
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 12 January 2023
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1048953
This article is part of the Research TopicMultiple Sclerosis and Neuroimmunology – Case Report Collection, Volume IIView all 35 articles
 Yuki Yokota1
Yuki Yokota1 Makoto Hara1*
Makoto Hara1* Natsuki Oshita1
Natsuki Oshita1 Tomotaka Mizoguchi1
Tomotaka Mizoguchi1 Haruna Nishimaki2
Haruna Nishimaki2 Hiroyuki Hao3
Hiroyuki Hao3 Hideto Nakajima1
Hideto Nakajima1Background: We herein detail our experience with a unique patient with a primary central nervous system (PCNS) B-cell lymphoma concomitant with anti-N-methyl-D-aspartate receptor (NMDAR) antibodies that satisfied the criteria of “probable anti-NMDAR encephalitis (ProNMDARE)” based on the Graus criteria 2016.
Case presentation: A 73-year-old Japanese woman presented with acute pyrexia, agitation, and disturbance of consciousness. She gradually developed a reduction in speech frequency and truncal dystonia causing abnormal posture. Brain magnetic resonance imaging (MRI) demonstrated high-intensity lesions in the bilateral frontal lobes, and her cerebrospinal fluid revealed mild pleocytosis. She was diagnosed with acute encephalitis and treated with acyclovir and intravenous dexamethasone; however, no improvement was observed. She was transferred to our hospital 6 weeks after the onset of her symptoms, and anti-NMDAR antibodies were identified in her cerebrospinal fluid through indirect immunolabeling with rat brain frozen sections and cell-based assays with NR1/NR2 transfected HEK cells. Follow-up MRI showed enlargement of the lesions in the right frontal lobe with gadolinium enhancement, suggesting a brain tumor. Stereotactic surgery was implemented, with subsequent pathological examination revealing that the tumor was consistent with diffuse large B-cell lymphoma (DLBCL) without evidence of systemic satellite lesions. Stereotactic irradiative therapies were then added to her treatment regimen, which partly improved her neurological symptoms with only mild cognitive dysfunction still remaining. A decrease in anti-NMDAR antibody titer was also confirmed after immunotherapy and tumor removal.
Conclusions: We herein report our experience with a novel case of PCNS-DLBCL masquerading as anti-NMDAR encephalitis that satisfied the diagnostic criteria of “proNMDARE.” Treatment, including tumor removal, ameliorated disease severity and antibody titers of the patient. Our findings suggest that anti-NMDAR antibody-associated autoimmunity can be triggered by PCNS B-cell tumors, although primary brain tumors need to be excluded before establishing a diagnosis of autoimmune encephalitis.
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is a well-characterized autoimmune encephalitis (AE) associated with immunoglobulin (Ig) G against the GluN1 subunit of the NMDAR (1). In 2016, Graus et al. (Graus criteria) proposed a syndrome-based diagnostic approach to AE that included NMDAR encephalitis (NMDARE) (2). The Graus criteria for probable NMDARE (ProNMDARE) comprises major groups of neuropsychiatric symptoms, laboratory study results, and reasonable exclusion of other disorders, whereas definite NMDARE requires the detection of the NMDAR IgG mainly in the CSF. In addition, associated teratoma, especially a systemic teratoma, is an important criterion for the diagnosis of NMDARE. Recently, primary central nervous system (PCNS) tumors, such as glioblastoma (3) and lymphomatosis cerebri (4), have been reported to occur concomitantly with NMDAR antibodies; however, the clinical significance of the antibodies associated with primary brain tumors has yet to be sufficiently discussed given that brain tumors need to be excluded before establishing a diagnosis of AE according to the criteria (2).
We herein report a patient with PCNS diffuse large B-cell lymphoma (DLBCL) concomitant with NMDAR antibodies in the CSF who developed acute encephalitis satisfying the diagnostic criteria of “proNMDARE.”
A 73-year-old Japanese woman was admitted to another hospital for pyrexia, agitation, and mild disturbance of consciousness. She was initially diagnosed with a urinary tract infection, for which antibiotics were administered. However, her neurological condition gradually deteriorated. Brain MRI showed high-intensity lesions in the right temporal and bilateral frontal lobes on fluid-attenuated inversion recovery (FLAIR) images. CSF examination showed mild pleocytosis (47/mm3, monocyte predominant). She was diagnosed with acute encephalitis and was started intravenous acyclovir and intravenous dexamethasone.
She was transferred to our hospital 6 weeks after the onset of her symptoms due to persistent disease despite treatment. Her medical history included hypertension. She had no family history of neuromuscular diseases. On admission, the patient had a height and weight of 154.0 cm and 56 kg, respectively. On physical examination, her body temperature was 37.4°C. Chest auscultation revealed normal respiratory sounds and a normal heart rate with no murmur. Neurological examination showed mild disturbance of consciousness: Glasgow Coma Scale (GCS) 13 (E4 V4 M5) with disorientation and agitation. She was able to answer some simple questions but showed a remarkable reduction in speech frequency. She presented with cervical and truncal dystonia causing abnormal posture. She did not experience seizures. Autonomic dysfunction and central hypoventilation were unremarkable. Electroencephalography (EEG) revealed a generalized slowness in activity (5–7 Hz) without epileptiform discharges. A cerebrospinal fluid (CSF) sample included 5 white blood cells/mm3, 18 mg/dl of total protein, and 77 mg/dl of glucose (149 mg/dl of serum glucose). Her IgG index (0.50) was within normal range (cut-off < 0.67). Her CSF sample came back positive for oligoclonal bands. Brain MRI revealed high-intensity lesions in the right temporal and bilateral frontal lobes on FLAIR images, with the right frontal lesion demonstrating gadolinium (Gd) enhancement, which can be considered to indicate CNS inflammation or a high-grade brain tumor. Magnetic resonance spectroscopy of the right frontal lesion revealed an increase in the choline/creatine ratio and a normal N-acetyl aspartate level (Figure 1). Whole-body contrast-enhanced computed tomography showed no associated tumor and swelling of systemic lymph nodes. In addition, 67Ga scintigraphy showed no abnormal uptake in the systemic organs and lymph nodes (Supplementary Figure 1).

Figure 1. Brain magnetic resonance (MR) fluid-attenuated inversion recovery (FLAIR) images (A, B), gadolinium (Gd)-enhanced T1-weighted image (C), and MR spectroscopy (D) findings. Brain MRI FLAIR 7 days before admission revealed high-intensity lesions in the bilateral frontal lobes (A). MRI FLAIR (B) and Gd-enhanced T1-weighted image (C) on admission revealed that high-intensity area in the right frontal lobe on MRI FLAIR showed round shaped on Gd enhancement. MR spectroscopy demonstrated an increase in choline/creatine ratio and decrease in N-acetyl aspartate levels in the Gd-enhanced lesion (D).
In-house screening for anti-neuronal antibodies (5, 6) was performed using the patient's CSF and sera samples via indirect immunohistochemistry with frozen sections of rat brain. Patient's CSF immunolabeled neuropil of the rat hippocampus (Figure 2) and granular cell layers in the cerebellum (Supplementary Figure 2), which was consistent with the immunolabeling pattern of anti-NMDAR antibodies. In contrast, the patient's serum did not immunolabel the rat brain section. A cell-based assay with NR1/NR2-transfected HEK293 cells (BIOCHIP, Euroimmun, Labor Berlin) confirmed anti-NMDAR antibodies in the CSF alone (Figure 2, antibody titers were 1:32). No onconeural antibodies that included amphiphysin, Hu, Yo, CV2, Ri, Ma2/Ta, recoverin, Tr, GAD65, and others measured via line blot (EUROLINE, Euroimmun, Lübeck, Germany) were detected (details regarding autoantibody detection using in-house and commercially available assays are described in the Supplementary methods). Furthermore, autoantibodies associated with other AE were not detected, including Bickerstaff's brainstem encephalitis (anti-GQ1b antibody in the serum), Hashimoto's encephalitis (antibodies against thyroid peroxidase and thyroglobulin in the serum), and demyelinating disorders of the CNS (antibodies against anti-myelin oligodendrocyte and aquaporin-4 in both the CSF and serum).
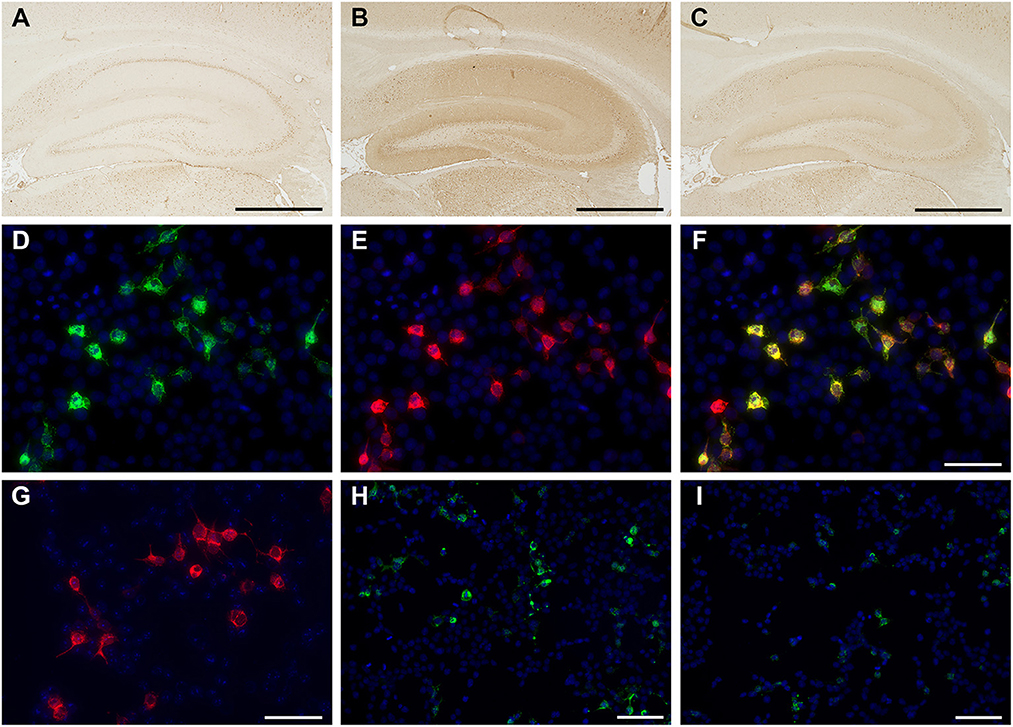
Figure 2. Immunolabeling of the patient's cerebrospinal fluid (CSF) sample with in-house tissue-based assay (TBA) (A–C) and cell-based assay (CBA) (D–I). Control CSF did not react with in-house TBA (A), whereas the initial patient's CSF immunolabeled neuropil on the rat hippocampus in the TBA (B). The patient's CSF showed reactivity [green, (D)], with human embryonic kidney (HEK) cells expressing the N-methyl-D-aspartate receptor (NMDAR). The commercial antibody against NMDAR1 (clone EPR2480Y, ab68144, Abcam) [red, (E)] colocalizes with that of the patient's CSF [yellow, (F)], whereas a control CSF is negative (G). Anti-NMDAR antibody titers (1:32) were confirmed using the fluorescent reactivity of CBA on the initial patient's CSF (H). Following tumor removal and whole-brain irradiation therapy, immunosignals in TBA (C) and CBA decreased, as did antibody titers of anti-NMDAR antibody (titers 1:4) (I). Nuclei were counterstained with 4′, 6-diamino-2-phenylindole. Bars = 1000 μm (A–C), 50 μm (D–G), and 100 μm (H, I).
The Gd-enhanced lesion accompanied by swelling of right frontal robe was required to rule out a high-grade brain tumor; thus, craniotomy was performed for tumor resection on day 15 of the hospitalization. Histopathological analyses showed a dense proliferation of atypical cells with large and irregular nuclei on hematoxylin-eosin (H&E) staining. Tumor cells were positive for CD20 (clone L26; Agilent Technologies, CA, US) and CD79a (clone JCB117; Agilent Technologies, CA, US) suggesting B-cell lymphoma with high Ki-67 (clone MIB-1; Agilent Technologies, CA, US) expression (90%). Immunological profiles of the tumor were consistent with primary central nervous system diffuse large B-cell lymphoma (PCNS-DLBCL) (Supplementary Figure 3). In addition, immunolabeling with biopsied brain sections using anti-NR1 antibodies (clone EPR23397-66; Abcam, Cambridge, UK) revealed that the tumor cells did not express the NR1 subunit of NMDAR (Figure 3).
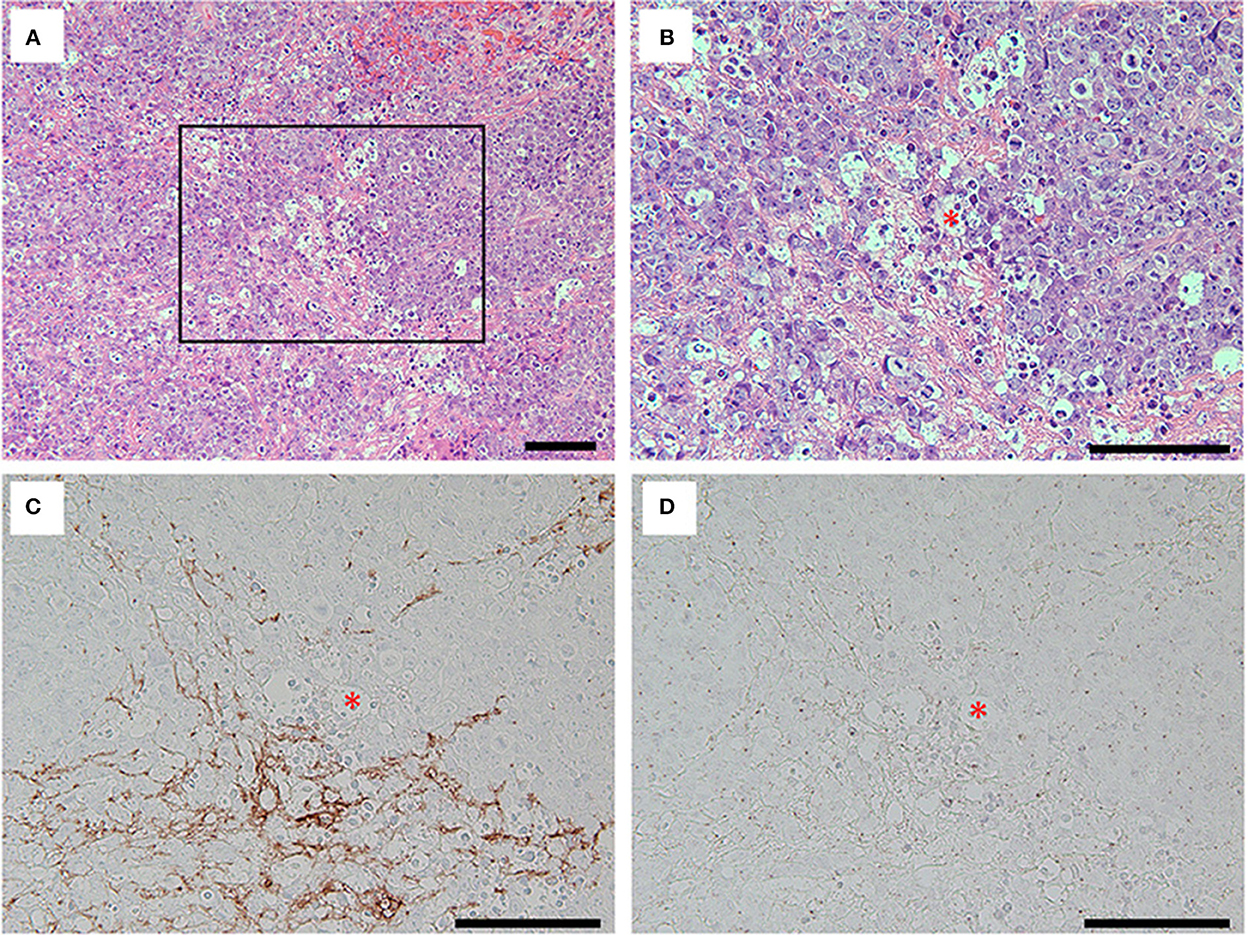
Figure 3. Hematoxylin and eosin (H&E) staining (A, B) and immunolabeling with the antibodies against grail fibrillary acidic protein (GFAP) (clone G-A-5; Sigma, MO, US) (C) and NR1 (D) for the tumor cells. Note that H&E staining of the removed brain specimens showed foci of highly proliferative atypical cells interposed with basophilic matrix (A). The tumor cells with irregular nuclei and frequent mitoses [(B), inset in (A)] were not immunolabeled with commercial antibodies against GFAP (C) nor NR1 subunit of NMDAR (D), while the basophilic matrix on H&E staining was immunolabeled with commercial antibody against GFAP (C). Asterisks in red (B–D) indicate the same blood vessel, and all bars = 100 μm.
After tumor removal, the patient underwent whole-brain stereotactic irradiation therapies (a total of 30 Gy). The antibody titers of anti-NMDAR antibodies in the CSF decreased as the volume of brain tumor reduced after surgical and irradiative therapies (Figure 2, titers were 1:4). Her consciousness and mental condition partially improved; however, she was discharged to another hospital for rehabilitation and long-term care due to limitations in performing activities of daily living. The clinical course of the patient is summarized in Supplementary Figure 4. Written informed consent was obtained from the patient and patient's kin for the publication of any potentially identifiable images or data included in this article.
We herein describe a case of PCNS-DLBCL that clinically manifested as acute encephalitis associated with anti-NMDAR antibody. Fortunately, a combination of tumor removal and irradiative therapies was able to decreased her antibody titers. The present case satisfied the criteria for proNMDARE (four of the six major symptoms and abnormal EEG finding) proposed by Graus et al. (2). In-house screening of the antibodies against neuronal surface antigens (5, 6), which included anti-NMDAR antibodies, could help with the early identification of antibodies in our patient. After testing positive for anti-NMDAR IgG in her CSF, she also satisfied the diagnostic criteria for definite NMDARE. However, systemic tumor analyses identified a DLBCL isolated in the CNS without radiological involvement of the systemic lymph nodes and organs. According to the aforementioned criteria (2), brain tumors are one of the diseases that need to be excluded before establishing a diagnosis of AE. Thus, we eventually diagnosed the patient with PCNS-DLBCL masquerading as NMDARE.
Recent studies have suggested the association between some types of neuronal surface antibodies (NSA) and hematologic neoplasms (7–10). For instance, Hodgkin lymphoma was associated with anti-NMDAR antibody (7) and mGluR5 antibody (8), whereas non-Hodgkin's lymphoma was associated with anti-DPPX antibody (9). Interestingly, Thomas et al. recently described a case with PCNS lymphoma mimicking anti-LGI1 limbic encephalitis. They mentioned that the case showed low serum anti-LGI1 antibody titers not detected in the CSF (10). In these reports (7–10), the patients were considered to have exhibited paraneoplastic syndrome considering that amelioration (8, 9) or deterioration (10) of the neurological syndrome was identified depending on the tumor condition. In addition, reports have also shown that the expression of the neuronal surface antigens was not confirmed on the surface of neoplastic lymphocytes (11, 12). In our case, the neurological syndrome partly improved, with only mild cognitive dysfunction remaining after the tumor removal following irradiative therapies. Furthermore, the tumor cells did not express NR1 subunits of NMDAR (Figure 3D), and no other associated tumors outside of the CNS (e.g., ovarian teratoma) were detected. Considering these facts and aforementioned evidence, it is likely that the PCNS lymphoma in our case induced paraneoplastic NMDARE. However, it seems unreasonable that an ectopic expression of NMDAR on the tumor cells could yield anti-NMDAR antibodies as observed in patients with systemic teratomas and other associated tumors (13, 14).
Conversely, it is wellknown that anti-NMDAR antibody titers in the CSF corresponded to the severity of the disorder and that most patients experienced a decrease in the titers following treatment that included tumor removal (for paraneoplastic cases) and intensive immunotherapies (15). The serial CSF antibody titers throughout the disease course in cases reported to be associated with NSA accompanied by hematologic neoplasms have not been thoroughly discussed (4, 7). Interestingly, in our case, a decrease in the antibody titers in the CSF was confirmed after treatments for the PCNS lymphoma. This finding also suggests that the neurological syndrome of the present case behaved as a paraneoplastic NMDARE.
Thereafter, we reviewed previous cases with primary brain tumors concomitant with anti-NMDAR antibodies (3, 4, 16) (Table 1). Fujii et al. (3) reported a 54-year-old woman with glioblastoma and ovarian teratoma who presented with complex partial seizures and impaired consciousness. Lu et al. (16) also reported a 67-year-old man with a brain astrocytoma who developed partial seizures. Interestingly, encephalitis worsened as the tumor progressed, although alterations in antibody titers were not examined. Mariotto et al. (4) reported a 54-year-old man who developed various types of cognitive impairment and was treated with plasma pheresis and cyclophosphamide. She was diagnosed with lymphomatosis cerebri following an autopsy. All previous cases developed limited syndrome (3, 4, 16) of NMDARE based on the Graus criteria (2). In contrast, our case developed typical anti-NMDAR syndrome and satisfied the criteria for “proNMDARE” (4 of the 6 major symptoms and abnormal EEG finding) (2). Moreover, in-house screening immediately detected anti-NMDAR antibody in the CSF. The prognosis of the cases depended on the malignancy potential of the associated brain tumors, which was relatively poor compared to a typical NMDARE (17). In our case, anti-NMDAR syndrome partly improved; however, she could not return to baseline after having been debilitated by PCNS-DLBCL, including a series of treatments for the tumors.
Regarding the mechanism for the association between brain tumors and production of autoantibodies, Mariotto et al. (4) suggested that dysregulated lymphoma cells or exposure to specific antigens caused by brain damage yielded autoantibodies. The aforementioned mechanism is similar to the mechanism proposed in NMDARE subsequent to herpes simplex encephalitis (post-HSE) (18, 19). Namely, viral-induced neuronal lysis of the CNS can induce autoimmune reaction to produce the NSAs that include anti-NMDAR antibodies in the patients with post-HSE (19–23). We challengingly speculated that the production of anti-NMDAR antibodies in the present case was mainly associated with antigen exposure of the damaged brain caused by tumor progression given that concomitant PCNS-DLBCL aggressively infiltrated the right frontal lobe with higher expression rate of Ki-67 (Supplementary Figure 3D), a marker for determining growth fraction (24). Further investigation is required to elucidate the precise mechanisms of NSA production induced by the brain tumors.
In conclusion, we herein report a unique case involving PCNS-DLBCL who developed acute encephalitis satisfying the diagnostic criteria for “probable NMDARE.” The present case indicated that anti-NMDAR antibody-associated autoimmunity can be triggered by PCNS B-cell tumors, which are involved in anti-NMDAR syndrome presenting as a paraneoplastic NMDARE. Furthermore, brain tumor assessment even for patients suspected of AE can help prevent delays in the induction of appropriate treatment.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) and/or minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
YY and MH drafted the manuscript. YY, MH, NO, TM, and HNa prepared patient's clinical data. HNi and HH performed the histological examination. MH, HH, and HNa supervised this study. All authors analyzed and interpreted the patient data and revised the manuscript for intellectual content. All authors approved the final manuscript.
This work was supported in part by MHLW Grant Number 22HA1003 and JSPS KAKENHI Grant Number JP20K07875 (MH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1048953/full#supplementary-material
1. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. (2011) 10:63–74. doi: 10.1016/S1474-4422(10)70253-2
2. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/s1474-4422(15)00401-9
3. Fujii H, Kubo S, Yunoki T, Sato K, Takamatsu K, Tanaka K, et al. Glioblastoma with ovarian teratoma having N-methyl-D-aspartate receptor (NMDAR) antibody in CSF—a case report. Rinsho Shinkeigaku. (2013) 53:712–5. doi: 10.5692/clinicalneurol.53.712
4. Mariotto S, Zamo A, Franchini E, Bonetti B, Parisi A, Hoftberger R, et al. Lymphomatosis cerebri and anti-NMDAR antibodies: a unique constellation. J Neurol Sci. (2019) 398:19–21. doi: 10.1016/j.jns.2019.01.014
5. Hara M, Martinez-Hernandez E, Arino H, Armangue T, Spatola M, Petit-Pedrol M, et al. Clinical and pathogenic significance of IgG, IgA, and IgM antibodies against the NMDA receptor. Neurology. (2018) 90:e1386–94. doi: 10.1212/WNL.0000000000005329
6. Mizoguchi T, Hara M, Hirose S, Nakajima H. Novel qEEG biomarker to distinguish anti-NMDAR encephalitis from other types of autoimmune encephalitis. Front Immunol. (2022) 13:845272. doi: 10.3389/fimmu.2022.845272
7. Zandi MS, Irani SR, Follows G, Moody AM, Molyneux P, Vincent A. Limbic encephalitis associated with antibodies to the NMDA receptor in Hodgkin lymphoma. Neurology. (2009) 73:2039–40. doi: 10.1212/WNL.0b013e3181c55e9b
8. Spatola M, Sabater L, Planaguma J, Martinez-Hernandez E, Armangue T, Pruss H, et al. Encephalitis with mGluR5 antibodies: symptoms and antibody effects. Neurology. (2018) 90:e1964–72. doi: 10.1212/WNL.0000000000005614
9. Hara M, Arino H, Petit-Pedrol M, Sabater L, Titulaer MJ, Martinez-Hernandez E, et al. DPPX antibody-associated encephalitis: main syndrome and antibody effects. Neurology. (2017) 88:1340–8. doi: 10.1212/WNL.0000000000003796
10. Thomas C, Lehrich C, Gross CC, Wiendl H, Meuth SG, Melzer N. Primary B cell lymphoma of the CNS mimicking anti-LGI1 limbic encephalitis. Front Neurol. (2018) 9:658. doi: 10.3389/fneur.2018.00658
11. Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. (2000) 342:21–7. doi: 10.1056/NEJM200001063420104
12. Graus F, Arino H, Dalmau J. Paraneoplastic neurological syndromes in Hodgkin and non-Hodgkin lymphomas. Blood. (2014) 123:3230–8. doi: 10.1182/blood-2014-03-537506
13. Dalmau J, Tuzun E, Wu HY, Masjuan J, Rossi JE, Voloschin A, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. (2007) 61:25–36. doi: 10.1002/ana.21050
14. Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. (2018) 378:840–51. doi: 10.1056/NEJMra1708712
15. Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, et al. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol. (2014) 13:167–77. doi: 10.1016/S1474-4422(13)70282-5
16. Lu J, Zhang JH, Miao AL, Yin JX, Zhu DL, Lin XJ, et al. Brain astrocytoma misdiagnosed as anti-NMDAR encephalitis: a case report. BMC Neurol. (2019) 19:210. doi: 10.1186/s12883-019-1436-x
17. Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. (2013) 12:157–65. doi: 10.1016/S1474-4422(12)70310-1
18. Pruss H, Finke C, Holtje M, Hofmann J, Klingbeil C, Probst C, et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann Neurol. (2012) 72:902–11. doi: 10.1002/ana.23689
19. Armangue T, Leypoldt F, Malaga I, Raspall-Chaure M, Marti I, Nichter C, et al. Herpes simplex virus encephalitis is a trigger of brain autoimmunity. Ann Neurol. (2014) 75:317–23. doi: 10.1002/ana.24083
20. Leypoldt F, Titulaer MJ, Aguilar E, Walther J, Bonstrup M, Havemeister S, et al. Herpes simplex virus-1 encephalitis can trigger anti-NMDA receptor encephalitis: case report. Neurology. (2013) 81:1637–9. doi: 10.1212/WNL.0b013e3182a9f531
21. Hacohen Y, Deiva K, Pettingill P, Waters P, Siddiqui A, Chretien P, et al. N-methyl-D-aspartate receptor antibodies in post-herpes simplex virus encephalitis neurological relapse. Mov Disord. (2014) 29:90–6. doi: 10.1002/mds.25626
22. Mohammad SS, Sinclair K, Pillai S, Merheb V, Aumann TD, Gill D, et al. Herpes simplex encephalitis relapse with chorea is associated with autoantibodies to N-methyl-D-aspartate receptor or dopamine-2 receptor. Mov Disord. (2014) 29:117–22. doi: 10.1002/mds.25623
23. Armangue T, Titulaer MJ, Malaga I, Bataller L, Gabilondo I, Graus F, et al. Pediatric anti-N-methyl-D-aspartate receptor encephalitis-clinical analysis and novel findings in a series of 20 patients. J Pediatr. (2013) 162:850–6.e852. doi: 10.1016/j.jpeds.2012.10.011
Keywords: primary central nervous system lymphoma, anti-NMDAR antibody, encephalitis, syndrome, diagnostic criteria
Citation: Yokota Y, Hara M, Oshita N, Mizoguchi T, Nishimaki H, Hao H and Nakajima H (2023) Case report: Anti-N-methyl-D-aspartate receptor antibody-associated autoimmunity triggered by primary central nervous system B-cell lymphoma. Front. Neurol. 13:1048953. doi: 10.3389/fneur.2022.1048953
Received: 20 September 2022; Accepted: 16 December 2022;
Published: 12 January 2023.
Edited by:
Shunya Nakane, Nippon Medical School Hospital, JapanReviewed by:
Ailiang Miao, Nanjing Brain Hospital Affiliated to Nanjing Medical University, ChinaCopyright © 2023 Yokota, Hara, Oshita, Mizoguchi, Nishimaki, Hao and Nakajima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Makoto Hara,  aGFyYS5tYWtvdG9Abmlob24tdS5hYy5qcA==
aGFyYS5tYWtvdG9Abmlob24tdS5hYy5qcA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.