- 1School of Health Preservation and Rehabilitation, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Preclinical Medicine, Chengdu University, Chengdu, China
Objective: Constraint-induced movement therapy (CIMT) is a common treatment for upper extremity motor dysfunction after a stroke. However, whether it can effectively improve lower extremity motor function in stroke patients remains controversial. This systematic review comprehensively studies the current evidence and evaluates the effectiveness of CIMT in the treatment of post-stroke lower extremity motor dysfunction.
Methods: We comprehensively searched randomized controlled trials related to this study in eight electronic databases (PubMed, Embase, The Cochrane Library, Web of Science, CBM, CNKI, WAN FANG, and VIP). We evaluated CIMT effectiveness against post-stroke lower extremity motor dysfunction based on the mean difference and corresponding 95% confidence interval (95% CI). We assessed methodological quality based on the Cochrane Bias Risk Assessment Tool. After extracting the general information, mean, and standard deviation of the included studies, we conducted a meta-analysis using RevMan 5.3 and Stata 16.0. The primary indicator was the Fugl-Meyer Assessment scale on lower limbs (FMA-L). The secondary indicators were the Berg balance scale (BBS), 10-meter walk test (10MWT), gait speed (GS), 6-min walk test (6MWT), functional ambulation category scale (FAC), timed up and go test (TUGT), Brunnstrom stage of lower limb function, weight-bearing, modified Barthel index (MBI), functional independence measure (FIM), stroke-specific quality of life questionnaire (SSQOL), World Health Organization quality of life assessment (WHOQOL), and National Institute of Health stroke scale (NIHSS).
Results: We initially identified 343 relevant studies. Among them, 34 (totaling 2,008 patients) met the inclusion criteria. We found that patients treated with CIMT had significantly better primary indicator (FMA-L) scores than those not treated with CIMT. The mean differences were 3.46 (95% CI 2.74–4.17, P < 0.01, I2 = 40%) between CIMT-treated and conventional physiotherapy-treated patients, 3.83 (95% CI 2.89–4.77, P < 0.01, I2 = 54%) between patients treated with CIMT plus conventional physiotherapy and patients treated only with conventional physiotherapy, and 3.50 (95% CI 1.08–5.92, P < 0.01) between patients treated with CIMT plus western medicine therapy and those treated only with western medicine therapy. The secondary indicators followed the same trend. The subgroup analysis showed that lower extremity CIMT with device seemed to yield a higher mean difference in FMA-L scores than lower extremity CIMT without device (4.52, 95% CI = 3.65–5.38, P < 0.01 and 3.37, 95% CI = 2.95–3.79, P < 0.01, respectively).
Conclusion: CIMT effectively improves lower extremity motor dysfunction in post-stroke patients; however, the eligible studies were highly heterogeneous.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=277466.
Introduction
Stroke has become a global health concern due to its extremely high mortality and disability rates. More than 20 million people suffer from stroke yearly, and only three-quarters survive (1). Limb motor dysfunction caused by stroke impairs patients' ability to live independently and their quality of life. Furthermore, patients may require long-term care, which burdens families and communities (2). Hemiplegia is a paralysis on one side of the body caused by pyramidal tract lesions and is a common post-stroke pathological manifestation. One of its main characteristics is the asymmetric motor pattern resulting from an excessive use of the unimpaired limb (3). Such a dependence can enhance the incoming information of the sensorimotor cortex and inhibit the use of the impaired limb, resulting in asymmetric and spasmodic patterns that complicate daily life (4).
Stroke can dramatically affect lower limb movement, resulting in abnormal movement patterns (5–7). Two-thirds of patients need help to walk 6 months after the stroke, and half cannot complete a 6-min walk test 1 year after the stroke (8–10). After effective rehabilitation, only one-third of patients return to work in the first year (11, 12). Therefore, it is necessary to help stroke patients recover lower limb motor function to enhance their independent living ability and allow them to rejoin society as soon as possible.
Constraint-induced movement therapy (CIMT), a motor rehabilitation technique, is often used to restore impaired limb motor function after a stroke to help bring back activities of daily living and reduce learned nonuse (13). The substantial positive results obtained with the upper extremity protocol have led to the development of CIMT for the lower extremity, an intervention to improve lower extremity function. Traditional CIMT involves a long period of limb limitation and intensive training, increasing patients' physical and mental pain (14). Unlike the traditional CIMT, the modified CIMT appropriately reduces the impaired limb's training intensity and the unimpaired limb's restriction time (15, 16), increasing the protocol's applicability, especially for older patients.
The lower extremity CIMT protocol includes (1) intensive practice of the functional activities, (2) limiting reliance on the unimpaired lower limb, (3) transfer of the gains from the training session to the family or community rehabilitation with a “transfer package” and (4) strong encouragement to use the impaired lower limb with improved coordination (17). However, unlike upper extremity CIMT, which uses a substantial constraint on the unimpaired lower limb (e.g., a padded mitt), the “constraint” for lower extremity CIMT can be behavioral, physical, or both (18). To stimulate walking ability and to overcome general inactivity, lower extremity CIMT consists of massed or repetitive practice of lower limb tasks (e.g., treadmill walking, over-ground walking, sit-to-stand, lie-to-sit, step climbing, and various balance and support exercises) (19). CIMT can improve gait parameters such as walking ability and gait speed, as well as the quantity and quality of movement (20–24).
A previous meta-analysis including six British randomized controlled trials (RCTs) of lower extremity CIMT intervention in stroke patients found that different CIMT protocols improved lower limb function (25). Silva and co-workers (26) observed that 2 weeks of CIMT (with the unimpaired ankle bearing a weight) did not improve lower limb motor function. Uswatte's study (27) also reported that upper limb restraint played a less critical role in CIMT benefits. Because gait training after stroke involves both the unimpaired and impaired legs, some studies (28, 29) do not recommend restricting the unimpaired leg with devices, as they can substantially alter leg inertia or normal gait patterns. Therefore, this systematic review and meta-analysis aimed to determine the effectiveness of CIMT on impaired lower limb function after stroke and to evaluate whether devices affect CIMT effectiveness.
Methods
The protocol of this systematic review and meta-analysis was registered in PROSPERO (CRD42021277466).
Search strategy
A systematic literature search was conducted in eight databases: PubMed, EMBASE, the Cochrane Library, Web of Science, China Biology Medicine (CBM), China National Knowledge Infrastructure (CNKI), Wan Fang Data, and the Chinese Science and Technology Periodical Database (VIP) from initiation up to December 2021. All the studies were limited to English and Chinese. All searches were based on the following keywords: “constraint induced movement therapy”, “stroke”, “lower extremity”, and “randomized controlled trial”. The complete search strategy of WOS is shown in Supplementary Figure S1.
Inclusion criteria
We included studies meeting the following criteria: (1) studies on patients with motor impairment of the lower limbs after a stroke (regardless of stroke type, duration, affected brain area, or hemiplegia side); (2) studies that evaluated CIMT, modified CIMT, and any interventions conforming to the lower extremity CIMT core strategy (intensive practice of the functional activities and restraint of the less-affected lower extremity by the device or behavioral procedures), regardless of the treatment's frequency, duration and length; (3) studies that were RCTs; (4) studies written in English or Chinese.
Exclusion criteria
We excluded studies that: (1) did not obtain results of interest; (2) were one of the following types: opinions, case reports, case series, conference papers, editorials, abstracts, or crossover studies; (3) were unavailable.
Outcome measures
The main outcome measure was the Fugl-Meyer Assessment scale on lower limbs (FMA-L), which is commonly used to assess lower extremity motor function. Secondary outcomes included the Berg balance scale (BBS), 10-meter walk test (10MWT), gait speed (GS), 6-min walk test (6MWT), functional ambulation category scale (FAC), timed up and go test (TUGT), Brunnstrom stage of lower limb function, weight-bearing, modified Barthel index (MBI), functional independence measure (FIM), stroke-specific quality of life questionnaire (SSQOL), World Health Organization quality of life assessment (WHOQOL), and National Institute of Health stroke scale (NIHSS).
Data extraction
We first imported the retrieved literature into EndNote X9 and automatically removed duplicate entries. Two reviewers (MZ and PG) screened the papers' titles and abstracts according to predefined inclusion and exclusion criteria. Then, two examiners reviewed the remaining full texts separately. They collected the following information: first author's name, year of publication, origin country, subjects' ethnicity, study design, sample size, mean age, stroke duration, grading criteria, interventions (including intensity and duration) in the experimental and control groups, and scores on the scale of interest (mean scores and standard deviation). Disagreements were resolved by consensus discussion between two authors (MZ and PG) or by consulting a third reviewer (YT).
Quality assessment
Two reviewers (MZ and YT) independently assessed the methodological quality of the included studies using the Cochrane Bias Risk Assessment Tool. It consists in rating the risk of bias of seven items (as “low”, “unclear”, or “high”). Next, we rated studies with seven low-risk items as “high-quality” and those with one or more high-risk or unclear items as “low-quality”.
Data analysis
We performed all statistical analyses using RevMan 5.3 and Stata 16.0. Because all variables included in the study were continuous, we analyzed the magnitude of the effects using the mean difference (MD) or standard MD and 95% confidence interval (CI). We also estimated the interstudy heterogeneity using the chi-squared test and the I2 statistic. A P < 0.05 or an I2 statistic > 50% indicated unaccepted variability among the included studies, and a random-effects model would be used to analyze the data. To explore the source of the heterogeneity, we performed sensitivity analyses. We estimated publication bias by constructing a funnel plot of each trial's MD and the standard error (Supplementary material). Next, we assessed the funnel plot asymmetry using Egger's test. Finally, we considered that P < 0.05 indicated significant publication bias.
Results
Literature search
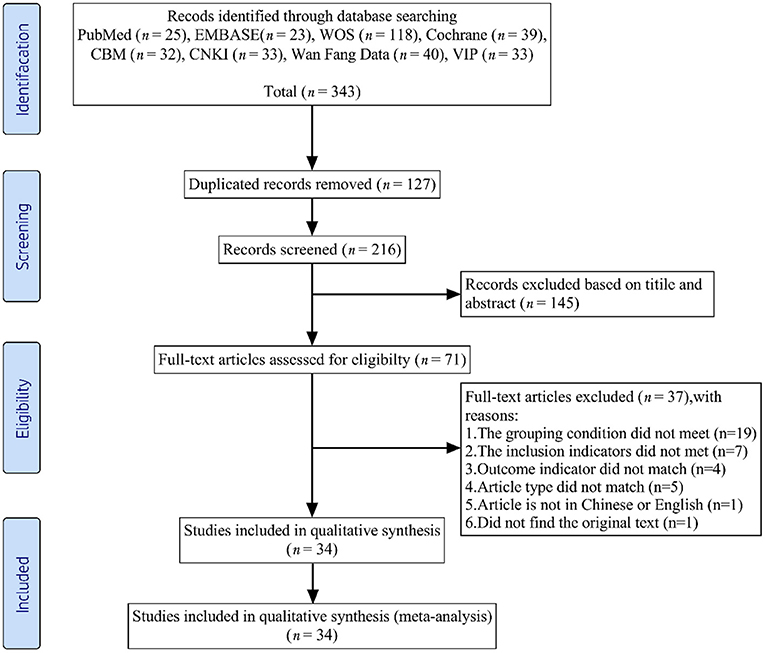
We initially extracted 343 records from the eight databases. After the first scanning stage, we removed 127 duplicates. Of the remaining 216 records, we excluded 182 articles that did not meet the predefined inclusion criteria. Ultimately, this systematic review and meta-analysis included 34 RCTs (26, 30–62). The literature selection process appears in Figure 1.
Main characteristics
Table 1 lists the main characteristics of the included studies. This meta-analysis included 34 studies with a total of 2008 participants (1,003 in the experimental group and 1,005 in the control group). All the articles were published between 2008 and 2021. The experimental group in these studies included 100 people and nine people.
In terms of intervention, 24 studies adopted CIMT, nine studies adopted CIMT in combination with conventional physiotherapy (such as muscle strengthening, facilitation, activity training, balance, gait training, or neurodevelopmental treatment), and one study adopted CIMT in combination with western medicine (including improving cerebral blood circulation, nerve nutrition, and blood pressure regulation). Table 2 lists the CIMT protocol details of the included studies.
Regarding outcome assessment, 18 studies assessed lower extremity motor function using the FMA scale (of which 16 assessed FMA-L). Additionally, 23 studies assessed the balance function through the BBS scale. Concerning walking speed evaluation, 13 studies measured 10MWT score, and 4 measured GS. Regarding mobility assessment, 11 studies used TUGT, 6 used FAC, 3 used 6MWT, and 1 used Brunnstrom Stage of lower limb function. To assess activities of daily living, 14 studies used MBI and 1 used FIM. As for quality of life assessment, two studies used SSQOL and one used WHOQOL. One study used the NIHSS to measure the severity of stroke damage. Only one study evaluated the weight bearing of affected limbs.
Methodological quality
The risk of bias in the 34 studies is shown in Supplementary Figures S2, S3. All 34 studies randomly divided the participants into different groups. Five studies (26, 31, 32, 39, 57) detailed assignment protocol concealment and four studies (31, 32, 44, 60) reported non-blindness by trial personnel (the first author performed the CIMT treatment). In addition, two studies (44, 53) were performed without blindness to the outcome assessment, and 16 (26, 31–34, 36, 37, 39, 40, 48, 50, 51, 55, 57, 61, 62) reported outcome assessments completed by therapists who did not participate in the experiment. Finally, 11 studies (34, 36, 40, 42, 45, 48, 53, 54, 56, 60, 62) did not clearly record whether the baseline was balanced and were, therefore, rated “unclear”. We assessed the quality of the included studies using the Cochrane Bias Risk Assessment tool and found methodological defects. Only four studies (26, 39, 57, 61) were rated “high quality”.
Effect of CIMT: Clinical evaluation
CIMT vs. conventional physiotherapy
Effect on lower extremity motor function
One study (56) used FMA as the outcome indicator (Figure 2A). It included 30 cases. Its MD value was 8.20 (95% CI 0.38–16.02, P = 0.04).
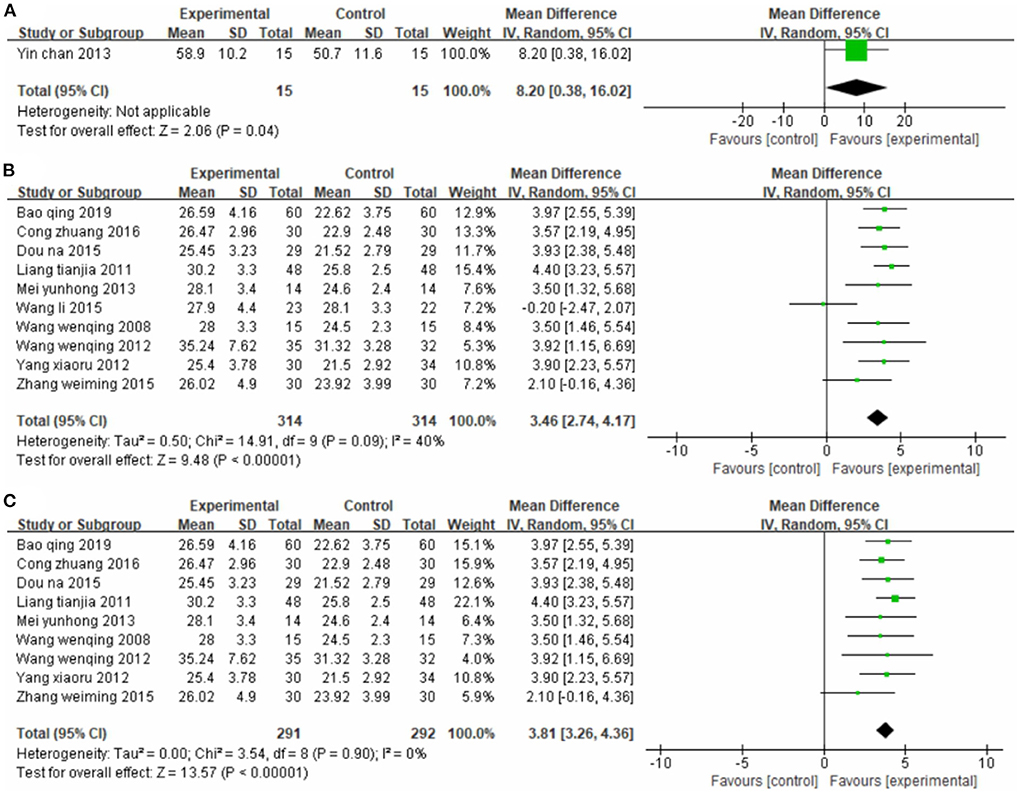
Figure 2. Forest plot. CIMT vs. conventional physiotherapy on the Fugl-Meyer Assessment total score (A) and Fugl-Meyer Assessment lower limb sub-scale (B,C).
Ten studies (33, 34, 42, 45, 47, 48, 50, 51, 53, 55), totaling 628 participants, reported the FMA-L (Figure 2B). Our pooled data analysis showed that the CIMT group had a higher FMA-L evaluation score than the conventional physiotherapy group, with an MD value of 3.46 (95% CI 2.74–4.17, P < 0.01, I2 = 40%). The sensitivity analysis showed that one study (48) was the main source of heterogeneity (Figure 2C). Removing this study notably reduced the interstudy heterogeneity (P = 0.90, I2 = 0%) but yielded similar analysis results (MD = 3.81, 95% CI 3.26–4.36, P < 0.01).
Effect on balance function
Sixteen studies (26, 31, 33, 34, 36, 42, 45, 46, 48, 50, 51, 53, 55, 56, 58, 62), totaling 847 cases, used BBS as the outcome indicator (Figure 3). The MD value was 7.63 (95% CI 5.49–9.77, P < 0.01, I2 = 94%), indicating that CIMT was superior to conventional physiotherapy in improving balance function. However, these studies were strongly heterogeneous. Unfortunately, neither the sensitivity analysis nor the meta-regression analysis of publication year and sample size reveals any find obvious sources of heterogeneity. We suspect publication bias because the funnel plot was asymmetric, and eight studies were outside it (Supplementary Figure S4). We also confirmed publication bias in this analysis using Egger's test (P = 0.02, Supplementary Figure S5).
Effect on walking speed
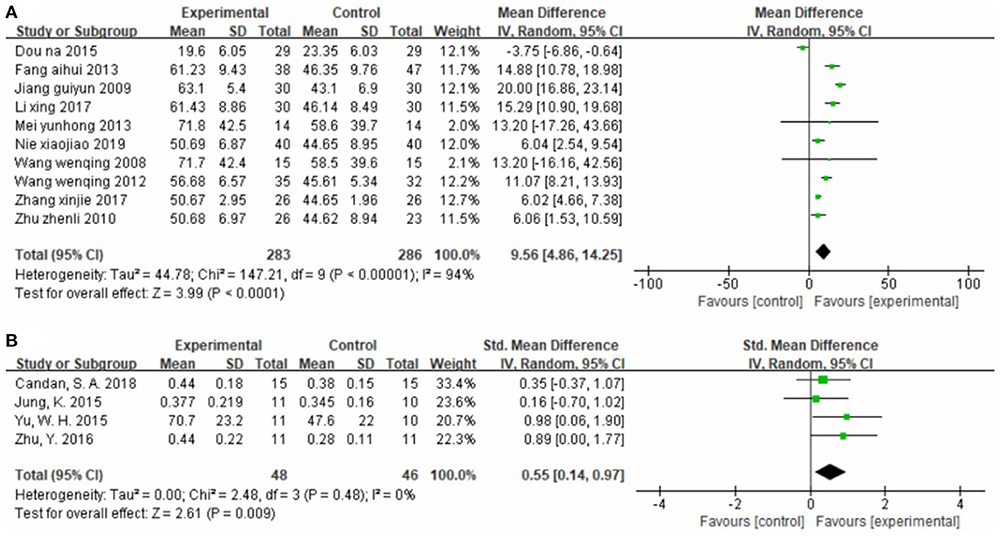
Ten studies (34–36, 41, 45, 46, 50, 51, 58, 62), totaling 569 cases, assessed motor function using 10MWT (Figure 4A) (MD = 9.56, 95% CI 4.86–14.25, P < 0.01, I2 = 94%). Neither the sensitivity analysis nor the meta-regression analysis of publication year and sample size revealed any obvious sources of heterogeneity. As shown in Supplementary Figure S6, the funnel plot for this analysis is symmetric. Egger's test confirmed the symmetry of the funnel plot, and we found no evident publication bias in this analysis (P = 0.351) (Supplementary Figure S7).
Four studies (31, 39, 57, 61), totaling 94 cases, measured GS as the outcome indicator (Figure 4B). The standard MD was 0.55 (95% CI 0.14–0.97, P < 0.01, I2 = 0%), indicating that CIMT is superior to conventional physiotherapy for improving walking speed.
Effect on mobility
Seven studies (26, 36, 46, 51, 57, 58, 62), totaling 367 cases, measured the TUGT score as the outcome indicator (Figure 5A). Our pooled data analysis showed that patients in the experimental group spent less time on the TUGT, with an MD value of −6.56 (95% CI −8.07 to −5.05, P < 0.01, I2 = 52%). The sensitivity analysis revealed that one study (36) was the main source of heterogeneity (Figure 5B). Removing it markedly reduced heterogeneity (P = 0.22, I2 = 29%), and yielded an MD value of −6.02 (95% CI −7.59 to −4.46, P < 0.01).

Figure 5. Forest plot. CIMT vs. conventional physiotherapy on the timed up and go test (A,B) and functional ambulation category scale (C).
Two studies (42, 49), totaling 190 cases, used FAC as the outcome indicator (Figure 5C). The MD value was 0.89 (95% CI 0.24–1.55, P < 0.01, I2 = 81%). The above results indicate that CIMT is better than conventional physiotherapy for improving patients' mobility, although there was some heterogeneity.
Effect on activities of daily living
Eight studies (33, 35, 41, 45, 47, 50, 53, 56), totaling 473 cases, used MBI as the outcome indicator (Figure 6A). The MD value was 9.12 (95% CI 7.20–11.04, P < 0.01, I2 = 52%). The meta-regression analysis of publication year and sample size did not reveal obvious sources of heterogeneity. However, the sensitivity analysis identified one study (47) as the main source of heterogeneity (Figure 6B). Removing it notably reduced heterogeneity (P = 0.11, I2 = 42%) and yielded an MD value of 9.86 (95% CI 7.59–12.13, P < 0.01).
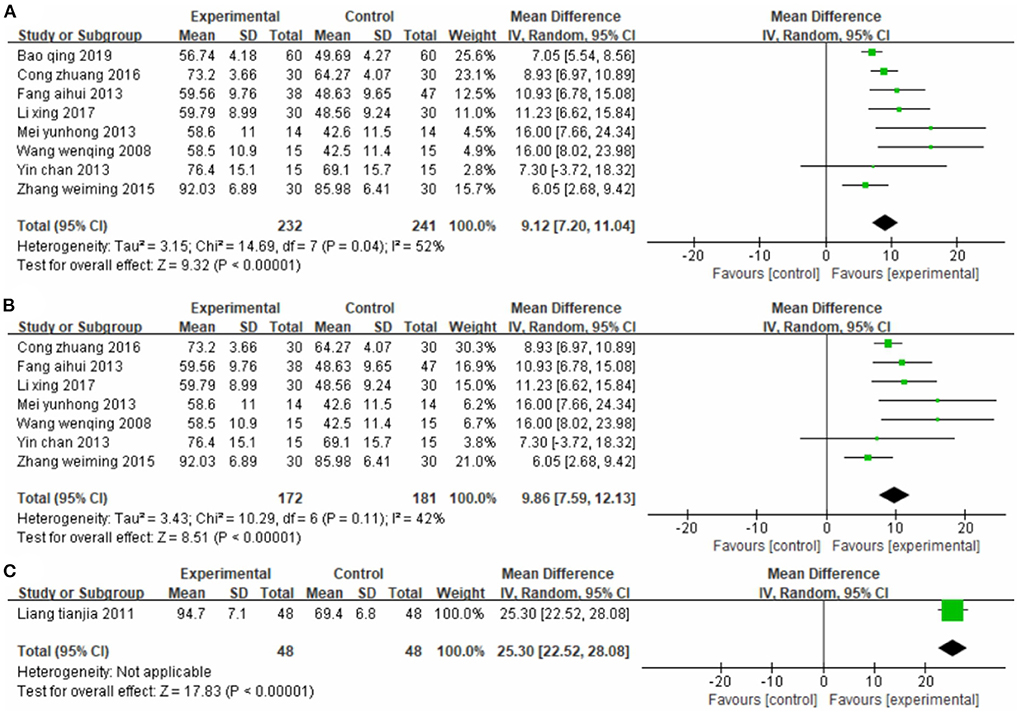
Figure 6. Forest plot. CIMT vs. conventional physiotherapy on the modified Barthel index (A,B) and functional independence measure (C).
One study (42), totaling 96 cases, used FIM as the outcome indicator (Figure 6C). The MD value was 25.30 (95% CI 22.52–28.08, P < 0.01). The above results indicate that patients in the CIMT group had higher scores than those in the conventional physiotherapy group.
Effect on quality of life
Two studies (32, 57), totaling 51 cases, used SSQOL as the outcome indicator (Figure 7A). The MD value was 20.76 (95% CI 1.72–39.80, P = 0.03, I2 = 59%). One study (47), totaling 120 cases, used WHOQOL as the outcome indicator (Figure 7B). The MD value was 18.70 (95% CI 13.73–23.67, P < 0.01). The above results indicate that patients treated with CIMT had higher Quality of Life scores than patients treated with conventional physiotherapy.
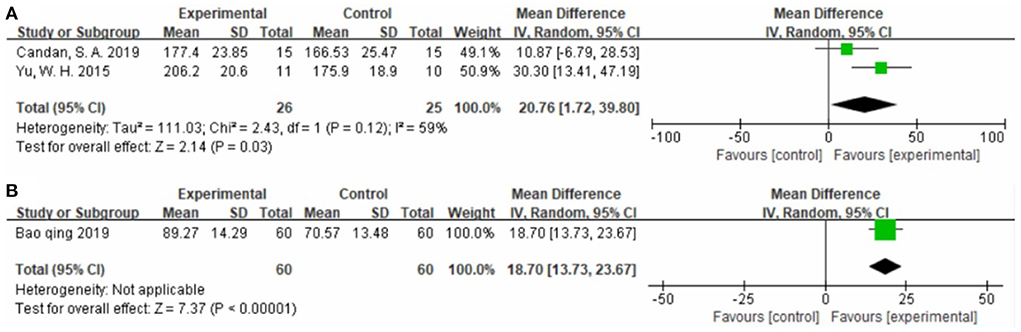
Figure 7. Forest plot. CIMT vs. conventional physiotherapy on the stroke-specific quality of life questionnaire (A) and World Health Organization quality of life assessment (B).
CIMT plus conventional physiotherapy vs. conventional physiotherapy
Effect on lower limb motor function
One study (30), totaling 18 cases, used FMA as the outcome indicator (Figure 8A). The MD value was 1.00 (95% CI −1.87 to 3.87, P = 0.49).

Figure 8. Forest plot. CIMT plus conventional physiotherapy vs. conventional physiotherapy alone on the Fugl-Meyer Assessment total score (A) and Fugl-Meyer Assessment lower limb sub-scale (B,C).
Five studies (37, 40, 43, 44, 60), totaling 388 participants, used the FMA-L as the outcome indicator (Figure 8B). The pooled analysis showed that CIMT combined with conventional physiotherapy was better than conventional physiotherapy alone for improving the motor function of the patients' lower limb (MD = 3.83, 95% CI 2.89–4.77, P < 0.01, I2 = 54%). The sensitivity analysis identified one study (43) as the main source of heterogeneity (Figure 8C). Removing it markedly reduced the heterogeneity (P = 0.61, I2 = 0%), and yielded similar results (MD = 3.35, 95% CI 2.78–3.93, P < 0.01).
Effect on balance function
Seven studies (30, 37, 40, 43, 44, 59, 60), totaling 510 cases, used BBS as the outcome indicator (Figure 9). The MD value was 6.37 (95% CI 3.74–8.99, P < 0.01, I2 = 93%), indicating that CIMT combined with conventional physiotherapy was superior to Conventional physiotherapy alone for improving the balance function. However, the studies were strongly heterogeneous and the meta-regression analysis did not reveal publication year and sample size as obvious sources of heterogeneity.
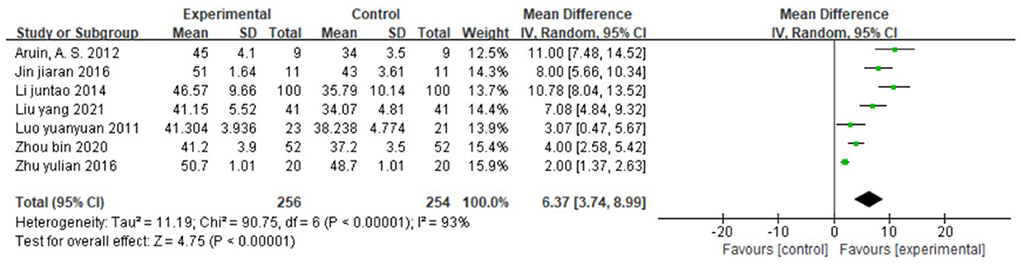
Figure 9. Forest plot. CIMT plus conventional physiotherapy vs. conventional physiotherapy alone on the Berg balance scale.
Effect on walking speed
Two studies (37, 60), totaling 62 cases, measured 10MWT score (s) as the outcome indicator (Figure 10A). The patients in the experimental group spent less time on the 10-MWT than patients in the control group (MD = −14.80, 95% CI −16.70 to −12.90, P < 0.01, I2 = 0%). One study (30), totaling 18 cases, measured 10MWT score (m/s) as the outcome indicator included (Figure 10B). The MD value was 0.13 (95% CI −0.56 to 0.82, P = 0.71). The above results indicate that CIMT combined with conventional physiotherapy was better than conventional physiotherapy alone for improving walking speed.
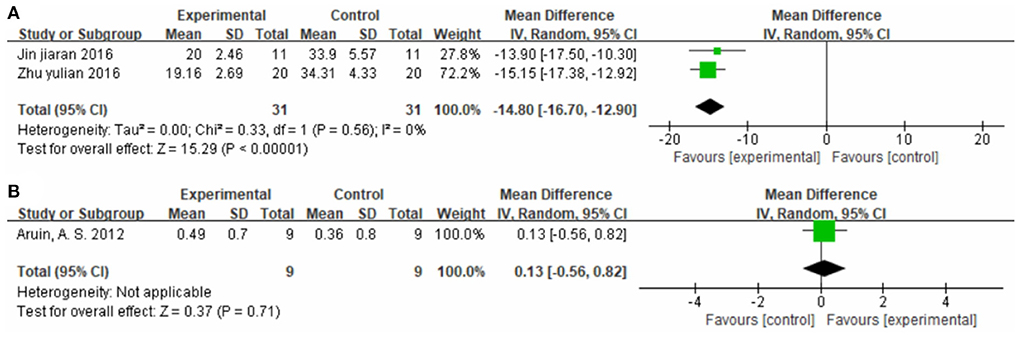
Figure 10. Forest plot. CIMT plus conventional physiotherapy vs. conventional physiotherapy alone on the 10-meter walk test [(A) s; (B) m/s].
Effect on mobility
Three studies (44, 52, 60), totaling 123 cases, used 6MWT score as the outcome indicator in Figure 11A. The experimental group walked longer distances within 6 min than the control group, with an MD value of 91.46 (95% CI 81.06–101.86, P < 0.01, I2 = 52%). The sensitivity analysis revealed that one study (44) was the main source of heterogeneity (Figure 11B). Removing it notably reduced the heterogeneity (P = 1.00, I2 = 0%) and yielded an MD value of 93.91 (95% CI 90.56–97.26, P < 0.01).
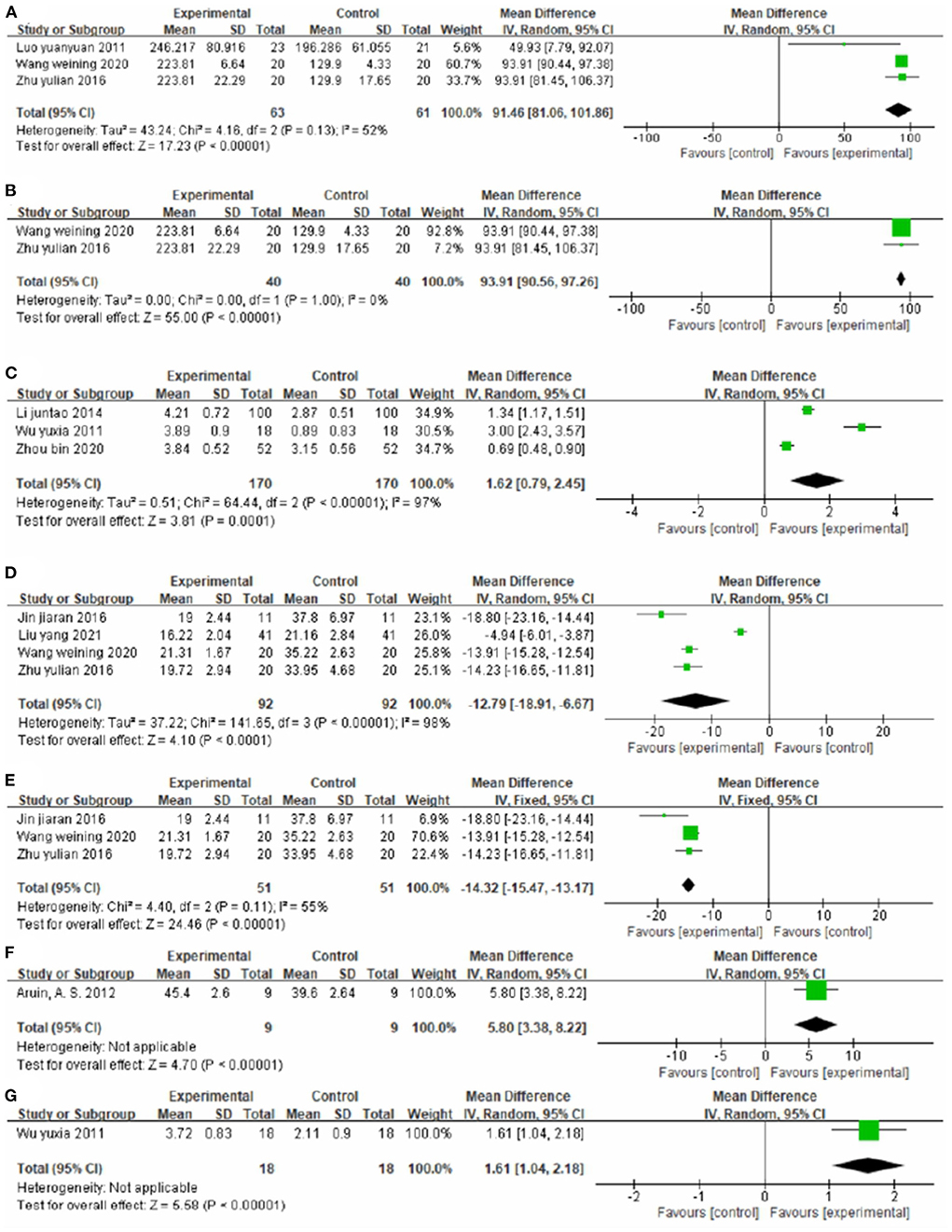
Figure 11. Forest plot. CIMT plus conventional physiotherapy vs. conventional physiotherapy alone on the 6-min walk test (A,B), functional ambulation category scale (C), timed up and go test (D,E), weight-bearing (F) and Brunnstrom stage of lower limb function (G).
Three studies (40, 54, 59), totaling 340 cases, used FAC as the outcome indicator (Figure 11C). The MD value was 1.62 (95% CI 0.79–2.45, P < 0.01, I2 = 97%). The meta-regression analysis of publication year and sample size did not identify obvious sources of heterogeneity.
Four studies (37, 43, 52, 60), totaling 184 cases, measured TUGT score as the outcome indicator (Figure 11D). The patients in the experimental group had lower scores than control patients (MD = −12.79, 95% CI −18.91 to −6.67, P < 0.01, I2 = 98%). The sensitivity analysis revealed that one study (43) was the main source of heterogeneity (Figure 11E). Removing it markedly reduced heterogeneity (P = 0.11, I2 = 55%) and yielded an MD value of −14.32 (95% CI −15.47 to −13.17, P < 0.01).
One study (30), totaling 18 cases, measured Weight Bearing as the outcome indicator (Figure 11F). The lower limb weight was more balanced in the experimental group than in the control group (MD = 5.80, 95% CI 3.38–8.22, P < 0.01).
One study (54), totaling 36 cases, used Brunnstrom stage of lower limb function as the outcome indicator (Figure 11G). The MD value was 1.61 (95% CI 1.04–2.08, P < 0.01). The above results indicate that CIMT combined with conventional physiotherapy is better than conventional physiotherapy alone for improving the mobility of patients.
Effect on activities of daily living
Six studies (40, 43, 44, 52, 54, 60), totaling 442 cases, used MBI as the outcome indicator (Figure 12). The patients in the experimental group had higher scores than control patients (MD = 10.41, 95% CI 6.45–14.36, P < 0.01, I2 = 88%). The meta-regression analysis of publication year and sample size did not find obvious sources of heterogeneity.

Figure 12. Forest plot. CIMT plus conventional physiotherapy vs. conventional physiotherapy alone on the modified Barthel index.
CIMT plus western medicine therapy vs. western medicine therapy
One study (38), totaling containing 61 cases compared the efficacy of CIMT plus western medicine therapy with western medicine therapy alone (Figure 13). The MD values for FMA-L, FAC, and NIHSS were 3.50 (95% CI 1.08–5.92, P < 0.01); 0.80 (95% CI 0.67–0.93, P < 0.01); and −2.40 (95% CI −3.22 to −1.58, P < 0.01), respectively.
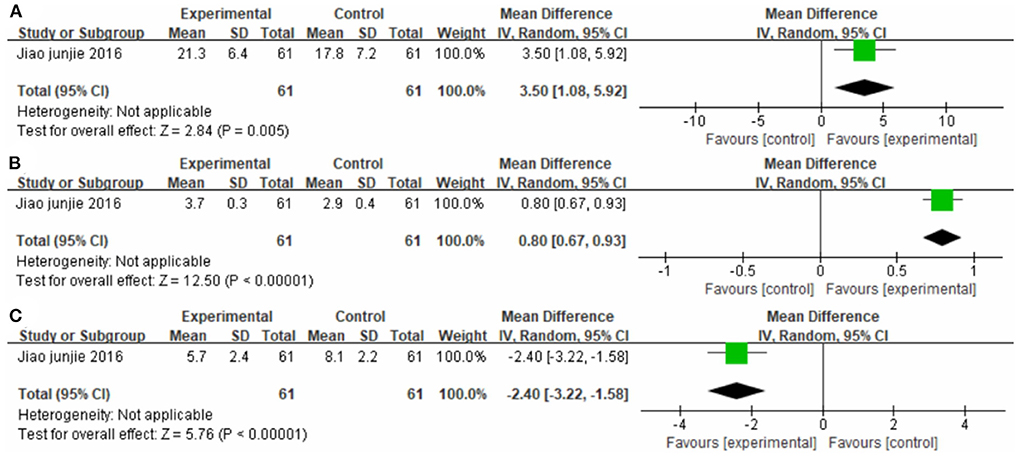
Figure 13. Forest plot. CIMT plus western medicine therapy vs. western medicine therapy alone on the Fugl-Meyer Assessment lower limb sub-scale (A), functional ambulation category scale (B) and National Institute of Health stroke scale (C).
Lower extremity CIMT with device vs. lower extremity CIMT without device
Figure 14 shows the synthetic results on FMA-L from 16 studies. Four studies (34, 43, 44, 55) evaluated lower extremity CIMT with device, and 12 studies (33, 37, 38, 40, 42, 45, 47, 48, 50, 51, 53, 60) evaluated lower extremity CIMT without device. The subgroup analysis suggests that lower extremity CIMT with device yields higher FMA-L scores than lower extremity CIMT without device (With device: MD = 4.52, 95% CI 3.65 to 5.38, P < 0.01; without device: MD = 3.37, 95% CI 2.95 to 3.79, P < 0.01).
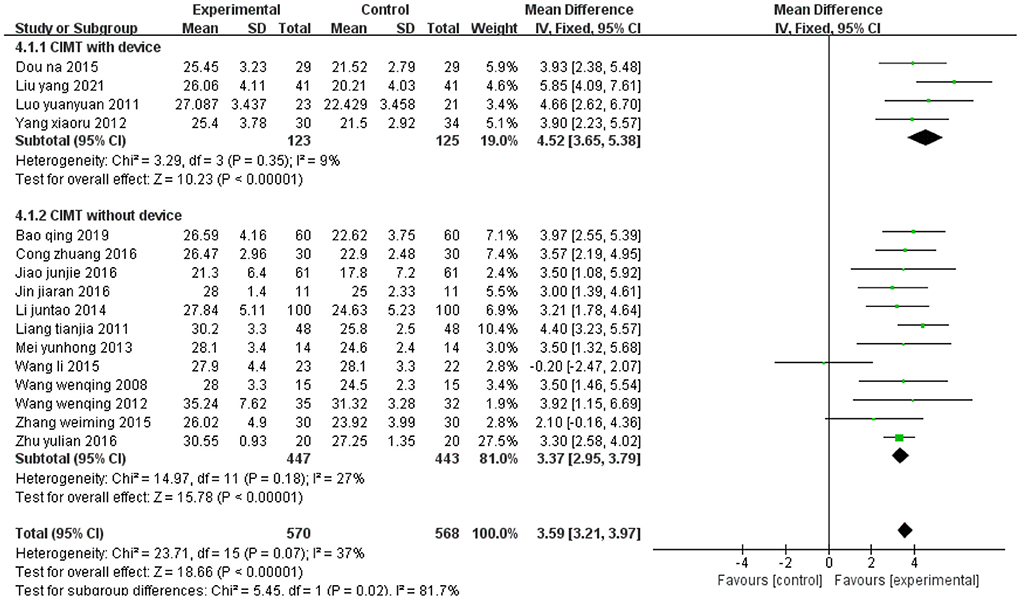
Figure 14. Forest plot. CIMT with device vs. CIMT without device on the Fugl-Meyer Assessment lower limb sub-scale.
Discussion
Although the effect of CIMT on post-stroke upper limb motor dysfunction has been widely studied, its effect on lower limbs remains incompletely understood. Fuzaro and co-workers (23) demonstrated that Forced Use Therapy and CIMT improve balance and gait, while lower extremity CIMT improves motor behavior patterns and scores on the functional scale. Silva and co-workers (26) observed that 2 weeks of treadmill gait training (with load to restrain the healthy lower limb), combined with home exercise, improved postural balance and functional flexibility in stroke patients. However, they concluded that the increased load in CIMT did not provide additional benefits for training. Auwal et al. (25) recently conducted a systematic evaluation and meta-analysis of CIMT in lower limbs, but their conclusion slightly differs from ours. They indicated that CIMT was superior to conventional treatment only for improving the quality of life. Although CIMT improved lower limb functions such as movement and balance, the advantage was insignificant compared with conventional treatment. However, their meta-analysis only included six RCTs published in English retrieved from five English language databases. We retrieved publications from four additional Chinese databases to avoid shortcomings due to insufficient sample size and ethnic limitations in individual studies. We conducted the present meta-analysis, which includes 2008 participants from 34 RCTs, to evaluate the effectiveness of CIMT in improving lower extremity motor function in post-stroke patients. We found that CIMT improved lower extremity motor function, balance function, walking speed, mobility, activities of daily living, and quality of daily life more substantially than conventional treatment. Moreover, participants who received CIMT had significantly higher FMA-L scores than those who received only conventional physiotherapy (MD1 = 3.46, 95% CI, 2.74–4.17, P1 < 0.01; MD2 = 3.83, 95% CI, 2.89–4.77, P2 < 0.01) and western medicine (MD3 = 3.50, 95% CI, 1.08–5.92, P3 < 0.01). These results suggest that CIMT is beneficial for patients with motor impairment of the lower limbs after stroke, and secondary indicators show the same trend.
In lower extremity CIMT, the constraint can be behavioral, physical, or both, and aims to improve walking ability and overcome general inactivity. The purpose of lower extremity CIMT is to improve the quality of leg use in the community, but the use of constraint devices remains controversial. For example, dos Anjos (63) noted that using a restraint device would induce yet another type of abnormal coordination pattern. For the legs, a physical restraint could be dangerous and unnecessary. Immobilizing the unimpaired leg (e.g., with knee splints or ankle weight bearing) may alter normal gait or increase inertia in the lower limbs (28, 29). Our results show that lower extremity CIMT with device seemed to yield higher FMA-L scores than lower extremity CIMT without device (with device: MD = 4.52, 95% CI 3.65 to 5.38, P < 0.01; with device: MD = 3.37, 95% CI 2.95 to 3.79, P < 0.01).
The studies included in this meta-analysis used different CIMT protocol and outcome measures, which may bias of CIMT efficacy assessment. A review (64) mentioned that differences in the types of restrictions, duration of restriction, intervention time, training intensity, and evaluation methods weaken the evidence for the clinical value of CIMT. First, included studies differed in CIMT intensity (ranging from 30 min to 6 h per day) and duration (ranging from 2 weeks to 3 months) because our inclusion criteria did not limit these parameters. One study (21) comparing the effectiveness of two different CIMT protocols pointed out that CIMT intensity can directly affect the patients' recovery time after a stroke. Therefore, it is necessary to develop a unified and effective CIMT protocol according to the different stages of stroke.
Neuroplasticity is typically achieved in response to about 300 highly repetitive tasks per day, typically completed in 1 h (65, 66). However, few of the included studies met that criterion. It is worth noting that, in most included studies, the experimental and control groups performed tasks with similar intensities and durations. Secondly, each study had a specific CIMT scheme. For example, some studies used splints or orthotics to force impaired limb use, some used elevated insoles to force a shift of the weight on the unimpaired lower limb, some used a cane providing auditory feedback to increase the weight on the impaired limb, and some used a 5% weight on the ankle joint. However, using an elevated insole in lower extremity CIMT to force a shift of the gravity center may alter the biomechanics of the lower extremity and impede functional recovery. Therefore, some reviews have opposed the use of shoe raises, advocating that patients should be actively encouraged to maximize the use of impaired limbs (25).
There are three limitations to this study. First, only 4 out of the 34 included studies were rated as “high-quality” through the Cochrane Bias Risk Assessment tool. Therefore, more high-quality studies are needed to provide more reliable evidence. Most researchers did not elaborate on their experimental process, preventing us from accurately judging biases on parameters such as random scheme, blinding method, and allocation hiding. Second, FMA-L, BBS, 10MWT, 6MWT, FAC, TUGT, MBI, and SSQOL, which are important lower extremity motor function indicators, showed pronounced interstudy heterogeneity. Fortunately, removing some studies did reduce heterogeneity. The meta-regression analysis did not identify sample size, publication time, or country as the sources of heterogeneity. As we outlined above, the inclusion criteria did not limit stroke type, duration, affected brain area, hemiplegic side, sample size, or the form, intensity, or duration of CIMT, potentially causing an overestimation or underestimation of CIMT efficacy on lower limb function. Third, to assess publication bias, we performed the Egger's test on more than 10 studies. The test revealed potential publication bias for BBS (P = 0.02) and 10MWT (P = 0.315).
Conclusion
This study suggests that CIMT improves lower extremity motor function more substantially than traditional physical therapy and western medicine. However, our results should be considered the considerable heterogeneity between studies in mind. Therefore, future studies with larger sample sizes and better quality are needed to demonstrate the benefits of CIMT on lower limb function after a stroke.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
MZ, TZ, and HL conceived the study. YT, HL, and TZ provided general guidance to draft the protocol. JW and YT designed the search strategy. MZ drafted the manuscript. MZ, TZ, HL, TY, JC, and PG reviewed and revised the manuscript. All the authors have read and approved the final version of the manuscript.
Funding
This research was funded by the Xinglin Scholar Research Promotion Project of Chengdu University of TCM (XSGG2019007).
Acknowledgments
Thanks to our team members for revising this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.1028206/full#supplementary-material
References
1. Pang MY, Harris JE, Eng JJ. A community-based upper-extremity group exercise program improves motor function and performance of functional activities in chronic stroke a randomized controlled trial. Arch Phys Med Rehabil. (2006) 87:1–9. doi: 10.1016/j.apmr.2005.08.113
2. Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. (1995) 26:982–9. doi: 10.1161/01.STR.26.6.982
3. Preetha K, Vimala U, Kamalakannan M. A study to compare task-based mirror therapy versus constraint induced movement therapy for hand function in hemiplegic subjects. Biomedicine (india). (2021) 41:665-668. doi: 10.51248/.v41i3.1204
4. Chiu HC, Ada L. Constraint-induced movement therapy improves upper limb activity and participation in hemiplegic cerebral palsy: a systematic review. J Physiother. (2016) 62:130–7. doi: 10.1016/j.jphys.2016.05.013
5. Hollerbach JM, Atkeson CG. Inferring limb coordination strategies from trajectory kinematics. J Neurosci Methods. (1987) 21:181–94. doi: 10.1016/0165-0270(87)90115-4
6. Yang JF, Gorassini M. Spinal and brain control of human walking implications for retraining of walking. Neuroscientist. (2006) 12:379–89. doi: 10.1177/1073858406292151
7. Schmidt RA. Motor schema theory after 27 years. reflections and implications for a new theory. Res Q Exerc Sport. (2003) 74:366–75. doi: 10.1080/02701367.2003.10609106
8. Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Recovery of walking function in stroke patients the Copenhagen stroke study. Arch Phys Med Rehabil. (1995) 76:27–32. doi: 10.1016/S0003-9993(95)80038-7
9. Patel AT, Duncan PW, Lai SM, Studenski S. The relation between impairments and functional outcomes poststroke. Arch Phys Med Rehabil. (2000) 81:1357–63. doi: 10.1053/apmr.2000.9397
10. Mayo NE, Wood-Dauphinee S, Côté R, Durcan L, Carlton J. Activity, participation, and quality of life 2002, 6 months poststroke. Arch Phys Med Rehabil. 83:1035–42. doi: 10.1053/apmr.2002.33984
11. Hendricks HT, van Limbeek J, Geurts AC, Zwarts MJ. Motor recovery after stroke a systematic review of the literature. Arch Phys Med Rehabil. (2002) 83:1629–37. doi: 10.1053/apmr.2002.35473
12. Myint JM, Yuen GF Yu TK, Kng CP, Wong AM, Chow KK, et al. A study of constraint-induced movement therapy in subacute stroke patients in Hong Kong. Clin Rehabil. (2008) 22:112–24. doi: 10.1177/0269215507080141
13. Taub E, Uswatte G. Constraint-induced movement therapy. bridging from the primate laboratory to the stroke rehabilitation laboratory. J Rehabil Med. (2003) 41:34–40. doi: 10.1080/16501960310010124
14. Numata K, Murayama T, Takasugi J, Oga M. Effect of modified constraint-induced movement therapy on lower extremity hemiplegia due to a higher-motor area lesion. Brain Inj. (2008) 22:898–904. doi: 10.1080/02699050802425436
15. Kallio K, Nilsson-Wikmar L, Thorsen AM. Modified constraint-induced therapy for the lower extremity in elderly persons with chronic stroke single-subject experimental design study. Top Stroke Rehabil. (2014) 21:111–9. doi: 10.1310/tsr2102-111
16. McCall M, McEwen S, Colantonio A, Streiner D, Dawson DR. Modified constraint-induced movement therapy for elderly clients with subacute stroke. Am J Occup Ther. (2011) 65:409–18. doi: 10.5014/ajot.2011.002063
17. dos Anjos Morris S, Taub DE. Constraint-induced movement therapy for lower extremity function: describing the LE-CIMT protocol. Physical Ther. (2020) 100:698–707. doi: 10.1093/ptj/pzz191
18. Mark VW, Taub E, Uswatte G, Bashir K, Cutter GR, Bryson CC, et al. Constraint-induced movement therapy for the lower extremities in multiple sclerosis. case series with 2013 4-year follow-up. Arch Phys Med Rehabil. (2013) 94:753–60. doi: 10.1016/j.apmr.2012.09.032
19. Taub E, Uswatte G, Pidikiti R. Constraint-Induced Movement Therapy a new family of techniques with broad application to physical rehabilitation–a clinical review. J Rehabil Res Dev. (1999) 36:237–51.
20. Bonnyaud C, Pradon D, Zory R, Bussel B, Bensmail D, Vuillerme N, Roche N. Effects of a gait training session combined with a mass on the non-paretic lower limb on locomotion of hemiparetic patients a randomized controlled clinical trial. Gait Posture. (2013) 37:627–30. doi: 10.1016/j.gaitpost.2012.09.010
21. Abdullahi A, Aliyu NU, Useh U, Abba MA, Akindele MO, Truijen S, Saeys W. Comparing two different modes of task practice during lower limb constraint-induced movement therapy in people with stroke a randomized clinical trial. Neural Plast. (2021) 2021:6664058. doi: 10.1155/2021/6664058
22. Billinger SA, Guo LX, Pohl PS, Kluding PM. Single limb exercise pilot study of physiological and functional responses to forced use of the hemiparetic lower extremity. Top Stroke Rehabil. (2010) 17:128–39. doi: 10.1310/tsr1702-128
23. Fuzaro AC, Guerreiro CT, Galetti FC, Jucá RB, Araujo JE. Modified constraint-induced movement therapy and modified forced-use therapy for stroke patients are both effective to promote balance and gait improvements. Rev Bras Fisioter. (2012) 16:157–65. doi: 10.1590/S1413-35552012005000010
24. Abdullahi A, Truijen S, Saeys W. Neurobiology of recovery of motor function after stroke the central nervous system biomarker effects of constraint-induced movement therapy. Neural Plast. (2020) 2020:9484298. doi: 10.1155/2020/9484298
25. Abdullahi A, Truijen S, Umar NA, Useh U, Egwuonwu VA, Van Criekinge T, Saeys W. Effects of lower limb constraint induced movement therapy in people with stroke a systematic review and meta-analysis. Front Neurol. (2021) 12:638904. doi: 10.3389/fneur.2021.638904
26. EMGS ES, Ribeiro TS TCC. da Silva, Costa MFP, Cavalcanti F, Lindquist ARR. Effects of constraint-induced movement therapy for lower limbs on measurements of functional mobility and postural balance in subjects with stroke. a randomized controlled trial. Topics Stroke Rehabilitat. (2017) 24:555–61. doi: 10.1080/10749357.2017.1366011
27. Uswatte G, Taub E, Morris D, Barman J, Crago J. Contribution of the shaping and restraint components of Constraint-Induced Movement therapy to treatment outcome. NeuroRehabilitation. (2006) 21:147–56. doi: 10.3233/NRE-2006-21206
28. Hsu CJ, Kim J, Roth EJ, Rymer WZ, Wu M. Forced use of the paretic leg induced by a constraint force applied to the nonparetic leg in individuals poststroke during walking. Neurorehabil Neural Repair. (2017) 31:1042–52. doi: 10.1177/1545968317740972
29. Coker P, Karakostas T, Dodds C, Hsiang S. Gait characteristics of children with hemiplegic cerebral palsy before and after modified constraint-induced movement therapy. Disabil Rehabil. (2010) 32:402–8. doi: 10.3109/09638280903171592
30. Aruin AS, Rao N, Sharma A, Chaudhuri G. Compelled body weight shift approach in rehabilitation of individuals with chronic stroke. Top Stroke Rehabil. (2012) 19:556–63. doi: 10.1310/tsr1906-556
31. Candan SA, Livanelioglu A. Effects of modified constraint-induced movement therapy for lower limb on motor function in stroke patients a randomized controlled study. Int J Physiotherapy. (2018) 4:277. doi: 10.15621/ijphy/2017/v4i5/159421
32. Candan SA, Livanelioglu A. Efficacy of modified constraint-induced movement therapy for lower extremity in patients with stroke. strength and quality of life outcomes. Turk J Physiother Rehabil. (2019) 30:23–32. doi: 10.21653/tfrd.406349
33. Cong Z, Zhao X, Dong F, Liu Y. Effect of forced exercise therapy on lower limb function and ADL in senile stroke patients health way. Health Way. (2016) 15:1. doi: 10.3969/j.issn.1671-8801.2016.06.001
34. Dou N, Li D, Ma S, Zhao Y, Chen C. Effect of modified constrained induced movement therapy on lower limb function of stroke patients with hemiplegia. Chin J Gerontol. (2015) 8. doi: 10.3969/j.issn.1005-9202.2015.08.017
35. Fang A. Effect of constrained induced movement therapy on rehabilitation of hemiplegia after stroke. J Clini Med. (2013) 10. doi: 10.3969/j.issn.1003-3548.2013.10.025
36. Guiyun J, Xiaolian Y, Wenqing W. The effects of constraint-induced movement therapy on the walking ability and balance function in stroke patients. Chin J Rehabil.Med. (2009) 8.
37. Jin J. The Effect of mCIMT on Walking Ability and EMG of Lower Limb in Stroke Patients. Shanghai: Shanghai University of Sport. (2016).
38. Junjie J, Hongliang G, Lijie L. Influence of constraint-induced movement therapy on the early neurologic function of hemiplegic patients after acute stroke. Chin General Pract. (2016) 19:1968–71. doi: 10.3969/j.issn.1007-9572.2016.16.026
39. Jung K, Kim Y, Cha Y, In TS, Hur YG, Chung Y. Effects of gait training with a cane and an augmented pressure sensor for enhancement of weight bearing over the affected lower limb in patients with stroke: a randomized controlled pilot study. Clini Rehabilitat. (2015) 29:135–42. doi: 10.1177/0269215514540923
40. Li J, Zhao X, Huo H, Cao L. Effect of constrained induced movement therapy on recovery of walking ability in stroke patients. Shandong Med. J. (2014) 54:30–1. doi: 10.3969/j.issn.1002-266X.2014.25.012
41. Li X. To Explore the Effect of Constrained Induced Movement Therapy in Rehabilitation of Hemiplegia After Stroke. Bipedal and Health Care Academic Edition. (2017).
42. Liang T, Long Y, Cao X. Modified constrained induced movement therapy for lower limb motor dysfunction after stroke. Chin J Rehabil Med. (2011) 26:5. doi: 10.3870/zgkf.2011.05.006
43. Liu Y, Yu K, Shi L, Li G. Effect of constrained induced movement therapy on rehabilitation of hemiplegia after stroke. World Latest Med Inf. (2021) 21:418–20.
44. Luo Y. The Investigation about Application of Constraint-Induced Movement Therapy in Rehabilitation Process of Lower Lamb Dysfunction after Stroke. Changchun: Jilin University. (2011).
45. Mei Y. Analysis on rehabilitation of lower limb motor function of stroke patients with constrained induced movement therapy. Road Health. (2013) 12:102. doi: 10.3969/j.issn.1671-8801.2013.04.097
46. Nie X. Effect analysis of constrained induced movement therapy in rehabilitation of hemiplegia after stroke. China Health Vision. (2019) 83−4. doi: 10.3969/j.issn.1005-0019.2019.03.101
47. Qing B, Yan L. Effects of constraint-induced movement therapy with family involvement on exercise capacity and quality of life in hemiplegia patients after stroke. Chin Med J. (2019) 10. doi: 10.14033/j.cnki.cfmr.2019.10.074
48. Wang L. Effect of modified constrained induced movement therapy on lower limb function recovery of stroke patients with hemiplegia. Chin J Integr Med. (2015) 24:11. doi: 10.13517/j.cnki.ccm.2015.11.012
49. Wang S. Effect of forced lower limb exercise on recovery of walking function in stroke patients. Chin J Rehabilitation Med. (2017) 8. doi: 10.3969/j.issn.1008-1879.2017.03.010
50. Wang W, Dai H, Xu L. Clinical analysis of constraint-induced movement therapy for motor function of lower-extremity in stroke patients. Chin J Integr Med. (2008) 10. doi: 10.3969/j.issn.1009-0126.2008.09.010
51. Wang W, Li X, Lu J. Influences of modified constraint-induced movement therapy on lower-extremity walking ability and blood flow of femoral artery among elderly patients with strok. Chin J Geriatr. (2012) 31. doi: 10.3760/cma.j.issn.0254-9026.2012.05.005
52. Wang W, Zhu Y, Liang S. Effects of modified constraint-induced movement therapy of the lower extremities on walking ability and activities of daily living in stroke patients with hemiplegia. Chin J Rehabilitation Med. (2020) 35:12. doi: 10.3969/j.issn.1001-1242.2020.12.008
53. Wei?ming Z, Shuai Y, Yi?jun W, Xin H, Jian?chun L, Qing X. Effect of modified constraint? induced movement therapy on the activities of daily living of patients with acute stroke. Chin.J Contemp Neurol Neurosurg. (2015) 4. doi: 10.3969/j.issn.1672-6731.2015.04.006
54. Wu Y, Wang X, Cai K, Wang T. Effect of constrained induced movement therapy on improving walking ability of hemiplegic patients. Chin J Rehabilitation Med. (2011) 26:766–7. doi: 10.3969/j.issn.1001-1242.2011.08.017
55. Xiaoru Y, Yuanyuan Y, Tong L. Clinical study on modified constraint-induced movement therapy on rehabilitation of motorfunction of lower-extremity in patients with stroke. J Hebei United University(Health Sciences). (2012) 14. doi: 10.19539/j.cnki.2095-2694.2012.06.008
56. Yin C, Liu X, Zeng M. Influence of CIMT on motor function and insulin resistance of stroke patients. Zhong Guo Kang Fu. (2013) 28. doi: 10.3870/zgkf.2013.02.007
57. Yu WH, Liu WY, Wong AM, Wang TC Li YC, Lien HY. Effect of forced use of the lower extremity on gait performance and mobility of post-acute stroke patients. J Phys Ther Sci. (2015) 27:421–5. doi: 10.1589/jpts.27.421
58. Zhang X. Effect analysis of constrained induced movement therapy on rehabilitation of hemiplegia after stroke. Diet Health. (2017) 4:35.
59. Zhou B. Effect of forced plastic training mode on comprehensive function of stroke patients with hemiplegia. Chin J Integr Med. 18:2.
60. Zhu Y. Effects of Modified Constraint-induced Movement Therapy on Walking Ability and Gait in Stroke Patients with Hemiplegia. Shanghai: Shanghai University of Sport. (2016).
61. Zhu Y, Zhou C, Liu Y, Liu J, Jin J, Zhang S, et al. Effects of modified constraint-induced movement therapy on the lower extremities in patients with stroke a pilot study. Disabil Rehabil. (2016) 38:1893–9. doi: 10.3109/09638288.2015.1107775
62. Zhu Z, Chen L, Zhang Q. Effect of constrained induced movement therapy on rehabilitation of hemiplegia after stroke. Shandong Medical J. (2010) 50.
63. Dos Anjos Morris SM, Taub DE. Constraint-induced movement therapy for improving motor function of the paretic lower extremity after stroke. Am J Phys Med Rehabil. (2020) 99:e75–e78. doi: 10.1097/PHM.0000000000001249
64. Wang D, Xiang J, He Y, Yuan M, Dong L, Ye Z, Mao W. The mechanism and clinical application of constraint-induced movement therapy in stroke rehabilitation. Front Behav Neurosci. (2022) 16:828599. doi: 10.3389/fnbeh.2022.828599
65. Chen HM, Hsieh CL, Sing Kai L, Liaw LJ, Chen SM, Lin JH. The test-retest reliability of 2007 2 mobility performance tests in patients with chronic stroke. Neurorehabil Neural Repair: 21:347–52. doi: 10.1177/1545968306297864
Keywords: constraint-induced movement therapy, post-stroke, lower extremity, motor dysfunction, meta-analysis
Citation: Zhou M, Tu Y, Cui J, Gao P, Yi T, Wang J, Hao Q, Li H and Zhu T (2022) Effect of constraint-induced movement therapy on lower extremity motor dysfunction in post-stroke patients: A systematic review and meta-analysis. Front. Neurol. 13:1028206. doi: 10.3389/fneur.2022.1028206
Received: 26 August 2022; Accepted: 01 November 2022;
Published: 21 November 2022.
Edited by:
Luigi Tesio, University of Milan, ItalyReviewed by:
Edward Taub, University of Alabama at Birmingham, United StatesAntonio Caronni, Italian Auxological Institute (IRCCS), Italy
Copyright © 2022 Zhou, Tu, Cui, Gao, Yi, Wang, Hao, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, dHRsaWh1aUAxNjMuY29t; Tianmin Zhu, dGlhbm1pbnpodUBjZHV0Y20uZWR1LmNu
 Mingze Zhou
Mingze Zhou Yang Tu1
Yang Tu1 Jiarui Cui
Jiarui Cui Ping Gao
Ping Gao Ting Yi
Ting Yi Tianmin Zhu
Tianmin Zhu