
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 16 December 2022
Sec. Neuroinfectious Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.1027615
Objectives: This study aimed to compare and rank the therapeutic effects of antivirals in treating hearing loss using a network meta-analysis approach.
Methods: We searched the PubMed, Embase, and Cochrane Library databases to identify eligible randomized controlled trials (RCTs) through April 2022. Placebo-controlled or head-to-head RCTs of three categories of antivirals for hearing loss were included, and pooled relative risks (RRs) with 95% confidence interval (CI) were calculated using pairwise and network meta-analyses.
Results: Six RCTs with 405 patients were included in the final analysis. The results showed that ganciclovir had relatively better effects on the incidence of hearing recovery (surface under the cumulative ranking: 88.8%) compared with other antivirals. However, pairwise comparison analyses found that the use of antivirals significantly increased the incidence of hearing recovery compared with the use of a placebo (RR: 1.27; 95% CI: 1.04–1.54; P = 0.017), while no significant difference was observed between any two categories of antivirals. Finally, the use of antivirals did not increase the risk of adverse events compared with the use of a placebo (RR: 1.27; 95% CI: 0.82–1.98; P = 0.285).
Conclusion: Antivirals are more efficacious than placebos for hearing recovery in patients with hearing loss, and ganciclovir is the most likely to increase the incidence of hearing recovery.
Hearing loss is characterized by the deterioration of hearing acuity and is associated with an elevated hearing threshold. Studies have demonstrated that hearing loss is significantly related to physical (1), cognitive (2), and psychosocial outcomes (3) and accounts for higher medical expenses (4). The World Health Organization indicates that more than 5% of individuals are affected by hearing loss, especially patients older than 65 years (5). The common risk factors for hearing loss include viral infections, microcirculatory disorders, autoimmune disorders, and labyrinthine hemorrhage (6–9). The disease spectrum of hearing loss, including sensorineural hearing loss (SNHL) and noise-induced hearing loss, could be preventable and treatable (9–11).
Nowadays, the use of antivirals has not been recommended for improving hearing loss in patients with isolated SNHL or asymptomatic congenital cytomegalovirus (CMV) disease (12, 13). A systematic review performed by De Cuyper et al. (14) identified 18 studies that revealed that the use of (val) ganciclovir could improve hearing loss and deterioration in children with symptomatic congenital CMV disease. A Cochrane review identified four randomized controlled trials (RCTs) with a low risk of bias and found that the use of antivirals had no significant effect on hearing improvement in idiopathic sudden SNHL (15). These studies focused on qualitative analyses, and the therapeutic effects of antivirals were neither compared nor ranked on the basis of direct and indirect evidence. Therefore, the current systematic review and network meta-analysis were conducted to update and expand previous systematic reviews to inform clinical practice by comparing different types of antivirals for managing hearing loss.
This systematic review and network meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines (16). Placebo-controlled or head-to-head RCTs of the three categories of antivirals for treating hearing loss were included in the analysis. The PubMed, Embase, and Cochrane Library databases were searched for trials that met the inclusion criteria through April 2022, and the publication language and status were not restricted. The following search terms were used as text words or medical subject heading terms: (“antiviral agent” OR “acyclovir” OR “valacyclovir” OR “ganciclovir”) AND (“cytomegalovirus infection” OR “hearing loss”). Additional trials that were completed but not published were searched on the ClinicalTrials.gov website (NIH, USA). We also manually reviewed reference lists of relevant reviews and articles to identify additional eligible trials.
The literature search and study selection were independently conducted by two authors, and conflicts between the authors were resolved through mutual discussion. Studies were included if they met the following criteria: (1) patients: all of patients diagnosed sudden sensorineural hearing loss or congenital CMV disease and were not restricted by age (17); (2) intervention: acyclovir, valacyclovir, or ganciclovir; (3) control: placebo or no treatment; (4) outcome: audiologic outcomes and adverse events; and (5) study design: had to have an RCT design.
The following items from each trial were abstracted by two authors: first author's name, publication year, region, sample size, mean age, proportion of males, inclusion criteria, intervention, control, treatment duration, and reported outcomes. Subsequently, these two authors assessed the methodological quality of the included trials using a risk of bias approach according to the methods described by the Cochrane Collaboration, which includes seven specified domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and other (18). Inconsistent results regarding data extraction and quality assessment between authors were settled by an additional author referring to the original article.
Therapeutic effects of antivirals were assigned as continuous and dichotomous data, and pooled RRs with 95% CIs were calculated as effect estimates. Indirect and mixed comparisons in network meta-analysis were analyzed to compare different antivirals (19), and the loop-specific approach was applied to assess differences between direct and indirect estimates for a specific comparison in the loop (20). The design-by-treatment interaction inconsistency model was used to assess the assumption of consistency in the entire network (19). In this study, an inconsistent model was applied because of the underlying heterogeneity across the included trials. To rank various antivirals for the investigated outcome, the surface under the cumulative ranking (SUCRA) probabilities were used (21). Publication bias was assessed using comparison-adjusted funnel plots (22). Subsequently, pairwise comparison analyses were performed using the random-effects model (23, 24). I2 and Q statistics were used to assess heterogeneity across the included trials, and significant heterogeneity was defined as I2 > 50.0% or P < 0.10 (25). The inspection level for the pooled results was two sided, and P values < 0.05 were considered statistically significant. Statistical analyses were performed using Software STATA (version 10.0; Stata Corporation, College Station, TX, USA).
A total of 1,543 articles were identified from the initial electronic search, and 1,079 articles were retained after duplicate topics were removed. Subsequently, 1,013 articles were excluded because they investigated irrelevant topics. The remaining 66 studies were retrieved for further evaluation, and 60 studies were excluded because of other interventions (n = 31), not being RCTs (n = 18), and having insufficient data (n = 11). Reviewing the reference lists of relevant studies yielded two potentially eligible trials, and these two trials were obtained through electronic searches. Finally, six RCTs were selected for the final quantitative analysis (Figure 1) (26–31).
The baseline characteristics of the included trials and recruited patients are presented in Table 1. In the six included trials, the sample size ranged from 42 to 96, and treatment duration ranged from 7.0 days to 6.0 weeks. Four trials included adult patients, whereas the remaining two trials included children. Three trials investigated the therapeutic effects of acyclovir, two trials assessed the therapeutic effects of valacyclovir, and the remaining trial evaluated the therapeutic effects of ganciclovir. Table 2 presents the methodological quality of the included trials; four of the included trials had a low risk of bias, while the remaining two trials had a relatively high risk of bias.
The network of eligible comparisons of hearing recovery is shown in Figure 2. The nodes were weighted based on the number of trials assessed for each treatment and the edges were weighted based on the precision of the direct estimate for each pairwise comparison. The treatment effects of the antivirals were ranked using SUCRA probabilities. We noted that ganciclovir had relatively better treatment effects on the incidence of hearing recovery (88.8%; Figure 3). The results of pairwise comparisons of the study agents are provided in Figure 4 and Table 3. There were no significant differences among the three antivirals in terms of the incidence of hearing recovery. The review of the funnel plot revealed no evidence of publication bias for the effect of antivirals on the incidence of hearing recovery (Figure 5).
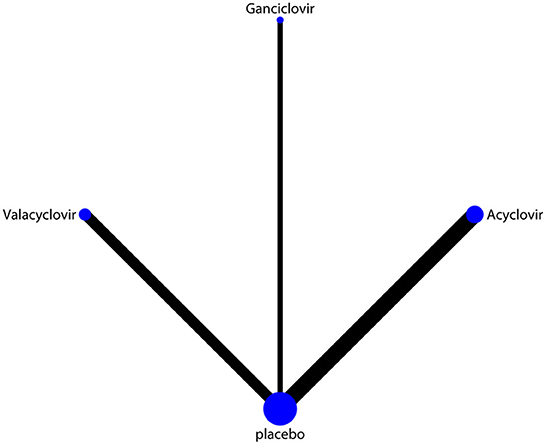
Figure 2. Network of comparisons for hearing recovery included in the analysis. The nodes were weighted by the number of trials, and the edges were weighted by the precision of the direct estimate for each pairwise comparison.
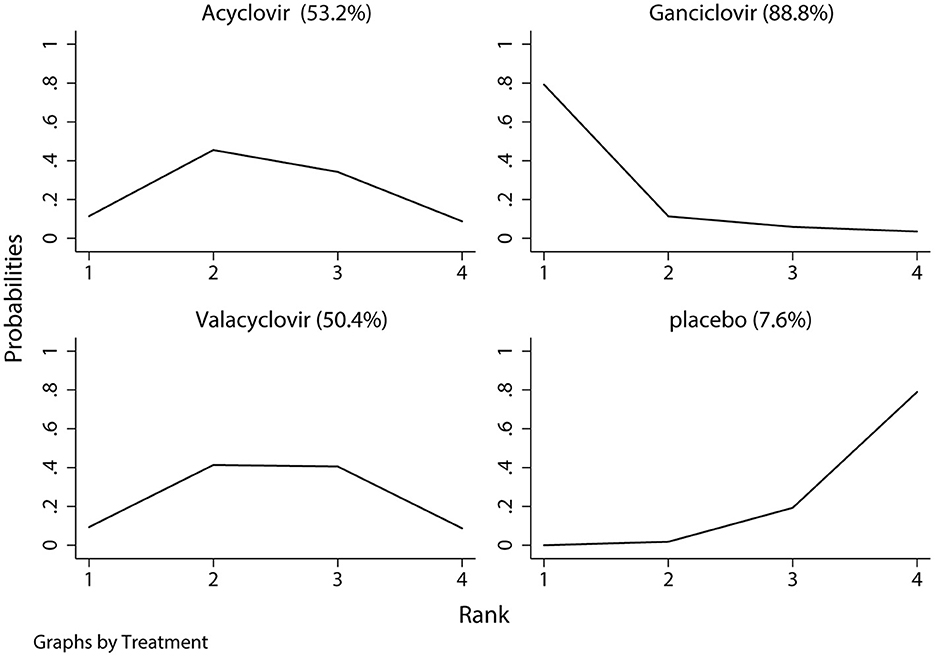
Figure 3. The surface under the cumulative ranking test for hearing recovery. Large area indicated better treatment effect on hearing recovery.
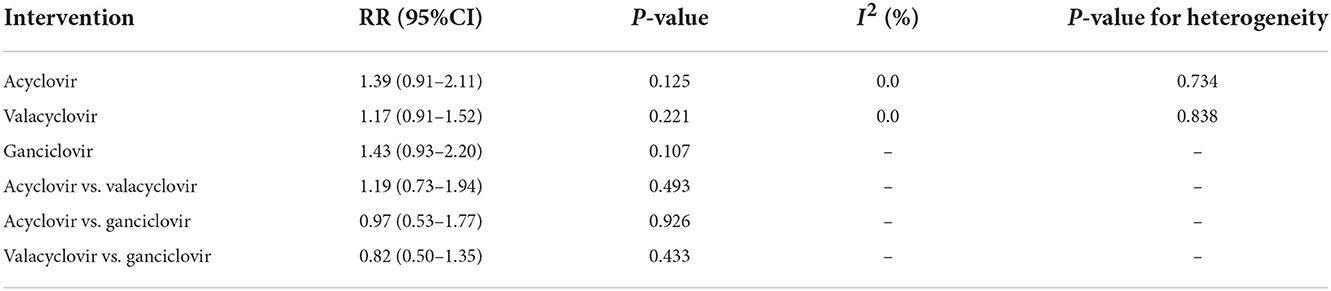
Table 3. Indirect comparison the therapeutic effects of antivirals on the incidence of hearing recovery.
After pooling all included trials, we noted that the use of antivirals significantly increased the incidence of hearing recovery compared with the use of a placebo (RR: 1.27; 95% CI: 1.04–1.54; P = 0.017; Figure 6), and no evidence of heterogeneity was observed (I2 = 0.0%; P = 0.919). Moreover, subgroup analyses found that acyclovir (RR: 1.39; 95% CI: 0.91–2.11; P = 0.125), valacyclovir (RR: 1.17; 95% CI: 0.91–1.52; P = 0.221), and ganciclovir (RR: 1.43; 95% CI: 0.93–2.20; P = 0.107) were not associated with the incidence of hearing recovery compared with the placebo.
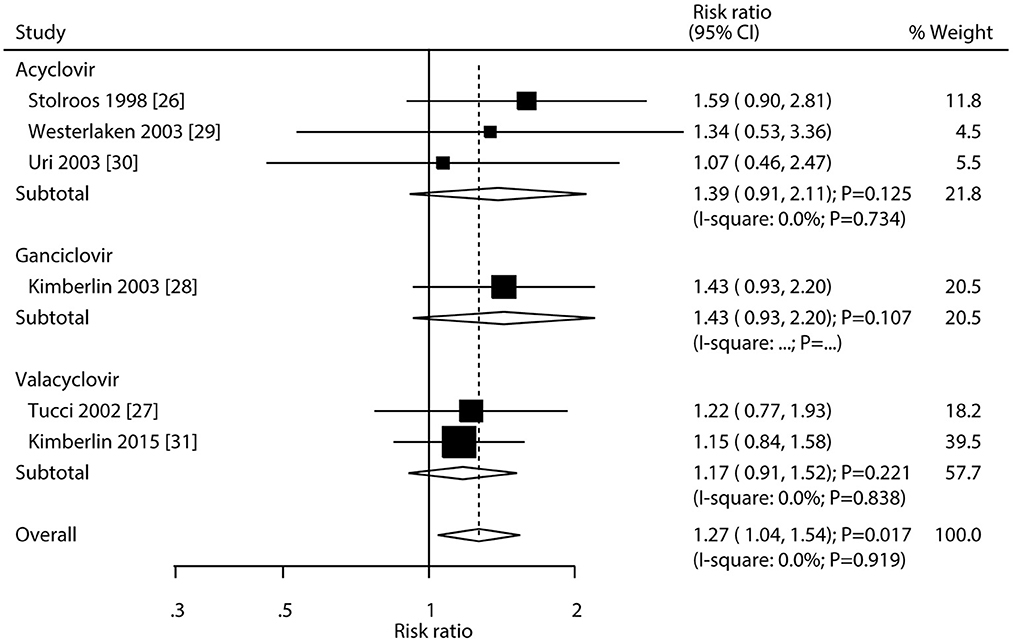
Figure 6. Forest plot of the meta-analysis of the incidence of hearing recovery between antivirals and placebo.
The summary results for adverse events are presented in Table 4. There were no significant differences in the risk of total adverse events between the antivirals and placebo (RR: 1.27; 95% CI: 0.82–1.98; P = 0.285). Moreover, the use of antivirals did not affect the risk of headache (RR: 2.86; 95% CI: 0.32–25.40; P = 0.345), nausea (RR: 0.95; 95% CI: 0.06–14.30; P = 0.973), stomach pain (RR: 0.32; 95% CI: 0.01–7.42; P = 0.476), elevated blood glucose (RR: 0.32; 95% CI: 0.01–7.42; P = 0.476), increased creatinine (RR: 2.87; 95% CI: 0.12–68.47; P = 0.515), increased total bilirubin (RR: 1.43; 95% CI: 0.61–3.31; P = 0.410), thrombopenia (RR: 1.37; 95% CI: 0.24–7.77; P = 0.725), neutropenia (RR: 1.57; 95% CI: 0.43–5.76; P = 0.493), and death (RR: 0.37; 95% CI: 0.10–1.37; P = 0.136).
To our knowledge, no previous systematic review and network meta-analysis has compared and ranked the efficacy and safety of antivirals in the treatment of hearing loss. This comprehensive, quantitative, systematic review and network meta-analysis was based on six RCTs involving 405 patients presenting with hearing loss who were randomly assigned to treatment with antivirals (acyclovir, valacyclovir, and ganciclovir) or placebos. Hearing recovery and any adverse events following treatment were investigated. The study results show that ganciclovir had relatively better treatment effects on hearing recovery. However, pairwise comparison analyses indicated that the incidence of hearing recovery was not statistically significant when comparing any two types of antivirals. A traditional meta-analysis found that the use of antivirals significantly increased the incidence of hearing recovery compared with the use of a placebo. Finally, the use of antivirals did not increase the risk of adverse events compared with the use of a placebo in patients with hearing loss.
Three studies reported the therapeutic effect of acyclovir on the incidence of hearing recovery (26, 29, 30). All three trials included adult patients with idiopathic sudden SNHL and reported an increased incidence of hearing recovery when using acyclovir. Moreover, the use of valacyclovir did not yield a benefit on hearing in adult patients with idiopathic sudden SNHL (27). The potential reason for the treatment effects of antiviral drugs in adults can be explained by the main cause of idiopathic sudden SNHL, including circulatory disturbance, membrane rupture, and viral loads, and might be an important factor for the role of antiviral drugs in the incidence of hearing recovery (29). Moreover, vestibular involvement, severity of initial hearing loss, audiogram shape, dose of antivirals, and interval between the onset of hearing loss and beginning of treatment could affect the prognosis of idiopathic sudden SNHL (32, 33).
Two of the included trials reported the treatment effects of antiviral drugs on hearing recovery in newborns (28, 31). Kimberlin et al. (28) found that ganciclovir therapy during the neonatal period could prevent hearing deterioration. Moreover, Kimberlin et al. (31) indicated that valacyclovir could improve long-term hearing and developmental outcomes in newborns. This network-analysis indicated that ganciclovir was associated with relatively better treatment effects on hearing recovery, while pairwise comparison analyses did not find significant differences among the three antiviral drugs. This result could be explained by only one trial addressing the treatment effect of ganciclovir on the incidence of hearing recovery, and the incidence of hearing recovery was lower with a smaller number of included trials, which caused the power to be insufficient to detect potentially significant differences.
A traditional meta-analysis found that the use of antivirals significantly increased the incidence of hearing recovery compared with the use of a placebo. Moreover, although the incidence of hearing recovery in patients treated with ganciclovir was superior to that in patients treated with acyclovir and valacyclovir, the differences were not statistically significant. However, the result should be cautiously recommended because of a relatively high risk of bias in the methodological quality of the trial performed by Kimberlin et al. (28). Finally, we noted that antivirals were associated with a higher risk of headache, increased creatinine and total bilirubin levels, thrombocytopenia, and neutropenia, while the risks of nausea, stomach pain, elevated blood glucose levels, and death were lower in patients treated with antivirals. However, the difference in the risk of any specific adverse events between antivirals and placebo was not statistically significant. These results could be explained as follows: (1) steroids were used as a background therapy, and most adverse events were related to the use of steroids (34) and (2) the incidence of adverse events was lower, and most adverse events were reported in only one trial, thus a broad 95% CI was always obtained.
This study had several limitations. First, the severity of hearing loss are differing among included trials, while stratified data according to severity of hearing loss were not available. Second, the background therapies are varies across the included trials, which could affect the treatment effectiveness of antivirals. Third, the main cause of hearing loss are differing between adult and pediatric patients (7), whereas stratified analyses were not performed owing to smaller number of included trials. Fourth, the cause of sudden sensorineural hearing loss across included studies were not available, and the treatment effectiveness of antivirals could affect; Fifth, the treatment duration, dose of antivirals (26, 29, 30), and viral load differed among the included trials, and further stratified analyses should be performed. Finally, inherent limitations of meta-analysis based on published articles, including inevitable publication bias and restricted detailed analysis.
We found that the use of antivirals significantly increased the incidence of hearing recovery in patients with hearing loss, and ganciclovir had relatively better effects on hearing recovery. Moreover, no significant differences were observed between any two types of antivirals, and the use of antivirals was well tolerated and did not increase the risk of adverse events compared with the use of a placebo. A large-scale RCT in the future should be conducted to directly compare the therapeutic effects of antivirals in the treatment of hearing loss.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Conception and design and administrative support: L-LX. Provision of study materials or patients, collection and assembly of data, data analysis and interpretation, and manuscript writing: L-ML. Final approval of manuscript: L-ML and L-LX. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
RCTs, randomized controlled trials; SNHL, sensorineural hearing loss; SUCRA, surface under the cumulative ranking.
1. Deal J, Richey Sharrett A, Bandeen-Roche K, Kritchevsky S, Pompeii L, Gwen Windham B, et al. Hearing impairment and physical function and falls in the atherosclerosis risk in communities hearing pilot study. J Am Geriatr Soc. (2016) 64:906–8. doi: 10.1111/jgs.14075
2. Deal JA, Sharrett AR, Albert MS, Coresh J, Mosley TH, Knopman D, et al. Hearing impairment and cognitive decline: a pilot study conducted within the atherosclerosis risk in communities neurocognitive study. Am J Epidemiol. (2015) 181:680–90. doi: 10.1093/aje/kwu333
3. Dalton DS, Cruickshanks KJ, Klein BE, Klein R, Wiley TL, Nondahl DM. The impact of hearing loss on quality of life in older adults. Gerontologist. (2003) 43:661–8. doi: 10.1093/geront/43.5.661
4. Reed NS, Altan A, Deal JA, Yeh C, Kravetz AD, Wallhagen M, et al. Trends in health care costs and utilization associated with untreated hearing loss over 10 years. JAMA Otolaryngol Head Neck Surg. (2019) 145:27–34. doi: 10.1001/jamaoto.2018.2875
5. WHO Global Estimates on Prevalence of Hearing Loss. Geneva: World Health Organization. (2012). Available online at: https://www.who.int/pbd/deafness/WHO_GE_HL.pdf (accessed August 10, 2022).
6. Lee HS, Lee YJ, Kang BS, Lee BD, Lee JS. A clinical analysis of sudden sensorineural hearing loss cases. Korean J Audiol. (2014) 18:69–75. doi: 10.7874/kja.2014.18.2.69
7. Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. (2014) 18:2331216514541361. doi: 10.1177/2331216514541361
8. Salomone R, Abu TAA, Chaves AG, Bocalini MCC, de Oliveira Vicente A, Riskalla PE. Sudden hearing loss caused by labyrinthine hemorrhage. Braz J Otorhinolaryngol. (2008) 74:776–9. doi: 10.1016/S1808-8694(15)31390-2
9. Kuhn M, Heman-Ackah SE, Shaikh JA, Roehm PC. Sudden sensorineural hearing loss: a review of diagnosis, treatment, and prognosis. Trends Amplif. (2011) 15:91–105. doi: 10.1177/1084713811408349
10. Conlin AE, Parnes LS. Treatment of sudden sensorineural hearing loss: I. A systematic review. Arch Otolaryngol Head Neck Surg. (2007) 133:573–81. doi: 10.1001/archotol.133.6.573
11. Daniel E. Noise and hearing loss: a review. J Sch Health. (2007) 77:225–31. doi: 10.1111/j.1746-1561.2007.00197.x
12. Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. (2017) 17:e177–88. doi: 10.1016/S1473-3099(17)30143-3
13. Luck SE, Wieringa JW, Blázquez-Gamero D, Henneke P, Schuster K, Butler K, et al. Congenital cytomegalovirus: a European expert consensus statement on diagnosis and management. Pediatr Infect Dis J. (2017) 36:1205–13. doi: 10.1097/INF.0000000000001763
14. De Cuyper E, Acke F, Keymeulen A, Dhooge I. The effect of (val)ganciclovir on hearing in congenital cytomegalovirus: a systematic review. Laryngoscope. (2022) 132:2241–50. doi: 10.1002/lary.30027
15. Awad Z, Huins C, Pothier DD. Antivirals for idiopathic sudden sensorineural hearing loss. Cochrane Database Syst Rev. (2012) 8:CD006987. doi: 10.1002/14651858.CD006987.pub2
16. Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. (2013) 8:e83138. doi: 10.1371/journal.pone.0083138
17. Li LY, Wang S, Wu C, Tsai C, Wu T, Lin Y. Screening for hearing impairment in older adults by smartphone-based audiometry, self-perception, HHIE screening questionnaire, and free-field voice test: comparative evaluation of the screening accuracy with standard pure-tone audiometry. JMIR Mhealth Uhealth. (2020) 8:e17213. doi: 10.2196/17213
18. Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Chichester, England: Cochrane Collaboration (2011).
19. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: model estimation using multivariate meta-regression. Res Synth Methods. (2012) 3:111–25. doi: 10.1002/jrsm.1045
20. Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. (1997) 50:683–91. doi: 10.1016/S0895-4356(97)00049-8
21. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
22. Trinquart L, Chatellier G, Ravaud P. Adjustment for reporting bias in network meta-analysis of antidepressant trials. BMC Med Res Methodol. (2012) 12:150. doi: 10.1186/1471-2288-12-150
23. DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. (2015) 45:139–45. doi: 10.1016/j.cct.2015.09.002
24. Ades AE, Lu G, Higgins JP. The interpretation of random-effects meta-analysis in decision models. Med Decis Making. (2005) 25:646–54. doi: 10.1177/0272989X05282643
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Stokroos RJ, Albers FW, Tenvergert EM. Antiviral treatment of idiopathic sudden sensorineural hearing loss: a prospective, randomized, double-blind clinical trial. Acta Otolaryngol. (1998) 118:488–95. doi: 10.1080/00016489850154603
27. Tucci DL, Farmer JC Jr, Kitch RD, Witsell DL. Treatment of sudden sensorineural hearing loss with systemic steroids and valacyclovir. Otol Neurotol. (2002) 23:301–8. doi: 10.1097/00129492-200205000-00012
28. Kimberlin DW, Lin CY, Sánchez PJ, Demmler GJ, Dankner W, Shelton M, et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: a randomized, controlled trial. J Pediatr. (2003) 143:16–25. doi: 10.1016/S0022-3476(03)00192-6
29. Westerlaken BO, Stokroos RJ, Dhooge IJ, Wit HP, Albers FW. Treatment of idiopathic sudden sensorineural hearing loss with antiviral therapy: a prospective, randomized, double-blind clinical trial. Ann Otol Rhinol Laryngol. (2003) 112:993–1000. doi: 10.1177/000348940311201113
30. Uri N, Doweck I, Cohen-Kerem R, Greenberg E. Acyclovir in the treatment of idiopathic sudden sensorineural hearing loss. Otolaryngol Head Neck Surg. (2003) 128:544–9. doi: 10.1016/S0194-59980300004-4
31. Kimberlin DW, Jester PM, Sánchez PJ, Ahmed A, Arav-Boger R, Michaels MG, et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N Engl J Med. (2015) 372:933–43. doi: 10.1056/NEJMoa1404599
32. Byl FM Jr. Sudden hearing loss: eight years' experience and suggested prognostic table. Laryngoscope. (1984) 94:647–61. doi: 10.1288/00005537-198405000-00014
33. Wood MJ, Johnson RW, McKendrick MW, Taylor J, Mandal BK, Crooks J. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster. N Engl J Med. (1994) 330:896–900. doi: 10.1056/NEJM199403313301304
Keywords: antivirals, hearing loss, systematic review, network meta-analysis, effectiveness
Citation: Liu L-M and Xia L-L (2022) Efficacy and safety of antivirals in treating hearing loss: A systematic review and network meta-analysis. Front. Neurol. 13:1027615. doi: 10.3389/fneur.2022.1027615
Received: 25 August 2022; Accepted: 29 November 2022;
Published: 16 December 2022.
Edited by:
Agnieszka J. Szczepek, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Mostafa Meshref, Al-Azhar University, EgyptCopyright © 2022 Liu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Li Xia, MzgxNzkxMDM0QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.