- 1Department of Pediatrics of Neurology Nursing, West China School of Nursing, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
- 3Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 4Key Laboratory of Obstetric and Gynecologic and Pediatric Diseases and Birth Defects of Ministry of Education, Sichuan University, Chengdu, Sichuan, China
- 5Key Laboratory of Development and Maternal and Child Diseases of Sichuan Province, Chengdu, Sichuan, China
Background and purpose: A growing body of research suggests that inflammation and maternal infections may lead to an increased risk of neurodevelopmental problems such as attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), cerebral palsy (CP), and epilepsy in offspring. The aim of this study was to observe the connection between prenatal antibiotic exposure and the risk of these neurodevelopmental disorders in offspring.
Patients and methods: A comprehensive search was conducted in the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Google Scholar, and Scopus databases for observational studies that looked into the link between prenatal exposure to antibiotics and the risk of neurodevelopmental problems in offspring, published from 1 January 1950 to 31 January 2022. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of the included studies. Data were analyzed using the STATA version 12 software, and an odds ratio (OR) with a 95% confidence interval (CI) was reported.
Results: A total of 15 studies were included in the meta-analysis. Prenatal antibiotic exposure was associated with the increased risk of ADHD (OR = 1.14; 95% CI = 1.13 to 1.15; I2 = 0%) and epilepsy (OR = 1.34; 95% CI = 1.02 to 1.66; I2 = 96.8%). The link between prenatal antibiotic exposure and the risk of ASD [OR = 1.09; 95 % CI = 0.88 to 1.31; I2 = 78.9%] and CP [OR = 0.99; 95% CI = 0.56 to 1.43; I2 = 91%] was found to be non-significant. In all of the included prospective cohort studies, subgroup analysis suggested a significant association between prenatal antibiotic exposure and the incidence of ASD [OR = 1.17; 95% CI = 1.03 to 1.31; I2 = 48.1%] and CP [OR = 1.18; 95% CI = 1.02 to 1.34; I2 = 0%].
Conclusion: Prenatal antibiotic exposure during pregnancy is linked to a higher incidence of ADHD and epilepsy in the offspring. Further prospective studies that compare prenatal antibiotic use and are adjusted for various confounders are needed to further assess the association of prenatal antibiotic exposure and neurological disorders in offspring.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42022306248.
Introduction
A higher risk of neurological disorders has been related to severe viral and bacterial infections, as well as infections caused by parasites, particularly in hospitalized patients (1–3). Continuous complex neurodevelopmental processes such as axonal and dendritic growth, neurogenesis, synaptogenesis, and refinement of these synaptic connections make the prenatal period and infancy particularly vulnerable to exposure (4). Antibiotics, which account for 80% of all drugs provided to pregnant women, are commonly used to treat infections during pregnancy (5). It is projected that 19–49% of pregnant women are given antimicrobials during their pregnancy and that this number will continue to rise. Antibiotic usage during pregnancy has been linked to immune system changes, childhood asthma, changes in gut microbiota, obesity, and functional deficits in the offspring (6–11).
Numerous studies demonstrated that maternal infections and inflammation influence the development of the fetal brain, raising the chance of mental disorders, with a focus on neurodevelopmental diseases (1, 12–14). While some studies have found that the use of antimicrobials during pregnancy is associated with the increased risk of neurodevelopmental disorders including cerebral palsy (CP), attention-deficit/hyperactivity deficit (ADHD), autism spectrum disorder (ASD), and epilepsy, other studies did not find such association (11, 15–20).
Antibiotic exposure during pregnancy has been linked to the likelihood of ASD (21) and ADHD (22) in two recent meta-analyses. These reviews, however, reported data from a small number of studies and could not cover all key neurodevelopmental disorders, such as cerebral palsy and epilepsy. The goal of this systematic review and meta-analysis is to look at the association between prenatal antibiotic exposure and the risk of these neurodevelopmental disorders in offspring.
Materials and methods
Study design
This study involves a systematic review and meta-analysis for critical assessment and evaluation of all published reports that evaluate the association of prenatal antibiotic exposure with the risk of neurodevelopmental problems in offspring.
Ethical clearance
No ethical approval is needed for this systematic review and meta-analysis, as it was based on already published data.
Protocol and registration
We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (23) to conduct the study, with a PROSPERO number (CRD42022306248).
Eligibility criteria
Inclusion criteria
(a) Observational studies investigating the effect of prenatal antibiotic exposure and the risk of neurodevelopmental disorders in offspring. (b) Children diagnosed with any one of the neurodevelopmental disorders including ASD, ADHD, CP, and epilepsy based on prenatal antibiotic exposure. (c) The Control group defined as Offspring with no diagnosis of any neurodevelopmental or other psychiatric or genetic disorders.
Exclusion criteria
(a) Duplicate studies, systematic reviews, editorials, case series, case reports, conference abstracts, and preprints. (b) Studies that do not describe required outcomes. (c) Unavailability of full texts. (d) Animal studies. (e) Gray literature (reports, conference proceedings, and theses) and ongoing studies.
Search strategy
This systematic review was performed following the guidelines of the PRISMA (23) and Cochrane (24). An electronic search in Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Google Scholar, and Scopus databases was performed for articles published from 1 January 1950 to 31 January 2022, with no language restrictions. The following key terms were used: “antibiotic” OR “antimedicine” OR “antibacterial” AND “Neurodevelopmental disorders” OR “ADHD” OR “Attention- deficit hyperactivity” OR “hyperactivity” OR “inattention” AND “Cerebral Palsy” AND “Autism” AND “Seizure disorders” OR Epilepsy. Reference lists of the retrieved papers, prior meta-analyses, and review articles were manually searched for additional relevant studies.
Data extraction
Data were collected independently by two reviewers and included author name, country, study period, publication year, study design, diagnostic criteria, exposure assessment, sample size, age at the time of antibiotic exposure, outcomes, and crude or adjusted estimates for disease occurrence.
Risk of bias assessment
Two authors independently assessed the risk of bias using the Newcastle Ottawa Scale (NOS) (25). This scale is used to assess the quality of non-randomized studies for systematic review and meta-analysis and has three important components: group comparability, study group selection, and determination of the target outcome or exposure. Each study may receive a maximum of eight stars (high quality) to a minimum of 0 stars (poor quality). Any disagreements among the authors on the quality scores were resolved by discussion.
Statistical analysis
Statistical analysis was done using the STATA version 12 software. For pooled estimates, the odds ratio (OR) with a 95% confidence interval (CI) was generated. The I2 statistic was used to calculate heterogeneity (26). This test determines how much of the difference in the study results is due to heterogeneity rather than sampling error. I2 of <50% is seen as inconsequential, and I2 of over 50% indicates moderate to significant heterogeneity. Publication bias was detected by funnel plot. By eliminating one study at a time, we performed sensitivity analyses to see how a single study affected the total risk estimates. P < 0.05 indicated statistical significance.
Results
Literature selection
As seen in Figure 1, the initial search yielded 287 results. Of them, 27 records were reviewed after duplicate removal, and 19 articles remained after the title and abstract screening. Finally, after evaluating full texts, four articles were removed due to insufficient data or overlapping data, and the remaining 15 studies were selected for the present meta-analysis.
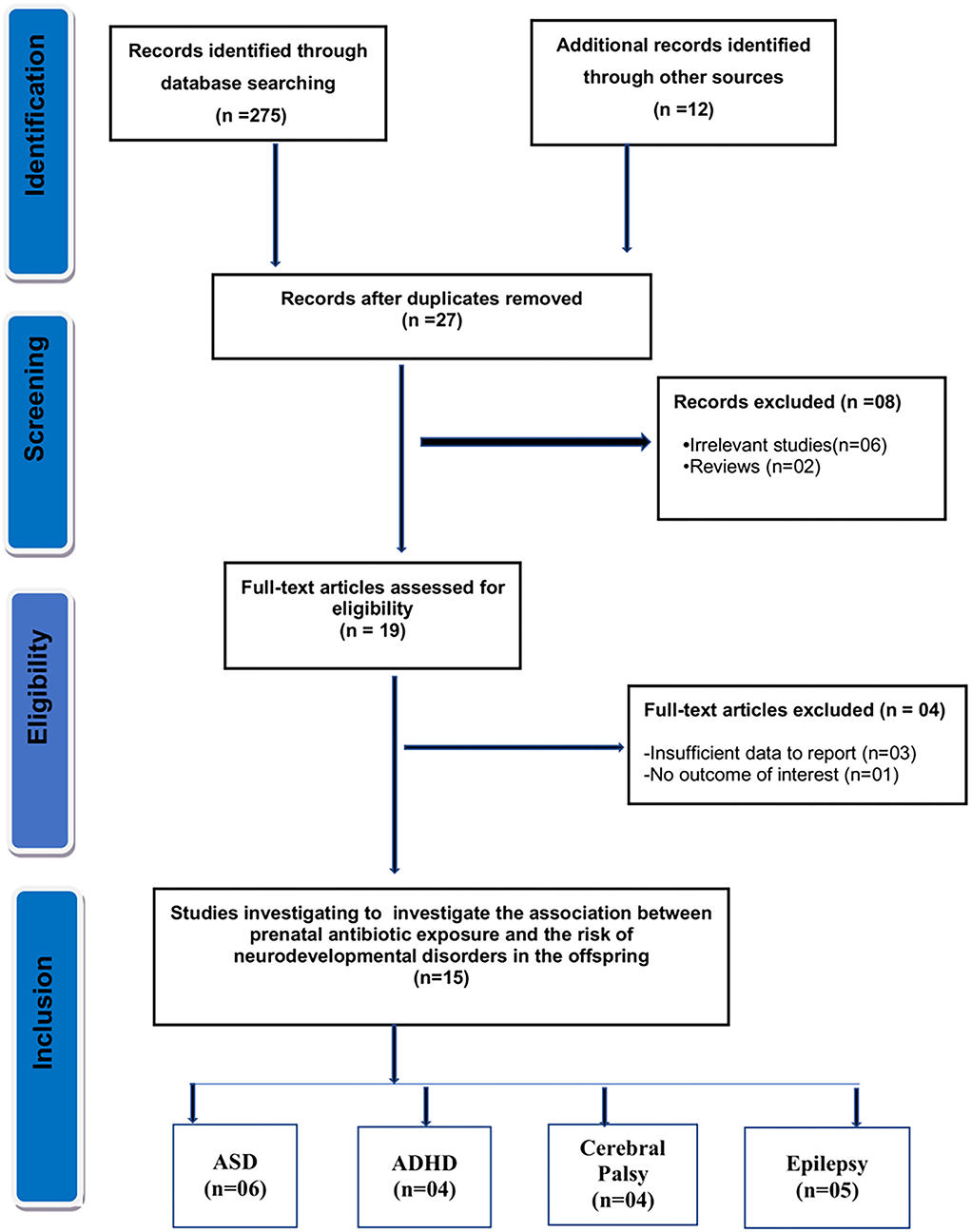
Figure 1. Flow diagram for the selection of studies and specific reasons for exclusion from the present meta-analysis.
Study characteristics
Fifteen observational studies (11, 15–20, 27–34) including six studies of ASD (11, 16, 19, 20, 29), four studies of ADHD (16, 17, 27, 28), four studies of CP (15, 16, 33, 34), and five studies of epilepsy (16, 30–32) were included in our systematic review. Three studies were retrospective cohort (15, 17, 30), three studies were case-control (11, 19, 34), one study was nested case-control (20), and the remaining eight studies were prospective cohort studies (16, 18, 27–29, 31–33). The publication year ranged between 1998 and 2021. The baseline and clinical characteristics for the included studies are shown in Table 1. Thirteen included studies were performed in the Caucasian population, and two studies were performed in the Asian population.
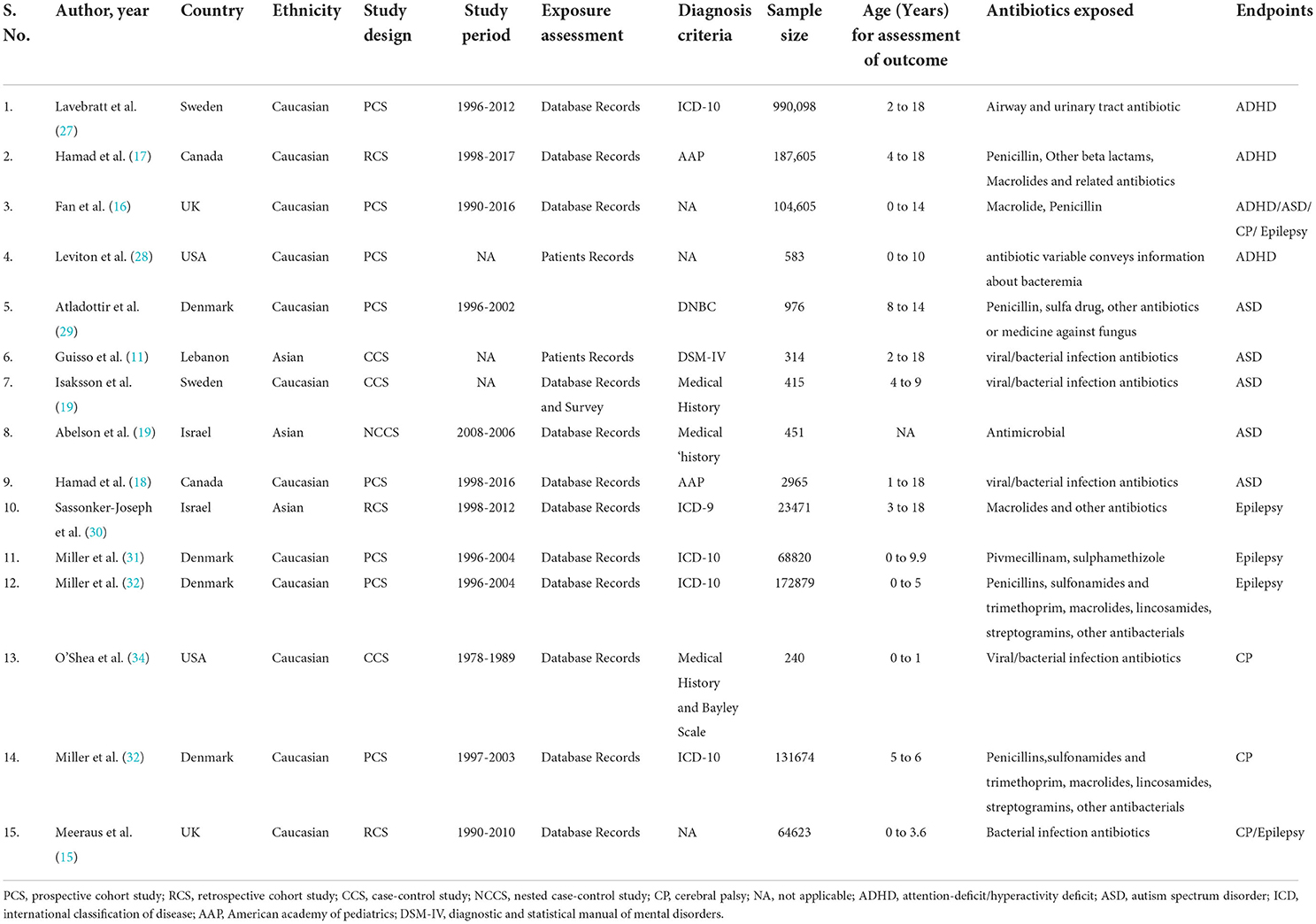
Table 1. Baseline characteristics of included studies in the meta-analysis investigating the association between prenatal antibiotic exposure and the risk of neurodevelopmental disorders in the offspring.
Four studies reported international disease classification (ICD)-10 criteria, one study used ICD-9, three studies used medical history, and two studies used American academy of pediatrics guidelines as the diagnostic criteria for defining neurodevelopmental disorders. The detailed classes for the antibiotic exposed in all the included studies are represented in Table 1. The classes of antibiotics include Penicillins, sulfonamides, trimethoprim, Pivmecillinam, sulphamethizole macrolides, lincosamides, streptogramins, and other antifungal/antiviral/antibacterial drugs. All included studies had quality levels ranging from moderate [NOS score 4–6] to high [NOS score 7–8] (Table 2). The included studies received quality scores ranging from 6 to 8. In addition, the included studies showed a high risk of comparability bias due to poor confounder adjustment. All included studies are of moderate to high quality, indicating the validity of our findings.
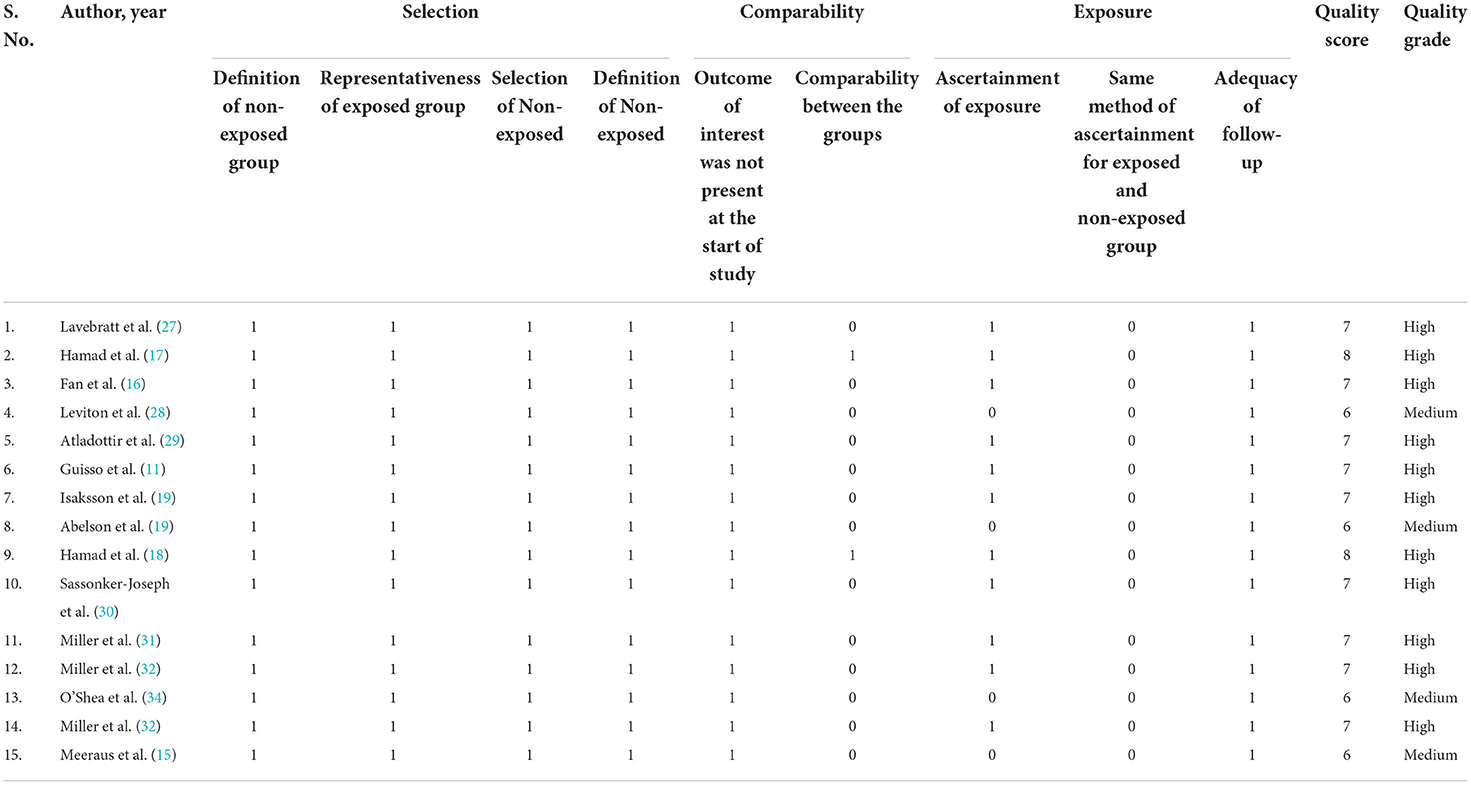
Table 2. Quality assessment of the included studies in the systematic review using the Newcastle Ottawa Scale.
Association between childhood ASD and prenatal antibiotic exposure
Six studies, including three prospective cohort studies (16, 18, 29), one nested case-control study (20), and two case-control studies (11, 19), explored the link between prenatal maternal antibiotic therapy and the risk of ASD in offspring. Our results showed no overall association between prenatal antibiotic exposure and the risk of ASD [OR = 1.09; 95% CI = 0.88 to 1.31, I2 = 78.9%]. Only prospective cohort studies found a significant relationship with the pooled OR of 1.17 (95% CI, 1.03–1.31; Figure 2A), with modest evidence of heterogeneity (P = 0.14, I2 = 48.1%) in the subgroup analysis based on the study design.
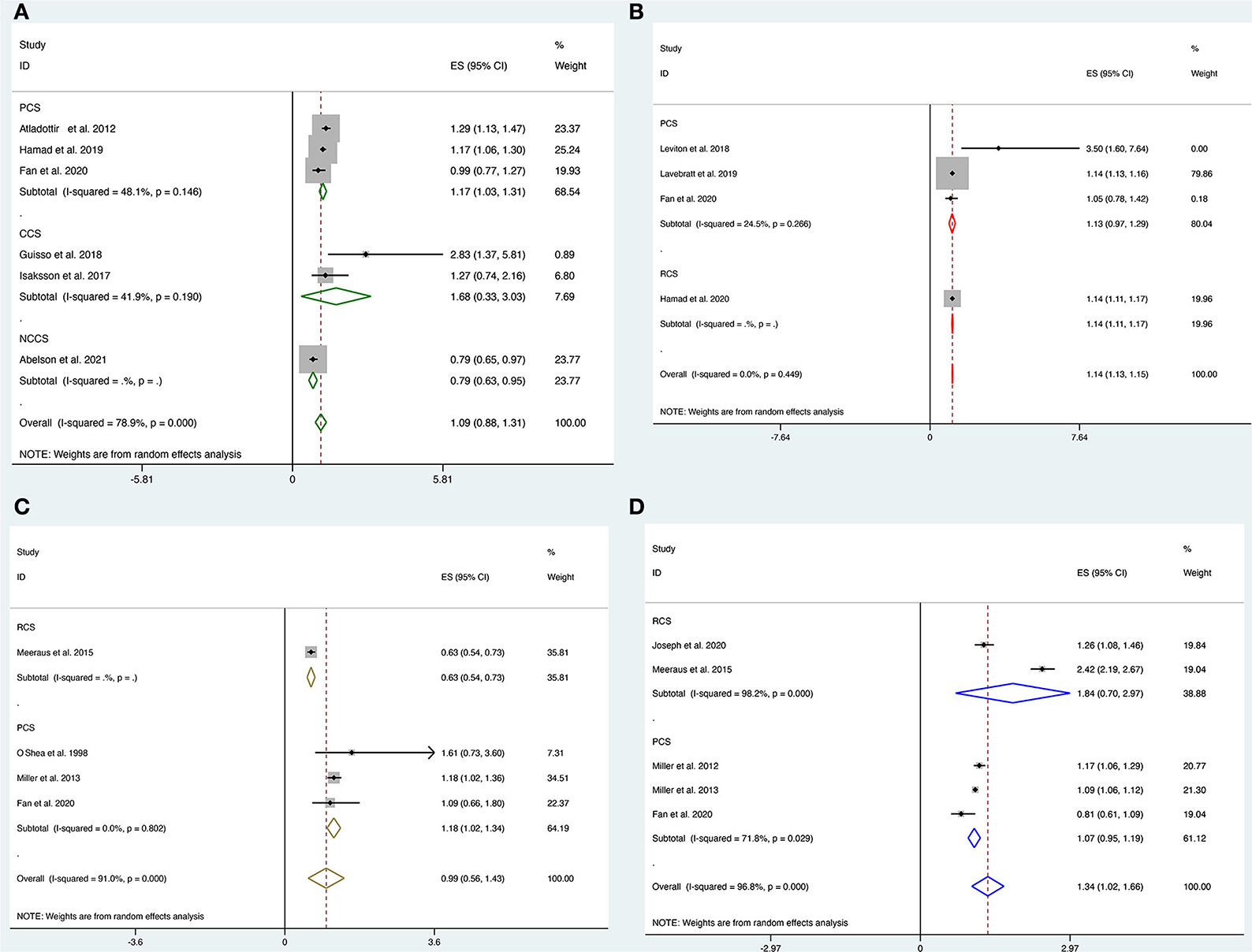
Figure 2. (A–D) Forest plot for the association of prenatal exposure to antibiotics and risk of neurodevelopmental disorders in the offspring. (A) Risk of ASD. (B) Risk of ADHD. (C) Risk of CP. (D) Risk of epilepsy.
Association between prenatal antibiotic exposure and childhood ADHD
Pooled analysis of four studies involving three prospective (16, 27, 28) and one retrospective (17) cohort studies showed a strong correlation between prenatal antibiotic exposure and the risk of ADHD [OR = 1.14; 95% CI = 1.13 to 1.15, I2 = 0%]. However, subgroup analysis showed a borderline risk of ASD in the prospective cohort [OR = 1.13; 95% CI = 0.97 to 1.29, I2 = 24.5%] and a strong association for the risk of ASD in the retrospective cohort [OR = 1.14; 95% CI = 1.11 to 1.17, Figure 2B].
Association between prenatal antibiotic exposure and childhood CP
Four studies (15, 16, 33, 34) looked at the link between prenatal antibiotic therapy and the risk of CP in offspring. There was no significant association between prenatal antibiotic exposure and the risk of CP [OR = 0.99, 95% CI = 0.56 to 1.43, I2 = 91%]. Subgroup analysis suggested a significant association between prenatal antibiotic exposure and the risk of CP [OR = 1.18, 95% CI = 1.02 to 1.34, I2 = 0%] in all three included prospective cohort studies but not in the retrospective studies [OR = 0.63, 95% CI = 0.54 to 0.73, Figure 2C].
Association between prenatal antibiotic exposure and childhood epilepsy during pregnancy
Five studies including three prospective cohort studies (16, 31, 32) and two retrospective cohort studies (15, 30) looked at the link between antibiotic therapy in pregnant women and the risk of epilepsy in offspring. Overall, prenatal antibiotic exposure was significantly associated with the risk of epilepsy [OR = 1.34, 95% CI = 1.02 to 1.66, I2 = 96.8%]. However, these associations were not significant in the subgroup analysis in both prospective [OR = 1.07, 95% CI = 0.95 to 1.19, I2 = 71.8%] and retrospective studies [OR = 1.84, 95% CI = 0.70 to 2.97, Figure 2D].
Publication bias
Egger test and Begg's funnel plot were used to analyzing publication bias for all 15 included studies in our meta-analysis. There was no discernible asymmetry in any included neurodevelopmental disorders based on the visual assessment of the funnel plot (Figures 3A–D). We found no evidence of publication bias in the included studies by Egger's test.
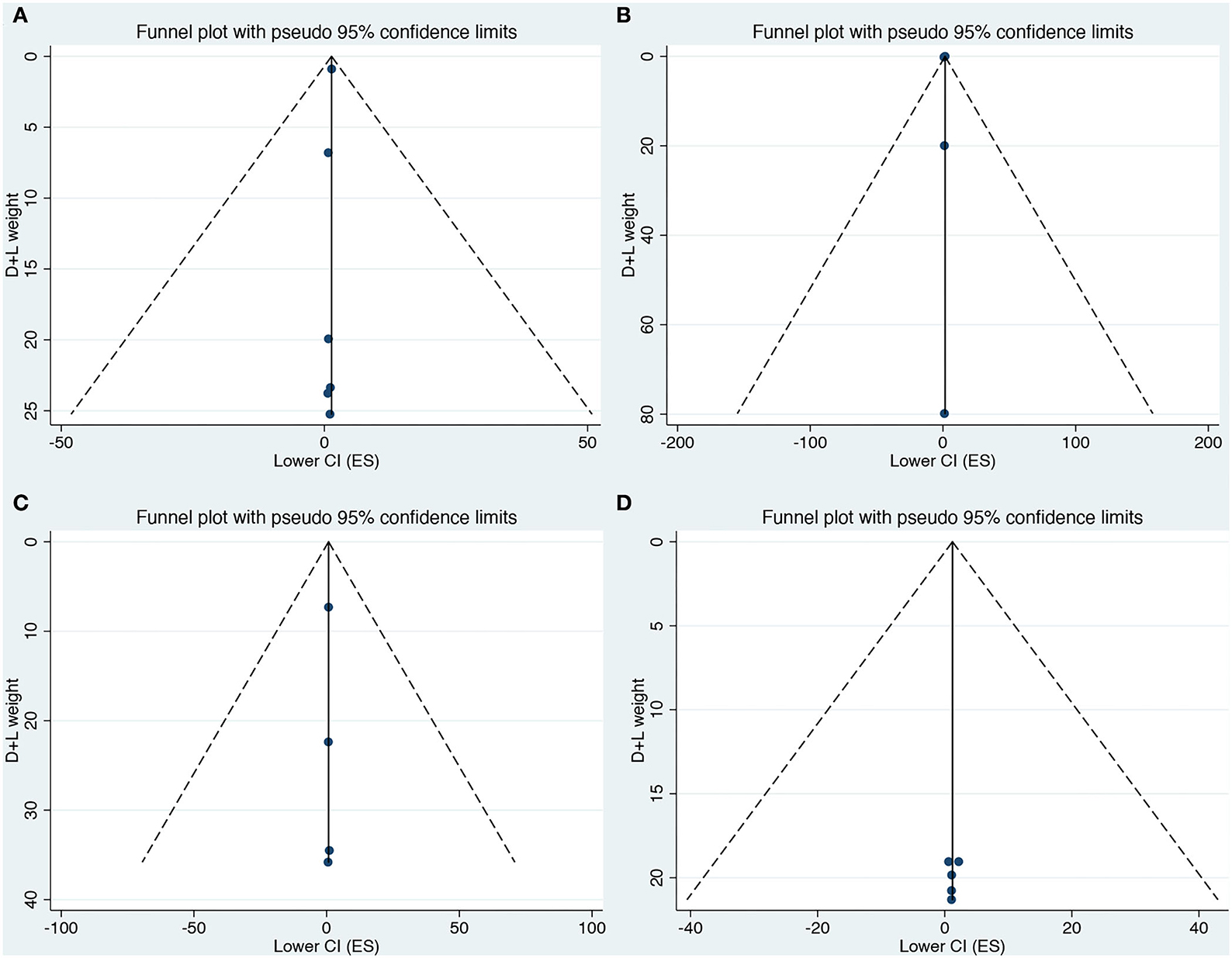
Figure 3. (A–D) Funnel plot for the association of prenatal exposure to antibiotics and risk of neurodevelopmental disorders in the offspring. (A) Risk of ASD. (B) Risk of ADHD. (C) Risk of CP. (D) Risk of epilepsy.
Sensitivity analyses
Sensitivity analyses were performed to see how each individual study of each neurodevelopmental disorder affected the pooled ORs by omitting individual included studies one at a time. Deleting any individual study did not significantly change the associated pooled ORs (Figures 4A–D), further verifying that the findings of the current meta-analysis are statistically sound and robust.
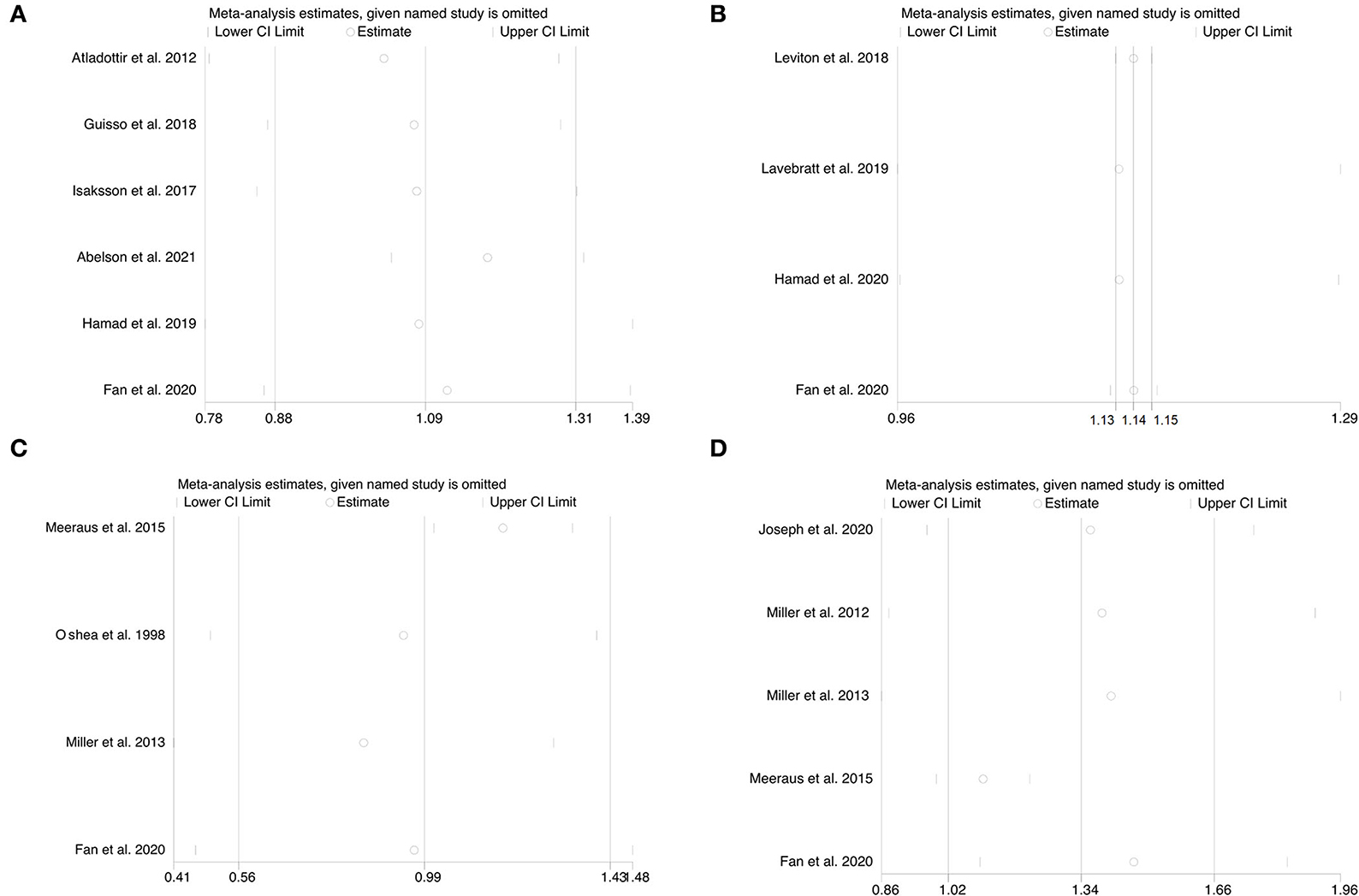
Figure 4. (A–D) Sensitivity analysis for the association of prenatal exposure to antibiotics and risk of neurodevelopmental disorders in the offspring. (A) Risk of ASD. (B) Risk of ADHD. (C) Risk of CP. (D) Risk of epilepsy.
Discussion
Infections have long been linked to aberrant prenatal brain development, and negative outcomes include cerebral palsy (35), developmental delay (36, 37), epilepsy (38), and schizophrenia (39). However, the reasons behind these associations are still unclear. Several studies suggested that certain maternal infections induce fetal brain impairment (38, 40). Since mothers with these infections are more likely to be treated with antibiotics, maternal infection was considered a confounder in the analyses, and accounting for infections can lead to erroneous results that reflect the infection rather than the therapy. Several meta-analyses have published data on infections that occurred prior to ASD diagnosis (41) and identified a significant positive association of ASD with infections that occurred during pregnancy (42, 43). While one systematic analysis found an elevated risk of ASD after prenatal exposure to various drugs, including antibiotics, the association of the diagnosis with individual drugs was not examined (43). Another meta-analysis revealed no evidence of a link between early postnatal infections and the likelihood of ASD later in life (44). On the other hand, several observational studies have discovered incidences of increased risk of mental illnesses in older children and adults following certain diseases (2, 45). The immune system can be influenced by infections and a dysfunctional gut microbiome. Numerous studies show the role of the immune system, inflammation, and autoimmunity in the etiology of neurodevelopmental and psychiatric disorders in children. Studies showed that the levels of pro-inflammatory cytokines are elevated in children with bipolar and major depressive disorders, posttraumatic stress disorder, obsessive-compulsive disorder, schizophrenia, ADHD, and Tourette's syndrome (46). Immune activation has also been linked to the pathogenesis of major depressive disorder (47) and schizophrenia (48) in adults. Recent studies suggest that immunomodulation plays a key role in orchestrating microbiota–gut–brain communication and has the potential to influence neurodevelopment (49–52).
To the best of our knowledge, this is the first systematic review and meta-analysis to examine the link between antibiotic exposure and the risk of neurodevelopmental disorders such as autism, ADHD, CP, and epilepsy. Our findings show a strong connection between prenatal antibiotic exposure and the risk of ADHD and epilepsy. However, we found no significant association between antibiotic exposure during pregnancy and the risk of ASD and CP. While further subgroup analysis based on the type of studies showed a significant correlation between prenatal antibiotic exposure and the risk of ASD and CP in the included prospective cohorts, no such association was found in retrospective studies.
Our findings are consistent with the previously published meta-analysis by Lee et al. (21) for ASD and by Ai et al. (22) for ADHD. There is no meta-analysis available for the possible association between prenatal antibiotic exposure and the risk of CP and epilepsy.
Lee et al. (21) concluded that prenatal antibiotic exposure may increase the risk of ASD in children. This study confirmed that while the use of antibiotics before and after birth raised the risk of ASD, only prenatal antibiotic use was substantially higher in children with ASD compared to children who were not exposed to prenatal treatment. However, the existing studies on the relationship between ASD risk and postnatal antibiotic use do not specifically focus on prenatal antibiotic exposure, making the comparison or evaluation of the risk levels of pre- and post-natal antibiotic use difficult. Another meta-analysis by Ai et al. (22) which included 11 studies suggested the association of prenatal antibiotic treatment with an increased risk of ADHD in children. However, there was a high degree of heterogeneity and publication bias in their analysis which may be due to the large disparity in population characteristics, sample size, timing of antibiotic administration, and antibiotic kind. The precision of the reported pooled estimations was also low which limits the applicability of their findings.
In the present meta-analysis, we found a substantial association between prenatal antibiotic exposure and the risk of CP and ASD, as well as an overall association with ADHD and epilepsy. Our results show a strong association between prenatal antibiotic exposure with the increased risk of ADHD and epilepsy development. While our combined results did not indicate that there is a substantial relationship between maternal antibiotic treatment and incidences of ASD and CP in offspring, this association was significant in prospective cohort studies. This could be because neurodevelopmental diseases have multiple causes. For example, non-genetic causes account for 70% of cerebral palsy cases, whereas genetic variables account for 60–70% of epilepsy, ADHD, and ASD cases (53–56).
Our systematic review and meta-analysis have some limitations, and therefore, results must be interpreted with caution. Only a limited number of published studies investigated a high degree of heterogeneity between study outcomes. This may be due to the inclusion of studies with different study designs leading to recall bias for the observed findings. Variability in the available outcomes, such as the type, number, and timing of antibiotic exposures among the included studies, limited our ability to perform further subgroup analyses. Due to the lack of genetic predisposition data, we were not able to correlate our findings with antibiotic exposure.
Conclusion
Our data show that prenatal antibiotic exposure during pregnancy is linked to a higher incidence of ADHD and epilepsy in children. Further prospective studies, comparing prenatal antibiotic use with adjustment for various confounders, are needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
QT and YS analyzed the data and contributed to the writing of the manuscript. YL, HL, and MY contributed to the data collection and analysis. JG contributed to the substantial review and editing of the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by a grant from the National Natural Science Foundation of China (No. 82071686), the Science and Technology Bureau of Sichuan province (No. 2021YFS0093), and the Research Fund of West China Second University Hospital (No. KL115, KL072).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Estes ML, McAllister AK. Maternal immune activation: implications for neuropsychiatric disorders. Science. (2016) 353:772–7. doi: 10.1126/science.aag3194
2. Benros ME, Waltoft BL, Nordentoft M, Ostergaard SD, Eaton WW, Krogh J, et al. Autoimmune diseases and severe infections as risk factors for mood disorders: a nationwide study. JAMA Psychiatry. (2013) 70:812–20. doi: 10.1001/jamapsychiatry.2013.1111
3. Khandaker GM, Dantzer R, Jones PB. Immunopsychiatry: important facts. Psychol Med. (2017) 47:2229–37. doi: 10.1017/S0033291717000745
4. Rice D, Barone S. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect. (2000) 108 Suppl 3:511–33. doi: 10.1289/ehp.00108s3511
5. Bookstaver PB, Bland CM, Griffin B, Stover KR, Eiland LS, McLaughlin M, et al. Review of antibiotic use in pregnancy. Pharmacotherapy. (2015) 35:1052–62. doi: 10.1002/phar.1649
6. Kuperman AA, Koren O. Antibiotic use during pregnancy: how bad is it? BMC Med. (2016) 14:91. doi: 10.1186/s12916-016-0636-0
7. Broe A, Pottegård A, Lamont RF, Jørgensen JS, Damkier P. Increasing use of antibiotics in pregnancy during the period 2000–2010: prevalence, timing, category, and demographics. BJOG Int J Obstet Gynaecol. (2014) 121:988–96. doi: 10.1111/1471-0528.12806
8. Stokholm J, Schjørring S, Pedersen L, Bischoff AL, Følsgaard N, Carson CG, et al. Prevalence and predictors of antibiotic administration during pregnancy and birth. PLoS ONE. (2013) 8:e82932. doi: 10.1371/journal.pone.0082932
9. Degroote S, Hunting DJ, Baccarelli AA, Takser L. Maternal gut and fetal brain connection: Increased anxiety and reduced social interactions in Wistar rat offspring following peri-conceptional antibiotic exposure. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 71:76–82. doi: 10.1016/j.pnpbp.2016.06.010
10. Hisle-Gorman E, Susi A, Stokes T, Gorman G, Erdie-Lalena C, Nylund CM. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr Res. (2018) 84:190–8. doi: 10.1038/pr.2018.23
11. Guisso DR, Saadeh FS, Saab D, El Deek J, Chamseddine S, Abou-El-Hassan H, et al. Association of autism with maternal infections, perinatal and other risk factors: a case-control study. J Autism Dev Disord. (2018) 48:2010–21. doi: 10.1007/s10803-017-3449-x
12. Blomström Å, Karlsson H, Gardner R, Jörgensen L, Magnusson C, Dalman C. Associations between maternal infection during pregnancy, childhood infections, and the risk of subsequent psychotic disorder–a swedish cohort study of nearly 2 million individuals. Schizophr Bull. (2016) 42:125–33. doi: 10.1093/schbul/sbv112
13. Dreier JW, Berg-Beckhoff G, Andersen AMN, Susser E, Nordentoft M, Strandberg-Larsen K. Fever and infections during pregnancy and psychosis-like experiences in the offspring at age 11. A prospective study within the Danish national birth cohort. Psychol Med. (2018) 48:426–36. doi: 10.1017/S0033291717001805
14. Flinkkilä E, Keski-Rahkonen A, Marttunen M, Raevuori A. prenatal inflammation, infections, and mental disorders. Psychopathology. (2016) 49:317–33. doi: 10.1159/000448054
15. Meeraus WH, Petersen I, Gilbert R. Association between antibiotic prescribing in pregnancy and cerebral palsy or epilepsy in children born at term: a cohort study using the health improvement network. PLoS ONE. (2015) 10:e0122034. doi: 10.1371/journal.pone.0122034
16. Fan H, Gilbert R, O'Callaghan F, Li L. Associations between macrolide antibiotics prescribing during pregnancy and adverse child outcomes in the UK: population based cohort study. BMJ. (2020) 368:m331. doi: 10.1136/bmj.m331
17. Hamad AF, Alessi-Severini S, Mahmud S, Brownell M, Kuo IF. Prenatal antibiotic exposure and risk of attention-deficit/hyperactivity disorder: a population-based cohort study. CMAJ Can Med Assoc J J Assoc Medicale Can. (2020) 192:E527–35. doi: 10.1503/cmaj.190883
18. Hamad AF, Alessi-Severini S, Mahmud SM, Brownell M, Kuo IF. Antibiotic exposure in the first year of life and the risk of attention-deficit/hyperactivity disorder: a population-based cohort study. Am J Epidemiol. (2019) 188:1923–31. doi: 10.1093/aje/kwz178
19. Isaksson J, Pettersson E, Kostrzewa E, Diaz Heijtz R, Bölte S. Brief report: association between autism spectrum disorder, gastrointestinal problems and perinatal risk factors within sibling pairs. J Autism Dev Disord. (2017) 47:2621–7. doi: 10.1007/s10803-017-3169-2
20. Abelson N, Meiri G, Solomon S, Flusser H, Michaelovski A, Dinstein I, et al. Association between antenatal antimicrobial therapy and autism spectrum disorder-A nested case-control study. Front Psychiatry. (2021) 12:771232. doi: 10.3389/fpsyt.2021.771232
21. Lee E, Cho J, Kim KY. The association between autism spectrum disorder and pre- and postnatal antibiotic exposure in childhood-a systematic review with meta-analysis. Int J Environ Res Public Health. (2019) 16:E4042. doi: 10.3390/ijerph16204042
22. Ai Y, Zhao J, Shi J, Zhu TT. Antibiotic exposure and childhood attention-deficit/hyperactivity disorder: systematic review and meta-analysis. Psychopharmacology. (2021) 238:3055–62. doi: 10.1007/s00213-021-05989-3
23. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. (2015) 4:1. doi: 10.1186/2046-4053-4-1
24. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
25. A S. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:9491. doi: 10.1007/s10654-010-9491-z
26. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
27. Lavebratt C, Yang LL, Giacobini M, Forsell Y, Schalling M, Partonen T, et al. Early exposure to antibiotic drugs and risk for psychiatric disorders: a population-based study. Transl Psychiatry. (2019) 9:317. doi: 10.1038/s41398-019-0653-9
28. Leviton A, Hooper SR, Hunter SJ, Scott MN, Allred EN, Joseph RM, et al. ELGAN study investigators. Antecedents of screening positive for attention deficit hyperactivity disorder in ten-year-old children born extremely preterm. Pediatr Neurol. (2018) 81:25–30. doi: 10.1016/j.pediatrneurol.2017.12.010
29. Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. (2012) 130:e1447–1454. doi: 10.1542/peds.2012-1107
30. Sassonker-Joseph N, Gorodischer R, Atar-Vardi M, Noyman I, Novack L. Prenatal exposure to antibiotics and development of epilepsy in children. J Clin Pharmacol. (2021) 61:18–24. doi: 10.1002/jcph.1674
31. Miller JE, Pedersen LH, Sun Y, Olsen J. Maternal use of cystitis medication and childhood epilepsy in a Danish population-based cohort. Paediatr Perinat Epidemiol. (2012) 26:589–95. doi: 10.1111/j.1365-3016.2012.01317.x
32. Miller JE, Pedersen LH, Vestergaard M, Olsen J. Maternal use of antibiotics and the risk of childhood febrile seizures: a Danish population-based cohort. PLoS One. (2013) 8:e61148. doi: 10.1371/journal.pone.0061148
33. Miller JE, Pedersen LH, Streja E, Bech BH, Yeargin-Allsopp M, Van Naarden Braun K, et al. Maternal infections during pregnancy and cerebral palsy: a population-based cohort study. Paediatr Perinat Epidemiol. (2013) 27:542–52. doi: 10.1111/ppe.12082
34. O'Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. (1998) 147:362–9. doi: 10.1093/oxfordjournals.aje.a009458
35. Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. JAMA. (1997) 278:207–11.
36. McDermott S, Callaghan W, Szwejbka L, Mann H, Daguise V. Urinary tract infections during pregnancy and mental retardation and developmental delay. Obstet Gynecol. (2000) 96:113–9. doi: 10.1016/s0029-7844(00)00823-1
37. McDermott S, Daguise V, Mann H, Szwejbka L, Callaghan W. Perinatal risk for mortality and mental retardation associated with maternal urinary-tract infections. J Fam Pract. (2001) 50:433–7.
38. Sun Y, Vestergaard M, Christensen J, Nahmias AJ, Olsen J. Prenatal exposure to maternal infections and epilepsy in childhood: a population-based cohort study. Pediatrics. (2008) 121:e1100–1107. doi: 10.1542/peds.2007-2316
39. Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. (2009) 166:1025–30. doi: 10.1176/appi.ajp.2009.08010031
40. Wu YW, Colford JM. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. JAMA. (2000) 284:1417–24. doi: 10.1001/jama.284.11.1417
41. Modabbernia A, Velthorst E, Reichenberg A. Environmental risk factors for autism: an evidence-based review of systematic reviews and meta-analyses. Mol Autism. (2017) 8:13. doi: 10.1186/s13229-017-0121-4
42. Jiang HY, Xu LL, Shao L, Xia RM, Yu ZH, Ling ZX, et al. Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav Immun. (2016) 58:165–72. doi: 10.1016/j.bbi.2016.06.005
43. Gardener H, Spiegelman D, Buka SL. Prenatal risk factors for autism: comprehensive meta-analysis. Br J Psychiatry J Ment Sci. (2009) 195:7–14. doi: 10.1192/bjp.bp.108.051672
44. Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics. (2011) 128:344–55. doi: 10.1542/peds.2010-1036
45. Köhler O, Petersen L, Mors O, Mortensen PB, Yolken RH, Gasse C, et al. Infections and exposure to anti-infective agents and the risk of severe mental disorders: a nationwide study. Acta Psychiatr Scand. (2017) 135:97–105. doi: 10.1111/acps.12671
46. Mitchell RHB, Goldstein BI. Inflammation in children and adolescents with neuropsychiatric disorders: a systematic review. J Am Acad Child Adolesc Psychiatry. (2014) 53:274–96. doi: 10.1016/j.jaac.2013.11.013
47. Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. (2008) 9:46–56. doi: 10.1038/nrn2297
48. Schwarcz R, Bruno JP, Muchowski PJ, Wu H-Q. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. (2012) 13:465–77. doi: 10.1038/nrn3257
49. Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. (2017) 20:145–55. doi: 10.1038/nn.4476
50. Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, et al. Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med. (2016) 22:586–97. doi: 10.1038/nm.4106
51. Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. (2014) 6:263ra158. doi: 10.1126/scitranslmed.3009759
52. Erny D, Hrabě de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. (2015) 18:965–77. doi: 10.1038/nn.4030
53. Hildebrand MS, Dahl H-HM, Damiano JA, Smith RJH, Scheffer IE, Berkovic SF. Recent advances in the molecular genetics of epilepsy. J Med Genet. (2013) 50:271–9. doi: 10.1136/jmedgenet-2012-101448
54. MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. (2015) 213:779–88. doi: 10.1016/j.ajog.2015.05.034
55. Faraone SV, Asherson P, Banaschewski T, Biederman J, Buitelaar JK, Ramos-Quiroga JA, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primer. (2015) 1:15020. doi: 10.1038/nrdp.2015.20
Keywords: prenatal antibiotic exposure, neurodevelopment disorder, autism, attention-deficit/hyperactivity deficit, cerebral palsy, epilepsy
Citation: Tao Q, Shen Y, Li Y, Luo H, Yuan M and Gan J (2022) Prenatal exposure to antibiotics and risk of neurodevelopmental disorders in offspring: A systematic review and meta-analysis. Front. Neurol. 13:1045865. doi: 10.3389/fneur.2022.1045865
Received: 16 September 2022; Accepted: 07 November 2022;
Published: 25 November 2022.
Edited by:
Siddharth Subhash Gaikwad, Tulane University, United StatesReviewed by:
Kiran Kumar Soni, Chonnam National University Medical School, South KoreaTej Nakashe, Kent State University, United States
Copyright © 2022 Tao, Shen, Li, Luo, Yuan and Gan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Gan, Z29yZG9ucmFjaGVsQHNjdS5lZHUuY24=; Z29yZG9ucmFjaGVsMUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Qiuji Tao1,2†
Qiuji Tao1,2† Yang Li
Yang Li Meng Yuan
Meng Yuan Jing Gan
Jing Gan