
This study was partially funded by grant Y133GI awarded by the University of Burgos (Spain) to the Social Inclusion and Quality of Life (SIQoL) research group. This work was partially supported by FEDER funds and the Spanish State Research Agency (Projects PID2019-104263RB-C44 and PDC2021-121021-C22) and the Regional Government of “Castilla y León” and FEDER funds (Project BU056P20).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1065335/full#supplementary-material
1. Han H-F, Yen H-C, Wu H-C, Tan H-Y, Xu W, Jiang H-S, et al. Ultrasensitive detection of Alzheimer's amyloids on a plasmonic-gold platform. ACS Appl Mater Interfaces. (2021) 13:57036–42. doi: 10.1021/acsami.1c19157
2. Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. (2009) 11:111–28. doi: 10.31887/DCNS.2009.11.2/cqiu
3. Bhushan I, Kour M, Kour G, Gupta S, Sharma S, Yadav A. Alzheimer's disease: causes and treatment—a review. Ann Biotechnol. (2018) 1:1002. doi: 10.33582/2637-4927/1002
4. Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. (2007) 3:186–91. doi: 10.1016/j.jalz.2007.04.381
5. Satizabal CL, Beiser AS, Chouraki V, Chêne G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N Engl J Med. (2016) 374:523–32. doi: 10.1056/NEJMoa1504327
6. Gottesman RT, Stern Y. Behavioral and psychiatric symptoms of dementia and rate of decline in Alzheimer's disease. Front Pharmacol. (2019) 10:1062. doi: 10.3389/fphar.2019.01062
7. Vickers JC, Mitew S, Woodhouse A, Fernandez-Martos C, Kirkcaldie MT, Canty AJ, et al. Defining the earliest pathological changes of Alzheimer's disease. Curr Alzheimer Res. (2016) 13:281–7. doi: 10.2174/1567205013666151218150322
8. Reisberg B, Jamil IA, Khan S, Monteiro I, Torossian C, Ferris S, et al. Staging dementia. Princ Pract Geriatr Psychiatry. (2010) 31:162–9. doi: 10.1002/9780470669600.ch31
9. American Psychiatric Association (APA). Diagnosis and Statistical Manual of Mental Disorders Fifth Edition, Text Revision (DSM-5-TR). (2022). Washington, DC: APA. doi: 10.1176/appi.books.9780890425787
10. Groves WC, Brandt J, Steinberg M, Warren A, Rosenblatt A, Baker A, et al. Vascular dementia and Alzheimer's disease: is there a difference? J Neuropsychiatry Clin Neurosci. (2000) 12:305–15. doi: 10.1176/jnp.12.3.305
11. Epperly TD, Dunay MA, Boice JL. Alzheimer disease: pharmacologic and nonpharmacologic therapies for cognitive and functional symptoms. Am Fam Physician. (2017) 95:771–8.
12. Orona CJ. Temporality and identity loss due to Alzheimer's disease. Soc Sci Med. (1990) 30:1247–56. doi: 10.1016/0277-9536(90)90265-T
13. Guarino A, Favieri F, Boncompagni I, Agostini F, Cantone M, Casagrande M. Executive functions in Alzheimer disease: a systematic review. Front Aging Neurosci. (2019) 10:437. doi: 10.3389/fnagi.2018.00437
14. Deutsch LH, Bylsma FW, Rovner BW, Steele C, Folstein MF. Psychosis and physical aggression in probable Alzheimer's disease. Am J Psychiatry (1991) 148, 1159–63. doi: 10.1176/ajp.148.9.1159
15. Giebel CM, Sutcliffe C, Challis D. Activities of daily living and quality of life across different stages of dementia: a UK study. Aging Ment Health. (2015) 19:63–71. doi: 10.1080/13607863.2014.915920
16. Castelli WP, Wilson PWF, Levy D, Anderson K. Cardiovascular risk factors in the elderly. Am J Cardiol. (1989) 63:12–9. doi: 10.1016/0002-9149(89)90110-0
17. Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Enfermedad de Alzheimer. Lancet. (2011) 377:1019–31. doi: 10.1016/S0140-6736(10)61349-9
18. Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Midlife vascular risk factors and Alzheimer's disease in later life: longitudinal, population based study. BMJ. (2001) 322:1447–51. doi: 10.1136/bmj.322.7300.1447
19. Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. (2005) 4:487–99. doi: 10.1016/S1474-4422(05)70141-1
20. Marfany A, Sierra C, Camafort M, Domenech M, Coca A. High blood pressure, Alzheimer disease and antihypertensive treatment. Panminerva Med. (2018) 60:8–16. doi: 10.23736/S0031-0808.18.03360-8
21. Silva JAD. Caracterização das abordagens farmacológicas usadas no tratamento das demências-análise de casos do CHCB (Doctoral dissertation) (2014).
22. Lennon MJ, Makkar SR, Crawford JD, Sachdev PS. Midlife hypertension and Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. (2019) 71:307–16. doi: 10.3233/JAD-190474
23. Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, et al. Midlife blood pressure and dementia: the Honolulu-Asia aging study? Neurobiol Aging. (2000) 21:49–55. doi: 10.1016/S0197-4580(00)00096-8
24. Posner HB, Tang M-X, Luchsinger J, Lantigua R, Stern Y, Mayeux R. The relationship of hypertension in the elderly to AD, vascular dementia, and cognitive function. Neurology. (2002) 58:1175–81. doi: 10.1212/WNL.58.8.1175
25. Morris MC, Scherr PA, Hebert LE, Glynn RJ, Bennett DA, Evans DA. Association of incident Alzheimer disease and blood pressure measured from 13 years before to 2 years after diagnosis in a large community study. Arch Neurol. (2001) 58:1640–6. doi: 10.1001/archneur.58.10.1640
26. Miyoshi K. What is 'early onset dementia'? Psychogeriatrics. (2009) 9:67–72. doi: 10.1111/j.1479-8301.2009.00274.x
27. Launer L, White L, Petrovitch H, Ross G, Curb J. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. (2001) 57:1447–52. doi: 10.1212/WNL.57.8.1447
28. Guo Z, Viitanen M, Fratiglioni L, Winblad B. Low blood pressure and dementia in elderly people: the Kungsholmen project. BMJ. (1996) 312:805–8. doi: 10.1136/bmj.312.7034.805
29. Euser SM, Van Bemmel T, Schram MT, Gussekloo J, Hofman A, Westendorp RG, et al. The effect of age on the association between blood pressure and cognitive function later in life. J Am Geriatr Soc. (2009) 57:1232–7. doi: 10.1111/j.1532-5415.2009.02264.x
30. Gillis EE, Sullivan JC. Sex differences in hypertension: recent advances. Hypertension. (2016) 68:1322–7. doi: 10.1161/HYPERTENSIONAHA.116.06602
31. Anstey KJ, Peters R, Mortby ME, Kiely KM, Eramudugolla R, Cherbuin N, et al. Association of sex differences in dementia risk factors with sex differences in memory decline in a population-based cohort spanning 20-76 years. Sci Rep. (2021) 11:7710. doi: 10.1038/s41598-021-86397-7
32. Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. (2018) 31:1247–54. doi: 10.1093/ajh/hpy148
33. Zhou B, Danaei G, Stevens GA, Bixby H, Taddei C, Carrillo-Larco RM, et al. Long-term and recent trends in hypertension awareness, treatment, and control in 12 high-income countries: an analysis of 123 nationally representative surveys. Lancet. (2019) 394:639–51. doi: 10.1016/S0140-6736(19)31145-6
34. Mills KT, Obst KM, Shen W, Molina S, Zhang H-J, He H, et al. Comparative effectiveness of implementation strategies for blood pressure control in hypertensive patients: a systematic review and meta-analysis. Ann Intern Med. (2018) 168:110–20. doi: 10.7326/M17-1805
35. Tibazarwa KB, Damasceno AA. Hypertension in developing countries. Can J Cardiol. (2014) 30:527–33. doi: 10.1016/j.cjca.2014.02.020
36. Unger T, Borghi C, Charchar F, Khan NA, Poulter NR, Prabhakaran D, et al. 2020 International society of hypertension global hypertension practice guidelines. Hypertension. (2020) 75:1334–57. doi: 10.1161/HYPERTENSIONAHA.120.15026
37. Stergiou GS, Parati G, McManus RJ, Head GA, Myers MG, Whelton PK. Guidelines for blood pressure measurement: development over 30 years. J Clin Hypertens. (2018) 20:1089–91. doi: 10.1111/jch.13295
38. Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol. (2014) 6:37. doi: 10.2147/CLEP.S37929
39. Tyas SL, Manfreda J, Strain LA, Montgomery PR. Risk factors for Alzheimer's disease: a population-based, longitudinal study in Manitoba, Canada. Int J Epidemiol. (2001) 30:590–7. doi: 10.1093/ije/30.3.590
40. Gao Y, Huang C, Zhao K, Ma L, Qiu X, Zhang L, et al. Retracted: Depression as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Int J Geriatr Psychiatry. (2013) 28:441–9. doi: 10.1002/gps.3845
41. Prins N, Den Heijer T, Hofman A, Koudstaal P, Jolles J, Clarke R, et al. Homocysteine and cognitive function in the elderly: the Rotterdam Scan Study. Neurology. (2002) 59:1375–80. doi: 10.1212/01.WNL.0000032494.05619.93
42. Nutaitis AC, Tharwani SD, Serra MC, Goldstein FC, Zhao L, Sher SS, et al. Diet as a risk factor for cognitive decline in African Americans and Caucasians with a parental history of Alzheimer's disease: a cross-sectional pilot study dietary patterns. J Prev Alzheimers Dis. (2019) 6:50–5. doi: 10.14283/jpad.2018.44
43. Lesener T, Gusy B, Wolter C. The job demands-resources model: a meta-analytic review of longitudinal studies. Work Stress. (2019) 33:76–103. doi: 10.1080/02678373.2018.1529065
44. Urrutia G, Bonfill X. PRISMA declaration: a proposal to improve the publication of systematic reviews and meta-analyses. Med Clín. (2010) 135:507–11. doi: 10.1016/j.medcli.2010.01.015
45. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's disease. Neurology. (1984) 34:939–939. doi: 10.1212/WNL.34.7.939
46. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. (2007) 7:10. doi: 10.1186/1471-2288-7-10
47. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. (2009) 62:1013–20. doi: 10.1016/j.jclinepi.2008.10.009
48. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta-Analysis. Hoboken, NJ: John Wiley and Sons (2011). p. 434.
49. Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-Analysis (CMA) Software (2007).
50. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
51. Xu W, Tan L, Wang H-F, Jiang T, Tan M-S, Tan L, et al. Meta-analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry. (2015) 86:1299–306. doi: 10.1136/jnnp-2015-310548
52. Meng X-F, Yu J-T, Wang H-F, Tan M-S, Wang C, Tan C-C, et al. Midlife vascular risk factors and the risk of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Dis. (2014) 42:1295–310. doi: 10.3233/JAD-140954
53. Guan J-W, Huang C-Q, Li Y-H, Wan C-M, You C, Wang Z-R, et al. No association between hypertension and risk for Alzheimer's disease: a meta-analysis of longitudinal studies. J Alzheimers Dis. (2011) 27:799–807. doi: 10.3233/JAD-2011-111160
54. Wang Z-T, Xu W, Wang H-F, Tan L, Tan C-C, Li J-Q, et al. Blood pressure and the risk of dementia: a dose-response meta-analysis of prospective studies. Curr Neurovasc Res. (2018) 15:345–58. doi: 10.2174/1567202616666181128114523
56. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
57. McGrath ER, Beiser AS, DeCarli C, Plourde KL, Vasan RS, Greenberg SM, et al. Blood pressure from mid-to late life and risk of incident dementia. Neurology. (2017) 89:2447–54. doi: 10.1212/WNL.0000000000004741
58. Chiang C-J, Yip P-K, Wu S-C, Lu C-S, Liou C-W, Liu H-C, et al. Midlife risk factors for subtypes of dementia: a nested case-control study in Taiwan. Am J Geriatr Psychiatry. (2007) 15:762–71. doi: 10.1097/JGP.0b013e318050c98f
59. Kimm H, Lee P, Shin Y, Park K, Jo J, Lee Y, et al. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr. (2011) 52:e117–22. doi: 10.1016/j.archger.2010.09.004
60. Ninomiya T, Ohara T, Hirakawa Y, Yoshida D, Doi Y, Hata J, et al. Midlife and late-life blood pressure and dementia in Japanese elderly: the Hisayama study. Hypertension. (2011) 58:22–8. doi: 10.1161/HYPERTENSIONAHA.110.163055
61. Verghese J, Lipton R, Hall C, Kuslansky G, Katz M. Low blood pressure and the risk of dementia in very old individuals. Neurology. (2003) 61:1667–72. doi: 10.1212/01.WNL.0000098934.18300.BE
62. Bermejo-Pareja F, Benito-León J, Louis ED, Trincado R, Carro E, Villarejo A, et al. Risk of incident dementia in drug-untreated arterial hypertension: a population-based study. J Alzheimers Dis. (2010) 22:949–58. doi: 10.3233/JAD-2010-101110
63. Huang C-C, Chung C-M, Leu H-B, Lin L-Y, Chiu C-C, Hsu C-Y, et al. Diabetes mellitus and the risk of Alzheimer's disease: a nationwide population-based study. PLoS ONE. (2014) 9:e87095. doi: 10.1371/journal.pone.0087095
64. Chu L-W, Tam S, Wong RL, Yik P-Y, Song Y, Cheung BM, et al. Bioavailable testosterone predicts a lower risk of Alzheimer's disease in older men. J Alzheimers Dis. (2010) 21:1335–45. doi: 10.3233/JAD-2010-100027
65. Luchsinger J, Reitz C, Honig LS, Tang M-X, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. (2005) 65:545–51. doi: 10.1212/01.wnl.0000172914.08967.dc
66. Forti P, Pisacane N, Rietti E, Lucicesare A, Olivelli V, Mariani E, et al. Metabolic syndrome and risk of dementia in older adults. J Am Geriatr Soc. (2010) 58:487–92. doi: 10.1111/j.1532-5415.2010.02731.x
67. Song X, Mitnitski A, Rockwood K. Nontraditional risk factors combine to predict Alzheimer disease and dementia. Neurology. (2011) 77:227–34. doi: 10.1212/WNL.0b013e318225c6bc
68. Raffaitin C, Gin H, Empana J-P, Helmer C, Berr C, Tzourio C, et al. Metabolic syndrome and risk for incident Alzheimer's disease or vascular dementia: the Three-City Study. Diabetes Care. (2009) 32:169–74. doi: 10.2337/dc08-0272
69. Muller M, Tang M-X, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Metabolic syndrome and dementia risk in a multiethnic elderly cohort. Dement Geriatr Cogn Disord. (2007) 24:185–92. doi: 10.1159/000105927
70. Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am J Epidemiol. (2002) 156:445–53. doi: 10.1093/aje/kwf074
71. Kivipelto M, Helkala E-L, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, et al. Apolipoprotein E ε4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. (2002) 137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006
72. Borenstein AR, Wu Y, Mortimer JA, Schellenberg GD, McCormick WC, Bowen JD, et al. Developmental and vascular risk factors for Alzheimer's disease. Neurobiol Aging. (2005) 26:325–34. doi: 10.1016/j.neurobiolaging.2004.04.010
73. Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, et al. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord. (2006) 20:93–100. doi: 10.1097/01.wad.0000213814.43047.86
74. Kuller LH, Lopez OL, Newman A, Beauchamp NJ, Burke G, Dulberg C, et al. Risk factors for dementia in the cardiovascular health cognition study. Neuroepidemiology. (2003) 22:13–22. doi: 10.1159/000067109
75. Rönnemaa E, Zethelius B, Lannfelt L, Kilander L. Vascular risk factors and dementia: 40-year follow-up of a population-based cohort. Dement Geriatr Cogn Disord. (2011) 31:460–6. doi: 10.1159/000330020
76. Annweiler C, Rolland Y, Schott AM, Blain H, Vellas B, Herrmann FR, et al. Higher vitamin D dietary intake is associated with lower risk of Alzheimer's disease: a 7-year follow-up. Gerontol Ser Biomed Sci Med Sci. (2012) 67:1205–11. doi: 10.1093/gerona/gls107
77. Wang K-C, Woung L-C, Tsai M-T, Liu C-C, Su Y-H, Li C-Y. Risk of Alzheimer's disease in relation to diabetes: a population-based cohort study. Neuroepidemiology. (2012) 38:237–44. doi: 10.1159/000337428
78. Qiu C, Winblad B, Marengoni A, Klarin I, Fastbom J, Fratiglioni L. Heart failure and risk of dementia and Alzheimer disease: a population-based cohort study. Arch Intern Med. (2006) 166:1003–8. doi: 10.1001/archinte.166.9.1003
79. Olazaran J, Trincado R, Bermejo-Pareja F. Cumulative effect of depression on dementia risk. Int J Alzheimer's Dis. (2013) 2013:457175. doi: 10.1155/2013/457175
80. Becker JT, Chang Y-F, Lopez OL, Dew MA, Sweet RA, Barnes D, et al. Depressed mood is not a risk factor for incident dementia in a community-based cohort. Am J Geriatr Psychiatry. (2009) 17:653–63. doi: 10.1097/JGP.0b013e3181aad1fe
81. Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer's disease. Ann Neurol. (2005) 57:381–7. doi: 10.1002/ana.20405
82. Harwood DG, Barker WW, Loewenstein DA, Ownby RL, George-Hyslop PS, Mullan M, et al. cross-ethnic analysis of risk factors for AD in white Hispanics and white non-Hispanics. Neurology. (1999) 52:551–551. doi: 10.1212/WNL.52.3.551
83. Wu C, Zhou D, Wen C, Zhang L, Como P, Qiao Y. Relationship between blood pressure and Alzheimer's disease in Linxian County, China. Life Sci. (2003) 72:1125–33. doi: 10.1016/S0024-3205(02)02367-6
84. Brayne C, Gill C, Huppert FA, Barkley C, Gehlhaar E, Girling DM, et al. Vascular risks and incident dementia: results from a cohort study of the very old. Dement Geriatr Cogn Disord. (1998) 9:175–80. doi: 10.1159/000017043
85. Mendez MF, Underwood KL, Zander BA, Mastri AR, Sung JH, Frey WH. Risk factors in Alzheimer's disease: a clinicopathologic study. Neurology. (1992) 42:770–770. doi: 10.1212/WNL.42.4.770
86. French LR, Schuman LM, Mortimer JA, Hutton JT, Boatman RA, Christians B, et al. case-control study of dementia of the Alzheimer type. Am J Epidemiol. (1985) 121:414–21. doi: 10.1093/oxfordjournals.aje.a114013
87. Kokmen E, Beard CM, Chandra V, Offord KP, Schoenberg BS, Ballard DJ. Clinical risk factors for Alzheimer's disease: a population-based case-control study. Neurology. (1991) 41:1393–1393. doi: 10.1212/WNL.41.9.1393
88. Foroughan M, Farahani ZG, Shariatpanahi M, Vaezinejad M, Akbari Kamerani AA, Sheikhvatan M. Risk factors of Alzheimer's disease among Iranian population. Curr Alzheimer Res. (2008) 5:70–2. doi: 10.2174/156720508783884594
89. Roberts RO, Cha RH, Knopman DS, Petersen RC, Rocca WA. Postmenopausal estrogen therapy and Alzheimer disease: overall negative findings. Alzheimer Dis Assoc Disord. (2006) 20:141–6. doi: 10.1097/00002093-200607000-00004
90. Kondo K, Niino M, Shido K, A. Case-control study of Alzheimer's disease in Japan—significance of life-styles. Dement Geriatr Cogn Disord. (1994) 5:314–26. doi: 10.1159/000106741
91. Suhanov AV, Pilipenko PI, Korczyn AD, Hofman A, Voevoda MI, Shishkin SV, et al. Risk factors for Alzheimer's disease in Russia: a case-control study. Eur J Neurol. (2006) 13:990–5. doi: 10.1111/j.1468-1331.2006.01391.x
92. Graves AB, White E, Koepsell TD, Reifler BV, Van Belle G, Larson EB, et al. case-control study of Alzheimer's disease. Ann Neurol Off J Am Neurol Assoc Child Neurol Soc. (1990) 28:766–74. doi: 10.1002/ana.410280607
93. Tsolaki M, Fountoulakis K, Chantzi E, Kazis A. Risk factors for clinically diagnosed Alzheimer's disease: a case-control study of a Greek population. Int Psychogeriatr. (1997) 9:327–41. doi: 10.1017/S104161029700447X
94. Imfeld P, Bodmer M, Jick SS, Meier CR. Metformin, other antidiabetic drugs, and risk of Alzheimer's disease: a population-based case-control study. J Am Geriatr Soc. (2012) 60:916–21. doi: 10.1111/j.1532-5415.2012.03916.x
95. Joas E, Bäckman K, Gustafson D, Östling S, Waern M, Guo X, et al. Blood pressure trajectories from midlife to late life in relation to dementia in women followed for 37 years. Hypertension. (2012) 59:796–801. doi: 10.1161/HYPERTENSIONAHA.111.182204
96. Qiu C, Xu W, Winblad B, Fratiglioni L. Vascular risk profiles for dementia and Alzheimer's disease in very old people: a population-based longitudinal study. J Alzheimers Dis. (2010) 20:293–300. doi: 10.3233/JAD-2010-1361
97. Li G, Rhew IC, Shofer JB, Kukull WA, Breitner JCS, Peskind E, et al. Age-varying association between blood pressure and risk of dementia in those aged 65 and older: a community-based prospective cohort study. J Am Geriatr Soc. (2007) 55:1161–7. doi: 10.1111/j.1532-5415.2007.01233.x
98. Ruitenberg A, Skoog I, Ott A, Aevarsson O, Witteman JC, Lernfelt B, et al. Blood pressure and risk of dementia: results from the Rotterdam study and the Gothenburg H-70 Study. Dement Geriatr Cogn Disord. (2001) 12:33–9. doi: 10.1159/000051233
99. Shah RC, Wilson RS, Bienias JL, Arvanitakis Z, Evans DA, Bennett DA. Relation of blood pressure to risk of incident Alzheimer's disease and change in global cognitive function in older persons. Neuroepidemiology. (2006) 26:30–6. doi: 10.1159/000089235
100. Stewart R, Xue Q-L, Masaki K, Petrovitch H, Ross GW, White LR, et al. Change in blood pressure and incident dementia: a 32-year prospective study. Hypertension. (2009) 54:233–40. doi: 10.1161/HYPERTENSIONAHA.109.128744
101. Treiber KA, Lyketsos CG, Corcoran C, Steinberg M, Norton M, Green RC, et al. Vascular factors and risk for neuropsychiatric symptoms in Alzheimer's disease: the Cache County Study. Int Psychogeriatr. (2008) 20:538–53. doi: 10.1017/S1041610208006704
102. Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, et al. Overweight in midlife and risk of dementia: a 40-year follow-up study. Int J Obes. (2009) 33:893–8. doi: 10.1038/ijo.2009.104
103. Tini G, Scagliola R, Monacelli F, La Malfa G, Porto I, Brunelli C, Rosa GM. Alzheimer's disease and cardiovascular disease: a particular association. Cardiol Res Pract. (2020) 2020:2617970. doi: 10.1155/2020/2617970
104. Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker III JC. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. (1995) 131:162–9. doi: 10.1016/0022-510X(95)00105-B
105. Murphy SJ, Werring DJ. Stroke: causes and clinical features. Medicine (Baltimore). (2020) 48:561–6. doi: 10.1016/j.mpmed.2020.06.002
106. Hachinski V, Einhäupl K, Ganten D, Alladi S, Brayne C, Stephan BC, et al. Preventing dementia by preventing stroke: the Berlin Manifesto. Alzheimers Dement. (2019) 15:961–84. doi: 10.1016/j.jalz.2019.06.001
107. Loera-Valencia R, Cedazo-Minguez A, Kenigsberg P, Page G, Duarte A, Giusti P, et al. Current and emerging avenues for Alzheimer's disease drug targets. J Intern Med. (2019) 286:398–437. doi: 10.1111/joim.12959
108. Kuljiš RO, Šalković-Petrišić M. Dementia, diabetes, Alzheimer's disease, and insulin resistance in the brain: progress, dilemmas, new opportunities, and a hypothesis to tackle intersecting epidemics. J Alzheimers Dis. (2011) 25:29–41. doi: 10.3233/JAD-2011-101392
109. Ponce-López T, Sorsby-Vargas AM, Bocanegra-López AP, Luna-Muñoz J, Ontiveros-Torres MA, Villanueva-Fierro I, et al. Diabetes mellitus and amyloid beta protein pathology in dementia. In: Amyloid Diseases. London: IntechOpen (2019) doi: 10.5772/intechopen.84473
110. Nelson L, Gard P, Tabet N. Hypertension and inflammation in Alzheimer's disease: close partners in disease development and progression! J Alzheimers Dis. (2014) 41:331–43. doi: 10.3233/JAD-140024
111. Skoog I, Gustafson D. Update on hypertension and Alzheimer's disease. Neurol Res. (2006) 28:605–11. doi: 10.1179/016164106X130506
112. Benetos A, Thomas F, Safar ME, Bean KE, Guize L. Should diastolic and systolic blood pressure be considered for cardiovascular risk evaluation: a study in middle-aged men and women. J Am Coll Cardiol. (2001) 37:163–8. doi: 10.1016/S0735-1097(00)01092-5
113. Katayama T, Hasebe N. Angiotensin-receptor blockers, hypertension and Alzheimer disease-the entangled relationship. Circ J. (2013) 77:315–6. doi: 10.1253/circj.CJ-12-1550
114. Iadecola C. Hypertension and dementia. Hypertension. (2014) 64:3–5. doi: 10.1161/HYPERTENSIONAHA.114.03040
116. Zhou B, Lu Y, Hajifathalian K, Bentham J, Di Cesare M, Danaei G, et al. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. (2016) 387:1513–30. doi: 10.1016/S0140-6736(16)00618-8
117. Turana Y, Tengkawan J, Chia YC, Hoshide S, Shin J, Chen C-H, et al. Hypertension and Dementia: a comprehensive review from the HOPE Asia Network. J Clin Hypertens. (2019) 21:1091–8. doi: 10.1111/jch.13558
118. Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control. Circulation. (2016) 134:441–50. doi: 10.1161/CIRCULATIONAHA.115.018912
Keywords: Alzheimer's disease, blood pressure, systo-diastolic hypertension, risk factor, meta-analysis
Citation: Sáiz-Vazquez O, Puente-Martínez A, Pacheco-Bonrostro J and Ubillos-Landa S (2023) Blood pressure and Alzheimer's disease: A review of meta-analysis. Front. Neurol. 13:1065335. doi: 10.3389/fneur.2022.1065335
Received: 09 October 2022; Accepted: 21 December 2022;
Published: 11 January 2023.
Edited by:
Anna Maria Lavezzi, University of Milan, ItalyReviewed by:
Alan Lerner, University Hospitals Cleveland Medical Center, United StatesCopyright © 2023 Sáiz-Vazquez, Puente-Martínez, Pacheco-Bonrostro and Ubillos-Landa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Ubillos-Landa,  c3ViaWxsb3NAdWJ1LmVz
c3ViaWxsb3NAdWJ1LmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.