
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 11 January 2023
Sec. Neuromuscular Disorders and Peripheral Neuropathies
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1039529
This article is part of the Research Topic Surgical Treatment of Peripheral Neuropathic Pain, Peripheral Nerve Tumors, and Peripheral Nerve Injury View all 25 articles
 Hu Yang1†
Hu Yang1† Yanzhao Dong1†
Yanzhao Dong1† Zewei Wang2
Zewei Wang2 Jingtian Lai2
Jingtian Lai2 Chenjun Yao2
Chenjun Yao2 Haiying Zhou1
Haiying Zhou1 Ahmad Alhaskawi1
Ahmad Alhaskawi1 Sohaib Hasan Abdullah Ezzi2
Sohaib Hasan Abdullah Ezzi2 Vishnu Goutham Kota2
Vishnu Goutham Kota2 Mohamed Hasan Abdulla Hasan Abdulla2
Mohamed Hasan Abdulla Hasan Abdulla2 Hui Lu1,3*
Hui Lu1,3*Traumatic neuromas are infrequent in clinical settings but are prevalent following trauma or surgery. A traumatic neuroma is not a true malignancy, rather, it is a hyperplastic, reparative nerve reaction after injury and typically manifests as a nodular mass. The most common clinical manifestations include painful hypersensitivity and the presence of a trigger point that causes neuralgic pain, which could seriously decrease the living standards of patients. While various studies are conducted aiming to improve current diagnosis and management strategies via the induction of emerging imaging tools and surgical or conservative treatment. However, researchers and clinicians have yet to reach a consensus regarding traumatic neuromas. In this review, we aim to start with the possible underlying mechanisms of traumatic neuromas, elaborate on the diagnosis, treatment, and prevention schemes, and discuss the current experiment models and advances in research for the future management of traumatic neuromas.
The history of neuromas dates back to 1634 when Ambroise Pare first described the painful neuroma and treated neuroma with massage and oil. In 1811, Odier (1) discovered that the bulbous stump tissue in the proximal ending of a transected nerve could be extremely sensitive. It was not until 1828 that the term “neuroma” was coined by Wood (2), who conducted the first pathological research on such nerve injuries. From then on, the understanding of traumatic neuromas grew steadily, including the introduction of Morton's neuroma by Morton (3), the elucidation of the actual mechanism forming these neuromas by Huber and Lewis (4), and the classification system put forward by Swanson (5), who also defined neuromas as cutaneous and post-traumatic.
A neuroma is not a conventional neoplasm that arises from malignant cells, but a local non-neoplastic proliferation of the injured nerve. Neuromas mostly occur when normal nerve conduction is damaged by injury, inadequate surgical repair, or in some cases, chronic fibro-inflammatory irritation. In this case, the reinnervation process is deterred when nerve reconstruction is interrupted by the interpolation of scar tissue. Depending on the nature of the trauma, traumatic neuromas can be largely categorized into terminal neuroma and neuroma-in-continuity (6). Terminal neuromas are generally observed in limb amputation, lower extremities in particular, where the nerve is completely transected without a distal end, rendering it impossible to reestablish innervation by the injured nerve. On the other hand, neuroma-in-continuity results from a fusiform swelling of the injured site following the trauma and is further divided into two subtypes: (1) total or partial transection of the nerve; (2) repeated blunt trauma to the nerve. The former type of trauma could cause fusiform neuromas, which are commonly seen in the digital nerve and median nerve, while the latter form of trauma causes thickening of the fibrous tissue surrounding the nerve, which is involved in cases of Morton neuroma, Bowler's thumb (7), and lateral femoral cutaneous neuropathy (8). It can be concluded that given the mechanism of traumatic neuromas, it is highly related to traumatic limb injury and occurs mostly in orthopedic patients.
The most common main complaint in patients suffering from peripheral traumatic neuromas remains paresthesia in the innervated area and a painful nodule at the site of injury. Pain, as mentioned before, is among the most common clinical symptoms, including painful hypersensitivity and the presence of a trigger point that causes neuralgic pain. Patients may feel burning, stabbing, raw, gnawing, or sickening sensations. These symptoms could lead to psychological distress and a severe decrease in the quality of life (9). In this review, we aimed to provide a comprehensive understanding of traumatic peripheral neuroma and its related research progress.
Traumatic neuromas, as infrequent as it is, are prevalent following trauma or surgery. As previously described, a traumatic neuroma is not a true malignancy. Instead, it is a hyperplastic, reparative nerve reaction after injury and typically manifests as a nodular mass. It is secondary to the abnormal growth of nerves and connective tissue attempting to reinnervate the region following an entire or partial nerve segment due to an accidental or surgical trauma (10, 11). As nerve lesions, traumatic neuromas are characterized as neuroma-in-continuity (NIC) following partial nerve transection or end-bulb neuromas (EBN) following the total disruption (12). Traumatic neuromas exhibit proximal continuity with the parent nerve, similar to the “tail sign” shown in peripheral nerve sheath tumors (PNSTs), indicating a neurogenic origin. On the other hand, EBNs do not present distal continuity with the parent nerve, whereas NICs are continuous both proximally and distally (13).
Many studies have found that inhibiting nerve growth factor (NGF) following nerve damage lowers neuroma growth and neuropathic pain in rat models (14). Furthermore, localized deactivation of the brain-derived nerve factor (BDNF) has been found to greatly reduce neuropathic pain and influence the regeneration of sensory fibers. In contrast, excessive concentrations of BDNF promote the growth of neuromas and neuropathic pain (15).
Traumatic damage to a peripheral nerve leads to multidimensional cell proliferation, regeneration failure, and deformed architecture of the nerve. Due to post-traumatic obstruction of axonal flow and subsequent Wallerian degeneration, the nerve segment distant to the site of damage has unique and complicated physiology (16).
Nevertheless, the ability of axons to regenerate and the growing support of Schwann cells (SCs) decrease with time and distance from a trauma (17). On condition that two severed nerve segments are distant respectively, or the proximal end is missing (amputations), axon regeneration occurs in an unstructured manner (18, 19). In some instances, the simultaneous proliferation of wound-healing cells and signaling molecules might result in collagen remodeling and scar formation and eventually forms a neuroma (9). Several studies and case reports have defined traumatic neuroma as having a tangled shape consisting of connective tissue, Schwann cells, and regenerated axons (20–23).
In painful neuromas, inflammatory signaling factors (22, 23) and myofibroblasts (24) have been reported. While the microscopic characteristics of a fully formed neuroma have been thoroughly recorded, little is known about the cellular structure of neuromas in their early stages of development, from nerve damage to initial neuroma formation. Further studies in this field is helpful to generate new and improved therapies that target the earliest stages of neuroma development and prevent the accompanying discomfort (25).
As a result of injuries or surgical procedures, traumatic neuromas generally present as a firm, oval, slow-growing, palpable nodule with a painful sensation, not larger than 2 cm in diameter. Common symptoms include pain, stiffness, pain hypersensitivity to light tactile stimuli, or neuralgic pain with a trigger point (26). It is vitally important that clinicians pay close attention to the previous medical history of patients with the above symptoms for an accurate initial diagnosis.
Regarding histopathology, Seddon's initial classification of peripheral nerve injuries is based on a three-tiered severity scale: neurapraxia, axonotmesis, and neurotmesis (27). In terms of Electrodiagnosis, axonotmesis and neurotmesis have the same characteristics. The symptoms of axonotmesis are reversible, but those of neurotmesis are irreversible due to disordered axon regeneration. In such instances, complete realignment of the sectioned fascicles and optimum neural tube repair necessitates surgery (28). Following Sudden, the traumatic damage to peripheral nerves was further classified into five classes by Sunderland (29). However, these categories are determined using a presumed prognosis without objective data on the anatomical damage. From this perspective, imaging may be useful for distinguishing between reversible axonotmesis and irreversible neurotmesis.
As trauma majorly affects working-age individuals, delayed management also causes economic and social harm (30, 31). While the classification systems mentioned proved effective in clinical practice, more often than not clinicians are faced with the dilemma that the injured nerve may branch into more regional nerves, even along the length of a nerve the degree of injury may vary. The localization of traumatic neuromas primarily depends on physical examination and medical history, and the application of medical imaging tools. Ultrasound (US) and magnetic resonance imaging (MRI) can be utilized to examine the anatomy and topography of peripheral nerves in order to determine the location, extent, and type of damage (32–37). In examining a limb trauma, it is crucial to examine the probability of nerve structure involvement. A thorough clinical history, physical examination, and electrodiagnostic testing (electromyography—EMG and nerve conduction velocity studies—NCVs) are sufficient to diagnose a nerve injury; nevertheless, a complete qualitative and quantitative assessment of the structural damage is not possible. Determining the kind of anatomical damage and the injury's severity is crucial in deciding whether surgical therapy is necessary since time is crucial for a successful prognosis (38). In addition, a comprehensive morphological diagnosis of a traumatic lesion is crucial in determining optimal care (conservative or surgical treatment) (32, 39). Therefore, the clinical examination strongly recommends morphological imaging using US or MRI (Table 1). The US is a useful utility not only to access the continuity of nerve but also in post-operative follow-up and detection of complications (39). MRI T2-weighted imaging provides high-resolution imaging of peripheral nerve anatomy in combination with fat and flow suppression (34, 41). Many researchers recommend MRI for examining anatomical nerve damage, considering it appropriate for a high-quality assessment (42–45). On the other hand, other researchers believe the two procedures are complementary, favoring the US as an initial examination and MRI for evaluating anatomical regions where US access is difficult or impossible or where US details seem insufficient or do not correspond with clinical suspicion and/or EMG results (33, 36).
Currently, few researchers have compared the diagnostic accuracy of the two techniques (36, 46), indicating that both US and MRI can detect the damaged region with a high level of anatomical details and pathological results that correspond with EMG testing. In research by Zaidman et al. (36), it was determined that the US had a greater sensitivity than MRI (93 vs. 67%, respectively), assuming the same level of specificity (86%). In contrast, Aggarwal et al. (46) observed that MRI is more sensitive than US (95 vs. 81%), attributing this to the deployment of a high-field (3 Tesla) MRI scanner; nevertheless, these scanners are not yet accessible for all clinicians. The advantages and limitations of US and MRI are listed in Table 2. The US offers a more affordable alternative, and while well-tolerated by patients, the US shows results in real time. Obtaining dynamic information is also achievable if the patient is requested to undertake certain actions while the physician performs the exam (Figure 1) (60). This dynamic examination provides essential objective data for an overall evaluation of nerve and surrounding tissue damage. On the other hand, high-resolution ultrasound (HRU) is now been identified as a useful tool in the diagnosis of lesions of traumatic neuromas (39, 61–63). HRU can achieve imaging of all main nerves running the limbs including the medial nerve, ulnar nerve, radial nerves, sciatic nerves, common peroneal nerves, and posterior tibial nerves, while still demonstrating the transections, lacerations, hematoma or neuroma formation clearly and accurately (35).
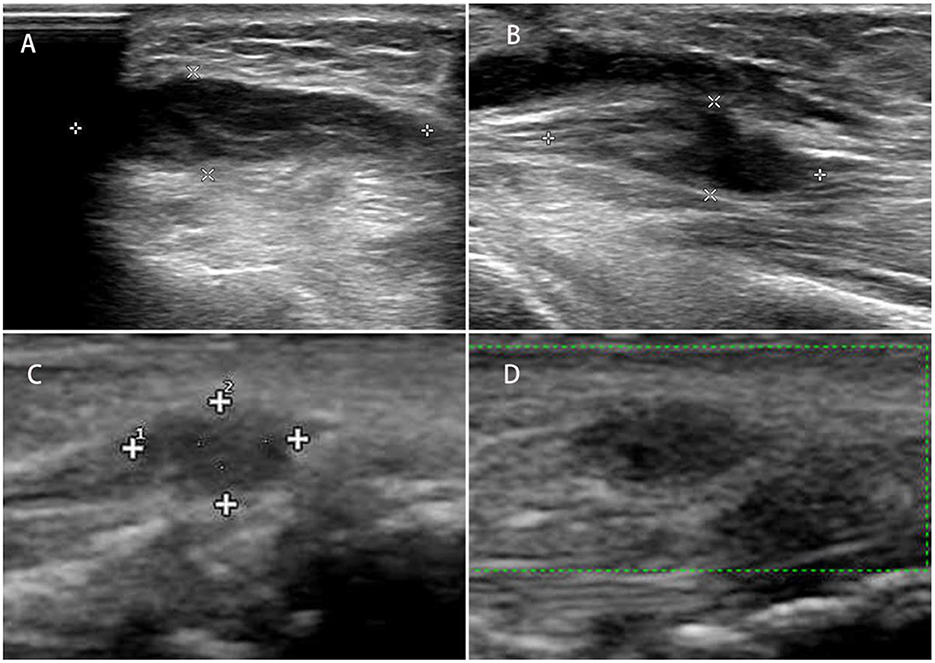
Figure 1. (A) Longitudinal and transverse ultrasound images of the left traumatic neuroma of the superficial branch of the radial nerve; (B) longitudinal and transverse images of the neuroma; (C, D) traumatic neuroma of the superficial branch of the radial nerve of the left wrist.
Early diagnosis of the location and type of a lesion is crucial for prognosis and treatment since there is a limited clear opportunity for efficient reinnervation and, if fails, the commencement of alternative treatment measures, such as nerve surgery. Nerve recovery begins immediately upon axonal transection when the denervated muscle fibers and skin regions begin producing neurotrophic signals that attract any adjacent surviving axons and induce sprouting of these axons into the denervated tissue (64). The rapid process of reinnervation is known as collateral reinnervation, where the surviving axons in the fascicle innervate the motor units and skin regions of their injured counterparts. In severe nerve damage, when over 75% of the axons in a fascicle is destroyed, the remaining axons in the bundle will be unable to reinnervate every motor unit and skin region, which calls for proximal ingrowth of new axons. The rate of proximal reinnervation is around 1 mm each day, which can be deterred if the length between the nerve lesion and target muscle is too great, as in the case of distal muscles of the lower leg and sciatic nerve injury, since over time there are irreversible alterations in the muscle and neural axon tubes that inhibit further abnormal growth and reinnervation (65). Moreover, injury to the connective tissue constructs of the nerves may result in perineural fibrosis and scarring that can inhibit future axon development. The therapeutic frame for nerve surgery focuses on an effective evaluation of the extent of the injury, which is typically performed clinically and may be enhanced by electromyography (EMG) via localizing the exact site of the lesion and the distance between it and the influenced muscle and skin, and identifying morphologic changes of the damaged nerve that indicate transaction or intraneural scarring. By analyzing the regeneration potential, this data will influence surgical decision-making (66).
The process of nerve reconstruction is accompanied by scar formation in the injured site, which eventually results in traumatic neuroma formation (67, 68). Treatment of traumatic neuromas is based on the removal of disturbed nervous tissue or neurolysis to improve the microenvironment surrounding axons and achieving partial or complete remission of painful symptoms (Table 3). Once the neuroma is formed, the priority for clinicians is to relieve symptoms and limit further disease development (54).
Surgical treatment is so far the most effective therapeutic method to manage peripheral traumatic neuromas (Figure 2). Various operative techniques have been invented for the management of peripheral traumatic neuroma occurring at different sites (69, 70), including nerve repair, distal nerve ending transposition to muscle, vein, or bone (71, 72), capping of distal nerve-ending with soft tissues or conduits (73–76). In the surgical management of traumatic neuroma, one predominant factor for the selection of operative technique is the continuity of the severed nerve, that is whether there is an adjacent distal nerve stump for future nerve reconstruction and reinnervation. As the described condition is vitally important and greatly affects the selection of operation method, conventional surgical treatment in this section is categorized following this criterion before the proposition of several emerging surgical approaches (Figure 3).
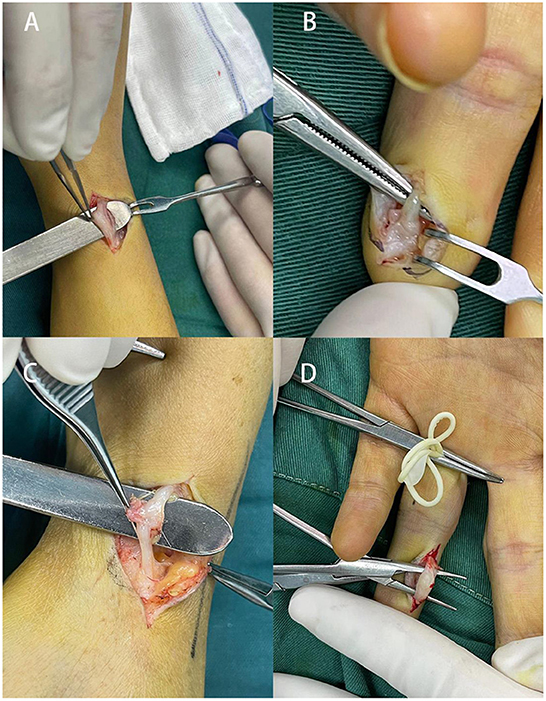
Figure 2. Cases of surgical management in four patients suffering from traumatic neuromas. (A) A 32-year-old female patient with a 2-year history of painful mass on the radial side of the right wrist; (B) a traumatic neuroma of the middle finger in a 34-year-old female patient as a result of previous finger tope amputation, (C) a 24-year-old patient with a traumatic neuroma on the wrist, (D) 33-year-old female presented a left index finger traumatic neuroma with local radiation pain for half a month, the patient had a history of left index finger injury by scissors several years ago.
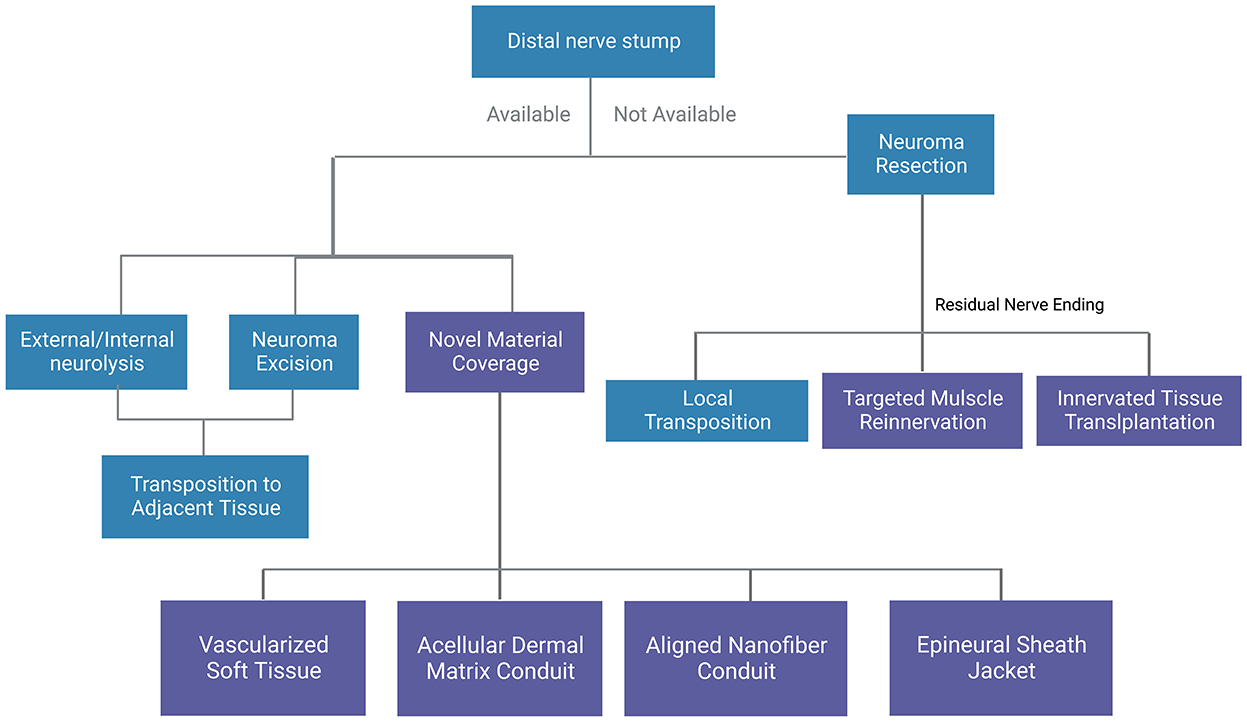
Figure 3. A general summary of surgical methods for traumatic neuromas. Blue boxes indicate conventional methods and purple boxes indicate novel methods.
For terminal peripheral neuroma where the distal nerve ending is not available, surgical neurectomy is considered where the target nerve is first identified via neurolysis. At this point, palpation of the target nerve should reproduce neuropathic pain in the patient, and resection proximal to the neuroma is performed, followed by transposition of the proximal nerve ending to nearby muscles, bones, and veins, thus reducing connective tissue formation around the nerve post-surgery to obtain a better pain relief effect. This surgical method has been tested out by various researchers and clinicians. In 1984, Mass et al. (77) transposed the hand neuroma into the bone, and 18 of 20 neuromas showed acceptable results. Laborde et al. (72) combined excision of the neuroma with transposition of the proximal palmar cutaneous branch of the median nerve to the pronator quadratus muscle, which freed the nerve from wrist motion and environmental stimulation. Transposition between the superficial and deep flexor muscles is also applicable. Koch et al. (78) studied the resection of the neuroma and transposition of the nerve stump into an adjacent vein, where 22 of 23 patients reported positive long-term results. While the above surgical methods are mainly aimed at nerve trunks such as radial nerve and ulnar nerve, smaller, unknown sensory nerve injuries require a more specific mapping technique. Conventionally, this is achieved by marking the painful area while the patients are awake and applying local anesthetic while incising progressively to identify discrete painful areas (79).
For traumatic peripheral neuroma-in-continuity where the distal ending is available, traditional treatments include external neurolysis, internal neurolysis, excision of the neuroma and transposition of the nerve, etc. External neurolysis or internal neurolysis is used to free the nerve from surrounding scar tissue while maintaining its integrity. Patients with an unsuccessful neurectomy as the primary treatment often suffer from a true neuroma. When the nerve is dispensable, the neuroma-in-continuity can be excised and transposed elsewhere.
Novel treatments targeting neuromas without distal ends mainly include transplantation of innervated tissue to cover the neuroma and targeted muscle reinnervation. In the former type, a muscle flap is always used, Reisman and Dellon (80) adopted a local transfer of the abductor digiti minimi muscle flap to treat palmar wrist pain. In the latter type, the cut, distal end of a peripheral nerve is transferred to a nearby muscle target or nerve target, for example, the deep peroneal nerve is transferred to the tibialis anterior muscle and the superficial peroneal nerve is transferred to the peroneus longus muscle (81), interdigital neurorrhaphy for treatment of digital neuromas. In addition to the techniques mentioned above, repeated intervention and higher amputations are rarely advised since it adds to the anxiety and pain of the patient (82). On the other hand, novel treatments for neuroma-in-continuity include the application of vascularized soft tissue or nerve conduits to cover the neuromas. A study by Krishnan et al. (83) showed that vascularized soft tissue coverage of painful peripheral nerve neuromas could be an effective method. Peterson and Adham (84) achieved satisfactory outcomes using an acellular dermal matrix conduit in the treatment of traumatic neuropathic pain at the wrist. Yan et al. (85) found that an aligned nanofiber conduit can significantly facilitate nerve regeneration, inhibit neuroma growth, and reduce traumatic neuropathic pain after neurectomy in a rat sciatic nerve model. Siemionow et al. (86) investigated the efficacy of the epineural sheath jacket as a novel technique for neuroma prevention in the rat sciatic nerve model and gained outcomes comparable to the muscle burying technique. Synthetic conduits may be a promising field in treating traumatic neuroma in the future. In general, traditional techniques combined with novel techniques are more effective in treating traumatic peripheral neuroma. Recently, treatment of traumatic peripheral neuroma has shifted from hiding injured nerves to attempting nerve healing (81). Some literature reviews support this conceptual shift (87, 88). However, more research is required in the future to match suitable operative methods with specific clinical settings.
The numerous techniques that have been attempted suggest that no one technique is completely effective or superior to other techniques, instead, each technique fits the certain situation of nerve injuries. Poppler et al. (89) and Ives et al. (90) compared various surgical treatments for neuroma of the extremities, including excision alone, excision and cap, excision and transposition, excision and repair, neurolysis and coverage, and found no difference in efficacy.
Treatment of traumatic peripheral neuroma usually starts with medication. Anti-neuropathic drugs include membrane-stabilizing agents (gabapentin, pregabalin, etc), anticonvulsants [carbamazepine (91), topiramate, phenytoin, lamotrigine, etc], antidepressants (amitriptyline, doxepin, etc), opioid analgesics (pethidine hydrochloride, etc) and muscle relaxants (baclofen, etc). Gabapentin and pregabalin (92) are considered the first-line effective medicine to inhibit central sensitization by affecting the calcium channels and reducing excessive neurotransmitter release. Although anti-neuropathic drugs were used as initial treatment, they often failed to obtain effective results while exposing the patients to side effects.
Other nonoperative management of traumatic peripheral neuroma includes physiotherapy, local injections (anesthetic, steroid, and alcohol), cryotherapy, radiofrequency ablation, shockwave therapy, and electrical stimulation. According to a systematic review from Samaila et al. (93), corticosteroid injections appear to be a safe treatment allowing good results with a very low complication rate. Alcohol injections in a study by Gurdezi et al. (94) showed that although the short-term results are encouraging, alcohol injection does not offer a permanent resolution of symptoms for most patients and can be associated with considerable morbidity. Ultrasound-guided radiofrequency ablation is a novel treatment modality, where a probe is inserted at the site of the neuroma, and the temperature is raised at 85°C for 90 s in an attempt to sever adjacent nerve endings and shows short-term success rates of 80%−85% (95–97).
Friedman et al. (98) performed a retrospective case series review in patients who had undergone sonographically guided cryotherapy for Morton's neuromas, postsurgical and posttraumatic neuromas, and idiopathic neuralgia, where 15 of 20 patients had a positive response to cryotherapy. On the other hand, Friedman et al. (99) did a study of extracorporeal shockwave therapy for an interdigital neuroma in 13 patients and obtained positive therapeutic results.
Continuous high-frequency electrical stimulation can be used to control the hypersensitive state of the injured nerve according to the gate control theory proposed by Wall et al. (100). A study by Stevanato et al. (101) showed that all patients experienced pain relief within a few minutes of treatment (>75 and >95% in most), with long-lasting pain relief with a reduction in mean Numerical Rating Scale of 76.2% after 6 months and of 71.5% after 12 months. No significant adverse events were observed.
Traumatic neuroma patients generally suffer from low life quality caused by function abnormalities, chronic pain syndrome (traumatic neuropathic pain) which may continue for weeks or years, and psychological issues (26, 102, 103). As prevention is better than cure, the first defense line against traumatic neuroma is the prevention of such events. Reconnecting the two ends of the injured nerve after nerve transection is important to obtain a better outcome and decrease the incidence of traumatic neuroma (104).
Several techniques have emerged to prevent traumatic neuroma based on better surgical treatment of peripheral nerve injuries. The incidence of developing traumatic neuroma increases along with the application of electrocoagulation or cryo-neurolysis, compared with other methods such as tight ligation or scissors cut and CO2 laser, indicating that applying the right neurectomy method helps to prevent traumatic neuroma (48, 49). Furthermore, some studies have investigated how the direction of nerve cutting can prevent traumatic neuroma formation, and they found that oblique nerve cutting displayed a better outcome compared with perpendicular or transverse cutting. This phenomenon occurred majorly because oblique nerve cutting leaves long and short nerve fibers, where a growth pathway will be formed between them (50). Amputated or untreated peripheral nerve injuries have a higher incidence of traumatic neuroma formation than neurorrhaphy (51). Direct nerve repair, on the other hand, can be divided into epineural repair and grouped fascicular repair and requires a tension-free environment and microscope magnification to obtain better recovery and prevention of traumatic neuromas (52).
NGF was experimentally proved to inhibit NGF decreasing neuroma formation, and minimizing neuropathic pain in traumatic neuroma formation after peripheral nerve injury (14), by applying an antibody against a specific receptor (trkA), or antibody against brain-derived neurotrophic factor (BDNF) which is usually upregulated after nerve injury by NGF (56). Fibrin glue proves its effectiveness in peripheral nerve repair by reducing the inflammatory response and improving axon regeneration. Also, Davis et al. showed that the continuous release of local tacrolimus (FK506) to repair site cloud actively inhibits neuroma formation (57). They used 3D-printing technology to create a polyethylene Y-shape conduit loop, where the nerve will be connected instead of direct connection to autografts. Other studies pointed out that the use of stem cells could improve nerve regeneration and possibly reduce neuroma formation, which could be a potential advanced traumatic neuroma prevention method (58, 59).
Experimental research attaches great importance to the treatment and management of traumatic neuromas. Over the years, with an enormous amount of neuroma models proposed in different animals, there is no model that could be of reference, for each model has its own strengths and weaknesses (105).
Rats are the most common species studied in animal models of traumatic neuroma, for the anatomy of rat nerves is well-established and similar to human anatomy (58, 86, 106–109). Besides, the model has a large number of standardized functional tests, making the experimental results easy to evaluate (110, 111). However, one of the major drawbacks of the rat models is that peripheral nerve regeneration is much faster in rodents than in humans, and made worse by the fact that only relative short nerve gaps can be obtained in rodents, making it difficult to compare this in vivo model with humans and to further apply treatment into clinical trials. Compared to rats, limiting further use of these mice models.
Rabbits used to be common traumatic neuroma models (112–115). Compared with mice models, rabbits yield longer regeneration time, worse nerve regeneration, and longer gaps which resembles humans more (116). However, the major disadvantage of the rabbit neuroma models is that rabbit nerve anatomy and limb muscle function differ significantly from human beings (117). Although similar to human beings, Primates are rarely used in research because of ethical concerns (118).
According to the research, the sciatic nerve, saphenous nerve (48), sural nerve (119), and tibial nerve (120, 121) can be used for modeling. Among these nerves, the sciatic nerve has gradually become the most common site for the animal model of a traumatic neuroma given the fact that the sciatic nerve is easy to expose and observe, while others are rarely used now (58, 86, 106, 107). After exposure of the sciatic nerve and its trifurcation under the microscope, the nerve is sharply dissected 3–10 mm distal from the trifurcation (108, 113, 114). To prevent spontaneous regeneration of the distal nerve stump, at least 10 mm of the distal nerve stumps should be removed and discarded (106–108). Finally, the presence or absence of a traumatic neuroma was determined by gross observation, ultrasound, hematoxylin-eosin staining, and immunofluorescence (106, 122). After reaching the predetermined time of the experiment or the growth and formation of the neuroma, the animals were sacrificed, and the nerve stump on the severed side and the contralateral normal nerve were harvested for neuroma adhesion evaluation, weight ratios, western blot analysis, and histological analysis.
In order to cover and cope with the recent advances and findings of traumatic neuromas of peripheral nerves, research has been conducted to improve and decrease the scientific gaps in this issue. Agarwal et al. (40) assessed the role of using imaging tools (high-resolution ultrasonography and MR neurography) and precise anatomical localization in diagnosing peripheral mononeuropathy. With IRB consent, a hospital-based prospective analytical investigation was conducted on 180 peripheral nerves in 131 individuals with symptoms of peripheral mononeuropathy in a context with limited resources. Generally, the proton density fat-saturated MR sequence had the best diagnosis accuracy (93.89%), followed by high-resolution ultrasonography (80%). The proton density fat-saturated sequence showed the maximum sensitivity, whereas the T1 MR sequence had the highest specificity. It was determined that the cumulative diagnostic accuracy of both modalities was 93.33%, with an 80% negative predictive value. Ultrasound and MRI revealed nerve interruption cases, but MRI was more effective at identifying neuromas. With the development of devices with a higher frequency and enhanced MR field strength, imaging of peripheral nerves is more accurate. Nerve imaging permits anatomical delineation and identification of the precise location of involvement. This comparative analysis highlights the significance of imaging in detecting peripheral nerve diseases, with an accuracy of 93.89% for MRI, which might act as the imaging gold standard. High-resolution ultrasonography can serve as a viable screening method since it is faster, more cost-effective, and has an accuracy of 80%, which is equivalent to other diagnostic techniques. The authors concluded that these two imaging techniques are not mutually exclusive. Instead, they complement one another and can be utilized in tandem to diagnose peripheral neuropathies using imaging.
Around 2.8% of hospitalized trauma patients suffer from acute peripheral nerve damage (123). Consequent significant disorder and socioeconomic effects have prompted continued research efforts on this subject (124). If a tension-free direct approximation of the nerve stumps is achievable, the epineural nerve suture is the treatment option. However, if tension-free coaptation is impossible, the current gold standard is autologous nerve transplantation (ANT) (125). Nevertheless, given the restricted accessibility of donor nerves and the morbidity associated with donor sites, new procedures are required to assist in peripheral nerve surgery. Nowadays, it is generally accepted that the material utilized to assist peripheral nerve regeneration should ideally consist of a totally biodegradable matrix that does not negatively impact regeneration during biodegradation (126). Despite significant advances in tissue engineering, no substance or bio-mimicking idea has yet demonstrated improved peripheral nerve regeneration results compared to the ANT, the current gold standard for bridging peripheral nerve deficits (125). In addition to the well-existing substances, chitosan is a relatively potential new substance in peripheral nerve regeneration. Due to its global availability, low cost, complete biodegradability, safe byproducts, and potentially compromising on the regeneration process (127). According to the literature, chitosan was shown to promote axonal regeneration [Kanazawa et al. (128), Stenberg et al. (129)], minimize severe scarring and enhance functional recovery (130), and inhibit further neuroma development peripheral nerve damage (131).
Relying on the processing of chitosan, the degree of acetylation (DOA) might vary, influencing both the molecular weights and solvent properties (132). In addition, the DOA has been found to be a factor that influences the survival, proliferation, and cellular activity of regeneration-supporting cells such as SC (133). However, the accurate adjustments of chitosan matrices remain problematic, as the mechanical rigidity, the biodegradation period, the spatial architecture, and the sterilization process all have the potential to influence the axonal regeneration process and must be considered across the manufacturing system (134). Furthermore, chitooligosaccharide (COS), a byproduct of chitosan, has been discovered to stimulate cell proliferation and inhibit apoptosis in SC, the essential cell for adequate axonal regeneration (135, 136). Furthermore, Wang et al. (137) contributed stimulating effects of COS to an expedited cell cycle leading to enhanced SC proliferation. Additionally, COS boosts the CCL2 production by down-regulating the miR-327 of the SC, resulting in improved migration to the damaged area (138). He et al. (139) examined the anti-apoptotic impacts of carboxymethylated chitosan (CMC) on SC by lowering caspase-3,−9, and Bax activities and enhancing Bcl-2 activities in CMC-treated SC. To protect the SC from oxidative stress, COS led to a decline in malondialdehyde activity and an increase in superoxide dismutase (SOD) activity. Subsequent in vivo tests on a rabbit model of axonotmesis revealed that daily intravenous injections of COS for 6 weeks dramatically enhanced peripheral nerve regeneration. Interestingly, the amount of regenerated myelinated nerve fibers, the thickness of the myelin sheath, and the compound muscle action potential (CMAP) as a measure of electrophysiological recovery were considerably greater in COS-treated individuals.
Traumatic neuroma-caused traumatic neuropathic pain has long bothered doctors and patients, scientists widely debate the reasons for traumatic neuropathic pain, and the therapy is difficult. Uncertain is the clinical therapy of painful neuroma. Specialists have developed numerous therapeutic methods in this discipline. However, there is currently no accepted standard treatment (26). Treatment strategies have been explored in animals and people, but pharmacotherapies (antidepressants, antiepileptics) continue to be the core of the care of traumatic neuropathic pain. Nerve stump transpositions into a muscle, vein, or bone are regarded as effective, traditional surgical treatments for persistent conditions, especially using soft tissue (83, 131, 140), and the usage of conduits (24, 131, 141, 142); which reflected an effective potential in traumatic neuropathic pain treatment. In recent decades, novel surgical procedures, including tube-guided nerve capping, electrical stimulation, and adipose autograft, have significantly increased the variety of treatments for traumatic neuropathic pain (143). Balcin et al. (144) hypothesized that nerve transplantation into a vein might limit the growth of painful neuromas. Contrasted the transposition of the nerve stump into a nearby vein or muscle as a surgical therapy for a painful neuroma. According to their pre-operative proportions in the muscle group 3 and 12 months after surgery, translocation into a vein resulted in decreased intensity and evaluating pain levels and enhanced sensory, as measured by the visual analog scale and the McGill pain score. This was connected with greater activity levels and enhanced function. In addition, the transposition of the nerve stump into an adjacent vein is favored over its relocation into a muscle. Myofibroblasts are strongly expressed in neuromas, and it is believed that they contribute to pain by compressing the collagen matrix around the sensitive non-myelinated fibers that proliferate to produce a neuromatous protuberance (83). Krishnan et al. (83) found that covering painful peripheral nerve neuromas with vascularized soft tissue might be an effective but difficult therapeutic strategy. For years, all types of nerve conduits have been used to repair nerve abnormalities (145), and have also been launched to treat painful neuromas (141).
Traumatic neuromas have long been a clinical challenge for doctors and researchers. While the standard classification system has been set based on Seddon's and Sunderland's theories, the imaging technology remains in dispute as opinions are divided in selecting the optimal tools among US, MRI, HRUS, EMG, and ect. The diagnosis of traumatic neuromas, with the utility of imaging tools, is based most generally on the previous medical history of nerve injury or operation and symptoms including pain hypersensitivity and the presence of trigger points that causes neuralgic pain. Concerning the management of traumatic neuromas, while surgical procedures are still the most effective treatment method, researchers are eager to develop more non-surgical methods including medication and physical therapy for treatment and prevention of traumatic neuromas. Although clinicians have yet to reach a consensus on a standardized management strategy of traumatic neuromas, it is clear that proper surgical procedures are vital for the prevention of traumatic neuromas. The future perspectives for management of traumatic neuromas, therefore, is most likely the prevention strategy during and post-operation, including an improved surgical approach, or the application of implants with a sustained release of medication that guides proper nerve regeneration.
HL and HY designed the study. YD, ZW, and JL performed data collection. HZ, AA, and SH analyzed the results. VK, MH, CY, and YD drafted the manuscript. The authors have read and approved the final manuscript.
The study was funded by the Zhejiang Provincial Natural Science Foundation (Grant number LS21H060001) and Alibaba Youth Studio Project (Grant number ZJU-032). The funding bodies had no role in the design of the study; in collection, analysis, and interpretation of data; and in drafting the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
2. Wood W. Observations on neuroma, with cases and histories of the disease. Trans Med Chir Soc Edinb. (1828) 3(Pt 2):367.
3. Morton TG. A peculiar and painful affection of the fourth metatarsophalangeal articulation. Am J Med Sci. (1876) 71:9. doi: 10.1097/00000441-187601000-00002
4. Huber GC, Lewis D. Amputation neuromas: their development and prevention. Arch Surg. (1920) 1:85–113. doi: 10.1001/archsurg.1920.01110010098007
5. Swanson HH. Traumatic neuromas. A review of the literature. Oral Surg Oral Med Oral Pathol. (1961) 14:317–26. doi: 10.1016/0030-4220(61)90297-3
6. Burchiel KJ, Ochoa JL. Surgical management of post-traumatic neuropathic pain. Neurosurg Clin N Am. (1991) 2:117–26. doi: 10.1016/S1042-3680(18)30761-7
7. Howell AE, Leach RE. Bowler's thumb. Perineural fibrosis of the digital nerve. J Bone Joint Surg Am. (1970) 52:379–81. doi: 10.2106/00004623-197052020-00024
8. Mukherjee K, Bassett IB. Meralgia paraesthetica. Br Med J. (1969) 2:55. doi: 10.1136/bmj.2.5648.55
9. Foltán R, Klíma K, Spacková J, Sedý J. Mechanism of traumatic neuroma development. Med Hypotheses. (2008) 71:572–6. doi: 10.1016/j.mehy.2008.05.010
10. Rainsbury JW, Whiteside OJH, Bottrill ID. Traumatic facial nerve neuroma following mastoid surgery: a case report and literature review. J Laryngol Otol. (2007) 121:601–5. doi: 10.1017/S0022215106004993
11. Li Q, Gao E-L, Yang Y-L, Hu H-Y, Hu X-Q. Traumatic neuroma in a patient with breast cancer after mastectomy: a case report and review of the literature. World J Surg Oncol. (2012) 10:35. doi: 10.1186/1477-7819-10-35
12. Chhabra A, Williams EH, Wang KC, Dellon AL, Carrino JA. MR neurography of neuromas related to nerve injury and entrapment with surgical correlation. AJNR Am J Neuroradiol. (2010) 31:1363–8. doi: 10.3174/ajnr.A2002
13. Ahlawat S, Belzberg AJ, Montgomery EA, Fayad LM. MRI features of peripheral traumatic neuromas. Eur Radiol. (2016) 26:1204–12. doi: 10.1007/s00330-015-3907-9
14. Kryger GS, Kryger Z, Zhang F, Shelton DL, Lineaweaver WC, Buncke HJ. Nerve growth factor inhibition prevents traumatic neuroma formation in the rat. J Hand Surg Am. (2001) 26:635–44. doi: 10.1053/jhsu.2001.26035
15. Marcol W, Kotulska K, Larysz-Brysz M, Kowalik JL. BDNF contributes to animal model neuropathic pain after peripheral nerve transection. Neurosurg Rev. (2007) 30:235–43. discussion 243. doi: 10.1007/s10143-007-0085-5
16. Carroll SL WS. Wallerian degeneration? In: Reference Module in Neuroscience and Biobehavioral Psychology. Netherlands: Elsevier. (2017), p. 1–8. doi: 10.1016/B978-0-12-809324-5.02077-0
17. Chan KM, Gordon T, Zochodne DW, Power HA. Improving peripheral nerve regeneration: from molecular mechanisms to potential therapeutic targets. Exp Neurol. (2014) 261:826–35. doi: 10.1016/j.expneurol.2014.09.006
18. Wood, M.D.and S.E., Mackinnon. Pathways regulating modality-specific axonal regeneration in peripheral nerve. Exp Neurol. (2015) 265:171–5. doi: 10.1016/j.expneurol.2015.02.001
19. Faroni A, Mobasseri SA, Kingham PJ, Reid AJ. Peripheral nerve regeneration: experimental strategies and future perspectives. Adv Drug Deliv Rev. (2015) 82–83:160–7. doi: 10.1016/j.addr.2014.11.010
20. Hirose T, Tani T, Shimada T, Ishizawa K, Shimada S, Sano T. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. (2003) 16:293–8. doi: 10.1097/01.MP.0000062654.83617.B7
21. Vora AR, Bodell SM, Loescher AR, Smith KG, Robinson PP, Boissonade FM. Inflammatory cell accumulation in traumatic neuromas of the human lingual nerve. Arch Oral Biol. (2007) 52:74–82. doi: 10.1016/j.archoralbio.2006.08.015
22. Egami S, Tanese K, Honda H, Kasai H, Yokoyama T, Sugiura M. Traumatic neuroma on the digital tip: immunohistochemical analysis of inflammatory signaling pathways. J Dermatol. (2016) 43:836–7. doi: 10.1111/1346-8138.13297
23. Khan J, Noboru N, Young A, Thomas D. Pro and anti-inflammatory cytokine levels (TNF-α, IL-1β, IL-6 and IL-10) in rat model of neuroma. Pathophysiology. (2017) 24:155–9. doi: 10.1016/j.pathophys.2017.04.001
24. Yan H, Zhang F, Kolkin J, Wang C, Xia Z, Fan C. Mechanisms of nerve capping technique in prevention of painful neuroma formation. PLoS ONE. (2014) 9:e93973. doi: 10.1371/journal.pone.0093973
25. Oliveira KMC, Pindur L, Han Z, Bhavsar MB, Barker JH, Leppik L. Time course of traumatic neuroma development. PLoS ONE. (2018) 13:e0200548. doi: 10.1371/journal.pone.0200548
26. Yao C, Zhou X, Zhao B, Sun C, Poonit K, Yan H. Treatments of traumatic neuropathic pain: a systematic review. Oncotarget. (2017) 8:57670–9. doi: 10.18632/oncotarget.16917
27. Seddon HJ. A classification of nerve injuries. Br Med J. (1942) 2:237–9. doi: 10.1136/bmj.2.4260.237
28. Griffin MF, Malahias M, Hindocha S, Khan WS. Peripheral nerve injury: principles for repair and regeneration. Open Orthop J. (2014) 8:199–203. doi: 10.2174/1874325001408010199
29. Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. (1951) 74:491–516. doi: 10.1093/brain/74.4.491
30. Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, et al. Traumatic peripheral nerve injuries: epidemiological findings, neuropathic pain and quality of life in 158 patients. J Peripher Nerv Syst. (2010) 15:120–7. doi: 10.1111/j.1529-8027.2010.00260.x
31. Dahlin LB, WibergM. Nerve injuries of the upper extremity and hand. EFORT Open Rev. (2017) 2:158–70. doi: 10.1302/2058-5241.2.160071
32. Koenig RW, Pedro MT, Heinen CPG, Schmidt T, Richter H-P, Antoniadis G, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. (2009) 26:E13. doi: 10.3171/FOC.2009.26.2.E13
33. Kermarrec E, Demondion X, Khalil C, Thuc VL, Boutry N, Cotten A. Ultrasound and magnetic resonance imaging of the peripheral nerves: current techniques, promising directions, and open issues. Semin Musculoskelet Radiol. (2010) 14:463–72. doi: 10.1055/s-0030-1268067
34. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intraoperative management of peripheral nerve lesions. J Neurosurg. (2011) 114:206–11. doi: 10.3171/2010.2.JNS091324
35. Zeidenberg J, Burks SS, Jose J, Subhawong TK, Levi AD. The utility of ultrasound in the assessment of traumatic peripheral nerve lesions: report of 4 cases. Neurosurg Focus. (2015) 39:E3. doi: 10.3171/2015.6.FOCUS15214
36. Zaidman CM, Seelig MJ, Baker JC, Mackinnon SE, Pestronk A. Detection of peripheral nerve pathology: comparison of ultrasound and MRI. Neurology. (2013) 80:1634–40. doi: 10.1212/WNL.0b013e3182904f3f
37. Ng ES, Vijayan J, Therimadasamy AK, Tan TC, Chan YC, Lim A, et al. High resolution ultrasonography in the diagnosis of ulnar nerve lesions with particular reference to post-traumatic lesions and sites outside the elbow. Clin Neurophysiol. (2011) 122:188–93. doi: 10.1016/j.clinph.2010.04.035
38. Karabay N, Toros T, Ademoglu Y, Ada S. Ultrasonographic evaluation of the iatrogenic peripheral nerve injuries in upper extremity. Eur J Radiol. (2010) 73:234–40. doi: 10.1016/j.ejrad.2008.10.038
39. Padua L, Di Pasquale A, Liotta G, Granata G, Pazzaglia C, Erra C, et al. Ultrasound as a useful tool in the diagnosis and management of traumatic nerve lesions. Clin Neurophysiol. (2013) 124:1237–43. doi: 10.1016/j.clinph.2012.10.024
40. Agarwal A, Chandra A, Jaipal U, Bagarhatta M, Mendiratta K, Goyal A, et al. Can imaging be the new yardstick for diagnosing peripheral neuropathy?-a comparison between high resolution ultrasound and MR neurography with an approach to diagnosis. Insights Imaging. (2019) 10:104. doi: 10.1186/s13244-019-0787-6
41. Chhabra A, Carrino J. Current MR neurography techniques and whole-body MR neurography. Semin Musculoskelet Radiol. (2015) 19:79–85. doi: 10.1055/s-0035-1545074
42. Koltzenburg M, Bendszus M. Imaging of peripheral nerve lesions. Curr Opin Neurol. (2004) 17:621–6. doi: 10.1097/00019052-200410000-00013
43. Gallagher TA, Simon NG, Kliot M. Diffusion tensor imaging to visualize axons in the setting of nerve injury and recovery. Neurosurg Focus. (2015) 39:E10. doi: 10.3171/2015.6.FOCUS15211
44. Chhabra A, Ahlawat S, Belzberg A, Andreseik G. Peripheral nerve injury grading simplified on MR neurography: as referenced to Seddon and Sunderland classifications. Indian J Radiol Imaging. (2014) 24:217–24. doi: 10.4103/0971-3026.137025
45. Hassanien OA, Younes RL, Dawoud RM, Younis LM, Hamoda IM. Reliable MRI and MRN signs of nerve and muscle injury following trauma to the shoulder with EMG and Clinical correlation. Egypt J Radiol Nucl Med. (2016) 47:929–36. doi: 10.1016/j.ejrnm.2016.03.015
46. Aggarwal A, Srivastava DN, Jana M, Sharma R, Gamanagatti S, Kumar A, et al. Comparison of different sequences of magnetic resonance imaging and ultrasonography with nerve conduction studies in peripheral neuropathies. World Neurosurg. (2017) 108:185–200. doi: 10.1016/j.wneu.2017.08.054
47. Visalli C, Cavallaro M, Concerto A, Torre DL, Di Salvo R, Mazziotti S, et al. Ultrasonography of traumatic injuries to limb peripheral nerves: technical aspects and spectrum of features. Jpn J Radiol. (2018) 36:592–602. doi: 10.1007/s11604-018-0765-9
48. Zeltser R, Beilin B-Z, Zaslansky R, Seltzer Z. Comparison of autotomy behavior induced in rats by various clinically-used neurectomy methods. Pain. (2000) 89:19–24. doi: 10.1016/S0304-3959(00)00342-0
49. Lewin-Kowalik J, Marcol W, Kotulska K, Mandera M, Klimczak A. Prevention and management of painful neuroma. Neurol Med Chir. (2006) 46:62–7. discussion 67–8. doi: 10.2176/nmc.46.62
50. Marcol W, Kotulska K, Larysz-Brysz M, Bierzyńska-Macyszyn G, Wlaszczuk P, Lewin-Kowalik J. Prevention of painful neuromas by oblique transection of peripheral nerves. J Neurosurg. (2006) 104:285–9. doi: 10.3171/jns.2006.104.2.285
51. van der Avoort DJJC, Hovius SER, Selles RW, van Neck JW, Coert JH. The incidence of symptomatic neuroma in amputation and neurorrhaphy patients. J Plast Reconstr Aesthet Surg. (2013) 66:1330–4. doi: 10.1016/j.bjps.2013.06.019
52. Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. (2014) 2014:698256. doi: 10.1155/2014/698256
53. Colen KL, Choi M, Chiu DTW. Nerve grafts and conduits. Plast Reconstr Surg. (2009) 124(6 Suppl):e386–94. doi: 10.1097/PRS.0b013e3181bf8430
54. Vernadakis AJ, Koch H, Mackinnon SE. Management of neuromas. Clin Plast Surg. (2003) 30:247–68, vii. doi: 10.1016/S0094-1298(02)00104-9
55. Pet MA, Ko JH, Friedly JL, Mourad PD, Smith DG. Does targeted nerve implantation reduce neuroma pain in amputees? Clin Orthop Relat Res. (2014) 472:2991–3001. doi: 10.1007/s11999-014-3602-1
56. Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. (1996) 7:134–42. doi: 10.1006/mcne.1996.0010
57. Davis B, Hilgart D, Erickson S, Labroo P, Burton J, Sant H, et al. Local FK506 delivery at the direct nerve repair site improves nerve regeneration. Muscle Nerve. (2019) 60:613–20. doi: 10.1002/mus.26656
58. Bolleboom A, de Ruiter GCW, Coert JH, Tuk B, Holstege JC, van Neck JW. Novel experimental surgical strategy to prevent traumatic neuroma formation by combining a 3D-printed Y-tube with an autograft. J Neurosurg. (2018) 130:184–96. doi: 10.3171/2017.8.JNS17276
59. Sullivan R, Dailey T, Duncan K, Abel N, Borlongan CV. Peripheral nerve injury: stem cell therapy and peripheral nerve transfer. Int J Mol Sci. (2016) 17:2101. doi: 10.3390/ijms17122101
60. Löfstedt T, Ahnlund O, Peolsson M, Trygg J. Dynamic ultrasound imaging—a multivariate approach for the analysis and comparison of time-dependent musculoskeletal movements. BMC Med Imaging. (2012) 12:29. doi: 10.1186/1471-2342-12-29
61. Lee TH, Hsieh ST, Chiang HY. Fibronectin inhibitor pUR4 attenuates tumor necrosis factor alpha-induced endothelial hyperpermeability by modulating beta1 integrin activation. J Biomed Sci. (2019) 26:37. doi: 10.1186/s12929-019-0529-6
62. Alaqeel A, Alshomer F. High resolution ultrasound in the evaluation and management of traumatic peripheral nerve injuries: review of the literature. Oman Med J. (2014) 29:314–9. doi: 10.5001/omj.2014.86
63. Beekman R, Visser LH. High-resolution sonography of the peripheral nervous system – a review of the literature. Eur J Neurol. (2004) 11:305–14. doi: 10.1111/j.1468-1331.2004.00773.x
64. Wijntjes J, Borchert A, van Alfen N. Nerve ultrasound in traumatic and iatrogenic peripheral nerve injury. Diagnostics. (2020) 11:30. doi: 10.3390/diagnostics11010030
65. Sulaiman W, Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: from bench top research to bedside application. Ochsner J. (2013) 13:100–8. doi: 10.1186/1749-7221-5-6
66. Antoniadis G, Kretschmer T, Pedro MT, König RW, Heinen CPG, Richter H-P. Iatrogenic nerve injuries: prevalence, diagnosis and treatment. Dtsch Arztebl Int. (2014) 111:273–9. doi: 10.3238/arztebl.2014.0273
67. Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg. (1985) 76:345–53. doi: 10.1097/00006534-198509000-00002
68. Dellon AL, Mackinnon SE, Pestronk A. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg. (1984) 12:30–40. doi: 10.1097/00000637-198401000-00006
69. Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. (1993) 78:714–9. doi: 10.3171/jns.1993.78.5.0714
70. Wu J, Chiu DT. Painful neuromas: a review of treatment modalities. Ann Plast Surg. (1999) 43:661–7. doi: 10.1097/00000637-199912000-00016
71. Herndon JH, Eaton RG, Littler JW. Management of painful neuromas in the hand. J Bone Joint Surg Am. (1976) 58:369–73. doi: 10.2106/00004623-197658030-00013
72. Laborde KJ, Kalisman M, Tsai TM. Results of surgical treatment of painful neuromas of the hand. J Hand Surg Am. (1982) 7:190–3. doi: 10.1016/S0363-5023(82)80086-5
73. Robbins TH. Nerve capping in the treatment of troublesome terminal neuromata. Br J Plast Surg. (1986) 39:239–40. doi: 10.1016/0007-1226(86)90089-5
74. Battista AF, Cravioto HM, Budzilovich GN. Painful neuroma: changes produced in peripheral nerve after fascicle ligation. Neurosurgery. (1981) 9:589–600. doi: 10.1097/00006123-198111000-00019
75. Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg Am. (1977) 2:70–8. doi: 10.1016/S0363-5023(77)80013-0
76. Yüksel F, Kişlaoglu E, Durak N, Uçar C, Karacaoglu E. Prevention of painful neuromas by epineural ligatures, flaps and grafts. Br J Plast Surg. (1997) 50:182–5. doi: 10.1016/S0007-1226(97)91367-9
77. Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES Jr. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. (1984) 74:182–5. doi: 10.1097/00006534-198408000-00002
78. Koch H, Haas F, Hubmer M, Rappl T, Scharnagl E. Treatment of painful neuroma by resection and nerve stump transplantation into a vein. Ann Plast Surg. (2003) 51:45–50. doi: 10.1097/01.SAP.0000054187.72439.57
80. Reisman NR, Dellon AL. The abductor digiti minimi muscle flap: a salvage technique for palmar wrist pain. Plast Reconstr Surg. (1983) 72:859–65. doi: 10.1097/00006534-198312000-00025
81. Lanier ST, Jordan SW, Ko JH, Dumanian GA. Targeted muscle reinnervation as a solution for nerve pain. Plast Reconstr Surg. (2020) 146:651e−63e. doi: 10.1097/PRS.0000000000007235
82. Grant GH. Methods of treatment of neuromata of the hand. J Bone Joint Surg Am. (1951) 33-A:841–8. doi: 10.2106/00004623-195133040-00003
83. Krishnan KG, Pinzer T, Schackert G. Coverage of painful peripheral nerve neuromas with vascularized soft tissue: method and results. Neurosurgery. (2005) 56(2 Suppl):369–78. discussion 369–78. doi: 10.1227/01.NEU.0000156881.10388.D8
84. Peterson SL, Adham MN. Acellular dermal matrix as an adjunct in treatment of neuropathic pain at the wrist. J Trauma. (2006) 61:392–5. doi: 10.1097/01.ta.0000224139.74143.f9
85. Yan H, Zhang F, Wang C, Xia Z, Mo X, Fan C. The role of an aligned nanofiber conduit in the management of painful neuromas in rat sciatic nerves. Ann Plast Surg. (2015) 74:454–61. doi: 10.1097/SAP.0000000000000266
86. Siemionow M, Bobkiewicz A, Cwykiel J, Uygur S, Francuzik W. Epineural sheath jacket as a new surgical technique for neuroma prevention in the rat sciatic nerve model. Ann Plast Surg. (2017) 79:377–84. doi: 10.1097/SAP.0000000000001117
87. Wolvetang NHA, Lans J, Verhiel SHWL, Notermans BJW, Chen NC, Eberlin KR. Surgery for symptomatic neuroma: anatomic distribution and predictors of secondary surgery. Plast Reconstr Surg. (2019) 143:1762–71. doi: 10.1097/PRS.0000000000005664
88. Guse DM, Moran SL. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: a 25-year comparative outcome study. Ann Plast Surg. (2013) 71:654–8. doi: 10.1097/SAP.0b013e3182583cf9
89. Poppler LH, Parikh RP, Bichanich MJ, Rebehn K, Bettlach CR, Mackinnon SE, et al. Surgical interventions for the treatment of painful neuroma: a comparative meta-analysis. Pain. (2018) 159:214–23. doi: 10.1097/j.pain.0000000000001101
90. Ives GC, Kung TA, Nghiem BT, Ursu DC, Brown DL, Cederna PS, et al. Current state of the surgical treatment of terminal neuromas. Neurosurgery. (2018) 83:354–64. doi: 10.1093/neuros/nyx500
91. Rizzo MA, Successful treatment of painful traumatic mononeuropathy with carbamazepine: insights into a possible molecular pain mechanism. J Neurol Sci. (1997) 152:103–6. doi: 10.1016/S0022-510X(97)00143-3
92. Alles SRA, Cain SM, Snutch TP. Pregabalin as a pain therapeutic: beyond calcium channels. Front Cell Neurosci. (2020) 14:83. doi: 10.3389/fncel.2020.00083
93. Samaila E, Colò G, Rava A, Negri S, Valentini R, Felli L, et al. Effectiveness of corticosteroid injections in Civinini-Morton's syndrome: a systematic review. Foot Ankle Surg. (2021) 27:357–65. doi: 10.1016/j.fas.2020.05.001
94. Gurdezi S, White T, Ramesh P. Alcohol injection for Morton's neuroma: a five-year follow-up. Foot Ankle Int. (2013) 34:1064–7. doi: 10.1177/1071100713489555
95. Cione JA, Cozzarelli J, Mullin CJ. A retrospective study of radiofrequency thermal lesioning for the treatment of neuritis of the medial calcaneal nerve and its terminal branches in chronic heel pain. J Foot Ankle Surg. (2009) 48:142–7. doi: 10.1053/j.jfas.2008.11.007
96. Moore JL, Rosen R, Cohen J, Rosen B. Radiofrequency thermoneurolysis for the treatment of Morton's neuroma. J Foot Ankle Surg. (2012) 51:20–2. doi: 10.1053/j.jfas.2011.10.007
97. Chuter GSJ, Chua YP, Connell DA, Blackney MC. Ultrasound-guided radiofrequency ablation in the management of interdigital (Morton's) neuroma. Skeletal Radiol. (2013) 42:107–11. doi: 10.1007/s00256-012-1527-x
98. Friedman T, Richman D, Adler R. Sonographically guided cryoneurolysis: preliminary experience and clinical outcomes. J Ultrasound Med. (2012) 31:2025–34. doi: 10.7863/jum.2012.31.12.2025
99. Friedman R, Cain JD, Weill L Jr. Extracorporeal shockwave therapy for interdigital neuroma: a randomized, placebo-controlled, double-blind trial. J Am Podiatr Med Assoc. (2009) 99:191–3. doi: 10.7547/0980191
101. Stevanato G, Devigili G, Eleopra R, Fontana P, Lettieri C, Baracco C, et al. Chronic post-traumatic neuropathic pain of brachial plexus and upper limb: a new technique of peripheral nerve stimulation. Neurosurg Rev. (2014) 37:473–79. discussion 479–80. doi: 10.1007/s10143-014-0523-0
102. Hanna SA, Catapano J, Borschel GH. Painful pediatric traumatic neuroma: surgical management and clinical outcomes. Childs Nerv Syst. (2016) 32:1191–4. doi: 10.1007/s00381-016-3109-z
103. Humberto C, Arthur B. Clinical and ultrastructural study of painful neuroma. Neurosurgery. (1981) 2:181–90.
104. Wang ML, Rivlin M, Graham JG, Beredjiklian PK. Peripheral nerve injury, scarring, and recovery. Connect Tissue Res. (2019) 60:3–9. doi: 10.1080/03008207.2018.1489381
105. Toia F, Giesen T, Giovanoli P, Calcagni M. A systematic review of animal models for experimental neuroma. J Plast Reconstr Aesthet Surg. (2015) 68:1447–63. doi: 10.1016/j.bjps.2015.05.013
106. He F-L, Qiu S, Zou J-L, Gu F-B, Yao Z, Tu Z-H, et al. Covering the proximal nerve stump with chondroitin sulfate proteoglycans prevents traumatic painful neuroma formation by blocking axon regeneration after neurotomy in Sprague Dawley rats. J Neurosurg. (2020) 134:1599–609. doi: 10.3171/2020.3.JNS193202
107. Weng W, Zhao B, Lin D, Gao W, Li Z, Yan H. Significance of alpha smooth muscle actin expression in traumatic painful neuromas: a pilot study in rats. Sci Rep. (2016) 6:23828. doi: 10.1038/srep23828
108. Yao C, Zhou X, Weng W, Poonit K, Sun C, Yan H. Aligned nanofiber nerve conduits inhibit alpha smooth muscle actin expression and collagen proliferation by suppressing TGF-β1/SMAD signaling in traumatic neuromas. Exp Ther Med. (2021) 22:1414. doi: 10.3892/etm.2021.10850
109. Tos P, Ronchi G, Papalia I, Sallen V, Legagneux J, Geuna S, et al. Chapter 4 Methods and protocols in peripheral nerve regeneration experimental research: part I—Experimental models. Int Rev Neurobiol. (2009) 87:47–79. doi: 10.1016/S0074-7742(09)87004-9
110. Bozkurt A, Tholl S, Wehner S, Tank J, Cortese M., O'Dey Dm, et al. Evaluation of functional nerve recovery with visual-SSI—a novel computerized approach for the assessment of the static sciatic index (SSI). J Neurosci Methods. (2008) 170:117–22. doi: 10.1016/j.jneumeth.2008.01.006
111. Nichols CM, Myckatyn TM, Rickman SR, Fox IK, Hadlock T, Mackinnon SE. Choosing the correct functional assay: a comprehensive assessment of functional tests in the rat. Behav Brain Res. (2005) 163:143–58. doi: 10.1016/j.bbr.2005.05.003
112. Song C, Zhang F, Zhang J, Mustain WC, Chen MB, Chen T, et al. Neuroma-in-continuity model in rabbits. Ann Plast Surg. (2006) 57:317–22. doi: 10.1097/01.sap.0000221512.06129.d3
113. Kim PS, Ko J, O'Shaughnessy KK, Kuiken TA, Dumanian GA. Novel model for end-neuroma formation in the amputated rabbit forelimb. J Brachial Plex Peripher Nerve Inj. (2010) 5:6.
114. Kim PS, Ko JH, O'Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am. (2012) 37:1609–16. doi: 10.1016/j.jhsa.2012.04.044
115. Ko JH, Kim PS, O'Shaughnessy KD, Ding X, Kuiken TA, Dumanian GA, et al. quantitative evaluation of gross versus histologic neuroma formation in a rabbit forelimb amputation model: potential implications for the operative treatment and study of neuromas. J Brachial Plex Peripher Nerve Inj. (2011) 6:e23–32.
116. Kerns JM, Sladek EH, Malushte TS, Bach H, Elhassan B, Kitidumrongsook P, et al. End-to-side nerve grafting of the tibial nerve to bridge a neuroma-in-continuity. Microsurgery. (2005) 25:155–64. discussion 164–6. doi: 10.1002/micr.20096
117. Angius D, Wang H, Spinner RJ, Gutierrez-Cotto Y, Yaszemski MJ, Windebank AJ, et al. systematic review of animal models used to study nerve regeneration in tissue-engineered scaffolds. Biomaterials. (2012) 33:8034–9. doi: 10.1016/j.biomaterials.2012.07.056
118. Arnason G. The emergence and development of animal research ethics: a review with a focus on nonhuman primates. Sci Eng Ethics. (2020) 26:2277–93. doi: 10.1007/s11948-020-00219-z
119. Chim H, Miller E, Gliniak C, Cohen ML, Guyuron B. The role of different methods of nerve ablation in prevention of neuroma. Plast Reconstr Surg. (2013) 131:1004–12. doi: 10.1097/PRS.0b013e3182879ec2
120. Adelson PD, Bonaroti EA, Thompson TP, Tran M, Nystrom NA. End-to-side neurorrhaphies in a rodent model of peripheral nerve injury: a preliminary report of a novel technique. J Neurosurg. (2004) 101:78–84. doi: 10.3171/ped.2004.101.2.0078
121. Huang H-L, Cendan C-M, Roza C, Okuse K, Cramer R, Timms JF, et al. Proteomic profiling of neuromas reveals alterations in protein composition and local protein synthesis in hyper-excitable nerves. Mol Pain. (2008) 4:33. doi: 10.1186/1744-8069-4-33
122. Jing W, Changfeng L, Jiang P, Chen Z, Wenjing X, Xiaoqing C, et al. Establishment and evaluation of traumatic neuroma model. Chin J Tissue Eng Res. (2020) 24:716–9. doi: 10.3969/j.issn.2095-4344.2431
123. Noble J, Munro CA, Prasad VS, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J Trauma. (1998) 45:116–22. doi: 10.1097/00005373-199807000-00025
124. Nicholson B, Verma S. Comorbidities in chronic neuropathic pain. Pain Med. (2004) 5(Suppl 1):S9–27. doi: 10.1111/j.1526-4637.2004.04019.x
125. Deumens R, Bozkurt A, Meek MF, Marcus MAE, Joosten EAJ, Weis J, et al. Repairing injured peripheral nerves: bridging the gap. Prog Neurobiol. (2010) 92:245–76. doi: 10.1016/j.pneurobio.2010.10.002
126. Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. (2003) 5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731
127. Freier T, Montenegro R, Koh HS, Shoichet MS. Chitin-based tubes for tissue engineering in the nervous system. Biomaterials. (2005) 26:4624–32. doi: 10.1016/j.biomaterials.2004.11.040
128. Kanazawa T, Akiyama F, Kakizaki S, Takashima Y, Seta Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials. (2013) 34:9220–6. doi: 10.1016/j.biomaterials.2013.08.036
129. Stenberg L, Kodama A, Lindwall-Blom C, Dahlin LB. Nerve regeneration in chitosan conduits and in autologous nerve grafts in healthy and in type 2 diabetic Goto-Kakizaki rats. Eur J Neurosci. (2016) 43:463–73. doi: 10.1111/ejn.13068
130. Neubrech F, Sauerbier M, Moll W, Seegmüller J, Heider S, Harhaus L, et al. Enhancing the outcome of traumatic sensory nerve lesions of the hand by additional use of a chitosan nerve tube in primary nerve repair: a randomized controlled bicentric trial. Plast Reconstr Surg. (2018) 142:415–24. doi: 10.1097/PRS.0000000000004574
131. Marcol W, Larysz-Brysz M, Kucharska M, Niekraszewicz A, Slusarczyk W, Kotulska K, et al. Reduction of post-traumatic neuroma and epineural scar formation in rat sciatic nerve by application of microcrystallic chitosan. Microsurgery. (2011) 31:642–9. doi: 10.1002/micr.20945
132. Chatelet C, Damour O, Domard A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials. (2001) 22:261–8. doi: 10.1016/S0142-9612(00)00183-6
133. Carvalho CR, López-Cebral R, Silva-Correia J, Silva JM, Mano JF, Silva TH, et al. Investigation of cell adhesion in chitosan membranes for peripheral nerve regeneration. Mater Sci Eng C Mater Biol Appl. (2017) 71:1122–34. doi: 10.1016/j.msec.2016.11.100
134. Stößel M, Wildhagen VM, Helmecke O, Metzen J, Pfund CB, Freier T, et al. Comparative evaluation of chitosan nerve guides with regular or increased bendability for acute and delayed peripheral nerve repair: a comprehensive comparison with autologous nerve grafts and muscle-in-vein grafts. Anat Rec. (2018) 301:1697–713. doi: 10.1002/ar.23847
135. Zhou S, Yang Y, Gu X, Ding F. Chitooligosaccharides protect cultured hippocampal neurons against glutamate-induced neurotoxicity. Neurosci Lett. (2008) 444:270–4. doi: 10.1016/j.neulet.2008.08.040
136. Huang H-C, Hong L, Chang P, Zhang J, Lu S-Y, Zheng B-W, et al. Chitooligosaccharides attenuate Cu2+-induced cellular oxidative damage and cell apoptosis involving Nrf2 activation. Neurotox Res. (2015) 27:411–20. doi: 10.1007/s12640-014-9512-x
137. Wang Y, Zhao Y, Sun C, Hu W, Zhao J, Li G, et al. Chitosan degradation products promote nerve regeneration by stimulating schwann cell proliferation via miR-27a/FOXO1 axis. Mol Neurobiol. (2016) 53:28–39. doi: 10.1007/s12035-014-8968-2
138. Zhao Y, Wang Y, Gong J, Yang L, Niu C, Ni X, et al. Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials. (2017) 134:64–77. doi: 10.1016/j.biomaterials.2017.02.026
139. He B, Tao HY, Liu SQ. Neuroprotective effects of carboxymethylated chitosan on hydrogen peroxide induced apoptosis in Schwann cells. Eur J Pharmacol. (2014) 740:127–34. doi: 10.1016/j.ejphar.2014.07.008
140. Ulrich D, van Doorn L, Hovius S. Fat injection for treatment of painful neuroma after episiotomy. Int J Gynaecol Obstet. (2011) 115:290–1. doi: 10.1016/j.ijgo.2011.07.023
141. Thomsen L, Bellemere P, Loubersac T, Gaisne E, Poirier P, Chaise F. Treatment by collagen conduit of painful post-traumatic neuromas of the sensitive digital nerve: a retrospective study of 10 cases. Chir Main. (2010) 29:255–62. doi: 10.1016/j.main.2010.07.004
142. Economides JM, DeFazio MV, Attinger CE, Barbour JR. Prevention of painful neuroma and phantom limb pain after transfemoral amputations through concomitant nerve coaptation and collagen nerve wrapping. Neurosurgery. (2016) 79:508–13. doi: 10.1227/NEU.0000000000001313
143. Koch H, Hubmer M, Welkerling H, Sandner-Kiesling A, Scharnagl E. The treatment of painful neuroma on the lower extremity by resection and nerve stump transplantation into a vein. Foot Ankle Int. (2004) 25:476–81. doi: 10.1177/107110070402500706
144. Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF, et al. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. (2009) 91:803–8. doi: 10.1302/0301-620X.91B6.22145
Keywords: traumatic neuroma, peripheral nerve, hand surgery, clinical management, diagnosis
Citation: Yang H, Dong Y, Wang Z, Lai J, Yao C, Zhou H, Alhaskawi A, Hasan Abdullah Ezzi S, Kota VG, Hasan Abdulla Hasan Abdulla M and Lu H (2023) Traumatic neuromas of peripheral nerves: Diagnosis, management and future perspectives. Front. Neurol. 13:1039529. doi: 10.3389/fneur.2022.1039529
Received: 08 September 2022; Accepted: 19 December 2022;
Published: 11 January 2023.
Edited by:
Federica Ginanneschi, University of Siena, ItalyReviewed by:
Luca Padua, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2023 Yang, Dong, Wang, Lai, Yao, Zhou, Alhaskawi, Hasan Abdullah Ezzi, Kota, Hasan Abdulla Hasan Abdulla and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Lu,  aHVpbHVAemp1LmVkdS5jbg==
aHVpbHVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.