- 1Department of Acupuncture and Moxibustion, The Third Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
- 2The Third Clinical Medical College, Zhejiang Chinese Medical University, Hangzhou, Zhejiang, China
Background: As a promising neuromodulation technique, vagus nerve stimulation (VNS) has been utilized to treat diverse diseases and the number of VNS studies has grown prosperously. Nonetheless, publication trends and research hotspots in this field remain unknown. This study aimed to perform a bibliometric analysis to systematically identify publication trends and research hotspots in VNS research within a 20-year panorama.
Methods: The Web of Science Core Collection (WoSCC) database was retrieved to screen eligible VNS-related publications from 2002 to 2021. The online analytic tool of the WoSCC database was used to analyze various bibliometric parameters, such as the number of annual publications, the output of countries/regions, journals, total citations, citations per publication, and the Hirsch index. Bibliometrics (http://bibliometric.com/) and CiteSpace (version 5.6.R3) were used to identify research trends and hotspots.
Results: A total of 7,283 publications were included for analysis. The annual number of publications increased stably but it increased significantly in recent years. The top five prolific countries were the United States, China, Germany, England, and France. The top five productive institutions were the University of California (Los Angeles), Harvard Medical School, Harvard University, University College London, and the University of Texas at Dallas. Notably, there was a geographical imbalance in countries and institutions. In addition, Epilepsy & Behavior, Epilepsia, and Plos One were the top three journals with the largest number of VNS publications. Michael P Kilgard was the most prolific author. Moreover, evolving research hotspots mainly included the effectiveness and mechanism of VNS on epilepsy, the role of VNS as an anti-inflammatory regulator, the application of VNS for psychiatric disorders, and the neuromodulation effect of VNS in headache management.
Conclusion: This study has revealed the overall publication trends and evolving research trends at a global level over a 20-year panorama. The potential collaborators, institutions, hotspots, and future research trends are also identified in this field, which will help guide new research directions of VNS.
Introduction
Vagus nerve stimulation (VNS) is a promising neuromodulation technique, administered via a minimally invasive or non-invasive approach. Invasive VNS can be implemented with surgically implanted electrodes, while non-invasive VNS (i.e., nVNS) can be performed by the transcutaneous auricular vagus nerve stimulation (taVNS) to stimulate the auricular branch of the vagus nerve with electrodes placed over specified sites (1). The rationale for selecting the vagus nerve as an attractive target for stimulation to treat a variety of diseases (especially, neurological and psychiatric disorders) is mainly because the wandering path of the vagus nerve intensively communicates with multiple brain structures and visceral organs. Dating back to 1997, VNS has been proven by the United States Food and Drug Administration (FDA) for treating epilepsy (2). Notably, as a non-pharmaceutical, effective, user-friendly, and well-tolerated therapy, taVNS has been widely used for various diseases with numerous benefits. In addition, taVNS has been investigated as a promising approach for treating a wide range of potential therapeutic indications over the past decades, including, but not limited to, headache (3), migraine (4), depression (5), insomnia (6), tinnitus (7), and post-stroke motor speech disorders (8).
Bibliometric analysis, as a well-established quantitative method for analyzing and quantifying literature information, has been adopted in diverse research fields to identify relevant bibliometric parameters (9), such as core scholars/institutions/countries and the cooperative association among them, co-occurrence analysis of keywords, the burst of keywords, and co-citation analysis (10). Such bibliometric parameters and information can significantly contribute to revealing the current status, topic hotspots, and research trends over time in a specific research field (9). Multiple research fields, such as pain medicine (11), anesthesiology (12), neurology (6), respiratory medicine (13), cancer medicine (14), and neuroimaging (15), have adopted this analysis approach to analyze the publication trend on a national or global level.
Although the number of VNS studies continues to grow rapidly in the research field of neuromodulation, to the best of our knowledge, an in-depth bibliometric analysis of VNS has not yet been conducted. Therefore, this study aimed to utilize a bibliometric analysis on VNS for the first time to systematically explore its publication trends and research hotspots over a 20-year panorama, which will further shed the light on future research in this field.
Materials and methods
Source of data and search strategy
Publications were retrieved from the Web of Science Core Collection (WoSCC) database with the following search strings: Topic = (Vagus Nerve Stimulation) OR (Auricular Vagus Nerve Stimulation) OR (Non-invasive Vagus Nerve Stimulation) OR (GammaCore) OR (Nemos), with the limited time frame set from 2002 to 2021. To reduce the deviations caused by daily updates of the database, publications retrieval and data downloads were all accomplished on 20th July 2022. Only original articles and reviews were included, and other study types, such as meeting abstracts, editorial materials, letters, and corrections, were excluded. However, there were no geographic or language restrictions.
Data collection and analysis
All data were retrieved by two authors (RRL and HTH) from the WoSCC. First, the retrieved data were analyzed using the WoSCC online analysis tool to yield various bibliometric parameters, mainly including the number of annual publications, the output of countries/regions, journals, total citations, citations per publication (CPP), and the Hirsch index (H-index). To the best of our knowledge, the H-index is widely used to quantify and standardize the scientific impact of researchers (16), and also accurately reflect the academic achievements of a country or an author; the influence of a study usually increases with the rise of the H-index (17). The impact factor (IF) and quartile of a journal category were obtained from the Journal Citation Reports (JCR) 2021. Any disagreements were resolved by judgment from a third senior reviewer (JQF).
Second, the website of bibliometrics (http://bibliometric.com/) and CiteSpace (version 5.6.R3) software were used to analyze and predict publication trends and hotspots in this field. Specifically, the website of bibliometrics was used to plot the collaboration analysis of countries/regions. CiteSpace (version 5.6.R3) is a tool designed for conducting a visual analytic study of scholarly literature in a specific research field, or a discipline, collectively known as a knowledge domain (18). All the downloaded data were exported into the CiteSpace (version 5.6.R3) to analyze diverse important bibliometric parameters, including co-citation analysis of the countries, journals, institutions, authors, and references, as well as the timeline view of co-cited references. In addition, the keywords with strong citation bursts and cluster maps of all items were captured.
The parameters of CiteSpace (version 5.6.R3) were set as follows: time-slicing was from 2002 to 2021, years per slice (1), all options in the term source were chosen, selected one node type at a time, and selection criteria were top 50 objects. Visualization knowledge figures consist of nodes and links. Each node in the map represented an element, including country, institution, co-cited author, co-cited reference, and keywords. The size of the node represented the frequency of appearance or citation, and the different colors of the nodes indicated the different years. The circles of the different colors from the inside to the outside of the node represented the years from 2002 to 2021. The nodes in the outermost area with a purple ring indicated high centrality, which was usually considered pivotal points or key points in a specific research field. In addition, lines between the nodes revealed cooperation, co-occurrence, or co-citation relationships.
Given that publicly available data were retrieved to perform the bibliometric analysis in this study, and no private information of individuals was involved, an ethical review by an institutional review board was not required.
Results
Annual number of publications and citations
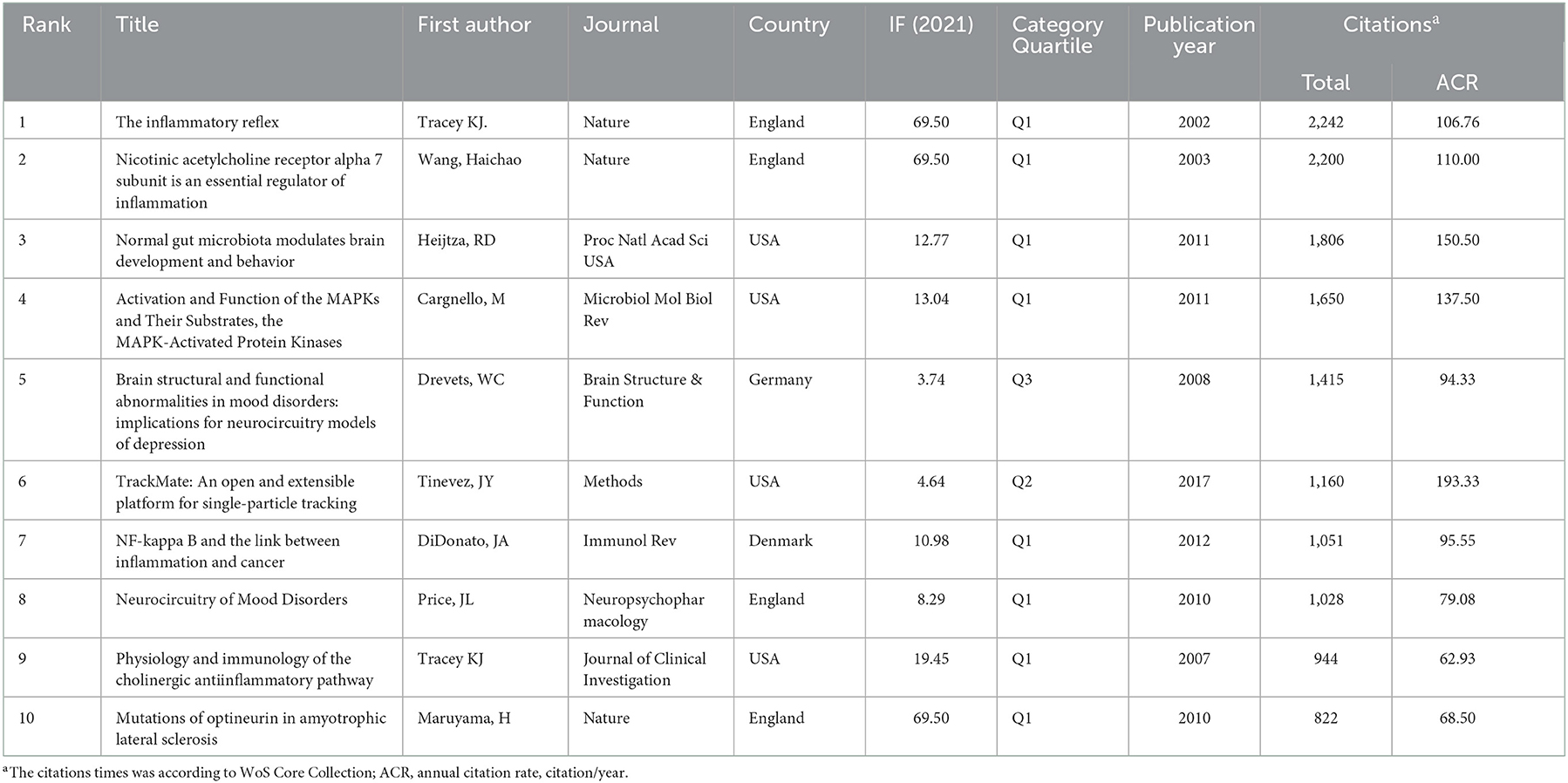
A total of 7,283 non-duplicate publications over the period from 2002 to 2021 were included in the bibliometric analysis, including 5,965 articles and 1,318 reviews. The growth of the annual publication output and the annual citations were shown in Figure 1. Compared with 2002 (n = 191), the growth rate of publication counts in this field was 100.05% in 2011 (n = 392). Notably, as shown in Figure 1, the annual number of articles and citations increased significantly in the past 10 years, reaching the highest point in 2021 (n = 697; citations = 35,972). In addition, the top 10 cited publications were shown in Table 1. The most frequently cited publication was a review published in Nature in 2002 entitled “The inflammatory reflex” with 2,242 citations (19). The annual citation rate (ACR), which was calculated as the ratio between the number of citations and the number of years since its publication, is 106.76 citations/year for this article. The second most frequently cited article (cited 2,200 times and 110.00 citations/year) was “Nicotinic acetylcholine receptor alpha 7 subunit is an essential regulator of inflammation” by HC Wang et al., published in Nature in 2003 (20).
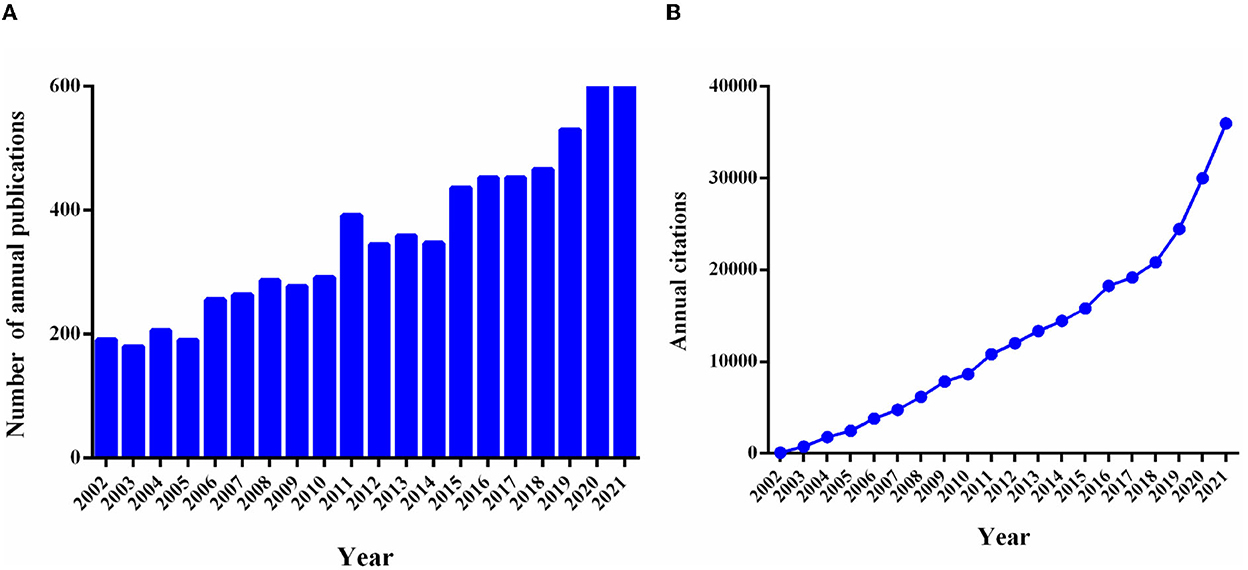
Figure 1. Trends in the number of annual publications and citations in VNS research from 2002 to 2021. (A) The trends of annual global publication outputs and the bar chart reflect the number of articles online per year. (B) The trends of annual global citations; exported the results from the GraphPad Prism (Version 6.01).
Distribution of countries/regions and institutions
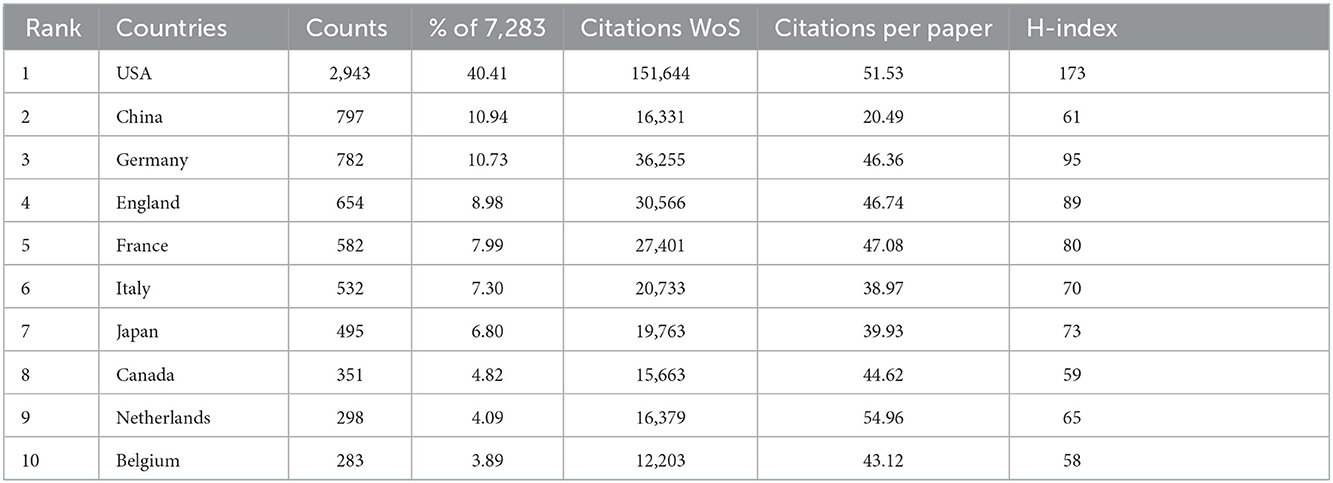
The distribution of all 7,283 publications covered 101 countries/regions. The top 10 countries of publications were demonstrated in Table 2. The United States ranked first in the number of publications (40.41% of 7,283 with 2,943 articles), followed by China (10.94%, with 797 articles), Germany (10.73%, with 782 articles), England (8.98%, with 654 articles), and France (7.99%, with 582 articles). Furthermore, the annual publications output of the 10 most productive countries/regions was identified. As shown in Figure 2A, the most prolific country for annual publications was the United States. It demonstrated that a leading position was maintained by the United States and it had a critical impact on the VNS research. Interestingly, since 2012, the fastest annual growth rate of publications has been in China, indicating that Chinese researchers had increased their enthusiasm in this research field in recent years and more relevant studies were carried out.
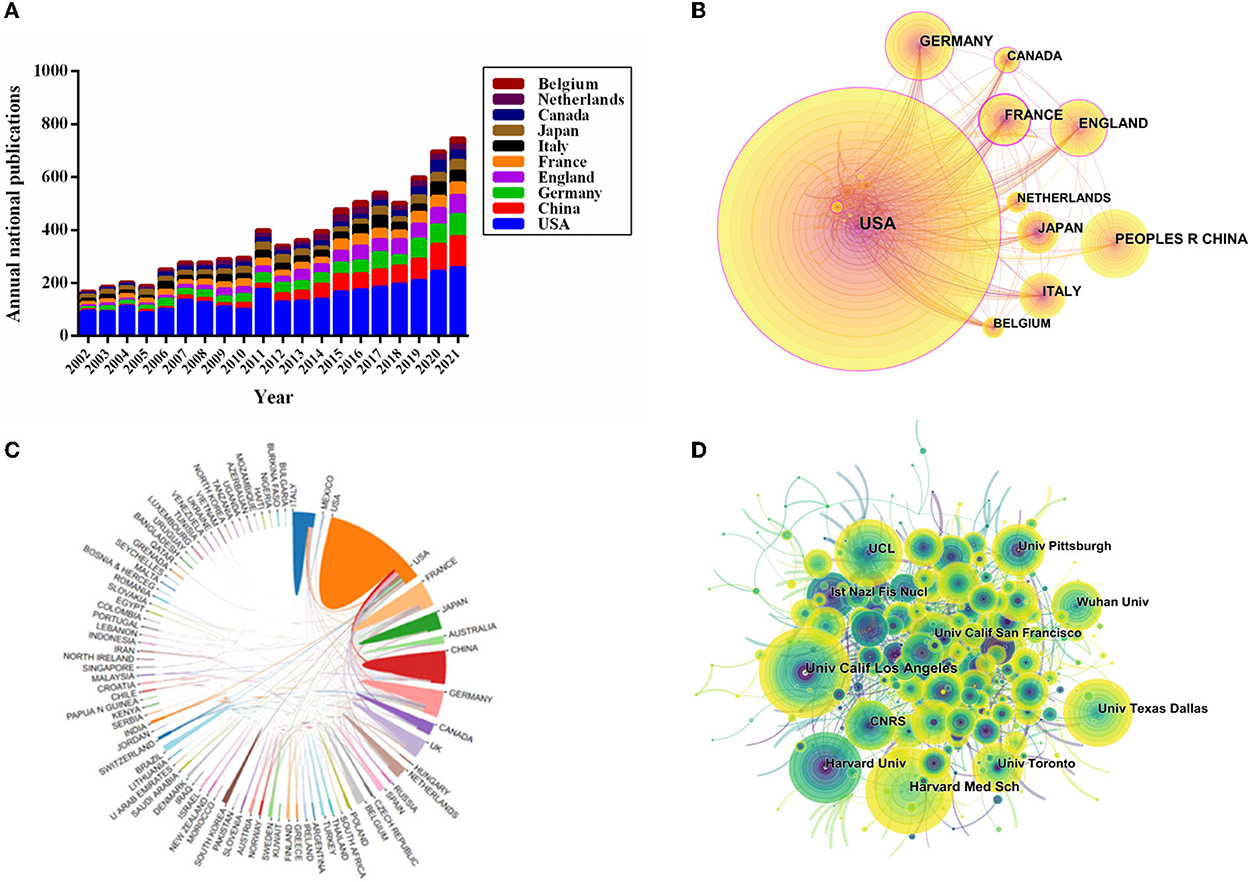
Figure 2. Visualization map of countries/regions and institutions related to VNS research from 2002 to 2021. (A) The annual national publication outputs and growth trends of the top 10 countries/regions via GraphPad Prism (Version 6.01). (B) CiteSpace collaboration network analysis of countries/regions. (C) The map of the international cooperation network in VNS research exported the results from the website of bibliometrics (http://bibliometric.com). (D) In collaboration network analysis of institutions via CiteSpace, the nodes in the map represent institutions, and the lines between the nodes represent collaboration relationships.
International cooperation network analysis among different countries/regions in the VNS research was shown in Figures 2B, C. Further analysis revealed that the closest international collaborative relationship with the United States was with China, followed by Germany, the United Kingdom, France, Canada, and Japan.
Among the top 10 countries/regions of citations, the United States had the highest number of citations (151,644) and the highest value of the H-index (173). China ranked second in publications output (797), nonetheless, the citations (16,331), CCP (20.49), and H-index (61) were still at the lower aspects (Table 2).
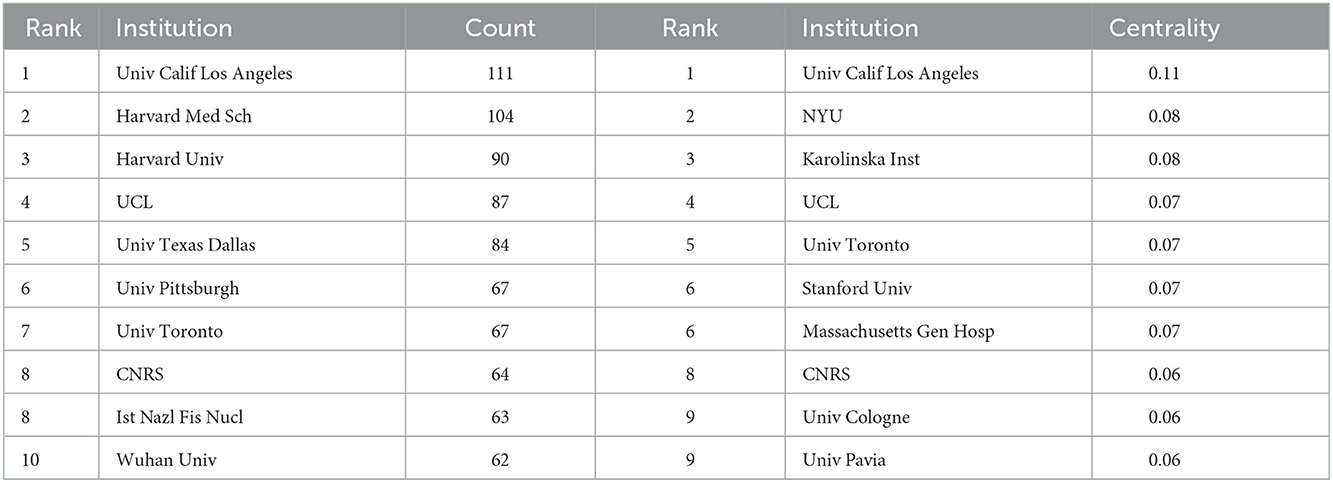
The distribution of institutions map consists of 612 nodes and 1,258 links (Figure 2D), and a total of 612 institutions were dedicated to the research of VNS. Based on Figure 2D, close collaboration among institutions was still inadequate. The leading top five institutions of publications were the University of California, Los Angeles (1.52% with 111 articles), Harvard Medical School (1.43% with 104 articles), Harvard University (1.24% with 90 articles), University College London (1.19% with 87 articles), and the University of Texas at Dallas (1.15% with 84 articles), respectively. The 10 most productive institutions were shown in Table 3. Interestingly, “University of California, Los Angeles” was not only the most prolific institution but also the highest centrality institution.
Distribution of journals
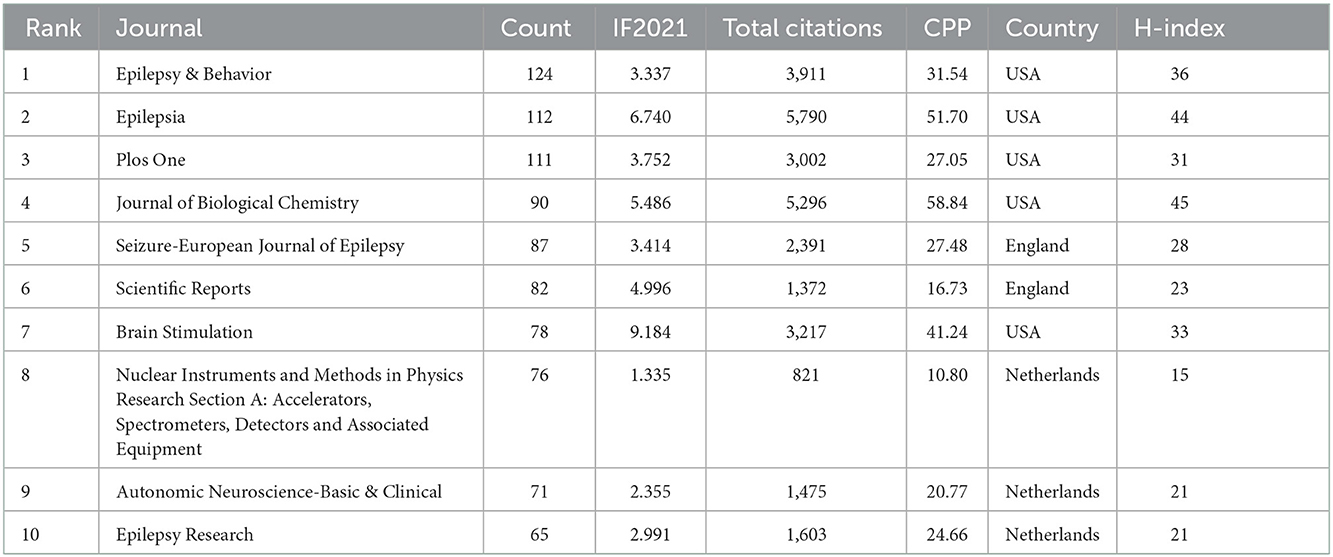
The retrieved publications in this study were published in 1,754 journals and the top 10 productive journals were shown in Table 4. Epilepsy & Behavior was the most productive journal for publishing VNS research (with 124 publications), which had an IF of 3.337 in 2021, followed by Epilepsia (112), Plos One (111), Journal of Biological Chemistry (90), and Seizure-European Journal of Epilepsy (87), respectively. Four journals were related to “epilepsy” among the top 10 prolific journals. Moreover, Epilepsia had the higher total citations (5,790), and the article published by Heck CN in the journal of Epilepsia had the most citations (356) (21), followed by Laxer KD published in Epilepsy & Behavior with 334 citations (22). Journal of Biological Chemistry listed the highest H-index (45), and the journal with the highest IF was Brain Stimulation (IF = 9.184) (Table 4).
Distribution of authors and cited authors
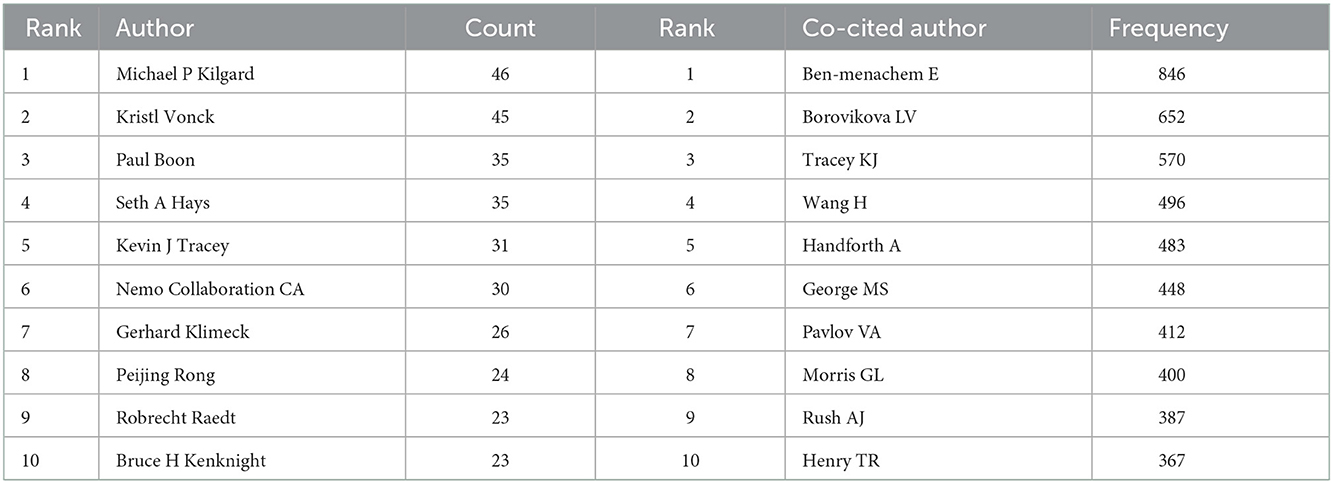
The authors of the 7,283 publications were analyzed, and this resulted in 947 nodes (Figure 3A). In terms of publications outputs, Michael P Kilgard was the most prolific author with 46 publications, followed by Kristl Vonck (45 publications), Paul Boon (35 publications), Seth A Hays (35 publications), and Kevin J Tracey (31 publications), and the top 10 productive authors are shown in Table 5. The map of authors revealed the closeness of collaborations among the authors, which could provide information on influential research groups and potential collaborators (Figure 3A). Based on Figure 3B, the closest cooperation was established in the teams of Ben-Menachem E and Borovikova LV as the core co-cited authors, respectively. Interestingly, they also had the most publication outputs in this field. In terms of the most co-cited authors, Ben-Menachem E ranked first with 846 citations, followed by Borovikova LV (652 citations), Tracey KJ (570 citations), Wang H (496 citations), and Handforth A (483 citations) (Table 5).
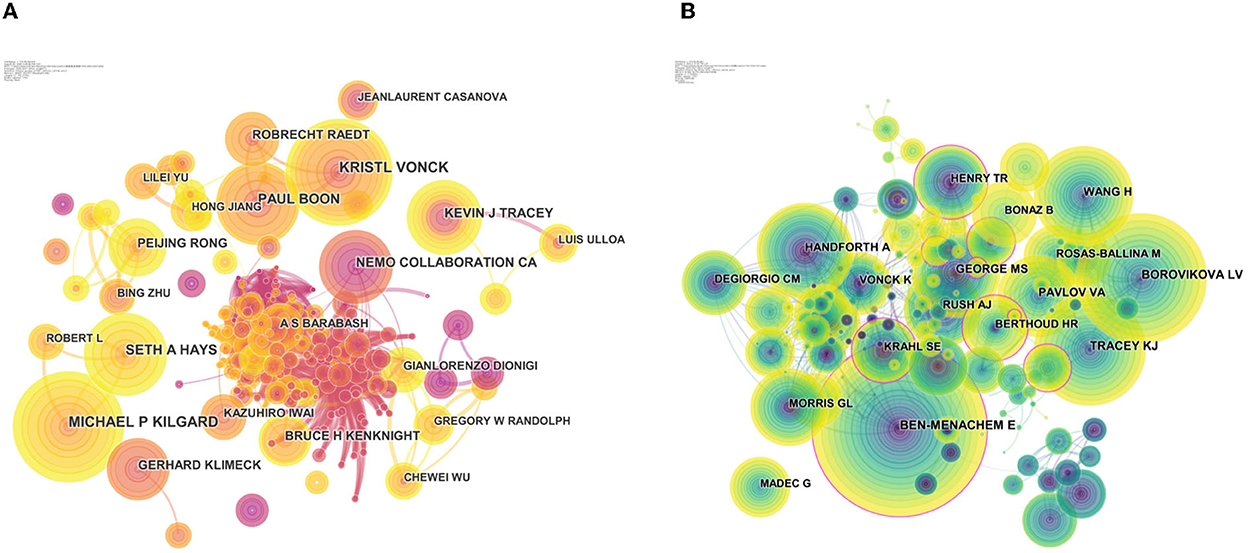
Figure 3. Visualization map of authors/cited authors. (A) CiteSpace visualization map of authors. (B) CiteSpace visualization map of cited authors. The nodes represent the authors and the lines between the nodes represent the collaboration relationships. The larger the node area, the larger the number of publications.
Distribution of cited references
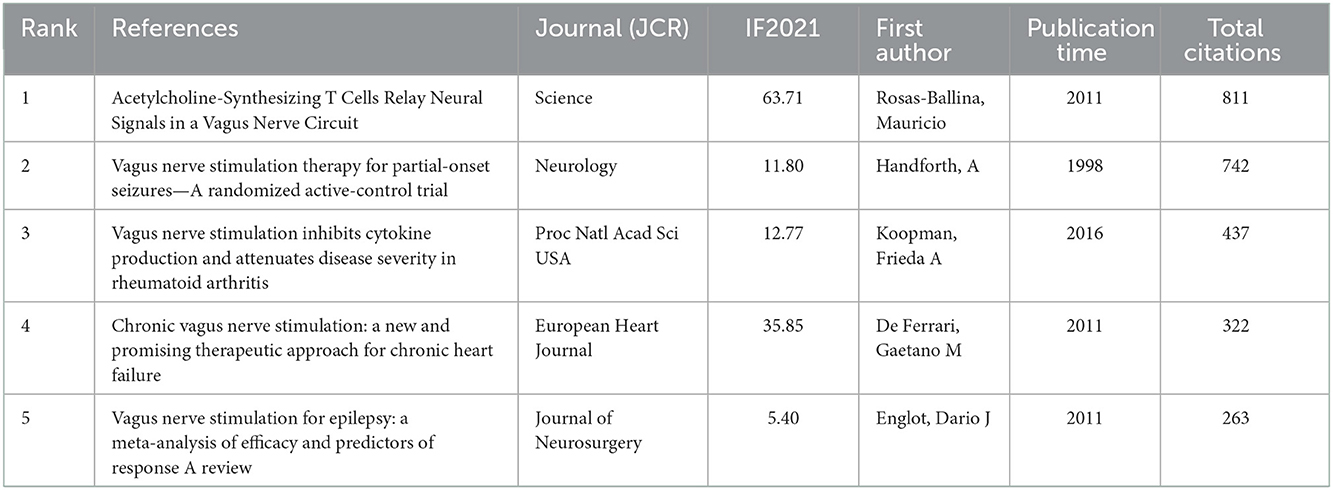
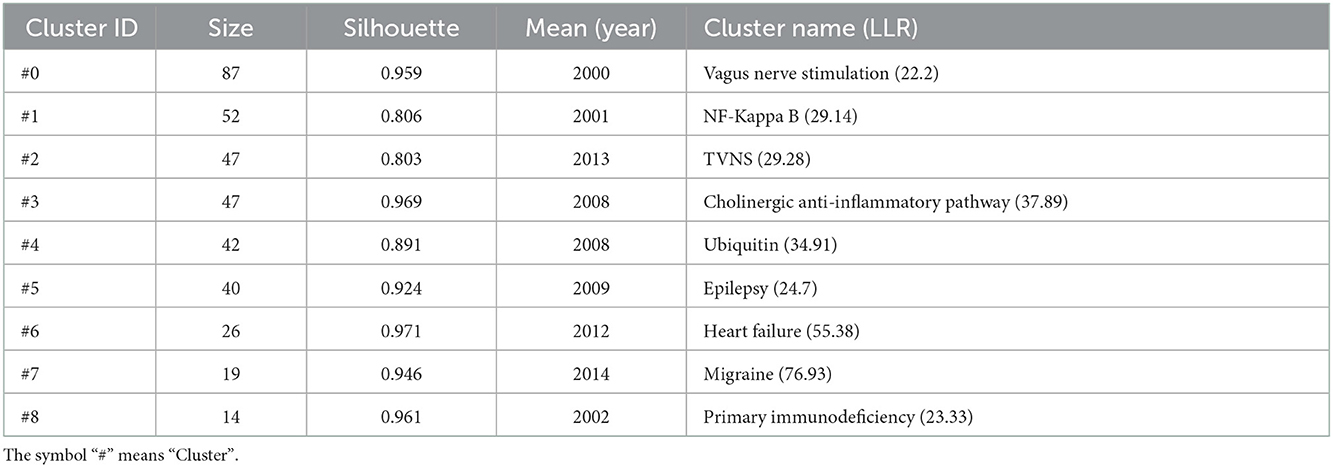
According to the five most frequently co-cited references (Table 6), the article entitled “Acetylcholine-Synthesizing T Cells Relay Neural Signals in a Vagus Nerve Circuit” by Rosas-Ballina and Mauricio was the most cited reference with 811 citations and it had the highest IF (63.71) as well (23). Additionally, the second most cited reference publication was “Vagus nerve stimulation therapy for partial-onset seizures—A randomized active-control trial” published in Neurology with 742 citations (24). Among the top five most frequently co-cited references, two other references were mainly related to anti-inflammatory (25) and improving the quality of life of patients with chronic heart failure (26). Furthermore, the timeline view map of co-cited references related to VNS research was executed by CiteSpace (version 5.6.R3) (Figure 4), and the log-likelihood rate (LLR) was used to find out the distribution of hotspots from references with nine clusters. The cluster with warmer colors indicated the latest research and larger nodes indicated more publications, indicating that this clustering issue was the hotspot in this field. Thus, cluster#2 (TVNS), cluster#3 (cholinergic anti-inflammatory pathway), cluster#5 (epilepsy), cluster#6 (heart failure), and cluster#7 (migraine) indicated the hotspots in VNS research during the past years. The structural characteristics of the cited references clusters were shown in Table 7, including the number of nodes in each cluster, silhouette, mean (year), and the name of clusters. It is generally believed that the cluster with a silhouette value >0.5 was reasonable, while a value >0.7 suggested that the cluster was convincing (27). The silhouette score was higher than 0.8 in all nine major clusters, which revealed the superior quality of clusters (Table 7). The highest silhouette was cluster #6 “heart failure” (0.971), with 26 publications. The biggest cluster was “vagus nerve stimulation”, including 87 nodes and its mean year of publication was 2000. Furthermore, the highest LLR was occupied by cluster #7 “migraine”, which contained 19 references with a rate of 76.93, followed by “heart failure” (55.38) and “cholinergic anti-inflammatory pathway” (37.89). In addition, the LLR of “epilepsy (24.7)” was ranked 7.
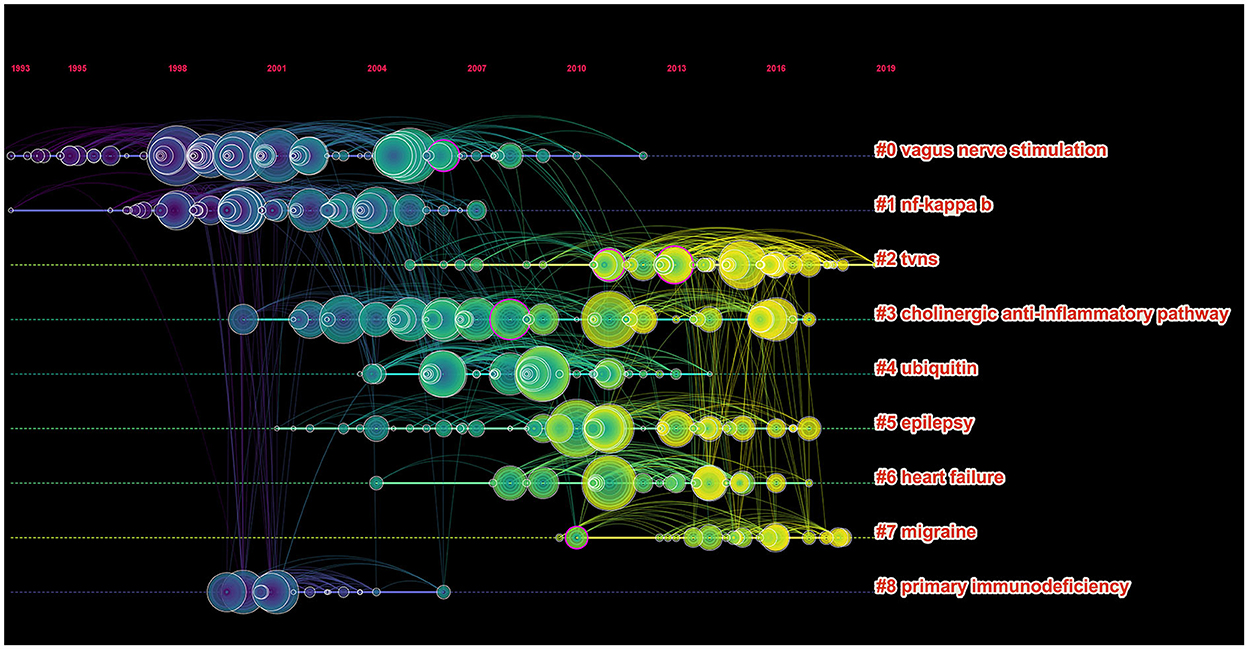
Figure 4. The timeline view clusters of co-cited references and their cluster labels in VNS research from 2002 to 2021, exported the results via CiteSpace, and the cluster with warmer colors and larger nodes contained more publications, indicating that this clustering issue was the hotspot in this field.
Distribution of keywords burst
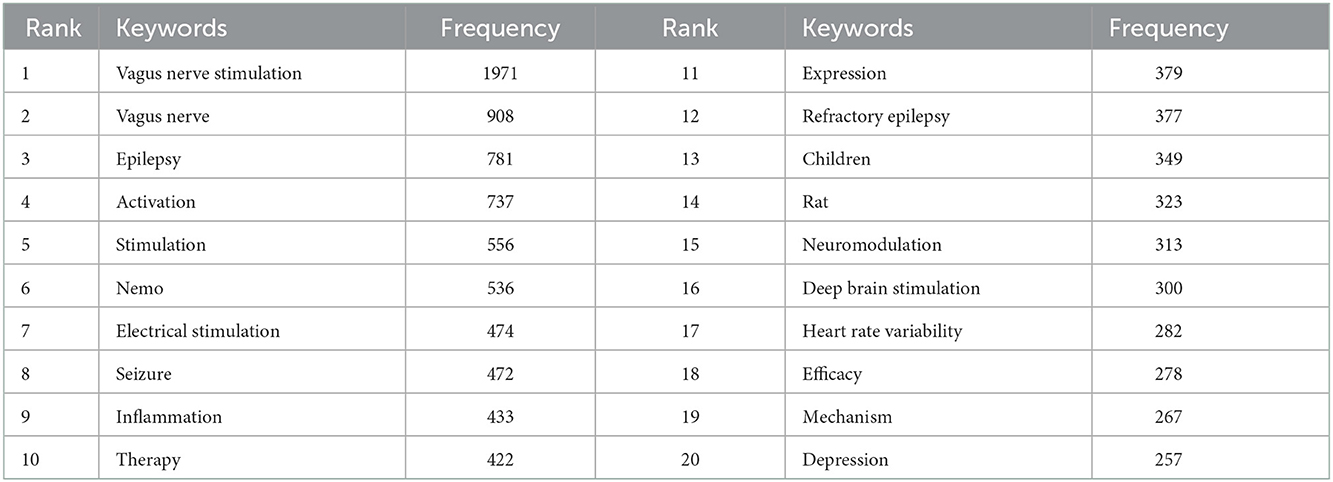
All of the 831 keywords were generated by using CiteSpace (version 5.6.R3). The top 20 keywords in terms of frequency are presented in Table 8. Notably, “epilepsy” was the most frequent keyword (a frequency of 781) in VNS research during the past 20 years, except for the keywords of “vagus nerve stimulation” and “vagus nerve” (28–30). In addition, among the top 100 keywords with the strongest citation bursts, we mainly focused on those keywords that have burst from the past 5 years (Figure 5A) to identify hotspots and research frontiers that change over time (27). The top five burst strength keywords were defined, including “neuromodulation”, with the highest burst strength of 39.9096, followed by “drug-resistant epilepsy” (21.8078), “locus coeruleus” (20.7256), “electrical stimulation” (18.0936), and “heart rate variability” (17.279). In addition, clustering analysis was implemented to reveal the emerging trends in the last 5 years. According to Figure 5B, 10 clusters were identified, including “inflammation”, “epilepsy”, “cluster headache”, “depression”, “anxiety”, “postoperative ileus”, “obesity”, etc.
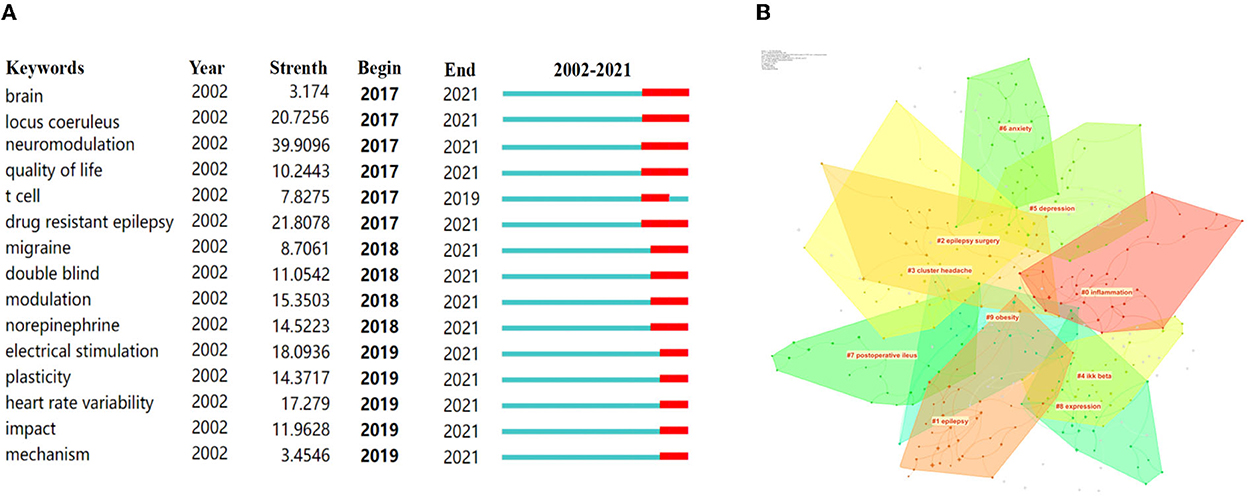
Figure 5. Keywords with the strongest citation burst and top 10 clusters over the past 5 years were exported via CiteSpace. (A) Keywords with the strongest citation bursts among the top 100 burst keywords related to VNS. (B) The 10 clusters of keywords related to VNS. The different colors mean different clusters.
Discussion
Although some previous reviews have examined the application of VNS, all of them were limited to exploring its application to specific diseases (31–35). Hence, the key questions concerning publication trends, research hotspots, and other major bibliometric outcomes in the VNS field need to be urgently solved. Given the urgency of the situation, this study conducted a bibliometric analysis that mainly aimed to quantify and visualize publication trends and research hotspots in the VNS field within a 20-year panorama for the first time.
General information
The annual number of publications and citations in the VNS field increased significantly. Notably, the United States was the main driving force with a high academic status in VNS research, which was confirmed by the highest number of annual publication outputs, as well as the highest citations and the H-index. However, the existence of geographical imbalance in this field should be noted. Only one developing country (i.e., the People's Republic of China) was featured in the top 10 prolific countries over the past 20 years. Therefore, there is an urgent need for developing countries to perform more close cooperation with developed countries to improve their academic status in this field.
In addition, the top three prolific institutions, including the “University of California Los Angeles,” “Harvard Medical School,” and “Harvard University,” pertained to the United States, given that the academic status of the United States in VNS research has been significantly enhanced through close cooperation between these institutions.
References analysis
Among the analysis of cited references, cholinergic anti-inflammatory pathways, heart failure, and migraine were the hotspots in the field of VNS research over the past years. It was considered that VNS could yield anti-inflammatory effects by modulating the production of TNF, thus suggesting its potential value in the treatment of autoimmune and auto-inflammatory diseases by analyzing the most cited references. Additionally, other cited references also have shown that VNS could decrease fatigue and pain in patients with systemic lupus erythematosus (36) and rheumatoid arthritis (37), and provide benefits for inflammation-related gastrointestinal diseases (33). However, it is noteworthy that some research questions remain inconclusive, such as optimal parameters, duration, and stimulation regions of VNS. Given this scenario, randomized controlled trials with large sample sizes are mandatory to yield definitive answers to these questions.
Evolution of research hotspots
The research hotspots are summarized below through an effective combination of top keywords, keywords burst, and cluster analysis. First, the effectiveness and mechanism of VNS for epilepsy are always the research hotspots in this field. As an optimized referral therapeutic option, VNS can reduce seizure frequency and severity in patients with refractory postencephalitic epilepsy (38), whereas the number of studies on optimized stimulation parameters remains insufficient (39). In addition, an updated guideline suggested that VNS may be considered for children with seizures (40). The mechanism of VNS on epilepsy possibly involves modulation of the locus coeruleus, thalamus, and limbic circuit through noradrenergic and serotonergic projections (41, 42). Nonetheless, the specific changes in these cortical circuits remain unknown. Therefore, future research perspectives can focus on (1) revealing the precise mechanism of VNS for epilepsy and (2) improving the efficacy and reducing the side effects of VNS for epilepsy.
Second, the role of VNS as an anti-inflammatory regulator is another emerging research hotspot. Increasing evidence demonstrated that VNS may attenuate inflammatory responses by activating the cholinergic anti-inflammatory pathway (CAP) (19, 43, 44). These findings support the critical role of VNS in the treatment of peripheral cholinergic release and its putative role in the inhibition of inflammation. Furthermore, accumulating evidence has revealed that VNS may be beneficial for multiple peripheral and central inflammatory disorders, such as rheumatic arthritis and irritable bowel syndrome (42, 45). However, further studies are urgently needed to fully elucidate the mechanism underlying the potential role of VNS in the treatment of these inflammatory diseases.
Third, recent research trends are evolving toward the application of VNS for psychiatric disorders, such as anxiety and depression. Nahas et al. revealed that VNS may have a long-term beneficial effect in patients with chronic or recurrent major depressive disorders (46). Additionally, the transmission of neurotransmitters in the medial and prefrontal cortex could be altered by VNS (including serotonin and norepinephrine), which exerts its anticonvulsive and antidepressant effects (47).
Fourth, the neuromodulation effect of VNS in headache management is another emerging research hotspot. As a major modality of neuromodulation techniques, VNS has been established as a safe and effective treatment option for migraine and cluster headaches (CH). It was identified that transcutaneous stimulation of the auricular branch of the vagal nerve (ta-VNS) at 1 Hz was effective for chronic migraine prevention over 3 months (48). In addition, the number of weekly attacks of cluster headaches was decreased significantly by daily nVNS treatment (49). Current widely accepted mechanisms underlying the effectiveness of nVNS in migraine and CH are mainly related to neurotransmitter regulation, nociceptive modulation, effects on autonomic nervous system functions, and inhibition of cortical spreading depression (CSD) (3). Notably, VNS may have therapeutic effects on migraine by inducing the release of norepinephrine and serotonin from the locus coeruleus and dorsal raphe nucleus, respectively, both of which are closely related to the pathogenesis of migraine (50). Given the accumulating evidence in recent years, VNS should be regarded as an important choice for headache management.
Finally, the relationship between VAS and acupuncture deserves attention. Interestingly, acupuncture, especially electroacupuncture (EA), has a certain comparability with VNS. As an important form of acupuncture, EA mainly exerts synergistic effects by needling acupoints and electrical stimulation to strengthen the therapeutic effects. Therefore, EA has similar neuromodulation effects as VNS and can be applied as a neuromodulation therapy. In addition, many classical acupoints are located in the suboccipital and lateral cervical regions, where the superficial branches of the vagus nerve are located (51). VNS has been introduced in the last two decades as a new treatment for refractory epilepsy and depression, while traditional Chinese medicine treats the same disorders by pricking the ear, mastoid, and suboccipital regions in anatomical proximity to the vagus nerve (51). Numerous studies indicated that acupuncture can modulate the activity of the parasympathetic nervous system directly or indirectly by stimulating the vagus nerve (52–54). Therefore, the fields of acupuncture research can refer to VNS studies for a more in-depth analysis of stimulation parameters and anatomical basis, thereby improving the scientific connotation of acupuncture research in future.
Limitations
First, the data were extracted only from the WoSCC, and articles published in other sources (e.g., PubMed and Scopus) may be missed. However, WoSCC is a comprehensive, multidisciplinary, core journal citation index database, which mainly includes the references of the core journals, and it contains six sub-databases, such as Science Citation Index-Expanded. Therefore, the findings of this study are robust. Second, to analyze the full annual data, the time frame of included publications was limited to 2002 and 2021, and publications published in 2022 were not considered. Therefore, hotspots in the VNS field in 2022 may be missed. Nonetheless, the general publication trends and evolving research hotspots in VNS were identified by analyzing publications within a 20-year panorama.
Conclusion
This bibliometric study has revealed the overall publication trends and evolving research trends at a global level over a 20-year panorama. The potential collaborators, institutions, hotspots, and future research trends are also identified in this field, which will help guide new research directions of VNS. All the findings will also guide clinicians, researchers, industry collaborators, policymakers, and other stakeholders.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data, took part in drafting the article or revising it critically for important intellectual content, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by the key plan of Zhejiang Province Traditional Chinese Medicine Prevention and Treatment of Major Diseases of the Health and Family Planning Commission of Zhejiang Province (No. 2018ZY008).
Acknowledgments
We thank all the publications and their authors involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Schoenen J, Ambrosini A. Update on non-invasive neuromodulation for migraine treatment-Vagus nerve stimulation. Prog Brain Res. (2020) 255:249–74. doi: 10.1016/bs.pbr.2020.06.009
2. Toffa DH, Touma L, Meskine TE, Bouthillier A, Nguyen DK. Learnings from 30 years of reported efficacy and safety of vagus nerve stimulation (VNS) for epilepsy treatment: a critical review. Seizure. (2020) 83:104–23. doi: 10.1016/j.seizure.2020.09.027
3. Silberstein SD, Yuan H, Najib U, Ailani J, Morais AL, Mathew PG, et al. Non-invasive vagus nerve stimulation for primary headache: a clinical update. Cephalalgia. (2020) 40:1370–84. doi: 10.1177/0333102420941864
4. Tassorelli C, Grazzi L, de Tommaso M, Pierangeli G, Martelletti P, Rainero I, et al. Non-invasive vagus nerve stimulation as acute therapy for migraine: the randomized PRESTO study. Neurology. (2018) 91:e364–73. doi: 10.1212/WNL.0000000000005857
5. Liu C-H, Yang M-H, Zhang G-Z, Wang X-X, Li B, Li M, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. (2020) 17:54. doi: 10.1186/s12974-020-01732-5
6. Ma Y, Dong M, Mita C, Sun S, Peng C-K, Yang AC. Publication analysis on insomnia: how much has been done in the past two decades? Sleep Med. (2015) 16:820–6. doi: 10.1016/j.sleep.2014.12.028
7. Hyvärinen P, Yrttiaho S, Lehtimäki J, Ilmoniemi RJ, Mäkitie A, Ylikoski J, et al. Transcutaneous vagus nerve stimulation modulates tinnitus-related beta- and gamma-band activity. Ear Hear. (2015) 36:e76–85. doi: 10.1097/AUD.0000000000000123
8. Morrison RA, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to rehabilitation for post-stroke motor speech disorders. Front Neurosci. (2021) 15:715928. doi: 10.3389/fnins.2021.715928
9. Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. (2015) 35:551–559. doi: 10.1002/phar.1586
10. Fazel S, Wolf A. What is the impact of a research publication? Evid Based Ment Health. (2017) 20:33–4. doi: 10.1136/eb-2017-102668
11. Ye J, Ding H, Ren J, Xia Z. The publication trend of neuropathic pain in the world and China: a 20-years bibliometric analysis. J Headache Pain. (2018) 19:110. doi: 10.1186/s10194-018-0941-4
12. Zheng H, Zheng B X, Lin X M. The trend of labor analgesia in the world and China: a bibliometric analysis of publications in recent 30 years. J Pain Res. (2020) 13:517–26. doi: 10.2147/JPR.S232132
13. Ma Y, Zhou R, Wu Q. Global research hotspots and research trends on idiopathic pulmonary fibrosis: a bibliometric and visualization analysis. Ann Palliat Med. (2021) 10:9057–68. doi: 10.21037/apm-21-1836
14. Cyclophosphamide M. Fluorouracil breast cancer treatments: implication for the role of inflammation in cognitive dysfunction. Front Mol Biosci. (2021) 8:683389. doi: 10.3389/fmolb.2021.683389
15. Zhang J, Zhang Y, Hu L, Huang X, Liu Y, Li J, et al. Global trends and performances of magnetic resonance imaging studies on acupuncture: a bibliometric analysis. Front Neurosci. (2020) 14:620555. doi: 10.3389/fnins.2020.620555
16. Larson AR. Implications of sex disparities in the h-index for academic medicine. JAMA Netw Open. (2021) 4:e2112877. doi: 10.1001/jamanetworkopen.2021.12877
17. Würtz M, Schmidt M. The stratified H-index makes scientific impact transparent. Ugeskr Laeger. (2017) 179:14.
18. Chen C. A Glimpse of the First Eight Months of the COVID-19 Literature on Microsoft Academic Graph: Themes, Citation Contexts, and Uncertainties. Front Res Metr Anal. (2020) 5:607286. doi: 10.3389/frma.2020.607286
20. Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. (2003) 421:384–8. doi: 10.1038/nature01339
21. Massey AD, Nair DR., Jobst BC, Barkley GL, et al. Two-year seizure reduction in adults with medically intractable partial onset epilepsy treated with responsive neurostimulation: final results of the RNS System Pivotal trial. Epilepsia. (2014) 55:432–41. doi: 10.1111/epi.12534
22. Laxer KD, Trinka E, Hirsch LJ, Cendes F, Langfitt J, Delanty N, et al. The consequences of refractory epilepsy and its treatment. Epilepsy Behav. (2014) 37:59–70. doi: 10.1016/j.yebeh.2014.05.031
23. Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, et al. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. (2011) 334:98–101. doi: 10.1126/science.1209985
24. DeGiorgio CM, Schachter SC., Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology. (1998) 51:48–55. doi: 10.1212/WNL.51.1.48
25. Koopman FA, Chavan SS., Miljko S, Grazio S, Sokolovic S, Schuurman PR, et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci USA. (2016) 113:8284–9. doi: 10.1073/pnas.1605635113
26. De Ferrari GM, Crijns HJGM., Borggrefe M, Milasinovic G, Smid J, Zabel M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. (2011) 32:847–55. doi: 10.1093/eurheartj/ehq391
27. Chen C. CiteSpace: A Practical Guide for Mapping Scientific Literature. Hauppauge, NY: Nova Science Publishers, Incorporated (2016).
28. Krahl SE, Senanayake SS, Handforth A. Destruction of peripheral C-fibers does not alter subsequent vagus nerve stimulation-induced seizure suppression in rats. Epilepsia. (2001) 42:586–9. doi: 10.1046/j.1528-1157.2001.09700.x
29. Schwartz TH, Spencer DD. Strategies for reoperation after comprehensive epilepsy surgery. J Neurosurg. (2001) 95:615–23. doi: 10.3171/jns.2001.95.4.0615
30. Boon P, Vonck K, Van Walleghem P, D'Havé M, Goossens L, Vandekerckhove T, et al. Programmed and magnet-induced vagus nerve stimulation for refractory epilepsy. J Clin Neurophysiol. (2001) 18:402–7. doi: 10.1097/00004691-200109000-00003
31. Dibué M, Greco T, Spoor JKH, Tahir Z, Specchio N, Hänggi D, et al. Vagus nerve stimulation in patients with Lennox-Gastaut syndrome: a meta-analysis. Acta Neurol Scand. (2021) 143:497–508. doi: 10.1111/ane.13375
32. Fetzer S, Dibué M, Nagel AM, Trollmann R. A systematic review of magnetic resonance imaging in patients with an implanted vagus nerve stimulation system. Neuroradiology. (2021) 63:1407–17. doi: 10.1007/s00234-021-02705-y
33. Lei W, Duan Z. Advances in the treatment of cholinergic anti-inflammatory pathways in gastrointestinal diseases by electrical stimulation of vagus nerve. Digestion. (2021) 102:128–38. doi: 10.1159/000504474
34. Wang Y, Zhan G, Cai Z, Jiao B, Zhao Y, Li S, et al. Vagus nerve stimulation in brain diseases: Therapeutic applications and biological mechanisms. Neurosci Biobehav Rev. (2021) 127:37–53. doi: 10.1016/j.neubiorev.2021.04.018
35. Wilks SJ, Melamed SE. Responsive vagus nerve stimulation for drug resistant epilepsy: a review of new features and practical guidance for advanced practice providers. Front Neurol. (2020) 11:610379. doi: 10.3389/fneur.2020.610379
36. Aranow C, Atish-Fregoso Y, Lesser M, Mackay M, Anderson E, Chavan S, et al. Transcutaneous auricular vagus nerve stimulation reduces pain and fatigue in patients with systemic lupus erythematosus: a randomised, double-blind, sham-controlled pilot trial. Ann Rheum Dis. (2021) 80:203–8. doi: 10.1136/annrheumdis-2020-217872
37. Rasmussen SE, Møller HJ., Brock B, Deleuran BW, et al. Short-term transcutaneous non-invasive vagus nerve stimulation may reduce disease activity and pro-inflammatory cytokines in rheumatoid arthritis: results of a pilot study. Scand J Rheumatol. (2021) 50:20–7. doi: 10.1080/03009742.2020.1764617
38. Sun Y, Chen J, Fang T, Wan L, Shi X, Wang J, et al. Vagus nerve stimulation therapy for the treatment of seizures in refractory postencephalitic epilepsy: a retrospective study. Front Neurosci. (2021) 15:685685. doi: 10.3389/fnins.2021.685685
39. Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimul. (2016) 9:356–63. doi: 10.1016/j.brs.2015.11.003
40. Morris GL, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the guideline development subcommittee of the American academy of neurology. Epilepsy Curr. (2013) 13:297–303. doi: 10.5698/1535-7597-13.6.297
41. de Oliveira TVF, Francisco AN. Junior ZD, Stebel SL. The role of vagus nerve stimulation in refractory epilepsy. Arq Neuropsiquiatr. (2017) 75:657–66. doi: 10.1590/0004-282x20170113
42. Johnson RL, Wilson CG. A review of vagus nerve stimulation as a therapeutic intervention. J Inflamm Res. (2018) 11:203–13. doi: 10.2147/JIR.S163248
43. Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. (2000) 405:458–62. doi: 10.1038/35013070
44. Matteoli G, Gomez-Pinilla PJ, Nemethova A, Di Giovangiulio M, Cailotto C, van Bree SH, et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. (2014) 63:938–48. doi: 10.1136/gutjnl-2013-304676
45. Han B, Li X, Hao J. The cholinergic anti-inflammatory pathway: an innovative treatment strategy for neurological diseases. Neurosci Biobehav Rev. (2017) 77:358–68. doi: 10.1016/j.neubiorev.2017.04.002
46. Marangell LB, Husain MM., Rush AJ, Sackeim HA, Lisanby SH, et al. Two-year outcome of vagus nerve stimulation (VNS) for treatment of major depressive episodes. J Clin Psychiatry. (2005) 66:1097–104. doi: 10.4088/JCP.v66n0902
47. Bajbouj M, Merkl A, Schlaepfer TE, Frick C, Zobel A, Maier W, et al. Two-year outcome of vagus nerve stimulation in treatment-resistant depression. J Clin Psychopharmacol. (2010) 30:273–81. doi: 10.1097/JCP.0b013e3181db8831
48. Straube A, Ellrich J, Eren O, Blum B, Ruscheweyh R. Treatment of chronic migraine with transcutaneous stimulation of the auricular branch of the vagal nerve (auricular t-VNS): a randomized, monocentric clinical trial. J Headache Pain. (2015) 16:543. doi: 10.1186/s10194-015-0543-3
49. Gaul C, Diener HC, Silver N, Magis D, Reuter U, Andersson A, et al. Non-invasive vagus nerve stimulation for PREVention and Acute treatment of chronic cluster headache (PREVA): a randomised controlled study. Cephalalgia. (2016) 36:534–46. doi: 10.1177/0333102415607070
50. D'Andrea G. Pathogenesis of chronic migraine: the role of neuromodulators. J Headache Pain. (2015) 16:A38. doi: 10.1186/1129-2377-16-S1-A38
51. Da SM, Dorsher PT. Neuroanatomic and clinical correspondences: acupuncture and vagus nerve stimulation. J Altern Complement Med. (2014) 20:233–40. doi: 10.1089/acm.2012.1022
52. Lim HD, Kim MH. Lee CY, Namgung U. Anti-inflammatory effects of acupuncture stimulation via the vagus nerve. PLoS ONE. (2016) 11:e151882. doi: 10.1371/journal.pone.0151882
53. Boehmer AA, Georgopoulos S, Nagel J, Rostock T, Bauer A, Ehrlich JR. Acupuncture at the auricular branch of the vagus nerve enhances heart rate variability in humans: an exploratory study. Heart Rhythm O2. (2020) 1:215–21. doi: 10.1016/j.hroo.2020.06.001
Keywords: vagus nerve stimulation, neuromodulation, neurophysiology, bibliometrics, visualization
Citation: Li R, Hu H, Luo N and Fang J (2022) Bibliometric analysis of publication trends and research hotspots in vagus nerve stimulation: A 20-year panorama. Front. Neurol. 13:1045763. doi: 10.3389/fneur.2022.1045763
Received: 16 September 2022; Accepted: 29 November 2022;
Published: 21 December 2022.
Edited by:
Bernhard Schaller, University of Zurich, SwitzerlandReviewed by:
Stefan Kampusch, AURIMOD GmbH, AustriaTaipin Guo, Yunnan University of Traditional Chinese Medicine, China
Xueling Ma, Beijing University of Chinese Medicine, China
Copyright © 2022 Li, Hu, Luo and Fang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianqiao Fang,  ZmFuZ2ppYW5xaWFvNzUzMkAxNjMuY29t
ZmFuZ2ppYW5xaWFvNzUzMkAxNjMuY29t
†These authors have contributed equally to this work
 Rongrong Li
Rongrong Li Hantong Hu
Hantong Hu Ning Luo
Ning Luo Jianqiao Fang1,2*
Jianqiao Fang1,2*